Abstract
The cytochrome P450 protein plays an important role in the synthesis of cholesterol and other lipid parameters. But little is known about the association of the single nucleotide polymorphism (SNP) of rs2068888 near cytochrome P450 26A1 gene (CYP26A1) and serum lipid profiles in the Chinese Maonan and Han populations. This study explored such association in the two populations. Genotyping of the CYP26A1 rs2068888 SNP was performed in 833 unrelated individuals of Maonan and 701 participants of Han by polymerase chain reaction and restriction fragment length polymorphism combined with gel electrophoresis, and confirmed by direct sequencing. The genotypic and allelic frequencies of the CYP26A1 rs2068888 SNP were significantly different between the Maonan and Han. The frequencies of AA, AG and GG genotypes were 75.51%, 23.41% and 1.08% in the Maonan population, and 64.62%, 33.10% and 2.28% in the Han population (P < 0.001). The frequency of the G allele was 12.78% in Maonan and 18.83% in Han (P < 0.001). The level of serum total cholesterol (TC) was lower in Maonan than in Han. The G allele carriers had higher serum TC level in Maonan than the G allele non-carriers. Subgroup analyses indicated that the G allele carriers had higher serum TC level in Maonan females. Serum lipid parameters in the two ethnic groups were also associated with several environmental factors. These findings revealed that there may be a racial/ethnic- and/or sex-specific association between the CYP26A1 rs2068888 SNP and serum lipid parameters in some populations.
Keywords: Lipids, cytochrome P450 26A1 gene, single nucleotide polymorphism, environmental factors
Introduction
Recent studies have found that cardiovascular disease (CVD) is the leading cause of disability among adults and becomes a huge burden in terms of disability, functional decline, and healthcare costs [1,2]. Great progresses have been made in the treatment of CVD during these decades, but the mortality and morbidity are still high. We are also unable to predict these kinds of health problems effectively [2,3]. It is widely acknowledged that dyslipidemia is one of the major risk factors for CVD from its prediction to development [4]. Dyslipidemia highly relates to the risk of coronary artery disease (CAD), which involves in elevated serum levels of total cholesterol (TC) [5], triglyceride (TG) [6], low-density lipoprotein cholesterol (LDL-C) [7], and apolipoprotein (Apo) B [8], combined with decreased levels of high-density lipoprotein cholesterol (HDL-C) [5], ApoA1 and the ApoA1/ApoB ratio [9]. It is recognized that abnormal serum lipid levels are concerned with genetic and multiple environmental factors and their interactions [10]. Twins and family studies demonstrated that there is a significant genetic inheritance component to CVD risk factors [11-13]. Therefore, the present study was to detect the association of the CYP26A1 rs2068888 SNP and several environmental factors with serum lipid parameters in the Chinese Maonan and Han populations.
Materials and methods
Subjects
A total of 833 unrelated participants (335 males, 40.22% and 498 females, 59.78%) of Maonan nationality and 701 unrelated subjects (270 males, 38.52% and 431 females, 61.48%) of Han nationality were randomly selected from our previous stratified randomized samples [14]. The participants were all agricultural workers from Huanjiang Maonan Autonomous County, Guangxi Zhuang Autonomous Region, People’s Republic of China. These participants’ age ranged from 22 to 92 years with the mean age of 57.16±15.07 years in Maonan and 57.36±13.96 years in Han, respectively. The age distribution and gender ratio were matched between the two ethnic groups. All participants were essentially healthy with no evidence of CVD such as CAD, stroke, diabetes, hyper- or hypo-thyroids, and chronic renal disease. They were free from any treatment which would affect serum lipid levels. This study design was approved by the Ethics Committee of the First Affiliated Hospital, Guangxi Medical University (No. Lunshen-2014-KY-Guoji-001, Mar. 7, 2014). Informed consent was obtained from all participants before study.
Epidemiological survey
The epidemiological survey was carried out using internationally standardized methods, following a common protocol [15]. Information on demographics, socioeconomic status, and lifestyle factors was collected with standardized questionnaires. The intake of alcohol was quantified as the number of liang (about 50 g) of rice wine, corn wine, rum, beer, or liquor consumed during the preceding 12 months. Alcohol consumption was categorized into groups of grams of alcohol per day: 0 (non-drinker), ≤ 25 and > 25. Smoking status was categorized into groups of cigarettes per day: 0 (non-smoker), ≤ 20 and > 20. Several parameters such as height, weight, blood pressure, and waist circumference were measured. Body mass index (BMI) was calculated as weight/height2 (kg/m2).
Biochemical measurements
A fasting venous blood sample of 5 ml was drawn from the participants. A part of the sample (2 mL) was collected into glass tubes and used to determine serum lipid levels. Another part of the sample (3 mL) was transferred to tubes with anticoagulants (4.80 g/L citric acid, 14.70 g/L glucose and 13.20 g/L tri-sodium citrate) and used to extract deoxyribonucleic acid (DNA). Measurements of serum TC, TG, HDL-C, and LDL-C levels in the samples were performed by enzymatic methods with commercially available kits (RANDOX Laboratories Ltd., Ardmore, Diamond Road, and Crumlin Co. Antrim, United Kingdom, BT29 4QY; Daiichi Pure Chemicals Co., Ltd., Tokyo, Japan). Serum ApoA1 and ApoB levels were detected by the immunoturbidimetric immunoassay using a commercial kit (RANDOX Laboratories Ltd.). All determinations were performed with an auto-analyzer (Type 7170A; Hitachi Ltd., Tokyo, Japan) in the Clinical Science Experiment Center of the First Affiliated Hospital, Guangxi Medical University [16].
DNA amplification and genotyping
Genomic DNA of the samples was isolated from peripheral blood leucocytes according to the phenol-chloroform method [17]. The extracted DNA was stored at 4°C until analysis. Genotyping of the CYP26A1 rs2068888 SNP was performed by polymerase chain reaction and restriction fragment length polymorphism (PCR-RFLP). PCR amplification was performed using 5’-CAGAGGAAAGGCAGTCTGGA-3’ as the forward and 5’-ACCCCTGGATTATGTCTGGC-3’ as reversed primer pair (Sangon, Shanghai, People’s Republic of China). Each amplification reaction was performed in a total volume of 25.0 μL, including 12.5 μL of 2 × Taq PCR MasterMix (constituent: 0.1 U Taq polymerase/μL, 500.0 μM dNTP each and PCR buffer), DNase/RNase-free water (ddH2O) 8.5 μL, 1.0 μL each primer (10 pmol/L) and 2.0 μL genomic DNA, processing started with 5 min of pre-denaturing at 95°C and followed by 45 s of denaturing at 95°C, 30 s of annealing at 58°C and 45 s of elongation at 72°C for 32 cycles. The amplification was completed by a final extension at 72°C for 7 min. Following electrophoresis on a 2.0% agarose gel with 0.5 μg/mL ethidium bromide, the amplification products were visualized under ultraviolet light. The restriction enzyme reaction consisted of 5.0 μL amplified DNA and 0.5 U of BcgI restriction enzyme together with 0.25 μL NEBuffer 3.1, 0.125 μL SAM [New England Biolabs (Beijing) Ltd.] and 0.9375 μL ddH2O. Each reaction system was digested at 37°C for 2.0 h. After restriction enzyme digestion of the amplified DNA, genotypes were identified by electrophoresis on 2% ethidium bromide stained agarose gels and visualized under ultraviolet light. Nine samples (each genotype in three; respectively) detected by the PCR-RFLP were also confirmed by direct sequencing. The DNA sequences were analyzed using an ABI Prism 3100 (Applied Biosystems) in Shanghai Sangon Biological Engineering Technology & Services Co., Ltd., People’s Republic of China.
Diagnostic criteria
The normal values of serum TC, TG, HDL-C, LDL-C, ApoA1, ApoB levels and the ApoA1/ApoB ratio in our Clinical Science Experiment Center were 3.10-5.17, 0.56-1.70, 1.16-1.42, 2.70-3.10 mmol/L, 1.20-1.60, 0.80-1.05 g/L and 1.00-2.50, respectively. The individuals with TC > 5.17 mmol/L and/or TG > 1.70 mmol/L were defined as hyperlipidemic [17,18]. Hypertension was diagnosed according to the 1999 and 2003 criteria of the World Health Organization-International Society of Hypertension Guidelines for the management of hypertension [19]. The diagnostic criteria of overweight and obesity were according to the Cooperative Meta-Analysis Group of China Obesity Task Force. Normal weight, overweight and obesity were defined as a BMI < 24, 24-28 and > 28 kg/m2, respectively [20].
Statistical analyses
The statistical analyses were performed with the statistical software package SPSS 21.0 (SPSS Inc., Chicago, Illinois). The quantitative variables were presented as mean ± standard deviation (serum TG levels were presented as medians and interquartile ranges). Allele frequency was determined via direct counting, and the Hardy-Weinberg equilibrium was verified with the standard goodness-of-fit test. The genotype distribution between the two groups was analyzed by the chi-square test. General characteristics between two ethnic groups were compared by the Student’s unpaired t-test. The association between genotypes and serum lipid parameters was tested by covariance analysis (ANCOVA) with gender, age, BMI, blood pressure, alcohol consumption and cigarette smoking as covariates. Multivariable linear regression analyses with stepwise modeling were used to determine the correlation between the genotypes (AA = 1, AG/GG = 2) and several environmental factors with serum lipid levels in males and females of Maonan and Han populations. Two-sided P value < 0.05 was considered statistically significant.
Results
General characteristics and serum lipid profiles
The general characteristics and serum lipid levels between the Maonan and Han populations are summarized in Table 1. The percentages of cigarette smoking, the levels of waist circumference, systolic blood pressure, diastolic blood pressure, pulse pressure, serum TG, ApoA1, and the ApoA1/ApoB ratio were higher in Maonan than in Han (P < 0.05-0.001), whereas the levels of serum TC and HDL-C were lower in Maonan than in Han (P < 0.001). There was no significant difference in the gender ratio, age structure, body height, weight, BMI, the percentage of alcohol consumption, blood glucose, LDL-C, and ApoB levels between the two ethnic groups (P > 0.05 for all).
Table 1.
Comparison of demographic, lifestyle characteristics and serum lipid levels between the Maonan and Han populations
| Parameter | Maonan | Han | t (χ2) | P |
|---|---|---|---|---|
| Number | 833 | 701 | ||
| Gender (Male/female) | 335/498 | 270/431 | 0.460 | 0.497 |
| Age (years) | 57.16±15.07 | 57.36±13.96 | 0.244 | 0.621 |
| Height (cm) | 153.80±8.07 | 154.43±8.02 | -1.500 | 0.134 |
| Weight (kg) | 53.23±10.67 | 53.62±9.41 | -0.770 | 0.441 |
| Body mass index (kg/m2) | 22.39±3.60 | 22.45±3.33 | -0.349 | 0.727 |
| Waist circumference (cm) | 76.79±9.22 | 75.72±8.43 | 2.364 | 0.018 |
| Cigarette smoking [n (%)] | ||||
| Non-smoker | 651 (78.2) | 545 (77.7) | ||
| ≤ 20 cigarettes per day | 79 (9.5) | 140 (20.0) | 79.219 | 0.000 |
| > 20 cigarettes per day | 103 (12.3) | 16 (2.3) | ||
| Alcohol consumption [n (%)] | ||||
| Non-drinker | 660 (79.2) | 570 (81.3) | ||
| ≤ 25 g per day | 113 (13.6) | 75 (10.7) | 2.883 | 0.237 |
| > 25 g per day | 60 (7.2) | 54 (8.0) | ||
| Systolic blood pressure (mmHg) | 136.00±24.33 | 129.89±19.59 | 5.416 | 0.000 |
| Diastolic blood pressure (mmHg) | 82.93±12.23 | 81.35±11.27 | 2.617 | 0.009 |
| Pulse pressure (mmHg) | 53.07±18.41 | 48.47±15.47 | 5.289 | 0.000 |
| Glucose (mmol/L) | 6.13±1.34 | 6.20±1.47 | -0.945 | 0.345 |
| Total cholesterol (mmol/L) | 4.97±1.05 | 5.01±1.07 | -2.892 | 0.003 |
| Triglyceride (mmol/L) | 1.30 (0.89) | 1.13 (0.76) | 4.585 | 0.000 |
| HDL-C (mmol/L) | 1.60±0.41 | 1.76±0.53 | -6.326 | 0.000 |
| LDL-C (mmol/L) | 2.86±0.83 | 2.86±0.77 | 0.013 | 0.990 |
| Apolipoprotein (Apo) A1 (g/L) | 1.38±0.31 | 1.34±0.25 | 2.870 | 0.004 |
| ApoB (g/L) | 0.88±0.20 | 0.90±0.24 | -1.535 | 0.125 |
| ApoA1/ApoB | 1.66±0.56 | 1.59±0.51 | 2.324 | 0.020 |
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol. The value of triglyceride was presented as median (interquartile range); the difference between the two ethnic groups was determined by the Wilcoxon-Mann-Whitney test.
Results of electrophoresis and genotyping
After the genomic DNA of the samples was amplified using PCR and visualized with 2% agarose gel electrophoresis, the products of 606 bp nucleotide sequences were observed in all samples (Figure 1). The genotypes identified were termed according to the presence (G allele) or absence (A allele) of the enzyme restriction sites. Thus, the AA genotype is homozygous for the absence of the site (bands at 606 bp), the AG genotype is heterozygous for the presence and absence of the site (bands at 606-, 354- and 252-bp) and the GG genotype is homozygous for the presence of the site (bands at 354 bp and 252 bp; Figure 2). The AA, AG and GG genotypes detected by PCR-RFLP were also confirmed by direct sequencing (Figure 3), respectively.
Figure 1.

Electrophoresis of polymerase chain reaction products of the samples. Lane M is the 100-600 bp marker ladder; Lanes 1-6 are samples, the 606 bp bands are the target genes.
Figure 2.
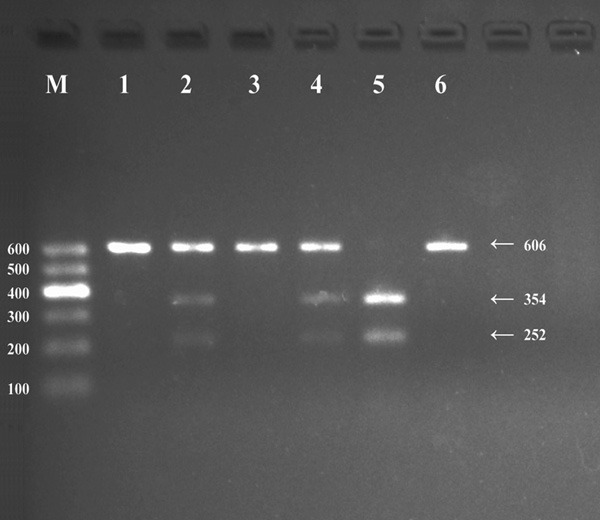
Genotyping of the CYP26A1 rs2068888 SNP. Lane M, 100-600 bp marker ladder; lanes 1, 3 and 6, AA genotype (606 bp); lanes 5, GG genotype (354 and 252 bp); lanes 2 and 4, AG genotype (606, 354 and 252 bp).
Figure 3.
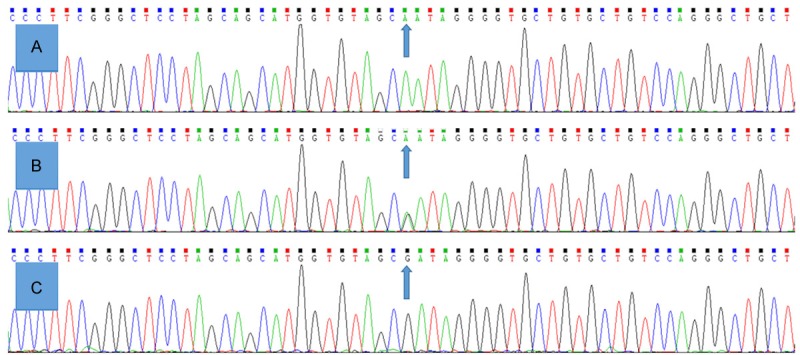
A part of the nucleotide sequence of the CYP26A1 rs2068888 SNP. A: AA genotype; B: AG genotype; C: GG genotype.
Genotypic and allelic frequencies
The genotypic and allelic frequencies of the CYP26A1 rs2068888 SNP are shown in Table 2. The genotype distribution of the Maonan and Han populations was consistent with the Hardy-Weinberg equilibrium (HWE, P > 0.05) by the chi-square test of the goodness of fit. The frequencies of A and G alleles were 87.22% and 12.78% in Maonan, and 81.17% and 18.83% in Han populations (P < 0.001), respectively. The frequencies of AA, AG, and GG genotypes were 75.51%, 23.41% and 1.08% in the Maonan population, and 64.62%, 33.10% and 2.28% in the Han population (P < 0.001), respectively. No difference in the genotypic and allelic frequencies was found between males and females in the two ethnic groups (P > 0.05 for all).
Table 2.
Comparison of the genotype and allele frequencies of the CYP26A1 rs2068888 SNP in the Maonan and Han populations [n (%)]
| Group | n | Genotype | Allele | P HWE | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| AA | AG | GG | A | G | |||
| Mannan | 833 | 629 (75.51) | 195 (23.41) | 9 (1.08) | 1453 (87.22) | 213 (12.78) | 0.331 |
| Han | 701 | 453 (64.62) | 232 (33.10) | 16 (2.28) | 1138 (81.17) | 264 (18.83) | 0.342 |
| χ2 | 22.603 | 21.189 | |||||
| P | 0.000 | 0.000 | |||||
| Mannan | |||||||
| Male | 335 | 251 (74.93) | 81 (24.18) | 3 (0.90) | 583 (87.01) | 87 (12.99) | 0.329 |
| Female | 498 | 378 (75.90) | 114 (22.89) | 6 (1.20) | 870 (87.35) | 126 (12.65) | 0.334 |
| χ2 | 0.345 | 0.040 | |||||
| P | 0.837 | 0.841 | |||||
| Han | |||||||
| Male | 270 | 175 (64.81) | 88 (32.59) | 7 (2.59) | 438 (81.11) | 102 (18.89) | 0.346 |
| Female | 431 | 278 (64.50) | 144 (33.41) | 9 (2.09) | 700 (81.21) | 162 (18.79) | 0.340 |
| χ2 | 0.221 | 0.002 | |||||
| P | 0.895 | 0.965 | |||||
HWE, the Hardy-Weinberg equilibrium. The genotype distribution between the two groups was analyzed by the chi-square test. The Hardy-Weinberg equilibrium was analyzed by the chi-square test of the goodness of fit.
Genotypes and serum lipid levels
Tables 3 and 4 showed the association between genotypes and serum lipid levels. Serum TC level was different between the AA and AG/GG genotypes (P < 0.05) in Maonan but not in Han, the G allele carriers had higher serum TC level than the G allele non-carriers. Subgroup analyses showed that the G allele carriers had higher serum TC level in Maonan females but not in males (P < 0.05). No significant difference in the remaining lipid parameters was found between the genotypes in the both populations or in the males and females of the two ethnic groups.
Table 3.
Comparison of the genotypes and serum lipid levels in the Maonan and Han populations
| Group/Genotype | n | TC (mmol/L) | TG (mmol/L) | HDL-C (mmol/L) | LDL-C (mmol/L) | ApoA1 (g/L) | ApoB (g/L) | ApoA1/ApoB |
|---|---|---|---|---|---|---|---|---|
| Maonan | ||||||||
| AA | 629 | 4.84±0.94 | 1.31 (0.83) | 1.61±0.41 | 2.89±0.87 | 1.39±0.33 | 0.89±0.20 | 1.66±0.59 |
| AG/GG | 204 | 5.06±1.10 | 1.29 (0.93) | 1.57±0.41 | 2.83±0.72 | 1.35±0.23 | 0.87±0.18 | 1.63±0.48 |
| F | 6.256 | -0.708 | 0.763 | 0.653 | 1.152 | 0.388 | 0.341 | |
| P | 0.013 | 0.479 | 0.383 | 0.419 | 0.283 | 0.533 | 0.559 | |
| Han | ||||||||
| AA | 453 | 4.99±1.01 | 1.14 (0.78) | 1.78±0.54 | 2.88±0.72 | 1.35±0.23 | 0.89±0.22 | 1.61±0.48 |
| AG/GG | 248 | 4.94±1.14 | 1.11 (0.77) | 1.73±0.48 | 2.83±0.86 | 1.32±0.29 | 0.90±0.27 | 1.57±0.53 |
| F | 0.405 | -0.440 | 1.280 | 0.186 | 1.785 | 0.583 | 1.117 | |
| P | 0.525 | 0.660 | 0.258 | 0.667 | 0.182 | 0.446 | 0.291 |
TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; ApoA1/ApoB, the ratio of apolipoprotein A1 to apolipoprotein B. The value of TG was presented as median (interquartile range), the difference between the genotypes was determined by the Wilcoxon-Mann-Whitney test.
Table 4.
Comparison of the genotypes and serum lipid levels between males and females in the Maonan and Han populations
| Ethnic/Genotype | n | TC (mmol/L) | TG (mmol/L) | HDL-C (mmol/L) | LDL-C (mmol/L) | ApoA1 (g/L) | ApoB (g/L) | ApoA1/ApoB |
|---|---|---|---|---|---|---|---|---|
| Maonan/Male | ||||||||
| AA | 251 | 5.00±1.00 | 1.37 (1.10) | 1.54±0.43 | 2.79±0.88 | 1.39±0.45 | 0.89±0.20 | 1.66±0.75 |
| AG/GG | 84 | 4.88±0.83 | 1.33 (1.00) | 1.53±0.46 | 2.81±0.75 | 1.33±0.24 | 0.89±0.18 | 1.59±0.50 |
| F | 0.971 | -1.428 | 0.043 | -0.156 | 0.884 | 0.195 | 0.836 | |
| P | 0.323 | 0.153 | 0.965 | 0.877 | 0.377 | 0.845 | 0.404 | |
| Maonan/Female | ||||||||
| AA | 378 | 4.81±1.01 | 1.25 (0.80) | 1.64±0.39 | 2.94±0.85 | 1.39±0.22 | 0.88±0.20 | 1.66±0.45 |
| AG/GG | 120 | 5.10±1.16 | 1.25 (0.90) | 1.59±0.38 | 2.83±0.70 | 1.37±0.21 | 0.87±0.18 | 1.67±0.45 |
| F | -2.368 | -0.197 | 1.172 | 1.156 | 0.592 | 0.670 | -0.136 | |
| P | 0.018 | 0.844 | 0.242 | 0.248 | 0.554 | 0.503 | 0.892 | |
| Han/Male | ||||||||
| AA | 175 | 5.04±1.10 | 1.20 (0.82) | 1.70±0.48 | 2.88±0.73 | 1.34±0.25 | 0.93±0.22 | 1.52±0.48 |
| AG/GG | 95 | 5.06±1.03 | 1.13 (1.02) | 1.67±0.53 | 2.91±0.84 | 1.34±0.30 | 0.95±0.24 | 1.51±0.53 |
| F | -0.141 | -0.074 | 0.370 | -0.358 | 0.088 | -0.427 | 0.218 | |
| P | 0.888 | 0.941 | 0.712 | 0.721 | 0.930 | 0.670 | 0.828 | |
| Han/Female | ||||||||
| AA | 278 | 4.95±0.94 | 1.10 (0.76) | 1.82±0.58 | 2.87±0.72 | 1.36±0.22 | 0.87±0.22 | 1.66±0.50 |
| AG/GG | 153 | 4.87±1.20 | 1.10 (0.70) | 1.76±0.48 | 2.81±0.86 | 1.31±0.28 | 0.89±0.28 | 1.59±0.51 |
| F | 0.875 | -0.424 | 1.220 | 0.795 | 1.685 | -0.755 | 1.273 | |
| P | 0.382 | 0.672 | 0.223 | 0.427 | 0.093 | 0.451 | 0.204 |
TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; ApoA1/ApoB, the ratio of apolipoprotein A1 to apolipoprotein B. The value of triglyceride was presented as median (interquartile range); the difference among the genotypes was determined by the Wilcoxon-Mann-Whitney test.
Relative factors for serum lipid parameters
Multiple linear regression analysis showed that serum TC level in Maonan and ApoB levels in Han were correlated with the genotypes of the CYP26A1 rs2068888 SNP (P < 0.05 for each; Table 5). When the correlation of serum lipid parameters and the genotypes was analyzed according to two genders, we found that serum TC level in Maonan female was correlated with the genotypes (P < 0.05; Table 6). Serum lipid parameters were also associated with age, gender, BMI, waist circumference, systolic and diastolic blood pressure, pulse pressure, fasting blood glucose, cigarette smoking and alcohol consumption in both ethnic groups or in males and females (P < 0.05-0.001; Tables 5 and 6).
Table 5.
Relationship between serum lipid parameters and relative factors in the Maonan and Han populations
| Lipid | Risk factor | B | Std. error | Beta | t | P |
|---|---|---|---|---|---|---|
| Maonan and Han | ||||||
| TC | Age | 0.009 | 0.002 | 0.122 | 3.774 | 0.000 |
| Waist circumference | 0.021 | 0.005 | 0.174 | 3.902 | 0.000 | |
| Genotype | -0.066 | 0.032 | -0.055 | -2.075 | 0.038 | |
| TG | Alcohol consumption | 0.332 | 0.087 | 0.119 | 3.826 | 0.000 |
| Cigarette smoking | 0.275 | 0.117 | 0.077 | 2.357 | 0.019 | |
| Waist circumference | 0.043 | 0.008 | 0.223 | 5.144 | 0.000 | |
| Glucose | 0.101 | 0.032 | 0.083 | 3.133 | 0.002 | |
| Height | -0.067 | 0.026 | -0.320 | -2.553 | 0.011 | |
| Weight | 0.091 | 0.036 | 0.538 | 2.544 | 0.011 | |
| Body mass index | -0.194 | 0.078 | -0.394 | -2.485 | 0.013 | |
| Diastolic blood pressure | 0.008 | 0.004 | 0.056 | 2.069 | 0.039 | |
| HDL-C | Waist circumference | -0.010 | 0.002 | -0.188 | -4.364 | 0.000 |
| Alcohol consumption | 0.123 | 0.023 | 0.162 | 5.234 | 0.000 | |
| Gender | 0.148 | 0.038 | 0.155 | 3.937 | 0.000 | |
| Age | 0.003 | 0.001 | 0.104 | 3.337 | 0.001 | |
| Pulse pressure | -0.002 | 0.001 | 0.005 | 0.177 | 0.860 | |
| Ethnic group | 0.165 | 0.024 | 0.176 | 6.760 | 0.000 | |
| LDL-C | Alcohol consumption | -0.099 | 0.042 | -0.076 | -2.369 | 0.018 |
| Age | 0.007 | 0.002 | 0.137 | 4.229 | 0.000 | |
| Waist circumference | 0.017 | 0.004 | 0.184 | 4.131 | 0.000 | |
| ApoA1 | Gender | 0.098 | 0.024 | 0.166 | 4.123 | 0.000 |
| Alcohol consumption | 0.105 | 0.015 | 0.225 | 7.072 | 0.000 | |
| Cigarette smoking | 0.074 | 0.020 | 0.124 | 3.717 | 0.000 | |
| Ethnic group | -0.039 | 0.015 | -0.067 | -2.497 | 0.013 | |
| ApoB | Waist circumference | 0.007 | 0.001 | 0.283 | 6.672 | 0.000 |
| Age | 0.002 | 0.000 | 0.128 | 4.159 | 0.000 | |
| Glucose | 0.012 | 0.004 | 0.081 | 3.102 | 0.002 | |
| Ethnic group | 0.029 | 0.011 | 0.066 | 2.574 | 0.010 | |
| ApoA1/ApoB | Waist circumference | -0.016 | 0.003 | -0.256 | -6.029 | 0.000 |
| Cigarette smoking | 0.112 | 0.036 | 0.100 | 3.126 | 0.002 | |
| Ethnic group | -0.082 | 0.028 | -0.076 | -2.958 | 0.003 | |
| Glucose | -0.026 | 0.010 | -0.069 | -2.653 | 0.008 | |
| Age | -0.003 | 0.001 | -0.079 | -2.557 | 0.011 | |
| Alcohol consumption | 0.137 | 0.027 | 0.157 | 5.153 | 0.000 | |
| Gender | 0.158 | 0.042 | 0.143 | 3.708 | 0.000 | |
| Maonan | ||||||
| TC | Gender | 0.247 | 0.114 | 0.112 | 2.171 | 0.030 |
| Age | 0.011 | 0.003 | 0.148 | 3.364 | 0.001 | |
| Height | -0.053 | 0.023 | -0.399 | -2.272 | 0.023 | |
| Weight | 0.073 | 0.032 | 0.723 | 2.290 | 0.022 | |
| Body mass index | -0.152 | 0.069 | -0.507 | -2.206 | 0.028 | |
| Genotypes | 0.015 | 0.007 | 0.128 | 2.183 | 0.029 | |
| TG | Alcohol consumption | 0.009 | 0.002 | 0.178 | 4.339 | 0.000 |
| Height | -0.126 | 0.034 | -0.613 | -3.650 | 0.000 | |
| Weight | 0.185 | 0.047 | 1.178 | 3.909 | 0.000 | |
| Body mass index | -0.359 | 0.102 | -0.770 | -3.507 | 0.000 | |
| Waist circumference | 0.030 | 0.010 | 0.165 | 2.955 | 0.003 | |
| HDL-C | Gender | 0.151 | 0.041 | 0.181 | 3.713 | 0.000 |
| Age | 0.002 | 0.001 | 0.082 | 1.972 | 0.049 | |
| Alcohol consumption | 0.003 | 0.000 | 0.245 | 6.023 | 0.000 | |
| Waist circumference | -0.012 | 0.002 | -0.264 | -4.772 | 0.000 | |
| Pulse pressure | -0.002 | 0.001 | -0.098 | -2.526 | 0.012 | |
| LDL-C | Age | 0.008 | 0.002 | 0.145 | 3.375 | 0.001 |
| Alcohol consumption | -0.004 | 0.001 | -0.184 | -4.367 | 0.000 | |
| Waist circumference | 0.017 | 0.005 | 0.189 | 3.304 | 0.001 | |
| ApoA1 | Gender | 0.090 | 0.032 | 0.141 | 2.774 | 0.006 |
| Alcohol consumption | 0.002 | 0.000 | 0.203 | 4.779 | 0.000 | |
| Waist circumference | -0.006 | 0.002 | -0.178 | -3.072 | 0.002 | |
| ApoB | Age | 0.002 | 0.001 | 0.146 | 3.485 | 0.001 |
| Waist circumference | 0.007 | 0.001 | 0.300 | 5.380 | 0.000 | |
| ApoA1/ApoB | Alcohol consumption | 0.003 | 0.001 | 0.207 | 5.106 | 0.000 |
| Waist circumference | -0.020 | 0.003 | -0.327 | -5.901 | 0.000 | |
| Han | ||||||
| TC | Alcohol consumption | 0.167 | 0.084 | 0.094 | 1.980 | 0.048 |
| Glucose | 0.067 | 0.029 | 0.098 | 2.324 | 0.020 | |
| Waist circumference | 0.034 | 0.009 | 0.267 | 3.740 | 0.000 | |
| TG | Cigarette smoking | 0.594 | 0.176 | 0.163 | 3.379 | 0.001 |
| Age | -0.012 | 0.005 | -0.109 | -2.308 | 0.021 | |
| Diastolic blood pressure | 0.017 | 0.015 | 0.334 | 4.762 | 0.000 | |
| Glucose | 0.142 | 0.047 | 0.121 | 3.012 | 0.003 | |
| Waist circumference | 0.070 | 0.015 | 0.334 | 4.762 | 0.000 | |
| HDL-C | Age | 0.004 | 0.002 | 0.128 | 2.642 | 0.008 |
| Alcohol consumption | 0.103 | 0.042 | 0.119 | 2.481 | 0.013 | |
| Weight | -0.036 | 0.016 | -0.650 | -2.157 | 0.031 | |
| LDL-C | Age | 0.006 | 0.002 | 0.117 | 2.404 | 0.017 |
| Waist circumference | 0.016 | 0.007 | 0.177 | 2.453 | 0.014 | |
| ApoA1 | Cigarette smoking | 0.078 | 0.026 | 0.146 | 3.022 | 0.003 |
| Gender | 0.075 | 0.031 | 0.144 | 2.427 | 0.015 | |
| Waist circumference | 0.005 | 0.002 | 0.169 | 2.403 | 0.017 | |
| Weight | -0.019 | 0.008 | -0.716 | -2.434 | 0.015 | |
| Alcohol consumption | 0.078 | 0.026 | 0.146 | 3.022 | 0.003 | |
| ApoB | Age | 0.001 | 0.001 | 0.093 | 2.072 | 0.039 |
| Waist circumference | 0.007 | 0.002 | 0.264 | 3.945 | 0.000 | |
| Diastolic blood pressure | 0.002 | 0.001 | 0.087 | 2.261 | 0.024 | |
| Glucose | 0.026 | 0.006 | 0.168 | 4.364 | 0.000 | |
| Genotype | 0.018 | 0.009 | 0.073 | 2.006 | 0.045 | |
| ApoA1/ApoB | Gender | 0.177 | 0.058 | 0.173 | 3.044 | 0.002 |
| Cigarette smoking | 0.104 | 0.048 | 0.100 | 2.163 | 0.031 | |
| Diastolic blood pressure | -0.003 | 0.002 | -0.077 | -1.986 | 0.047 | |
| Glucose | -0.046 | 0.013 | -0.138 | -3.560 | 0.000 | |
| Alcohol consumption | 0.117 | 0.038 | 0.140 | 3.106 | 0.002 |
TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; ApoA1/ApoB, the ratio of apolipoprotein A1 to apolipoprotein B; B, unstandardized coefficient; Beta, standardized coefficient.
Table 6.
Relationship between serum lipid parameters and relative factors in the males and females of the Han and Maonan populations
| Lipid | Risk factor | B | Std. error | Beta | t | P |
|---|---|---|---|---|---|---|
| Maonan/Male | ||||||
| TC | Glucose | 0.100 | 0.043 | 0.131 | 2.300 | 0.022 |
| TG | Alcohol consumption | 0.009 | 0.003 | 0.180 | 3.055 | 0.002 |
| HDL-C | Alcohol consumption | 0.003 | 0.001 | 0.332 | 5.882 | 0.000 |
| Waist circumference | -0.021 | 0.004 | -0.457 | -4.746 | 0.000 | |
| LDL-C | Age | 0.008 | 0.004 | 0.151 | 2.141 | 0.033 |
| Alcohol consumption | -0.005 | 0.001 | -0.289 | -4.771 | 0.000 | |
| ApoA1 | Alcohol consumption | 0.002 | 0.001 | 0.222 | 3.655 | 0.000 |
| Waist circumference | -0.015 | 0.005 | -0.351 | -3.375 | 0.001 | |
| ApoB | Age | 0.002 | 0.001 | 0.153 | 2.258 | 0.025 |
| Alcohol consumption | -0.001 | 0.000 | -0.126 | -2.165 | 0.031 | |
| Glucose | 0.017 | 0.008 | 0.114 | 2.083 | 0.038 | |
| ApoA1/ApoB | Alcohol consumption | 0.004 | 0.001 | 0.244 | 4.197 | 0.000 |
| Waist circumference | -0.024 | 0.007 | -0.321 | -3.216 | 0.001 | |
| Maonan/Female | ||||||
| TC | Age | 0.012 | 0.004 | 0.158 | 2.844 | 0.005 |
| Genotypes | 0.023 | 0.009 | 0.166 | 2.499 | 0.013 | |
| TG | Waist circumference | 0.027 | 0.006 | 0.283 | 4.449 | 0.000 |
| Systolic blood pressure | 0.005 | 0.002 | 0.149 | 2.231 | 0.026 | |
| HDL-C | Age | 0.003 | 0.001 | 0.122 | 2.233 | 0.026 |
| Waist circumference | -0.007 | 0.003 | -0.155 | -2.371 | 0.018 | |
| Systolic blood pressure | -0.003 | 0.001 | -0.185 | -2.704 | 0.007 | |
| LDL-C | Age | 0.009 | 0.003 | 0.159 | 2.915 | 0.004 |
| Alcohol consumption | 0.012 | 0.006 | 0.092 | 2.011 | 0.045 | |
| Waist circumference | 0.023 | 0.006 | 0.238 | 3.662 | 0.000 | |
| ApoA1 | Systolic blood pressure | -0.002 | 0.001 | -0.169 | -2.406 | 0.017 |
| ApoB | Age | 0.002 | 0.001 | 0.151 | 2.881 | 0.004 |
| Waist circumference | 0.008 | 0.002 | 0.349 | 5.560 | 0.000 | |
| Systolic blood pressure | 0.001 | 0.001 | 0.170 | 2.582 | 0.010 | |
| ApoA1/ApoB | Waist circumference | -0.019 | 0.003 | -0.350 | -5.566 | 0.000 |
| Systolic blood pressure | -0.004 | 0.001 | -0.211 | -3.214 | 0.001 | |
| Han/Male | ||||||
| TC | Waist circumference | 0.035 | 0.015 | 0.280 | 2.354 | 0.019 |
| Diastolic blood pressure | 0.014 | 0.006 | 0.156 | 2.329 | 0.021 | |
| TG | Waist circumference | 0.126 | 0.035 | 0.421 | 3.621 | 0.000 |
| Cigarette smoking | 0.635 | 0.268 | 0.155 | 2.365 | 0.019 | |
| Diastolic blood pressure | 0.028 | 0.014 | 0.131 | 2.007 | 0.046 | |
| HDL-C | Age | 0.004 | 0.002 | 0.153 | 1.994 | 0.047 |
| Weight | -0.045 | 0.019 | -0.916 | -2.367 | 0.019 | |
| Alcohol consumption | 0.091 | 0.039 | 0.158 | 2.361 | 0.019 | |
| ApoA1 | Alcohol consumption | 0.114 | 0.022 | 0.328 | 5.169 | 0.000 |
| Cigarette smoking | 0.075 | 0.028 | 0.166 | 2.678 | 0.008 | |
| Weight | -0.024 | 0.011 | -0.807 | -2.196 | 0.029 | |
| ApoB | Glucose | 0.026 | 0.009 | 0.192 | 2.971 | 0.003 |
| ApoA1/ApoB | Alcohol consumption | 0.115 | 0.040 | 0.182 | 2.865 | 0.005 |
| Weight | -0.044 | 0.020 | -0.820 | -2.235 | 0.026 | |
| Glucose | -0.049 | 0.018 | -0.167 | -2.680 | 0.008 | |
| Han/Female | ||||||
| TC | Age | 0.016 | 0.005 | 0.219 | 3.363 | 0.001 |
| Waist circumference | 0.029 | 0.012 | 0.222 | 2.422 | 0.016 | |
| TG | Waist circumference | 0.035 | 0.012 | 0.278 | 3.021 | 0.003 |
| Diastolic blood pressure | 0.011 | 0.005 | 0.116 | 2.108 | 0.036 | |
| Glucose | 0.091 | 0.040 | 0.120 | 2.266 | 0.024 | |
| LDL-C | Age | 0.015 | 0.003 | 0.277 | 4.266 | 0.000 |
| Waist circumference | 0.017 | 0.009 | 0.182 | 1.991 | 0.047 | |
| ApoB | Waist circumference | 0.008 | 0.003 | 0.259 | 3.008 | 0.003 |
| Age | 0.003 | 0.001 | 0.165 | 2.697 | 0.007 | |
| Glucose | 0.023 | 0.009 | 0.135 | 2.706 | 0.007 | |
| ApoA1/ApoB | Age | -0.004 | 0.002 | -0.132 | -2.086 | 0.037 |
| Cigarette smoking | 0.362 | 0.164 | 0.112 | 2.201 | 0.028 | |
| Glucose | -0.040 | 0.019 | -0.110 | -2.143 | 0.033 |
TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; ApoA1/ApoB, the ratio of apolipoprotein A1 to apolipoprotein B; B, unstandardized coefficient. Beta, standardized coefficient. The correlation among serum lipid parameters and the genotypes and several environmental factors was determined by multivariable linear regression analyses with stepwise modeling.
Discussion
The results of the present study showed that the serum lipid profiles were different between the Maonan and Han populations. The levels of TG and ApoA1, and the ratio of ApoA1 to ApoB were higher in Maonan than in Han (P < 0.05-0.001), whereas the levels of TC and HDL-C were lower in Maonan than in Han (P < 0.01). There was no significant difference in the levels of LDL-C and ApoB levels between the two ethnic groups (P > 0.05 for each). It was widely realized that dyslipidemia as a serious risk factor for CAD is caused by various elements, mainly including genetic and environmental factors and their interaction [21,22]. Maonan nationality belongs to a mountain ethnic minority and is mainly occupied with cereal and miscellaneous grain crops. The history of Maonan can retrospect to the 11th century. According to the statistics in 2000, the numbers of Maonan population were 107166, mainly engaged in agriculture and were good at raising beef cattle and prepare the bamboo hat. The main food for them was rice, along with corn, sorghum, millet, sweet potatoes and pumpkin which are also important complements. Thus, they were enjoyed a very special lifestyle and dietary habits compared with the other nationalities. Maonan people were keen on spicy and acid food. Parents usually took charge of their children’s marriages. Maonan stays endogamy; intermarriage with Han or Zhuang people is seldom happened. Therefore, it is considered that the hereditary characteristics and genotypes of certain lipid metabolism-related genes in this population might be different from those in the Han population.
To the best of our knowledge, the genotypic and allelic frequencies of the CYP26A1 rs2068888 SNP have not been reported previously in different ethnic groups. In the present study, we firstly showed that the G allele frequency of the CYP26A1 rs2068888 SNP was lower in Maonan than in Han populations (12.78% vs. 18.83%, P < 0.001). The distribution of the genotypes was also different between the two ethnic groups (P < 0.001), the frequencies of AG and GG genotypes were lower in Maonan than in Han groups, respectively. No significant difference was observed in the genotypic and allelic frequencies between males and females in the two ethnic groups. These results indicate that the prevalence of CYP26A1 rs2068888 SNP may have racial/ethnic specificity.
The potential association of the CYP26A1 rs2068888 SNP and serum lipid levels has not been previously reported in different racial/ethnic groups. A previous associated study indicated that the CYP26A1 encodes a member of the cytochrome P450 superfamily of enzymes. The cytochrome P450 protein is monooxygenases which catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids and other lipids [23]. Recently, another genome-wide association study (GWAS) has also identified that genetic variant of the CYP26A1 rs2068888 SNP was associated with serum TG levels [24,25]. In the current study, we firstly showed that the G allele carriers in Maonan had higher serum TC level than the G allele non-carriers. Subgroup analyses showed that the G allele carriers had higher serum TC level in Maonan females than the G allele non-carriers. These findings suggest that there may be an ethnic- and gender-specific association of the CYP26A1 rs2068888 SNP and serum lipid levels.
It is well known that environmental factors such as dietary patterns, lifestyle and physical inactivity are all strongly related with serum lipid levels [26]. In the present study, multivariate linear regression analysis also showed that serum lipid parameters were correlated to age, sex, waist circumference, BMI, blood pressure, blood glucose, alcohol consumption, and cigarette smoking in both ethnic groups. These findings suggest that the environmental factors also play an important role in determining serum lipid levels in our study populations. The dietary habits are different between the Mannan and Han populations. Rice is the Maonan people’s staple food supplemented with corn, sweet potato and other grains. Maonan people prefer to very strong flavor food, such as eating spicy and acid food with lots of oil and salt, for instance. This preference of high in carbohydrates may be related to the higher blood glucose levels, weight, BMI and waist circumference in Maonan than in Han people. In the meantime, rich oil and salt can give rise to higher blood pressure, serum TC, LDL-C and ApoB levels in Maonan than in Han people. Many previous studies proved that diet alone could account for the variability on serum lipid levels.
In addition, we also noticed that the percentage of cigarette smoking was higher in Maonan than in Han. In multiple linear regression analysis, we could find that alcohol consumption and cigarette smoking may influence serum TC, TG, HDL-C, ApoA1 levels and the ApoA1/ApoB ratio (P < 0.05). Several case-control and cohort studies have described a J- or U-shaped association between alcohol intake and atherogenesis [27]. A moderate intake of alcohol when taken on a regular amount has been showed to protect against CAD death, which has been attributed to the changes in serum HDL-C, TG and ApoA1 levels [28]. However, alcohol consumption was also associated with worse hematological values of TC and LDL-C levels. Another research indicated that the effects of alcohol consumption on LDL-C appear to vary by specific patient types or patterns of alcohol intake, and sex as well as genetic variants [29]. When it comes to cigarette smoking, one of previous study described that cigarette smoking could be a key factor in potential changes in lipid profile, which in the future may give rise to the onset of atherosclerosis and CAD [30]. Another study showed that there was difference between two cigarette smoking habits: the length period of smoking and a number of cigarettes smoked daily, and made a conclusion that more reflection to the status of lipids has the bigger number of smoked cigarettes daily than the length of the period of cigarette smoking [31]. Therefore, the results of exposure to different lifestyle and environmental factors probably further modify the association of genetic variations and serum lipid levels in our study populations.
Limitations
There are several potential limitations in our study. Firstly, we were not able to alleviate the effect of diet and some environmental factors during the statistical analysis. Secondly, we could not completely exclude asymptomatic disorders, atherosclerosis, for instance, which could create a potentially significant bias due to poor field study condition. Thirdly, there are still many unmeasured environmental and genetic factors which should be considered, although we have observed significant association of the CYP26A1 rs2068888 SNP and serum lipid levels. In addition, the interactions of gene-gene, gene-environment, and environment-environment on serum lipid levels are remained to be determined. In addition, the relevance of this finding has to be defined in further high caliber of studies including incorporating the genetic information of the CYP26A1 rs2068888 SNP and in vitro functional studies to confirm the impact of a variant on a molecular level.
Conclusions
The present study showed that the genotypic and allelic frequencies of the CYP26A1 rs2068888 SNP were different between the Maonan and Han populations. The associations of the CYP26A1 rs2068888 SNP and serum lipid levels were also different between the two ethnic groups and between males and females in the Maonan population. There may be a racial/ethnic- and/or sex-specific association of the CYP26A1 rs2068888 SNP and serum lipid levels.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No: 81460169).
Disclosure of conflict of interest
None.
References
- 1.Yazdanyar A, Newman AB. The burden of cardiovascular disease in the elderly: morbidity, mortality, and costs. Clin Geriatr Med. 2009;25:563–577. doi: 10.1016/j.cger.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De FS, Metzinger L, Serra R. The discovery of novel genomic, transcriptomic, and proteomic biomarkers in cardiovascular and peripheral vascular disease: the state of the art. Bio Med Res Int. 2016;2016:7829174. doi: 10.1155/2016/7829174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martín-Ventura JL, Blanco-Colio LM, Tuñón J, Muñoz-García B, Madrigal-Matute J, Moreno JA, Vega de Céniga M, Egido J. Biomarkers in cardiovascular medicine. Rev Esp Cardiol. 2009;62:677–688. doi: 10.1016/s1885-5857(09)72232-7. [DOI] [PubMed] [Google Scholar]
- 4.D’Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 5.Sin HY, Jin YK, Jung KH. Total cholesterol, high density lipoprotein and triglyceride for cardiovascular disease in elderly patients treated with metformin. Arch Pharm Res. 2011;34:99–107. doi: 10.1007/s12272-011-0112-5. [DOI] [PubMed] [Google Scholar]
- 6.Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Borén J, Catapano AL, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease. Eur Heart J. 2015;32:1345–1361. doi: 10.1093/eurheartj/ehr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okamura T, Kokubo Y, Watanabe M, Higashiyama A, Miyamoto Y, Yoshimasa Y, Okayama A. Low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol and the incidence of cardiovascular disease in an urban Japanese cohort study: the suita study. Atherosclerosis. 2009;203:587–592. doi: 10.1016/j.atherosclerosis.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Berkinbayev S, Rysuly M, Mussayev A, Blum K, Baitasova N, Mussagaliyeva A, Dzhunusbekova G, Makhatov B, Mussayev AA, Yeshmanova A. Apolipoprotein gene polymorphisms (APOB, APOC111, APOE) in the development of coronary heart disease in ethnic groups of Kazakhstan. J Genet Syndr Gene Ther. 2014;5:216. doi: 10.4172/2157-7412.100021610.4172/2157-7412.1000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walldius G, Jungner I. The apoB/apoA-I ratio: a strong, new risk factor for cardiovascular disease and a target for lipid-lowering therapy--a review of the evidence. J Intern Med. 2006;259:493–519. doi: 10.1111/j.1365-2796.2006.01643.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee YC, Lai CQ, Ordovas JM, Parnell LD. A database of gene-environment interactions pertaining to blood lipid traits, cardiovascular disease and type 2 diabetes. J Data Mining Genomics Proteomics. 2011;2:106. doi: 10.4172/2153-0602.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Superko HR, Roberts R, Garrett B, Pendyala L, King S 3rd. Family coronary heart disease: a call to action. Clin Cardiol. 2010;33:E1–6. doi: 10.1002/clc.20684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heller DA, Pedersen NL, De FU, Mcclearn GE. Genetic and environmental correlations among serum lipids and apolipoproteins in elderly twins reared together and apart. Am J Hum Genet. 1994;55:1255–1267. [PMC free article] [PubMed] [Google Scholar]
- 13.Zdravkovic S, Wienke A, Pedersen NL, Marenberg ME, Yashin AI, Faire UD. Heritability of death from coronary heart disease: a 36-year follow-up of 20966 Swedish twins. J Intern Med. 2002;252:247–254. doi: 10.1046/j.1365-2796.2002.01029.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Aung LHH, Tan JY, Yin RX, Hu XJ, Long XJ, Wu DF, Miao L, Yang DZ, Pan SL. Prevalence of dyslipidemia and its risk factors in the Chinese Maonan and Han populations. Int J Clin Exp Pathol. 2016;9:10603–10616. [Google Scholar]
- 15.An epidemiological study of cardiovascular and cardiopulmonary disease risk factors in four populations in the People’s Republic of China. Baseline report from the P.R.C.-U.S.A. Collaborative study. People’s Republic of China--United States cardiovascular and cardiopulmonary epidemiology research group. Circulation. 1992;85:1083–1096. doi: 10.1161/01.cir.85.3.1083. [DOI] [PubMed] [Google Scholar]
- 16.Guo T, Yin RX, Lin QZ, Wu J, Shen SW, Sun JQ, Shi GY, Wu JZ, Li H, Wang YM. Polymorphism of rs873308 near the transmembrane protein 57 gene is associated with serum lipid levels. Biosci Rep. 2014;34:69–81. doi: 10.1042/BSR20130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang QH, Yin RX, Gao H, Huang F, Wu JZ, Pan SL, Lin WX, Yang DZ. Association of the SPTLC3 rs364585 polymorphism and serum lipid profiles in two Chinese ethnic groups. Lipids Health Dis. 2017;16:1. doi: 10.1186/s12944-016-0392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grönroos P, Raitakari OT, Kähönen M, Hutri-Kähönen N, Juonala M, Marniemi J, Viikari J, Lehtimäki T. Relation of apolipoprotein E polymorphism to markers of early atherosclerotic changes in young adults--the cardiovascular risk in young Finns study. Circ J. 2008;72:29–34. doi: 10.1253/circj.72.29. [DOI] [PubMed] [Google Scholar]
- 19.Whitworth JA World Health Organization, International Society of Hypertension Writing Group. 2003 world health organization (WHO)/international society of hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–1992. doi: 10.1097/00004872-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Zhou B Coorperative Meta-Analysis Group Of China Obesity Task Force. Predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese adult population. Zhonghua Liu Xing Bing Xue Za Zhi. 2002;23:5–10. [PubMed] [Google Scholar]
- 21.Zhang L, Yin RX, Liu WY, Miao L, Wu DF, Aung LH, Hu XJ, Cao XL, Wu JZ, Pan SL. Association of methylenetetrahydrofolate reductase C677T polymorphism and serum lipid levels in the Guangxi Bai Ku Yao and Han populations. Lipids Health Dis. 2010;9:123. doi: 10.1186/1476-511X-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin SS, Blaha MJ, Blankstein R, Agatston A, Rivera JJ, Virani SS, Ouyang P, Jones SR, Blumenthal RS, Budoff MJ, Nasir K. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease: implications for statin therapy from the multi-ethnic study of atherosclerosis. Circulation. 2014;129:77–86. doi: 10.1161/CIRCULATIONAHA.113.003625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weng Y, DiRusso CC, Reilly AA, Black PN, Ding X. Hepatic gene expression changes in mouse models with liver-specific deletion or global suppression of the NADPH-cytochrome P450 reductase gene. Mechanistic implications for the regulation of microsomal cytochrome P450 and the fatty liver phenotype. J Biol Chem. 2005;280:31686–31698. doi: 10.1074/jbc.M504447200. [DOI] [PubMed] [Google Scholar]
- 24.Jeemon P, Pettigrew K, Sainsbury C, Prabhakaran D, Padmanabhan S. Implications of discoveries from genome-wide association studies in current cardiovascular practice. World J Cardiol. 2011;3:230–247. doi: 10.4330/wjc.v3.i7.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansen CT, Kathiresan S, Hegele RA. Genetic determinants of plasma triglycerides. J Lipid Res. 2011;52:189–206. doi: 10.1194/jlr.R009720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson D, Kawamura T, Hinohara S, Sakamoto Y, Takahashi T. Levels of cardiovascular risk factors in Japanese people living in the UK. J Cardiovasc Risk. 1995;2:449–458. doi: 10.1177/174182679500200510. [DOI] [PubMed] [Google Scholar]
- 27.Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999;319:1523–1528. doi: 10.1136/bmj.319.7224.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Oliveira E Silva ER, Foster D, McGee Harper M, Seidman CE, Smith JD, Breslow JL, Brinton EA. Alcohol consumption raises HDL cholesterol levels by increasing the transport rate of apolipoproteins A-I and A-II. Circulation. 2000;102:2347–2352. doi: 10.1161/01.cir.102.19.2347. [DOI] [PubMed] [Google Scholar]
- 29.Rees K, Hartley L, Flowers N, Clarke A, Hooper L, Thorogood M, Stranges S. ‘Mediterranean’ dietary pattern for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013:Cd009825. doi: 10.1002/14651858.CD009825.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Sliwinska-Mosson M, Mihulka E, Milnerowicz H. Assessment of lipid profile in non-smoking and smoking young health persons. Przegl Lek. 2014;71:585–587. [PubMed] [Google Scholar]
- 31.Bisanovic S, Mehic B, Sivic S. Status of lipids and the frequency diseases of cardiovascular origin in smokers according to the length period of smoking and a number of cigarettes smoked daily. Bosn J Basic Med Sci. 2011;11:46–51. doi: 10.17305/bjbms.2011.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]


