Abstract
The present study aimed to investigate whether miR-30b plays a pivotal role in the progression of esophageal squamous cell carcinoma (ESCC) as well as to elucidate its possible regulatory mechanism. The expression levels of miR-30b in the ESCC tissues and cells were determined. The TE-1 cells were transfected with the miR-30b mimic, the mimic control, the miR-30b inhibitor, the inhibitor control, the pCDNA3.1-Chromobox 3 (pCDNA3.1-CBX3) and/or the blank vector, while the TE-2 cells were transfected with the miR-30b mimic and/or the mimic control. Cell proliferation, cell apoptosis, and cell migration of the different transfected groups were evaluated. The luciferase reporter assay was performed to detect the relationship between miR-30b, and CBX3. Furthermore, the relationship between miR-30b, CBX3, and the JAK2/STAT3 signaling pathway was explored. Significant downregulation of miR-30b was observed in the ESCC tissues and cells, while CBX3 was upregulated in the ESCC tissues and cells. The inhibition of miR-30b promoted cell proliferation, inhibited cell apoptosis, and enhanced cell migration in ESCC, which was similar to the effects of CBX3 overexpression. In fact, CBX3 was confirmed to be a direct target of miR-30b. The overexpression of miR-30b decreased the expression levels of p-JAK2/JAK2 and p-STAT3/JAK3 significantly, which was obviously reversed after the simultaneous overexpression of CBX3. Our results revealed that the downregulation of miR-30b may increase cell proliferation, inhibit cell apoptosis, and promote cell migration in ESCC by targeting CBX3 and activating the JAK2/STAT3 signaling pathway. Thus, miR-30b may serve as a useful marker for predicting the progression of ESCC.
Keywords: Esophageal squamous cell carcinoma, miR-30b, Chromobox 3, JAK2/STAT3 pathway
Introduction
Esophageal cancer is one of the common upper gastrointestinal tract cancers with a high mortality across the world [1,2]. Esophageal squamous cell carcinoma (ESCC) is the most predominant type of esophageal cancer, accounting for more than 90% of the esophageal cancer cases [3,4]. ESCC is characterized by a high rate of metastasis, limited therapeutic options, and poor prognosis [5]. Moreover, the molecular mechanisms, leading to the progression and poor prognosis of ESCC remain to be elucidated [6]. Therefore, a better understanding of the key mechanisms will be helpful in designing a promising therapeutic approach for ESCC.
MicroRNAs (miRNAs) are a class of small, non-coding RNAs, whose dysregulation has been implicated in numerous diseases, including almost all types of cancers [7-10]. Several miRNAs have been identified as key regulators in the development and progression of ESCC. For instance, miR-9 can function as an activator to promote tumor metastasis in ESCC via E-cadherin repression, miR-143 can suppress the migration and invasion of the ESCC cells via the inhibition of the extracellular signal-regulated kinase 5 (ERK5) activity, and the overexpression of miR-211 causes a significant downregulation of SOX2OT and SOX2, thereby playing a vital role in the pluripotency and tumorigenesis of ESCC [11-13]. Recently, alterations in the expression of miR-30b have been shown to lead to the progression of several types of cancers, including gastric cancer, colorectal cancer, and non-small cell lung cancer (NSCLC) [14-16]. Although a very recent study has reported that the low level of expression of miR-30b is correlated with lymph node metastasis, pathological stage, and poor overall survival in patients with esophageal cancer, the roles and possible regulatory mechanisms of miR-30b in ESCC are largely unknown [17].
In the present study, the expression levels of miR-30b in the ESCC tissues and cells were determined. The expression of miR-30b in the ESCC cells was then altered in vitro, in order to elucidate further the effects of miR-30b on cell proliferation, cell apoptosis, and cell migration. In addition, the potential targets of miR-30b were identified and the relationship between miR-30b and the JAK2/STAT3 signaling pathway was explored. All these efforts were expected to provide some theoretical basis for the better understanding of key mechanisms underlying the development of ESCC.
Materials and methods
Tissue sampling
A total of 20 ESCC patients, hospitalized in our hospital from June 2015 to January 2017 were included in this study. The diagnosis of ESCC was pathologically confirmed. Matched ESCC tissues and their adjacent normal tissues were collected from clinically diagnosed surgical specimens, snap-frozen in liquid nitrogen, and stored at -80°C. All the patients gave informed consent and this study was approved by the ethics committee of our hospital.
Cell culture
The normal esophageal cell line (NEEC) as well as the highly and poorly differentiated ESCC cell lines (TE-1 and TE-2, respectively) were purchased from the American Type Culture Collection (Manassas, VA, USA), cultured in RPMI 1640 medium (Gibco, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS) (Gibco, Carlsbad, CA, USA), and incubated at 37°C in a humidified chamber with 5% CO2.
Cell transfection
The miR-30b mimic, the mimic control, the miR-30b inhibitor, the inhibitor control, the overexpressed pCDNA3.1-CBX3 vector, and the blank vector with no CBX3 sequence (used as a negative control) were synthesized by RiboBio Corporation, China. The TE-1 cells were transfected with the miR-30b mimic, the mimic control, the miR-30b inhibitor, the inhibitor control, the pCDNA3.1-CBX3 vector, and the blank vector, using the Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA, USA), according to the instructions of the manufacturer, while the TE-2 cells were transfected with the miR-30b mimic and the mimic control, using the same method. After 48 h of incubation, the transfection efficiency was determined by quantitative real-time PCR (qRT-PCR).
The MTT assay
The viability of the transfected cells was measured using the MTT assay. Briefly, the cells (5×103 cells per well) were seeded in triplicate into 96-well microplates. Following different times of transfection, 20 μL of 10 mg/mL MTT solution was added and the plates were incubated at 37°C for 4 h. The culture medium was then removed and 150 μL of dimethyl sulfoxide (DMSO) was added to each well, in order to incubate the cells for 15 min. The absorbance (optical density) values at 490 nm were analyzed with a microplate reader (BioTek, Winooski, VT, USA).
The colony formation assay
In order to detect cell proliferation, the colony formation assay was performed. Briefly, a total of 100 cells from each group, after 48 h of transfection were seeded in triplicate into 6-well dishes and incubated for an additional 14 days in RPMI 1640 medium with 10% FBS. Subsequently, the colonies were fixed in 10% formalin for 10 min and stained with 0.1% crystal violet (Sigma-Aldrich Chemical Company, St. Louis, MO, USA). Finally, the colonies were counted under an Olympus IX83 microscope (Olympus Inc., Tokyo, Japan).
Flow cytometry analysis
Flow cytometry analysis was performed to detect apoptosis in the transfected cells. Briefly, the transfected cells were harvested and suspended in cold PBS buffer. Subsequently, the cells were double stained with Annexin V-FITC (fluorescein isothiocyanate) and PI (propidium iodide) for 20 min at room temperature. The percentage of the apoptotic cells was then analyzed in a flow cytometer (FACSCalibur, Becton-Dickinson, San Jose, CA, USA), equipped with the CellQuest software (Becton-Dickinson, Franklin Lakes, NJ, USA).
The transwell migration assay
The migration of the transfected cells was measured by the transwell migration assay. Briefly, the cells in different groups were seeded into the upper layer of a transwell chamber of pore size 8 μm (Corning Inc., NY, USA), supplemented with serum-free RPMI 1640 medium. The RPMI 1640 medium, containing 10% FBS was added to the lower layer of the chamber as a chemoattractant. Following 48 h of incubation, the transwell chamber was washed with PBS buffer, fixed in 4% paraformaldehyde, and stained with 0.1% crystal violet. Finally, the migrated cells were counted under an Olympus IX83 microscope (Olympus Inc., Tokyo, Japan). Each experiment was carried out in triplicate.
The luciferase reporter assay
The CBX3 3’UTR sequence, containing putative binding sites for miR-30b (CBX3 3’UTR-wt) was amplified from human genomic DNA and inserted into the pGL3- Basic luciferase reporter plasmid, creating a luciferase reporter construct. The mutated CBX3 3’UTR sequence in the complementary site of miR-30b (CBX3 3’UTR-mut) was used as a negative control. The cells were then cotransfected with the miR-30b mimic and the luciferase reporter constructs, comprising either CBX3 3’UTR-wt or CBX3 3’UTR-mut. Subsequently, the transfected cells were serum shocked (20%) for 2 h and incubated for 24 h with 2% media. Luciferase activity was then detected with the Dual Luciferase Assay kit (Promega Corporation, Madison, WI, USA), using a beta-counter or luminometer. The ratio of the raw firefly luciferase activity to the Renilla luciferase activity was calculated as the relative luciferase activity.
qRT-PCR analysis
Total RNA was isolated from the ESCC tissues and cells, using the TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) and the quality of the extracted RNA was detected with a SMA4000 UV-VIS spectrophotometer (Merinton, Shanghai, China). The synthesis of complementary DNA (cDNA) was then carried out from the extracted RNA, using the PrimeScript First Strand cDNA Synthesis Kit (Invitrogen, Carlsbad, CA, USA). In order to detect the relative expressions of miR-30b, RT-qPCR was performed using the SYBR ExScript RT-PCR kit (Takara, Shiga, Japan)in accordance with the protocols provided by the manufacturer. The sequences of the primers used in this study were as follow:miR-30b: sense, 5’-TGAAAGAGAGAACGATAAATGTT-3’ and antisense, 5’-ACTTCTGAATCAAAATATTGGTA-3’; U6: sense, 5’-CTGGTAGGGTGCTCGCTTCGGCAG-3’ and antisense, 5’-CAACTGGTGTCGTGGAGTCGGC-3’. The U6 gene was used as an endogenous control and the relative fold change in the expression of miR-30b was calculated using the comparative 2-ΔΔCT method. Each reaction was performed in triplicate.
Western blot assay
The cells were lysed in radioimmunoprecipitation (RIPA) buffer (Sangon Biotech, Shanghai, China) and the resulting cell lysates were quantitated using the bicinchoninic acid (BCA) assay (Pierce, Rochford, IL, USA). Equal amounts of the cell lysates were then subjected to a 10% SDS-PAGE, followed by blotting onto a Polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA, USA). The primary antibodies to the CBX3, BAX, BCL2, JAK2, p-JAK2, STAT3, p-STAT3, p-STAT5, and GAPDH (1:1000) genes were purchased from Cell Signaling Technology (Beverly, MA, USA) and were used for incubating the membranes overnight at 4°C, followed by incubation with a horseradish peroxidase (HRP)-labeled secondary antibody (1:1000 dilution) at 37°C for 1 h. Subsequently, the protein bands were detected by an enhanced chemiluminescence (ECL) detection system (GE Healthcare, Piscataway, NJ, USA). The GAPDH gene was used as an internal control.
Statistical analysis
Data obtained from the multiple experiments were presented as mean ± standard deviation (SD). Student’s t-test was employed for analyzing the significant differences between two groups and the one-way ANOVA was used for comparing three groups or more. All statistical analyses were performed using the SPSS 19.0 software (IBM, SPSS, Chicago, IL, USA) and the results were considered statistically significant at P<0.05.
Results
miR-30b is downregulated in the ESCC tissues and cells
As shown in Figure 1, the qRT-PCR analysis was performed to detect the expression of miR-30b in the ESCC tissues and cells. The significant downregulation of miR-30b was observed in the ESCC tissues, compared to that in the adjacent normal tissues (P<0.05, Figure 1A). Similarly, miR-30b was downregulated in the TE-1 and TE-2 cells, relative to that in the NEEC cells (P<0.05, Figure 1B). Moreover, the expression of miR-30b in the TE-2 cells was significantly lower than that in the TE-1 cells (P<0.05, Figure 1B). Taken together, these data indicated that the expression of miR-30b was associated with the progression of ESCC.
Figure 1.
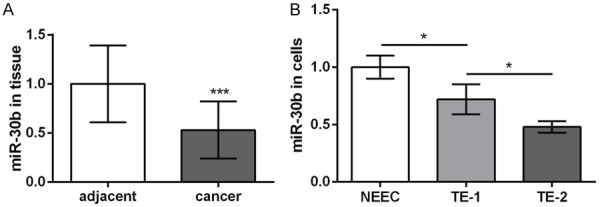
The expression levels of miR-30b in esophageal squamous cell carcinoma (ESCC) tissues and their adjacent normal tissues (A), as well as in ESCC cell lines (TE-1 and TE-2) and normal esophageal cell line NEEC (B). *P<0.05, ***P<0.001 compared with corresponding control.
The inhibition of miR-30b promotes cell proliferation, inhibits cell apoptosis, and enhances cell migration in ESCC
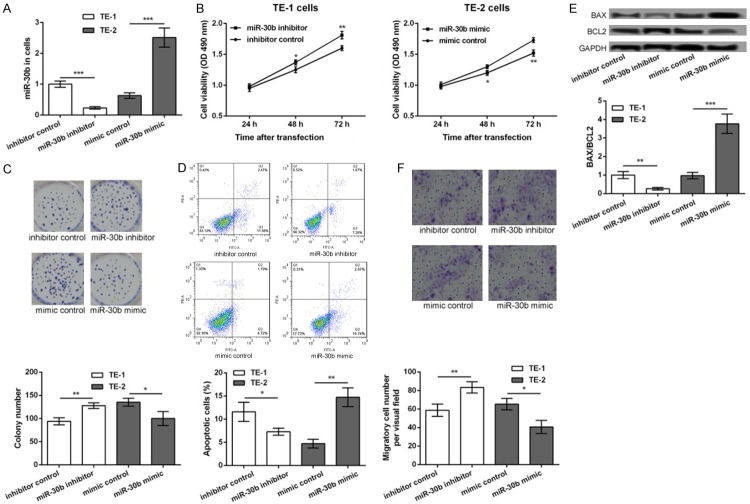
In order to detect the role of miR-30b in the development of ESCC, the TE-1 cells were transfected with the miR-30b inhibitor and the inhibitor control, while the TE-2 cells were transfected with the miR-30b mimic and the mimic control. After 48 h of transfection, the expression of miR-30b was significantly decreased in the miR-30b inhibitor transfected TE-1 cells but was markedly increased in the miR-30b mimic transfected TE-2 cells, compared to their corresponding controls (P<0.001, Figure 2A), indicating that the transfection efficiency was high and could be used for further analysis. After 24, 48, and 72 h of transfection, the cell viability of the different transfection groups was assessed. The transfection with the miR-30b inhibitor markedly promoted the viability of the TE-1 cells, whereas transfection with the miR-30b mimic suppressed the viability of the TE-2 cells (P<0.05, Figure 2B). The colony formation assay also showed that transfection with the miR-30b inhibitor significantly increased the number of colonies of the TE-1 cells, while transfection with the miR-30b mimic decreased the number of colonies of the TE-1 cells (P<0.05, Figure 2C). Furthermore, the results of the flow cytometry analysis showed that the apoptosis of the TE-1 cells was significantly inhibited after transfection with the miR-30b inhibitor, while the apoptosis of the TE-2 cells was markedly inhibited after transfection with the miR-30b mimic (P<0.05, Figure 2D). Similar changes in the expression levels of Bax/Bcl2 were obtained from the Western blot assay. The expression levels of Bax/Bcl2 were significantly decreased in the miR-30b inhibitor transfected TE-1 cells and were markedly increased in the miR-30b mimic transfected TE-2 cells, compared to their corresponding controls (P<0.05, Figure 2E). Besides, the results of the transwell migration assay showed that the number of the migratory TE-1 cells was significantly decreased after transfection with the miR-30b inhibitor but was increased after transfection with the miR-30b mimic (P<0.05, Figure 2E), indicating that the inhibition of miR-30b promoted cell migration.
Figure 2.
The inhibition of miR-30b increased esophageal cancer cell proliferation, inhibited cell apoptosis, and promoted cell migration. TE-1 cells were transfected with miR-30b inhibitor and inhibitor control, while TE-2 cells were transfected with miR-30b mimic and mimic control. A: The expression levels of miR-30b in different transfected cells. B: MTT assay showing the cell viability of different transfected cells. C: Colony formation assay showing the colony formation number of different transfected cells. D: Flow cytometry showing the apoptotic cells in different transfected groups. E: The Western blot showing the expression of BAX/BCL2 in different transfected cells. F: Transwell assay showing the number of migratory cells in different transfected groups. Compared with corresponding control, *P<0.05, **P<0.01 and ***P<0.001.
CBX3 is a target of miR-30b
According to the information provided by the TargetScan software, CBX3 was predicted to be a potential target of miR-30b (Figure 3A). The results of the luciferase reporter assay further confirmed that the miR-30b mimic significantly inhibited the luciferase reporter activity of the CBX3 3’UTR-wt construct (P<0.05), compared to the CBX3 3’UTR-mut construct (Figure 3B). The Western blot assay, performed to detect the expression of the CBX3 protein in the ESCC tissues and cells, showed that CBX3 was significantly upregulated in the ESCC tissues, relative to the adjacent normal tissues (P<0.05, Figure 3C). The expression of CBX3 was also upregulated in the TE-1 and TE-2 cells compared to the NEEC cells (P<0.05, Figure 3D). The expression of CBX3 in the TE-2 cells was significantly higher than that in the TE-1 cells (P<0.05, Figure 3D). Furthermore, the expression of CBX3 was significantly increased in the miR-30b inhibitor transfected TE-1 cells but was decreased in the miR-30b mimic transfected TE-2 cells, compared to their corresponding controls, following 48 h of transfection (P<0.05, Figure 3E). All these data indicated that CBX3 was upregulated in the ESCC cells and its expression was inhibited by miR-30b.
Figure 3.
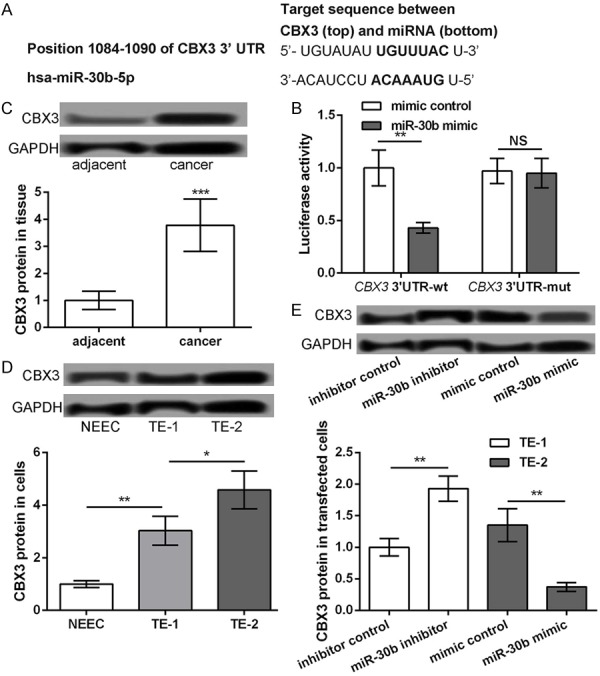
CBX3 as a target of miR-30b. A: The predicted sequence of CBX3 and miR-30b. B: Luciferase reporter assay showing the luciferase report activity of CBX3 3’UTR-wt and CBX3 3’UTR-mut after transfection with miR-30b mimic or mimic control. C: Western blot showing the protein expression level of CBX3 in ESCC tissues and their adjacent normal tissues. D: Western blot showing the protein expression level of CBX3 in ESCC cell lines (TE-1 and TE-2) and normal esophageal cell line NEEC. E: TE-1 cells transfected with miR-30b inhibitor and inhibitor control, while TE-2 cells transfected with miR-30b mimic and mimic control. The Western blot showing the protein expression level of CBX3 in different transfected groups. *P<0.05, **P<0.01 and ***P<0.001 compared with corresponding control.
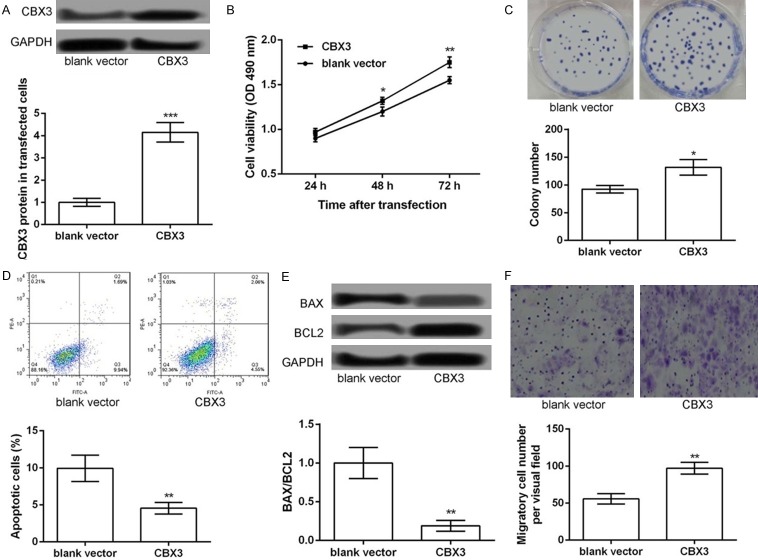
The overexpression of CBX3 promotes cell proliferation, inhibits cell apoptosis, and strengthens cell migration in the TE-1 cells
In order to detect whether CBX3 plays a key role in ESCC, the TE-1 cells were transfected with the pCDNA3.1-CBX3 vector for the overexpression of CBX3. As shown in Figure 4A, the expression of CBX3 was significantly increased after transfection with the pCDNA3.1-CBX3 vector (P<0.001, Figure 4A), indicating that CBX3 was successfully overexpressed in the TE-1 cells. The overexpression of CBX3 significantly promoted the viability (P<0.05, Figure 4B) and colony formation ability of the TE-1 cells (P<0.05, Figure 4C). In addition, the results of the flow cytometry analysis showed that the apoptosis of the TE-1 cells was significantly inhibited after the overexpression of CBX3 (P<0.05, Figure 4D) and the expression levels of Bax/Bcl2 were significantly decreased after the overexpression of CBX3 (P<0.05, Figure 4E). Furthermore, the results of the transwell migration assay showed that the number of the migratory TE-1 cells was significantly decreased after the overexpression of CBX3 (P<0.05, Figure 4F). Taken together, our data indicated that the overexpression of CBX3 promoted cell proliferation, inhibited cell apoptosis, and strengthened cell migration in the TE-1 cells, which was similar to the effects of miR-30b inhibition.
Figure 4.
Overexpression of CBX3 promoted TE-1 cell proliferation, inhibited cell apoptosis, and strengthened cell migration. TE-1 cells were transfected with pc-CBX3 and blank control. A: The expression levels of CBX3 in different transfected cells. B: MTT assay showing the cell viability of different transfected cells. C: Colony formation assay showing the colony formation number of different transfected cells. D: Flow cytometry showing the apoptotic cells in different transfected groups. E: Western blot showing the expression of BAX/BCL2 in different transfected cells. F: Transwell assay showing the number of migratory cells in different transfected groups. Compared with corresponding control, *P<0.05, **P<0.01 and ***P<0.001.
The JAK2/STAT3 pathway may be a key mechanism to mediate the role of miR-30b/CBX3 in ESCC
As shown in Figure 5A, the expression of p-JAK2/JAK2 and p-STAT3/JAK3 was significantly downregulated in the miR-30b mimic groups, compared to that in the mimic controls but was markedly reversed after the cells were cotransfected with the miR-30b mimic and the pCDNA3.1-CBX3 vector (P<0.05). However, there was no significant difference in the expression of p-STAT5/STAT5 among the different groups. Therefore, these data indicated that the JAK2/STAT3 signaling pathway might be a key mechanism to mediate the role of miR-30b/CBX3 in ESCC.
Figure 5.
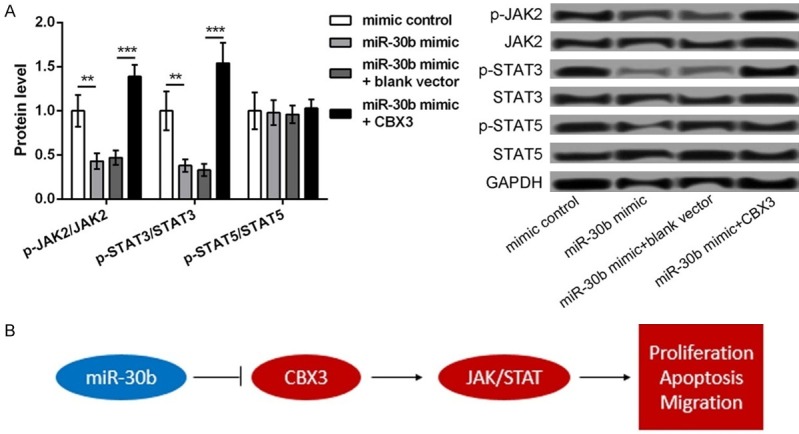
The mechanism of miR-30b targeted CBX3 in activating JAK2/STAT3 pathway, thus regulating ESCC cell proliferation, apoptosis, and migration. A. The Western blot showing the protein expression of p-JAK2/JAK2, p-STAT3/JAK3, and p-STAT5/STAT5 in TE-1 cells transfected with miR-30b mimic, mimic control, miR-30b mimic plus blank control, and miR-30b mimic plus pc-CBX3. B. The regulatory mechanism. Red nodes indicate upregulation while blue nodes indicate downregulation. **P<0.01 and ***P<0.001 compared with corresponding control.
Discussion
The present study found the reverse expression of miR-30b and CBX3 in the ESCC tissues and cells. The study showed that miR-30b was downregulated and CBX3 was upregulated. In fact, CBX3 was confirmed to be a direct target of miR-30b. The inhibition of miR-30b promoted cell proliferation, inhibited cell apoptosis, and enhanced cell migration in ESCC, which was similar to the effects of CBX3 overexpression. In addition, the overexpression of miR-30b significantly decreased the expression levels of p-JAK2/JAK2 and p-STAT3/JAK3, which was markedly reversed after the simultaneous overexpression of CBX3. The mechanism of miR-30b in the development of ESCC is shown in Figure 5B, suggesting a regulatory role for miR-30b in the development of ESCC.
In a previous study, miR-30b was found to be decreased in the esophageal cancer tissues, suppressing the growth and migration of tumor cells [17]. Similarly, in this study, we found that miR-30b was downregulated in the ESCC tissues and downregulation of miR-30b promoted the proliferation and migration of the ESCC cells. In addition, numerous studies have exposed that miRNAs contribute to the development of esophageal cancer via the regulation of their target genes [18,19]. In this study, CBX3 was confirmed to be a direct target of miR-30b. Accumulating evidence has confirmed the overexpression of CBX3 in various cancer cells, thereby contributing to tumorigenesis [20]. Saini et al. revealed that a higher expression of CBX3 was observed in spheres compared to monolayers, suggesting that CBX3 might be a marker for Tumor Stem Cell (TSC) enrichment in osteosarcoma [21]. Again, Fan et al. reported that CBX3 could promote cell cycle and cell proliferation in colon cancer [22]. Furthermore, the increased expression of CBX3 may be a promising marker for NSCLC [23]. In this study, we found that the overexpression of CBX3 promoted cell proliferation, inhibited cell apoptosis, and strengthened cell migration in the TE-1 cells, which was similar to the effects of miR-30b inhibition. Given the key roles of CBX3 in multiple cancers, we speculate that the downregulation of miR-30b may regulate cell proliferation, cell apoptosis, and cell migration in ESCC by targeting CBX3.
In order to further elucidate the possible mechanism of miR-30b in the development of ESCC, the relationship between miR-30b and the JAK2/STAT3 signaling pathway was investigated. Several studies have confirmed that the activation of the JAK2/STAT3 signaling pathway widely occurs with high frequency in ESCC and disruption of this pathway can repress the tumorigenesis and progression of ESCC [24,25]. Thus, blocking the JAK2/STAT3 signaling pathway can inhibit tumor growth in ESCC by regulating cell growth, cell cycle, and angiogenesis [26]. In addition, the inactivation of the STAT3 signaling pathway promotes the inhibitory effect of metformin on the growth of tumor cells in ESCC [27]. Furthermore, the depletion of the transmembrane protein, B7-H4 can repress the secretion of the cytokine, IL-6 via the inactivation of the JAK2/STAT3 signaling pathway, thereby suppressing cell proliferation in ESCC [28]. In this study, the overexpression of miR-30b resulted in the decreased expression of p-JAK2/JAK2 and p-STAT3/JAK3, which was reversed after the simultaneous overexpression of CBX3. Taken together, these data indicate that the overexpression of miR-30b may inhibit the development of ESCC via the inactivation of the JAK2/STAT3 signaling pathway. Thus, the miR-30b/CBX3/JAK2/STAT3 signaling pathway may play a key role in regulating the progression of ESCC.
In conclusion, our results reveal that the downregulation of miR-30b may increase cell proliferation, inhibit cell apoptosis, and promote cell migration in ESCC by targeting CBX3 and activating the JAK2/STAT3 signaling pathway. Thus, miR-30b may serve as a useful marker for predicting the progression of ESCC.
Acknowledgements
This work was supported by Shandong Medical and Health Science and Technology Development program, Grant No: 2016WS0329.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014:65. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2–9. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 5.Lento A, Long A, Giroux V, Tang Q, Sammons M, Klein-Szanto A, et al. Investigating the role of mutant p53 in esophageal squamous cell carcinoma. AACR. 2017 [Google Scholar]
- 6.Qin HD, Liao XY, Chen YB, Huang SY, Xue WQ, Li FF, Ge XS, Liu DQ, Cai Q, Long J. Genomic characterization of esophageal squamous cell carcinoma reveals critical genes underlying tumorigenesis and poor prognosis. The American Journal of Human Genetics. 2016;98:709–727. doi: 10.1016/j.ajhg.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Svoronos AA, Engelman DM, Slack FJ. OncomiR or tumor suppressor? The duplicity of microRNAs in cancer. Cancer Res. 2016;76:3666–3670. doi: 10.1158/0008-5472.CAN-16-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trang P, Weidhaas JB, Slack FJ. The molecular basis of human cancer. Springer; 2017. MicroRNAs and Cancer; pp. 277–286. [Google Scholar]
- 9.Abba ML, Patil N, Leupold JH, Moniuszko M, Utikal J, Niklinski J, Allgayer H. MicroRNAs as novel targets and tools in cancer therapy. Cancer Lett. 2017;387:84–94. doi: 10.1016/j.canlet.2016.03.043. [DOI] [PubMed] [Google Scholar]
- 10.Adams BD, Kasinski AL, Slack FJ. Aberrant regulation and function of microRNAs in cancer. Curr Biol. 2014;24:R762–R776. doi: 10.1016/j.cub.2014.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song Y, Li J, Zhu Y, Dai Y, Zeng T, Liu L, Li J, Wang H, Qin Y, Zeng M. MicroRNA-9 promotes tumor metastasis via repressing E-cadherin in esophageal squamous cell carcinoma. Oncotarget. 2014;5:11669. doi: 10.18632/oncotarget.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ni Y, Meng L, Wang L, Dong W, Shen H, Wang G, Liu Q, Du J. MicroRNA-143 functions as a tumor suppressor in human esophageal squamous cell carcinoma. Gene. 2013;517:197–204. doi: 10.1016/j.gene.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 13.Shafiee M, Aleyasin SA, Vasei M, Semnani SS, Mowla SJ. Down-regulatory effects of miR-211 on long non-coding RNA SOX2OT and SOX2 genes in esophageal squamous cell carcinoma. Cell Journal (Yakhteh) 2016;17:593. doi: 10.22074/cellj.2016.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu ED, Li N, Li BS, Li W, Zhang WJ, Mao XH, Guo G, Zou QM, Xiao B. miR-30b, downregulated in gastric cancer, promotes apoptosis and suppresses tumor growth by targeting plasminogen activator inhibitor-1. PLoS One. 2014;9:e106049. doi: 10.1371/journal.pone.0106049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao H, Xu Z, Qin H, Gao Z, Gao L. miR-30b regulates migration and invasion of human colorectal cancer via SIX1. Biochem J. 2014;460:117–129. doi: 10.1042/BJ20131535. [DOI] [PubMed] [Google Scholar]
- 16.Zhong K, Chen K, Han L, Li B. MicroRNA-30b/c inhibits non-small cell lung cancer cell proliferation by targeting Rab18. BMC Cancer. 2014;14:703. doi: 10.1186/1471-2407-14-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q, Zhang X, Li N, Liu Q, Chen D. miR-30b inhibits cancer cell growth, migration, and invasion by targeting homeobox A1 in esophageal cancer. Biochem Biophys Res Commun. 2017;485:506–512. doi: 10.1016/j.bbrc.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Ren L, Chen W, Li S, He X, Zhang Z, Li M, Cao R, Hao B, Zhang H, Qiu H. MicroRNA-183 promotes proliferation and invasion in oesophageal squamous cell carcinoma by targeting programmed cell death 4. Br J Cancer. 2014;111:2003. doi: 10.1038/bjc.2014.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng H, Wang K, Chen X, Guan X, Hu L, Xiong G, Li J, Bai Y. MicroRNA-330-3p functions as an oncogene in human esophageal cancer by targeting programmed cell death 4. Am J Cancer Res. 2015;5:1062. [PMC free article] [PubMed] [Google Scholar]
- 20.Takanashi M, Oikawa K, Fujita K, Kudo M, Kinoshita M, Kuroda M. Heterochromatin protein 1γ epigenetically regulates cell differentiation and exhibits potential as a therapeutic target for various types of cancers. Am J Pathol. 2009;174:309–316. doi: 10.2353/ajpath.2009.080148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saini V, Hose CD, Monks A, Nagashima K, Han B, Newton DL, Millione A, Shah J, Hollingshead MG, Hite KM. Identification of CBX3 and ABCA5 as putative biomarkers for tumor stem cells in osteosarcoma. PLoS One. 2012;7:e41401. doi: 10.1371/journal.pone.0041401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan Y, Li H, Liang X, Xiang Z. CBX3 promotes colon cancer cell proliferation by CDK6 kinase-independent function during cell cycle. Oncotarget. 2017;8:19934. doi: 10.18632/oncotarget.15253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han SS, Kim WJ, Hong Y, Hong SH, Lee SJ, Ryu DR, Lee W, Cho YH, Lee S, Ryu YJ. RNA sequencing identifies novel markers of non-small cell lung cancer. Lung Cancer. 2014;84:229–235. doi: 10.1016/j.lungcan.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Du XL, Wang CJ, Lin DC, Ruan X, Feng YB, Huo YQ, Peng H, Cui JL, Zhang TT. Reciprocal activation between PLK1 and Stat3 contributes to survival and proliferation of esophageal cancer cells. Gastroenterology. 2012;142:521–530. e523. doi: 10.1053/j.gastro.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 25.You Z, Xu D, Ji J, Guo W, Zhu W, He J. JAK/STAT signal pathway activation promotes progression and survival of human oesophageal squamous cell carcinoma. Clin Transl Oncol. 2012;14:143–149. doi: 10.1007/s12094-012-0774-6. [DOI] [PubMed] [Google Scholar]
- 26.Fang J, Chu L, Li C, Chen Y, Hu F, Zhang X, Zhao H, Liu Z, Xu Q. JAK2 inhibitor blocks the inflammation and growth of esophageal squamous cell carcinoma in vitro through the JAK/STAT3 pathway. Oncol Rep. 2015;33:494–502. doi: 10.3892/or.2014.3609. [DOI] [PubMed] [Google Scholar]
- 27.Feng Y, Ke C, Tang Q, Dong H, Zheng X, Lin W, Ke J, Huang J, Yeung SC, Zhang H. Metformin promotes autophagy and apoptosis in esophageal squamous cell carcinoma by downregulating Stat3 signaling. Cell Death Dis. 2014;5:e1088. doi: 10.1038/cddis.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X, Wang L, Wang W, Zhao L, Shan B. B7-H4 facilitates proliferation of esophageal squamous cell carcinoma cells through promoting interleukin-6/signal transducer and activator of transcription 3 pathway activation. Cancer Sci. 2016;107:944–954. doi: 10.1111/cas.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]