Abstract
Objective: The PD-1/PD-L1 pathway plays a key role in the immune evasion of tumor cells from the host immune system. This study aimed to examine PD-L1 expression and prevalence of infiltrating T cellin gastric diseases, and elucidate the relevance of PD-L1 and prevalence of infiltrating T cell in the pathogenesis of gastric carcinoma. Methods: Immunohistochemistry and HE staining were used to investigate the in situ expression of PD-L1, CD4 and CD8 in paraffin-embedded gastric tissues from patients with gastric ulcer (n=21), intestinal metaplasia/atrophic gastritis (n=26) and gastric carcinoma tissues (n=38). Results: The expression of PD-L1 was found to be most prevalent in gastric carcinoma and, by comparison, decreased in gastric tissues from patients with gastric ulcer or intestinal metaplasia/atrophic gastritis. The overexpression of PD-L1 was notably more prevalent in cancer tissues and tissues from cases of intestinal metaplasia/atrophic gastritis, with the highest prevalence observed in intestinal metaplasia/atrophic gastritis. Meanwhile, decreasing prevalence was observed for CD4 and CD8 expression in gastric carcinoma. Therefore, PD-L1 expression in gastric carcinoma appeared to be inversely correlated with prevalence of infiltrating T cell. Conclusion: PD-L1 expression was more prevalent while CD4 and CD8 expression were decreased in gastric carcinoma compared with gastric ulcer and intestinal metaplasia/atrophic gastritis. As the activated immunological status in gastric ulcerappears to switch to one of immunological suppression in intestinal metaplasia/atrophic gastritis and gastric carcinoma, this may suggest that the immune evasion associated with the PD-L1 pathway may be triggered in the pathogenesis of human gastric carcinoma.
Keywords: Programmed death-1 ligand-1, CD4, CD8, infiltrating T cell, gastric carcinoma
Introduction
Gastric cancer is a solid malignant tumor with a rising rate of incidence and a high rate of mortality. As for other solid tumors, the tumor microenvironment, particularly the local infiltrating T cell, is crucial in the pathogenesis of gastric carcinoma.
Programmed death-1 ligand 1 (PD-L1, also known as CD274 or B7-H1), is a member of the B7 family of cell-surface immune-regulatory proteins [1]. Overexpression of PD-L1 by tumor cells has been observed in various types of human cancer, including melanoma, glioblastoma, and carcinomas of the colon, breast, lung, ovary, and renal cells, and has been demonstrated to impair anti-tumor T-cell immunity [2]. Additionally, in mouse models of cancer such as renal cell carcinoma, multiple myeloma, and pancreatic cancer, blockade of PD-L1 has been shown to produce an anti-tumor response [3-5]. Furthermore, through interaction with its receptor, programmed death-1 (PD-1), it has been indicated that PD-L1 may act as a negative regulatory molecule of the anti-tumor response [6-8]. Therefore, examining the expression of PD-L1 in human gastric carcinoma tissues via immunohistochemistry may provide a better understanding of how this co-inhibitory signaling molecule contributes to the suppression of anti-tumor immunity in the gastric carcinoma microenvironment. Such data may aid in identifying potential immunological therapeutic targets of PD-L1 in gastric carcinoma.
In the present study, we hypothesized that PD-L1 expression in the gastric tumor microenvironment may play a role in immune regulation, triggered the immune evasion associated with the PD-L1 pathway, and thus participate in the pathogenesis of gastric carcinoma. To verify this hypothesis, samples of paraffin-embedded gastric tissues were collected from 85 patients with gastric ulcer, intestinal metaplasia/atrophic gastritis (precursor lesions to gastric carcinoma), or gastric carcinoma, and the expression levels of PD-L1, CD4 and CD8 in the gastric tissues were examined. Additionally, the relevance of PD-L1 expression and prevalence of infiltrating T cell in the human gastric tissues was evaluated. The results indicated that the PD-L1 expression was more prevalent while CD4 and CD8 expression were decreased in gastric carcinoma compared with gastric ulcer and intestinal metaplasia/atrophic gastritis, and that the immune evasion associated with the PD-L1 pathway may be triggered in the pathogenesis of human gastric carcinoma.
Materials and methods
Subjects
The research protocol was approved by the Institutional Review Board of West China Second University Hospital, Sichuan University (Chengdu, China). In this study, 85 individual samples of paraffin-embedded gastric tissue were collected from 21 patients with gastric ulcer, 26 patients with intestinal metaplasia and atrophy, and 38 patients with gastric carcinoma, all of whom were treated at the No. 4 West China Teaching Hospital. The histological diagnosis, differentiation and stage of all 3 diseases were evaluated according to the World Health Organization classification [9]. Tumors were staged via the TNM classification and clinical staging systemsof the American Joint Committee on Cancer (AJCC) 2009 [10]. The clinical characteristics of all subjects were shown in Table 1.
Table 1.
Clinical characteristics of the subjects in the study
| Characteristics | Patients with gastric ulcer | Patients with intestinal metaplasia/atrophic gastritis | Patients with gastric carcinoma |
|---|---|---|---|
|
| |||
| N=21 | N=26 | N=38 | |
| Mean age (range), years | 57.47±11.61 (31-83) | 56.67±12.84 (37-79) | 58.00±13.43 (37-84) |
| Gender | |||
| Male | 13 | 20 | 29 |
| Female | 8 | 6 | 9 |
| Histology | |||
| Gastric ulcer | 21 | / | / |
| Intestinal metaplasia and atrophy | / | 26 | / |
| Carcinoma, poor differentiated | / | / | 5 |
| Carcinoma, moderate differentiated | / | / | 33 |
| Depth of tumor invasion | |||
| T1 | / | / | 0 |
| T2 | / | / | 1 |
| T3 | / | / | 19 |
| T4 | / | / | 18 |
| N stage | / | ||
| N0 | / | / | 2 |
| N1 | / | / | 10 |
| N2 | / | / | 13 |
| N3 | / | / | 13 |
| M stage | |||
| M0 | / | / | 28 |
| M1 | / | / | 10 |
Immunohistochemistry
The immunohistochemical (IHC) staining was performed on serial tissue sections and all samples were routinely processed as described previously [11]. Mouse anti-human CD4 monoclonal antibody (1:100; IgG1; catalog no., MAB379; R&D Systems, Inc., Dallas, TX), rabbit anti-human CD8 polyclonal antibody (1:100; IgG; catalog no., ab85792; Abcam, Cambridge, UK), and mouse anti-human PD-L1 antibody (1:100; IgG1; catalog no., GTX104763; GeneTex, Inc., Irvine, CA) were used as the primary antibodies for IHC staining. Negative controls were established by using isotype control antibodies of goat serum (Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China), mouse IgG1 or rabbit IgG for primary antibody substitution according to the source of primary antibody. All antibodies were diluted in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (Roche Applied Science, Rotkreuz, Switzerland) and 0.01% sodium azide. For IHC staining, in brief, 4-μm tissue sections were deparaffinized for 15 min in xylene, hydrated in an ethyl alcohol gradient of 100, 95, 85, 80 and 75%, and then blocked for endogenous peroxidase activity with 3% hydrogen peroxidase for 10 min at room temperature. After antigen retrieval in 0.1 mol/l citrate buffer (pH 6.0) for 15 min at 95°C in a pressure cooker, the sections were incubated with the diluted primary antibodies at 4°C overnight and rewarmed to room temperature for 30 min, and then incubated with HRP-conjugated goat anti-rabbit/mouse secondary antibody (1:100; catalog no., ab6720; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA). Subsequently, the sections were developed with diaminobenzidine (DAB) at room temperature for 3 min for color development and counterstained with Mayer’s hematoxylin, and mounted in Permount (ZSGB-BIO, Beijing, China). Finally, microscopic observation (magnification, ×4,000; 5 fields of view) was performed, and the intensities of immunostaining for PD-L1, CD4 and CD8 in the tissues were determined.
The IHC staining was evaluated by two individual pathologists. We determined the IHC staining intensity scores with a 3-tier system, as follows: 0, no discernible staining; 1, weak or moderate staining; 2, strong staining. A staining intensity score of 1 or 2 was defined as a positive result. Samples exhibiting 2 was considered as “positive for overexpression” [12].
Statistical analysis
The percentages of IHC staining intensity scores were calculated and analyzed. The statistical significance of data was assessed by Pearson’s Chi squared test using a computer software SPSS (SPSS for windows, version 19.0.0, Chicago, IL, USA). P value < 0.05 was considered statistically significant.
Results
Expression of PD-L1 in gastric tissue
To study the expression of PD-L1 in gastric tissue, an anti-PD-L1 antibody was used as a primary antibody for IHC staining. A negative control was established using an isotype control of mouse IgG1 as the primary antibody substitute.
A staining intensity score of 1 or 2 was considered to indicate positive expression. As shown in Table 2 and Figure 1, the positive rates of PD-L1 expression in gastric ulcer tissue, intestinal metaplasia/atrophic gastritis tissue, and cancer tissue were 80.95, 84.62, and 86.84%, respectively. A staining intensity score of 2 was considered to indicate overexpression. The rates of PD-L1 overexpression in gastric ulcer tissue, intestinal metaplasia/atrophic gastritis tissue, and cancer tissue were determined to be 9.52, 50.00, and 21.05%, respectively. Thus, it was seemingly higher in cancer tissue and intestinal metaplasia/atrophic gastritis tissue, with the highest level observed in intestinal metaplasia/atrophic gastritis tissue at a rate of 50.00%, while significant difference was not shown.
Table 2.
IHC staining intensity scores of CD4, CD8 and PD-L1 in the different histologically classified gastric tissues
| Protein | Score | The percentage of IHC staining intensity score, n (%) | P value | ||
|---|---|---|---|---|---|
|
| |||||
| Gastric ulcer (N=21) | Intestinal metaplasia/atrophic gastritis (N=26) | Gastric carcinoma (N=38) | |||
| PD-L1 | 0 | 4 (19.05%) | 4 (15.38%) | 5 (13.16%) | 0.805 |
| 1 | 15 (71.43%) | 9 (34.62%) | 25 (65.79%) | ||
| 2 | 2 (9.52%) | 13 (50.00%) | 8 (21.05%) | ||
| 1+2 | 17 (80.95%) | 22 (84.62%) | 33 (86.84%) | ||
| CD4 | 0 | 4 (19.05%) | 8 (30.77%) | 16 (42.11%) | 0.871 |
| 1 | 13 (61.90%) | 12 (46.15%) | 15 (39.47%) | ||
| 2 | 4 (19.05%) | 6 (23.08%) | 7 (18.42%) | ||
| 1+2 | 17 (80.95%) | 18 (69.23%) | 22 (57.89%) | ||
| CD8 | 0 | 3 (14.29%) | 7 (26.92%) | 7 (18.42%) | 0.906 |
| 1 | 14 (66.67%) | 10 (38.46%) | 24 (63.16%) | ||
| 2 | 4 (19.05%) | 9 (34.62%) | 7 (18.42%) | ||
| 1+2 | 18 (85.72%) | 19 (73.08%) | 31 (81.58%) | ||
A staining intensity score of 0 was defined as a negative result; a score of 1 or 2 was defined as a positive result; and a score of 2 was considered to indicate overexpression.
Figure 1.
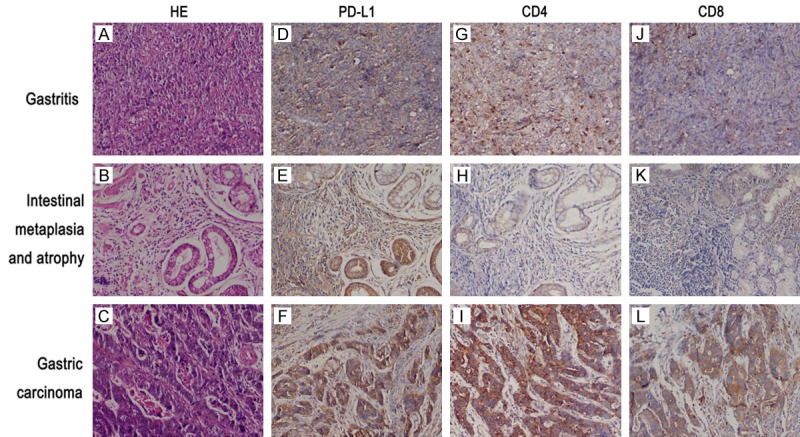
Detection of PD-L1, CD4 and CD8 expression by immunohistochemistry in the different histologically classified gastric tissues. HE staining was also performed. A-C. HE staining of gastric ulcer tissues, intestinal metaplasia/atrophic gastritis tissues, and gastric carcinoma tissues. D-F. PD-L1 expression in gastric ulcer tissues, intestinal metaplasia/atrophic gastritis tissues, and gastric carcinoma tissues. G-I. CD4 expression in gastric ulcer tissues, intestinal metaplasia/atrophic gastritis tissues, and gastric carcinoma tissues. J-L. CD8 expression in gastric ulcer tissues, intestinal metaplasia/atrophic gastritis tissues, and gastric carcinoma tissues (original magnification ×4,000).
Expression of CD4 and CD8 in gastric tissue
As shown in Table 2 and Figure 1, the positive rates of CD4 expression in gastric ulcer tissue, intestinal metaplasia/atrophic gastritis tissue, and cancer tissue were 80.95, 69.23, and 57.89%, respectively. The overexpression of CD4 was detected to be at the highest level in intestinal metaplasia/atrophic gastritis tissue at a rate of 23.08%.
The positive rates of CD8 expression in gastric ulcer tissue, intestinal metaplasia/atrophic gastritis tissue, and cancer tissue were 85.72, 73.08, and 81.58%, respectively. The overexpression of CD8 was detected to be at the highest level in intestinal metaplasia/atrophic gastritis tissue at a rate of 34.62%.
Similar decreasing trends were observed for CD4 and CD8 expression with the worsening pathologies. As the activated immunological status (indicated with increasing of infiltrating CD4+ and CD8+ T lymphocytes) in gastric ulcer appears to switch to one of immunological suppression (indicated with decreasing of infiltrating CD4+ and CD8+ T lymphocytes) in intestinal metaplasia/atrophic gastritis and gastric carcinoma, this may suggest that the immune evasion associated with the PD-L1 pathway may be triggered in the pathogenesis of human gastric carcinoma.
Discussion
Programmed death 1 (PD-1) is a receptor of the CD28/CTLA-4 family, and expressed mainly on the surface of peripheral T cells, B cells and monocytes [13]. As a ligand of PD-1, PD-L1 belongs to the B7 family, and is widely expressed by activated T cells, B cells, DCs, macrophages and non-hematopoietic derived cells [1,14]. The PD-1/PD-L1 axis is well established as a T-cell co-inhibitory pathway in vivo [15-17]. Notably, the apoptosis of activated T cells, particularly memory T cells, has been found to induce the PD-1/PD-L1 pathway to maintain T cell balance and homeostasis under various circumstances, including in autoimmune disease and tolerance induction in transplantation [18-22]. In recent years, the immune-regulatory function of PD-1/PD-L1 in the tumor microenvironment has become a topic of interest for researchers [19,23].
Previous studies have reported that the immune evasion mediated by the PD-L1 pathway in the local environment plays an important role in the carcinogenesis of various tumor types, including non-small cell lung cancer, melanoma, and colorectal, renal cell, ovarian, pancreatic, gastric and breast cancers [24-28]. It has been observed that T-cell apoptosis may be induced by tumor-associated PD-L1, and that the blockade of interactions between PD-1 and PD-L1 enhances immune function in vitro and mediates anti-tumor activity in preclinical models [29,30]. Furthermore, antibody-mediated blockade of PD-L1 has been reported to induce durable tumor regression (objective response rate 6 to 17%) and prolonged stabilization of disease (12 to 41% at 24 weeks) in patients with advanced cancers, including non-small cell lung cancer, melanoma, and renal cell cancer. Inhibitory anti-PD-L1 antibodies are therefore being evaluated in clinical trials for various solid tumors in humans, including melanoma and cancers of the lung, kidney, colon, pancreas and stomach. These findings suggest the potential of PD-L1 as a therapeutic target in human solid tumors.
Many factors have been reported to be involved in the carcinogenesis of gastric tumors. However, few studies have investigated the role of PD-L1 in gastric cancer. In this study, we assessed the role of PD-L1 in the evasion of gastric tumor cells from the host immune system, and aimed to elucidate the role of PD-L1 in the pathogenesis of gastric carcinoma.
Our data indicated that immune evasion involving the PD-L1 pathway may be triggered in the pathogenesis of human gastric carcinoma. With worsening pathologies, from gastric ulcer to intestinal metaplasia and atrophy, and further to gastric carcinogenesis, the activated immunological status was indicated to switch to an immunological suppressive status in intestinal metaplasia/atrophic gastritis tissue and gastric carcinoma.
Acknowledgements
This study was funded by National Natural Science Foundation of China (grant No. 81272821 and No. 81172440).
Informed consent was obtained from all individual participants included in the study.
Disclosure of conflict of interest
None.
References
- 1.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 2.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumorassociated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 3.Hirayama Y, Gi M, Yamano S, Tachibana H, Okuno T, Tamada S, Nakatani T, Wanibuchi H. Anti-PD-L1 treatment enhances antitumor effect of everolimus in a mouse model of renal cell carcinoma. Cancer Sci. 2016;107:1736–44. doi: 10.1111/cas.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hallett WH, Jing W, Drobyski WR, Johnson BD. Immunosuppressive effects of multiple myeloma are overcome by PD-L1 blockade. Biol Blood Marrow Transplant. 2011;17:1133–45. doi: 10.1016/j.bbmt.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Okudaira K, Hokari R, Tsuzuki Y, Okada Y, Komoto S, Watanabe C, Kawaguchi A, Nagao S, Azuma M, Yagita H, Miura S. Blockade of B7- H1 or B7-DC induces an anti-tumor effect in a mouse pancreatic cancer model. Int J Oncol. 2009;35:741–9. doi: 10.3892/ijo_00000387. [DOI] [PubMed] [Google Scholar]
- 6.Liu SM, Meng Q, Zhang QX, Wang SD, Liu ZJ, Zhang XF. [Expression and significance of B7-H1 and its receptor PD-1 in human gastric carcinoma] . Zhonghua Zhong Liu Za Zhi. 2008;30:192–5. [PubMed] [Google Scholar]
- 7.Mandai M. PD-1/PD-L1 blockage in cancer treatment-from basic research to clinical application. Int J Clin Oncol. 2016;21:447. doi: 10.1007/s10147-016-0969-x. [DOI] [PubMed] [Google Scholar]
- 8.Hamanishi J, Mandai M, Matsumura N, Abiko K, Baba T, Konishi I. PD-1/PD-L1 blockade in cancer treatment: perspectives and issues. Int J Clin Oncol. 2016;21:462–73. doi: 10.1007/s10147-016-0959-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. International statistical classification of diseases and health related problems.10th revision. 15th edition. World Health Organization; 2016. [Google Scholar]
- 10.Edge SB, Compton CC. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 11.Ema A, Yamashita K, Ushiku H, Kojo K, Minatani N, Kikuchi M, Mieno H, Moriya H, Hosoda K, Katada N, Kikuchi S, Watanabe M. Immunohistochemical analysis of RTKs expression identified HER3 as a prognostic indicator of gastric cancer. Cancer Sci. 2014;105:1591–600. doi: 10.1111/cas.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diabassaf M, Aboukhouzam R, Saadallahzeidan N, Habib K, Bitar N, Karam W, Liagre B, Harakeh S, Azar R. Expression of eukaryotic initiation factor 4E and 4E binding protein 1 in colorectal carcinogenesis. Int J Clin Exp Pathol. 2015;8:404–13. [PMC free article] [PubMed] [Google Scholar]
- 13.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–72. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses. Int Immunol. 1998;10:1563–72. doi: 10.1093/intimm/10.10.1563. [DOI] [PubMed] [Google Scholar]
- 15.Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH. PD-L1-deficient mice show that PDL1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci U S A. 2004;101:10691–6. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol. 2007;19:309–14. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–53. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okazaki T, Wang J. PD-1/PD-L pathway and autoimmunity. Autoimmunity. 2005;38:353–7. doi: 10.1080/08916930500124072. [DOI] [PubMed] [Google Scholar]
- 19.Zha Y, Blank C, Gajewski TF. Negative regulation of T-cell function by PD-1. Crit Rev Immunol. 2004;24:229–37. doi: 10.1615/critrevimmunol.v24.i4.10. [DOI] [PubMed] [Google Scholar]
- 20.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–22. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 21.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–51. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 22.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–24. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 23.Dong H, Chen L. B7-H1 pathway and its role in the evasion of tumor immunity. J Mol Med (Berl) 2003;81:281–7. doi: 10.1007/s00109-003-0430-2. [DOI] [PubMed] [Google Scholar]
- 24.Ma W, Gilligan BM, Yuan J, Li T. Current status and perspectives in translational biomarker research for PD-1/PD-L1 immune checkpoint blockade therapy. J Hematol Oncol. 2016;9:47. doi: 10.1186/s13045-016-0277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300–9. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 26.Qorraj M, Bruns H, Bottcher M, Weigand L, Saul D, Mackensen A, Jitschin R, Mougiakakos D. The PD-1/PD-L1 axis contributes to immune metabolic dysfunctions of monocytes in chronic lymphocytic leukemia. Leukemia. 2017;31:470–8. doi: 10.1038/leu.2016.214. [DOI] [PubMed] [Google Scholar]
- 27.Beckermann KE, Johnson DB, Sosman JA. PD-1/PD-L1 blockade in renal cell cancer. Expert Rev Clin Immunol. 2017;13:77–84. doi: 10.1080/1744666X.2016.1214575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheel AH, Dietel M, Heukamp LC, Johrens K, Kirchner T, Reu S, R¨¹schoff J, Schildhaus HU, Schirmacher P, Tiemann M, Warth A, Weichert W, Fischer RN, Wolf J, Buettner R. Harmonized PD-L1 immunohistochemistry for pulmonary squamous-cell and adenocarcinomas. Mod Pathol Inc. 2016;29:1165–72. doi: 10.1038/modpathol.2016.117. [DOI] [PubMed] [Google Scholar]
- 29.Gentzler R, Hall R, Kunk PR, Gaughan E, Dillon P, Slingluff CL Jr, Rahma OE. Beyond melanoma: inhibiting the PD-1/PD-L1 pathway in solid tumors. Immunotherapy. 2016;8:583–600. doi: 10.2217/imt-2015-0029. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Li F, Jiang F, Lv X, Zhang R, Lu A, Zhang G. A mini-review for cancer immunotherapy: molecular understanding of PD-1/PD-L1 pathway & Translational blockade of immune checkpoints. Int J Mol Sci. 2016:17. doi: 10.3390/ijms17071151. [DOI] [PMC free article] [PubMed] [Google Scholar]


