Abstract
The aim of this study is to explore the influence of miR-146a on cisplatin resistance in non-small cell lung cancer (NSCLC) cells and the related molecular mechanism. The expression of miR-146a in NSCLC tumor samples and cell lines was measured by qRT-PCR. The DDP (cisplatin) cytotoxicity was detected by CCK-8 assay. The protein expressions of TRAF6, IRAK1, p50, p-p65, p65 in normal DDP-resistant cells were determined by western blot analysis. Luciferase reporter assay was used to investigate the relationship between miR-146a and NF-κB pathway activity. The expression of miR-146a in DDP-resistant NSCLC tumor samples was significantly lower than that in DDP-sensitive ones. Its expression in DDP-resistant cell lines was much lower as well. The protein levels of TRAF6, IRAK1 and p50 were up-regulated in A549/DDP and Calu-1/DDP cells compared to parental cells, and phosphorylation of p65 was also increased, indicating the activation of NF-κB signaling pathway. Furthermore, NF-κB activity was conversely related with miR-146a level in NSCLC. It was revealed that miR-146a expression in NSCLC was negatively correlated with activation of of NF-κB pathway. In conclusion, the study indicates that miR-146a regulates the DDP sensitivity by inhibiting NF-κB signaling pathway in NSCLC cells.
Keywords: MiR-146a, NSCLC, DDP-resistance, NF-κB
Introduction
Lung cancer is a leading cause of cancer-related deaths in the world, despite improvement in detection methods and treatment. And non-small cell lung cancer accounts for nearly 80% of all lung cancers [1]. Non-small cell lung cancer (NSCLC) are the leading cause of cancer mortality, and the overall 5-year survival rate of NSCLC patients is not more than 15% [2]. Although surgical resection and adjuvant therapy can effectively treat NSCLC, patients with tumor metastasis are mostly incurable because of its systemic nature and the resistance of disseminated tumor cells to existing therapeutic agents, including chemotherapy [3]. And chemotherapy is in the first line treatment of lung cancer. Due to the multi-drug resistance, it has become a major obstacle in the present tumor treatment. Cisplatin (DDP), which is one of the most broadly used chemotherapeutic agents in the treatment of cancer, especially in NSCLC. The cytotoxic effects of cisplatin are mediated by its interaction with DNA, resulting in the formation of DNA adducts which activates several signal transduction pathways and culminate in the activation of apoptosis.
Despite the well-defined clinical manifestation of NSCLC, a given stage is often associated with wide-ranging survival rates and outcomes, even the same histological type of NSCLC might have different treatments. This inconsistency has resulted in an increased demand for the underlying mechanism and identifying novel clinical biomarkers. Somatic cell produced reprogramming requires reconfiguration of cellular signaling pathways and gene expression profiles, including microRNAs. MiRNAs (microRNAs) are small non-coding RNAs that play important roles in posttranscriptional gene transcription. Mis-regulations of miRNA expression often result in abnormal tissue homeostasis and cancers [4]. MiRNAs are short noncoding RNA molecules with 18~25 nucleotides in length which regulate specific messenger RNA (mRNA) translation post-transcriptionally in many cellular processes. Increasing reports indicate that miRNAs play important roles in regulating the drug sensitivity of tumor cells. MiRNAs have also been reported to be crucial factors in regulation of inflammation [5]. Recent studies show that microRNAs might regulate chemotherapy resistance. Hence, finding a novel biomarker with increasing the sensitivity of cancer cells to chemotherapy is necessary. MiR-146 and miR-155 were the most relevant microRNAs involving in the inflammation. Studies have indicated that miR-146a regulates inflammation through TLR4-mediated pathway [5-7]. For example, expression of miR-146a was positively associated with those of IRAK, TRAF and TLR4 [8]. The induction of miR-146a was indicated to be NF-Κb-dependent, and besides, miR-146a is directly to down-regulate TNF receptor-associated factor 6 (TRAF6) and IL-1 receptor-associated kinase 1 (IRAK1), two of the signal transducers in the NF-κB activation pathway [9]. In vitro experiments demonstrated that miR-146a suppressed NF-κB pathway and shifted the balance of cytokines in the cord blood toward a repertoire of pro-inflammatory outcomes by downregulating IRAK1 and TRAF6 [10].
However, little is known about the role of miR-146a and its mechanism of action. It has been reported that restoration of miR-146a expression inhibited NF-κB by targeting NF-κB pathway [11]. Furthermore, NF-κB pathway was recently suggested to be targeted by miR-146a as a part of an NF-κB induced negative feedback loop [9,12]. In this study, we found that miR-146a expression was down-regulated in cisplatin-resistant non-small cell lung cancer cells, and tested the hypothesis that miR-146a are important modulators of cisplatin resistance by targeting NF-κB-mediated signaling.
Materials and methods
Samples and cell culture
A total of 28 NSCLC and adjacent non-tumor tissues were collected from patients at Second Hospital of Lanzhou University (Lanzhou, China) between February 2016 and January 2017. Informed consent was acquired from all subjects and this study was approval by the Clinical Research Ethics Committee of Second Hospital of Lanzhou University. NSCLC cell lines A549 (adenocarcinoma), Calu-1 and DDP-resistant cell strain A549/DDP, Calu-1/DDP were also purchased from ATCC. For all cell lines, media was supplemented with 10% fetal bovine serum (FBS). MiR-146a mimics and negative control mimics (NC) were purchased from Genepharma. Total RNA and protein were prepared 72 h after transfection and were used for qRT-PCR or Western blot analysis.
Cell viability assay
Cells were seeded into 96-well plates at a density of 5×103 cells per well. 24 h later, cells were added serially diluted cisplatin at 1.6-100 μM. After 48 h incubation, 10 μl CCK-8 solution was added to each well after treatment, followed by another 2 h incubation, cell viability was assessed by absorbance at 450 nm.
Quantitative real-time PCR of miRNA
It was verified the expression of selected miRNAs in A549/DDP, Calu-1/DDP and parental cells by qRT-PCR. Total RNA of each cell line was extracted by Trizol reagent. 1 μg of total extracted RNA sample was reverse transcribed into cDNA using PrimeScriptTM RT reagent Kit following the manufacturer’s protocol (Takara, Otsu, Japan). Real-time qRT-PCR was applied in a volume of 20 μl reaction on the ABI Stepone plus instrument. The primers used for miRNA qPCR are shown as follow, RT primer GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAACCCA, Forward primer TGAGAACTGAATTCCA and Reverse primer GTGCAGGGTCCGAGGT for miR-146a, RT primer AACGCTTCACGAATTTGCGT, Forward primer CTCGCTTCGGCAGCACA and Reverse primer AACGCTTCACGAATTTGCGT for U6. The cycling conditions were as follow: initial denaturation at 95°C for 30 s, 95°C for 60 s, 57°C for 30 s, 35 cycles. Relative miRNA expression was evaluated by the 2-ΔΔCt method. All qRT-PCRs were performed in triplicates.
Western blot assay
About 1×106 A549 and Calu-1 cells transfected for 72 h and total protein was collected. An equal quantity of proteins extraction was separated in 12% SDS-PAGE and transferred onto a PVDF membrane. The membrane was block with 5% non-fat milk for 1 hour, incubated with primary antibodies overnight at 4°C, followed by incubation of horse radi shper oxidase conjugated goat anti-rabbit IgG antibody (Abcam, Cat no. ab6721; 1:2000 dilution). Signals intensity were measured by the ECL-PLUS/Kit (Amersham, Cat no. RPN2132) following the manufacturer’s protocol. The blots were quantified by densitometry using Quantity One software (Bio-Rad, CA, USA).
Actin antibody was used as an internal control. The primary antibodies were as follow: rabbit anti-TRAF6 (Cat. no. ab33915, 1:2000 dilution), IRAK1 (Cat. no. ab19234, 1:1000 dilution), p50 (Cat. no. ab194729, 1:500 dilution), mouse anti-p-p65 (Cat. no. ab6503, 1:16000 dilution), P65 (Cat. no. ab32536, 1:50000 dilution), Actin (Cat. no. ab2302, 1:200 dilution) were provided by Abcam (MA, USA).
Luciferase reporter assay
DDP-sensitive and -resistant cells were transfected with PGL3-NFκB luciferase reporter plasmid and PRL-TK reference plasmid (SA Biosciences, Frederick, MD) for 48 hours. Cells were washed with PBS and lysed with reporter lysis buffer. After centrifugation for 10 min, supernatant was harvested. Relative NF-κB luciferase activity was measured by the Dual-Luciferase Reporter Assay System (Promega, Madison, WI).
Annexin V-fluorescein isothiocyanate (FITC)/propidium Iodide (PI) apoptosis assay
An Annexin V-FITC Apoptosis Detection kit (Beyotime Institute of Biotechnology, Haimen, China) was used to detect apoptosis strictly according to the manufacturer’s protocol. Cell apoptosis analysis was applied a FACS can instrument (Becton Dickinson, Mountain view, CA, US) equipped with Cell Quest software.
Statistical analysis
The experimental data are presented as the mean ± standard deviation (SD). All statistical analyses were performed using SPSS 13.0 (Chicago, IL, USA). P < 0.05 was considered to be statistically significant. Data were analyzed by two-tailed-unpaired t-test or one-way ANOVA where appropriate.
Results
Quantitative analysis of miR-146a expression in DDP-resistant NSCLC tumor samples
To investigate the association between miR-146a and DDP sensitivity, 28 clinical tumor tissue samples were obtained from patients with advanced lung adenocarcinoma. As illustrated in Figure 1, miR-146a expression was significantly decreased in DDP-resistant group compared to DDP-sensitive group. It was divided into “sensitive” and “resistance” groups according to the patient’s response to cisplatin-based chemotherapy. A one-way ANOVA assay was used to detect the miRNA-146a expression in 28 lung cancer tissue samples.
Figure 1.
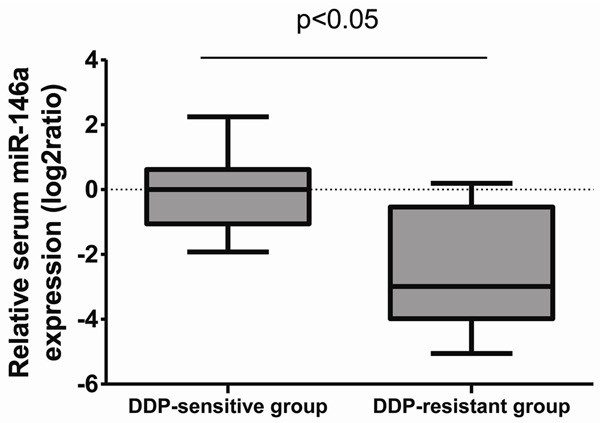
The expression of miR-146a in NSCLC samples. Relative expression levels of miR-136a were detected in cisplatin-sensitive and resistance NSCLC tissue samples via qRT-PCR.
Expression of miR-146a was decreased in DDP-resistant NSCLS cell lines
To investigate the relationship between mir-146a and DDP resistance, two NSCLC cell lines A549, Calu-1 and their DDP-resistant strains were used. Cell sensitivities to DDP were certified by CCK-8 assay (Figure 2). Expression of miR-146a was significantly decreased in DDP-resistant A549 (Figure 2A) and Calu-1 (Figure 2B) compared to parental cells, which is consistent to the result from clinical samples.
Figure 2.
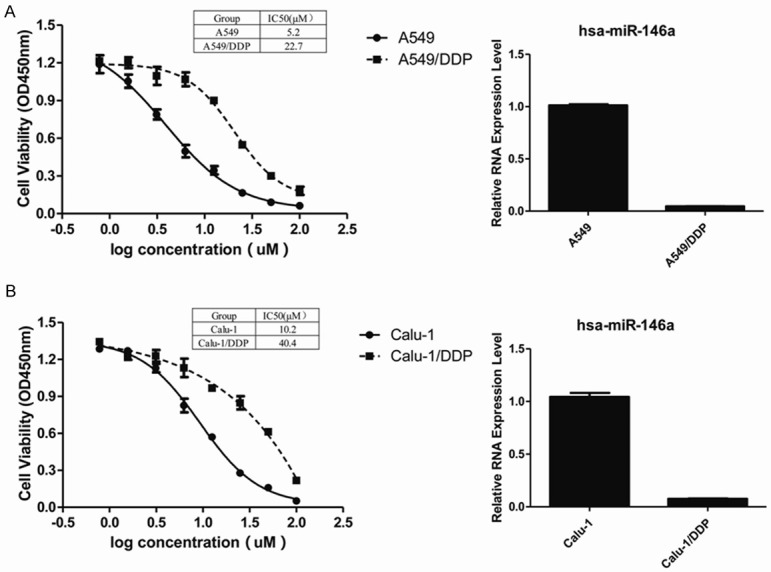
Different expression level of miR-146a in DDP-sensitive or -resistant NSCLC cell lines. The cell viability was evaluated by CCK-8 assay, A549/DDP transfected with NC treated with serially diluted DDP (A). The cell viability was evaluated by CCK-8 assay, Calu-1/DDP cells transfected with NC treated with serially diluted DDP (B).
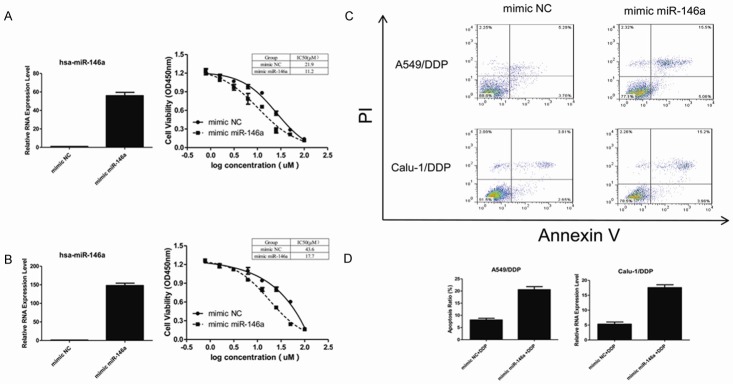
MiR-146a restored cisplatin activity in DDP-resistant cells
To better understand the roles of miR-146a in the DDP resistance NSCLC cells, we transfected the cells with miR-146a mimic or negative control. As shown in Figure 3, the expression of miR-146a significantly (P < 0.05) increased and sensitized A549/DDP (Figure 3A) and Calu-1/DDP (Figure 3B) cells to cisplatin induced inhibition of cell viability, which was determined by CCK-8 assay. In Figure 3C and 3D, expression of miR-146a significantly increased DDP induced apoptosis. A549/DDP and Calu-1/DDP cells were transfected with 50 nM mimic miR-146a or mimic NC for 48 hours and followed by 24-hours treatment of 10 μM DDP. Cell apoptosis was detected using FITC-Annexin V/PI staining. Annexin V-FITC/PI apoptosis detection by flow cytometry revealed that, miR-146a could significantly (P < 0.05) increase apoptosis rate of A549/DDP (20.6 vs 8.1%) or Calu-1/DDP (17.6 vs 5.3%) cells compared with control group.
Figure 3.
Transfection with mimic miR-146a restored miR-192 expression level compared with the negative control groups. Expression of miR-146a significantly increased and sensitized A549/DDP (A) and Calu-1/DDP (B) cells to cisplatin induced inhibition of proliferation. In (C) and (D), expression of miR-146a significantly increased DDP induced apoptosis. Cell apoptosis was detected by FITC-Annexin V/PI staining using flow cytometry.
MiR-146a directly targeted at NFκB1
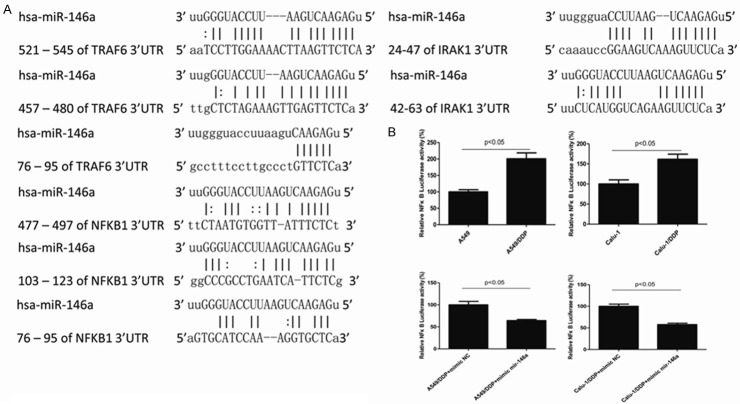
TRAF6, IRAK1 and NFκB1 were chosen as preferred candidates target gene of miR-146a predicted by open access online tools miRTar Base and microRNA.org (Figure 4A).
Figure 4.
miR-146a directly targeted at NF-κB. The predicted target genes of miR-146a (A). DDP-sensitive and -resistant cells were transfected with PGL3-NFκB luciferase reporter plasmid and PRL-TK reference plasmid, relative NF-κB luciferase activity was mesured by luciferase reporter assay (B).
To further determine whether miR-146a regulate DDP by targeting at the NF-κB activity, we conducted luciferase reporter assay. As shown in Figure 4B, NF-κB activity was increased in DDP-resistant cells and could be eliminated by miR-146a. DDP-sensitive and -resistant cells were transfected with PGL3-NFκB luciferase reporter plasmid and PRL-TK reference plasmid, relative NF-κB luciferase activity was measured. NF-κB activity was dramatically increased in DDP-resistant cells than in DDP-sensitive cells. Transfection of miR-146a in DDP-resistant cells could downregulate NF-gB activity (Figure 4B).
Many researchers have reported TRAF6 and IRAK1 as targets of miR-146a in various cell types, including monocytes and macrophages [13]. Also TRAF6 and IRAK1 are upstream signal transducers of NF-κB activation [14], therefore, we investigated TRAF6, IRAK1 and NF-κB1, of which NF-κB1 was proven to be a direct target gene of miR-146a.
MiR-146a inhibits NF-κB signaling pathway by down-regulating TRFA6, IRAK1 and NFκB1 (P105/p50)
As shown in Figure 5, the protein level of TRAF6, IRAK1 and p50 were upregulated in A549/DDP and Calu-1/DDP cells compared with parental cells, and phosphorylation of p65 was also increased indicating the activation of NF-κB signaling pathway. Transfection of miR-146a mimic recovered TRAF6, IRAK1 and p50 to normal level and the eliminated phosphorylation of p65.
Figure 5.
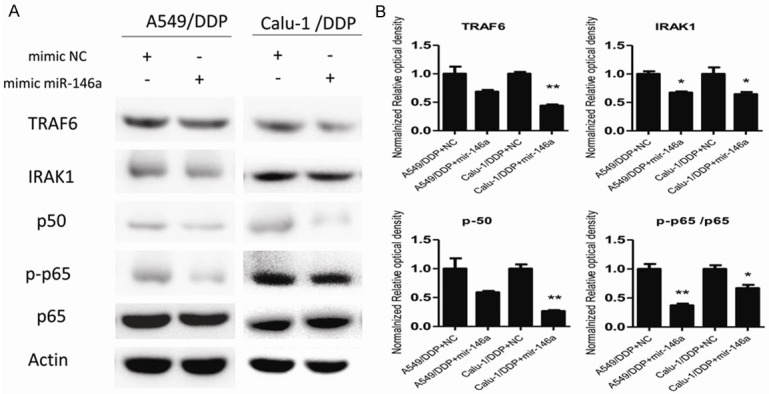
The protein levels of TRAF6, IRAK1, p50, p-p65, p65 and Actin were analyzed in A549/DDP and Calu-1/DDP cells stably transfected with mimic miR-146a or mimic NC by western blotting (A) and qPCR (B). All data presented as mean value ± SD from 3 independent experiments. N.S. = not significant, **P < 0.01, ***P < 0.05.
Taken together (Figures 4 and 5), the miR-146a inhibits NF-κB signaling pathway by luciferase assay and western blotting. These results suggest that miR-146a directly targets NF-κB in NSCLC cells, and miR-146a may negatively regulate the activation of F-κB pathway.
Discussion
The expression of miRNAs varies with the development of lung cancer, and most of these miRNA variations emerge during tumorigenesis [15].
MiRNAs are more stable than mRNAs, which enables them to be readily detected in formalin-fixed paraffin-embedded tissue. miR-146a is located at the chromosome 11 and has been shown to regulate inflammation through TLR4-mediated pathway [16-18]. In additional, increasing evidences suggested the involvement of miR-146a in the pathogenesis of multiples diseases, including atherosclerosis, bacterial infection and cancer [19]. Although a great number of studies reported the induction of miR-146a expression by pro-inflammatory mediators [20,21], there is still little information about its role and mechanism.
Herein, we further verified the expression of miR-146a in lung cancer tissue. Consistent with our expectations, miR-146a is highly expressed in NSCLC and can specifically detect the high expression of miR-146a in DDP-resistant lung cancer tissues compared to DDP-sensitive ones. In the present study, we demonstrated that miR-146a is a critical cisplatin resistance regulator in non-small cell lung cancer via NF-κB pathway. Furthermore, down-upregulation of miR-146a is correlated with cisplatin resistance and poor prognosis of NSCLC patients. Moreover, miR-146a seems to play an important role in mediating drug resistance as shown in this study. MiR-146a modulated cisplatin-resistance via NF-κB pathway. These findings provide novel insights into the molecular functions of miR-146a and the role of NF-κB in cisplatin-resistance.
Chemo-resistance is a major issue occurred during the treatment in the majority of human tumors, including lung cancer. And chemotherapy is the most common method to treat against the tumor. Therefore, exploring biomarkers to increase chemotherapy sensitivity and screening for novel targets to regulate multi-drug resistance for cancer treatment are urgent. A particular miRNA can affect the expression of proteins involved in many cellular pathways, and possibly serving as novel therapeutic target or biomarker for clinical outcome than single proteins [22]. Several miRNAs have shown used as predictors of chemo-resistance in cancers [23].
Therefore, our study illustrated that miR-146a regulates the DDP sensitivity by inhibiting NF-κB signaling pathway. Our results demonstrated that miR-146 could modulate cisplatin resistance, which offer novel mechanistic insights into the role of miR-146a/NF-κB pathway in the cisplatin resistance of NSCLC cells.
Acknowledgements
This study was supported by Natural Science Foundation of Gansu Province (Grant No.: 17JR5RA243).
Disclosure of conflict of interest
None.
References
- 1.Bishop JA, Benjamin H, Cholakh H, Chajut A, Clark DP, Westra WH. Accurate classification of non-small cell lung carcinoma using a novel microrna-based approach. Clin Cancer Res. 2010;16:610–619. doi: 10.1158/1078-0432.CCR-09-2638. [DOI] [PubMed] [Google Scholar]
- 2.Cho WC. [Promises and challenges in developing MiRNA as a molecular diagnostic tool for lung cancer] . Expert Rev Mol Diagn. 2011;11:763–766. doi: 10.1586/erm.11.71. [DOI] [PubMed] [Google Scholar]
- 3.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monsalve E, Pérez MA, Rubio A, Ruiz-Hidalgo MJ, Baladrón V, García-Ramírez JJ, Gómez JC, Laborda J, Díaz-Guerra MJ. Notch-1 upregulation and signaling following macrophage activation modulates gene expression patterns known to affect antigen-presenting capacity and cytotoxic activity. J Immunol. 2006;176:5362–5373. doi: 10.4049/jimmunol.176.9.5362. [DOI] [PubMed] [Google Scholar]
- 5.Echavarria R, Mayaki D, Neel JC, Harel S, Sanchez V, Hussain SN. Angiopoietin-1 inhibits toll-like receptor 4 signalling in cultured endothelial cells: role of miR-146b-5p. Cardiovasc Res. 2015;106:465–477. doi: 10.1093/cvr/cvv120. [DOI] [PubMed] [Google Scholar]
- 6.Fan G, Jiang X, Wu X, Fordjour PA, Lin M, Zhang H, Zhu Y, Gao X. Anti-inflammatory activity of tanshinone IIA in LPS-stimulated RAW264.7 macrophages via miRNAs and TLR4-NF-κB pathway. Inflammation. 2016;39:375–384. doi: 10.1007/s10753-015-0259-1. [DOI] [PubMed] [Google Scholar]
- 7.Xie YF, Shu R, Jiang SY, Liu DL, Ni J, Zhang XL. MicroRNA-146 inhibits pro-inflammatory cytokine secretion through IL-1 receptor-associated kinase 1 in human gingival fibroblasts. J Inflamm (Lond) 2013;10:20. doi: 10.1186/1476-9255-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi Y, Satoh M, Minami Y, Tabuchi T, Itoh T, Nakamura M. Expression of miR-146a/b is associated with the toll-like receptor 4 signal in coronary artery disease: effect of renin-angiotensin system blockade and statins on miRNA-146a/b and toll-like receptor 4 levels. Clin Sci. 2010;119:395–405. doi: 10.1042/CS20100003. [DOI] [PubMed] [Google Scholar]
- 9.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang W, Kong L, Ni Q, Lu Y, Ding W, Liu G, Pu L, Tang W, Kong L. MiR-146a ameliorates liver ischemia/reperfusion injury by suppressing IRAK1 and TRAF6. PLoS One. 2014;9:e101530. doi: 10.1371/journal.pone.0101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhaumik D, Scott GK, Schokrpur S, Patil CK, Campisi J, Benz CC. Expression of microRNA-146 suppresses NF-κB activity with reduction of metastatic potential in breast cancer cells. Oncogene. 2008;27:5643–5647. doi: 10.1038/onc.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taganov KD, Boldin MP, Baltimore D. MicroRNAs and immunity: tiny players in a big field. Immunity. 2007;26:133–137. doi: 10.1016/j.immuni.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Hou J, Wang PL. MicroRNA-146a feedback inhibits RIG-I-dependent type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J Immunol. 2009;183:2150–2158. doi: 10.4049/jimmunol.0900707. [DOI] [PubMed] [Google Scholar]
- 14.Boldin MP, Taganov KD, Rao DS, Yang L, Zhao JL, Kalwani M, Garciaflores Y, Luong M, Devrekanli A, Xu J, Sun G, Tay J, Linsley PS, Baltimore D. MiR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med. 2011;208:1189–201. doi: 10.1084/jem.20101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Yang H, Li Y, Liu Y, Chen S, Qi C, Zhang Q, Lan T, He X, Guan XY. MicroRNA-146 up-regulation predicts the prognosis of non-small cell lung cancer by miRNA in situ hybridization. Annu Rev Pathol. 2014;96:195–199. doi: 10.1016/j.yexmp.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Echavarria R, Mayaki D, Neel JC, Harel S, Sanchez V, Hussain SN. Angiopoietin-1 inhibits tolllike receptor 4 signalling in cultured endothelial cells: role of miR-146b-5p. Cardiovasc Res. 2015;106:465–477. doi: 10.1093/cvr/cvv120. [DOI] [PubMed] [Google Scholar]
- 17.Elton TS, Selemon H, Elton SM, Parinandi NL. Regulation of the MIR155 host gene in physiological and pathological processes. Gene. 2013;532:1–12. doi: 10.1016/j.gene.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Fan G, Jiang X, Wu X, Fordjour PA, Miao L, Zhang H, Zhu Y, Gao X. Anti-inflammatory activity of tanshinone IIA in LPS-stimulated RAW264.7 macrophages via miRNAs and TLR4-NF-κB pathway. Inflammation. 2016;39:375–384. doi: 10.1007/s10753-015-0259-1. [DOI] [PubMed] [Google Scholar]
- 19.Li Z, Wang S, Zhao W, Sun Z, Yan H, Zhu J. Oxidized low-density lipoprotein upregulates microRNA-146a via JNK and NF-κB signaling. Mol Med Rep. 2016;13:1709–16. doi: 10.3892/mmr.2015.4729. [DOI] [PubMed] [Google Scholar]
- 20.Roos J, Enlund E, Funcke JB, Tews D, Holzmann K, Debatin KM, Wabitsch M, Fischerposovszky P. MiR-146a-mediated suppression of the inflammatory response in human adipocytes. Sci Rep. 2016;6:38339. doi: 10.1038/srep38339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olivieri F, Lazzarini R, Recchioni R, Marcheselli F, Rippo MR, Di Nuzzo S, Albertini MC, Graciotti L, Babini L, Mariotti S, Spada G, Abbatecola AM, Antonicelli R, Franceschi C, Procopio AD. MiR-146a as marker of senescence-associated pro-inflammatory status in cells involved in vascular remodelling. Age. 2013;35:1157–1172. doi: 10.1007/s11357-012-9440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pandima Devi K, Rajavel T, Daglia M, Nabavi SF, Bishayee A, Nabavi SM. Targeting miRNAs by polyphenols: novel therapeutic strategy for cancer. Semin Cancer Biol. 2017;46:146–157. doi: 10.1016/j.semcancer.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Tan G, Dong L, Cheng L, Li K, Wang Z, Luo H. Circulating MiR-125b as a marker predicting chemoresistance in breast cancer. PLoS One. 2012;7:e34210. doi: 10.1371/journal.pone.0034210. [DOI] [PMC free article] [PubMed] [Google Scholar]