Abstract
Aim: This study aimed to investigate the relationship between the changes of muscle fibers and the changesof peripheral nerve functions (CMAP) with aging. Methods: Lewis rats enrolled in this study. Muscle biopsy and CMAP of tibialis anterior (TA) were measured. Results: CMAP amplitudes increased significantly in 6 months (P<0.05). CMAP latencies of peroneal nerves found to be significantly higher in older rats (P<0.05). In 6 months these two muscle fibers went through a significant decrease (P<0.05). Type IIb and type IIx muscle fibers increased significantly in 6 months when compared with those in 1 month (P<0.05). After 6 months, the percentages of type I and type IIa muscle fibers increased as the month increased (P<0.05). The percentages of type IIb muscle fibers and type IIx muscle fibers dropped significantly as the month increased (P<0.05). Type I and type IIa muscle fibers were negatively related to CMAP amplitudes (P<0.05). Type IIb muscle fibers were positively related to CMAP amplitudes (P<0.05). Conclusion and discussion: There is a shift from the fast types (Type IIx and IIb) to slow types (Type I and IIa) in muscle fibers with agingwhich is consistent with changes in CMAP amplitude of peripheral nerves.
Keywords: Aging, CMAP, muscle type
Introduction
Skeletal muscle atrophy is inevitable with aging. Age related skeletal muscle atrophy and force decline, which is also called sarcopenia, limits daily living activities and leads to morbidity and mortality in the elderly. Atrophy is characterized by active degradation, removal of contractile proteins, and a shift in muscle fiber isoforms. In other words, the transitions in muscle fiber isoforms with aging is usually accompanied with decrease in strength and power [1,2], as well as an increase in muscle weakness (the strength per unit of cross-sectional area of muscle) and fatigability [3,4].
Skeletal muscle composes of muscle fibers that differ in their speed of contraction and predominant type of energy metabolism [4]. Currently, four major fiber types, which include a slow-type fiber (Type I) and three fasttype fibers (Types IIA, IIB and IIX), are generally recognized in mature skeletal muscle. Muscle fibers can be classified as type I, slow-twitch fibers, and type II, fast-twitch fibers, base on their predominant myosin heavy chain (MHC) isoform content. Muscle fibers can adapt to different usage by transformation from one type to another, and such transformation can also occur with aging. It is known that type I can transform into type IIx fiber as illustrated: I↔I/IIa ↔IIa ↔IIa/X ↔IIa ↔IIb/x ↔IIb [5]. The proportion of each muscle fiber type in skeletal muscle defines the overall functional capabilities which are also affected by neuromuscular activity, exercise training, thyroid hormone, and mechanical loading [6,7].
To date, several questions with respect to the mechanisms underlying age-related muscle atrophy remain unresolved. Changes in each type of muscle fibers especially their relationships with peripheral nerves degeneration have not been totally investigated yet.
In this study, it was hypothesized that the changes in peripheral nerves were correlated with the fast-to-slow type transformations during aging. Aging has definite effects on neuromuscular functions. Compound muscle action potential (CMAP) is a valuable assessment of routine nerve conduction (NCS). And NCS can directly reflect peripheral motor and sensory functions [7]. Commonly measured parameters of CMAP include latency and amplitude.
Materials and methods
Experimental research has been approved by the Ethics Committee of Shanghai Tenth People’s Hospital. And the use of animals in experiments has observed the Interdisciplinary Principles and Guidelines for the Use of Animals in Research, Testing, and Education by the New York Academy of Sciences, Ad Hoc Animal Research Committee. 60 female Lewis rats were used (obtained from Central Lab of Shanghai TenthPeople’sHospital, Shanghai, China) with an average body weight of 100±5 gat the beginning of the experiments. All rats were housed in a temperature and humidity controlled room with 12 h light and 12 h dark cycles. The room temperature was controlled at 26°C and rat body temperature maintained at around 35°C by a heating pad. The animals were offered standard chow and water ad libitum. All rats were housed two weeks before the experiments to adapt to the new environment. 10 rats were picked randomly every time at 1, 3, 6, 12, 18 and 24 months for CMAP and histological analysis of tibialis anterior (TA) muscle.
CMAP measurement
CMAP was recorded (Nicolet Viking IV; Shanghai Tenth People’s Hospital, Shanghai, China) in the peroneal muscles of rats at the age of 1, 3, 6, 12, 18 and 24 months. The ground electrode was inserted subcutaneously at the heel, the reference electrode was inserted subcutaneously at the fifth metatarsal joint, and the active recording electrode was inserted subcutaneously in the dorsum of the foot.
The transcutaneous stimulating electrodes were placed along the course of the sciatic nerve. Supramaximum stimulation was applied to elicit a response. All tests were repeated twice to ensure reproducibility. ANOVA was performed to compare CMAP values among different groups. Tukey’s post hoc test was performed when P<0.05.
Muscle fiber analysis by biopsy with triple immunostaining method
Frozen muscle specimens were transferred to the -20°C cryostat (Leica CM3050S, German) by dry ice. 10 um frozen sections were cut and fixed in absolute acetone at 4°C for 5 min. Four monoclonal primary antibodies including anti-myosin A4.74 IgG1, anti-myosin BA-F8 IgGb2, anti-myosin BF-F3 IgM, and anti myosin BF-F35 IgG1 (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) were used in this study.
For single immunohistochemicalimmunostaining, frozen section was incubated with one primary antibody (1/5 dilution) for 1 hour at room temperature. After washed, the section was incubated in the peroxidase conjugated second antibody which was included in the ImmPRESS REAGENT Anti-Mouse Ig kit (Vector Lab) followed by respective detection kits (Vector Lab) as follows: DAB kit (brown) for A4.74 and BF-F35, SG kit (grey) for BA-F8. The sections with BF-F3 antibody were incubated in the second antibody followed by ABC complex from the Alkaline Phosphatase Mouse IgM ABC kit (Vector Lab) and visualized using the Vulcan fast Red chromogen kit 2 (red) from Biocare Medical, CA. All immunostaining using the detection kit were performed following the manufacture’s instructions. The triple immunostaining was performed using the polymerized reporter enzyme staining system for the first two antigens and alkaline phosphatase labeling for the third antigen to avoid the nonspecific cross reactions between the different detection systems. All the sections were scanned by NDP machine (Nanozoomei Digital Pathology 2.0HT, HAMAMATSU, Inc). We systematically sampled and counted 33% of the whole muscle fibers on the scanned sections by the Image J software (NIH). The result showed in Figure 1.
Figure 1.
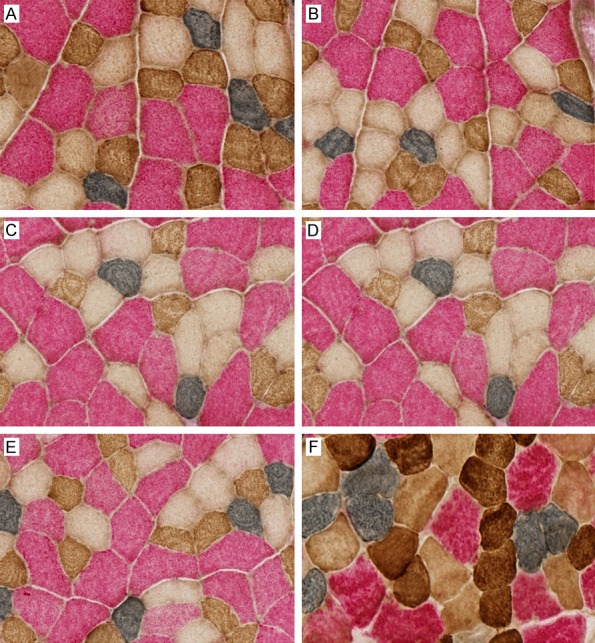
Different muscle fibers’ distribution by triple immunostaining method. A. Different muscle fiber proportion in 1 month. B. Different muscle fiber proportion in 3 months. C. Different muscle fiber proportion in 6 months. D. Different muscle fiber proportion in 12 months. E. Different muscle fiber proportion in 18 months. F. Different muscle fiber proportion in 24 months.
Statistical analysis
Data collected were first entered in the Microsoft Excel Worksheet and then statistically analyzed by using SPSS 20.0 version. Due to the non-normal distribution of data, Mann Whitney U test was applied to analyze NCS variables. P<0.05 was considered as statistically significant.
Results
Effect of aging on motor nerve conduction study variables
CMAP amplitudes of peroneal nerves in 3 months were not significantly different from the first month (P>0.05). However CMAP amplitudes increased significantly in 6 months when compared with that in 1 month (P<0.05). After that CMAP amplitudes showed a decreasing trend with aging, but without significance (P>0.05). CMAP latencies of peroneal nerves didn’t show any converse result with CMAP amplitude. CMAP latencies of peroneal nerves showed no significant changes in 3 months compared with 1 month (P>0.05). Only were CMAP latencies of peroneal nerves found to be significantly higher in older rats (6-24 months) than that in 1 months (P<0.05). The datesareshowed in Tables 1, 2 and 3.
Table 1.
Changes of CMAP during aging process
| CMAP | Amplitude (mv) | Latencies (ms) |
|---|---|---|
| 1 month | 17.35±1.17 | 0.91±0.38 |
| 3 months | 18.40±1.2 | 1.12±0.21 |
| 6 months | 18.51±1.27 | 1.33±0.31 |
| 12 months | 17.10±4.24 | 1.22±0.18 |
| 18 months | 16.67±1.43 | 1.28±0.11 |
| 24 months | 16.37±0.97 | 1.70±0.38 |
Table 2.
P value of CMAP amplitude comparison between different time points
| vs. | 3 | 6 | 12 | 18 | 24 |
|---|---|---|---|---|---|
| 1 | 0.064 | 0.048* | 0.863 | 0.262 | 0.058 |
| 3 | 0.837 | 0.375 | 0.01 | 0.001 | |
| 6 | 0.337 | 0.007 | 0.001 | ||
| 12 | 0.767 | 0.608 | |||
| 18 | 0.592 |
P<0.05.
Table 3.
P value of CMAP latencies comparison between different time points
| vs. | 3 | 6 | 12 | 18 | 24 |
|---|---|---|---|---|---|
| 1 | 0.151 | 0.014* | 0.036* | 0.014* | 0.000* |
| 3 | 0.085 | 0.242 | 0.044* | 0.001* | |
| 6 | 0.345 | 0.639 | 0.029* | ||
| 12 | 0.391 | 0.003* | |||
| 18 | 0.007* |
P<0.05.
Effect of aging on muscle fiber proportion
The percentages of type I and type IIa muscle fibers in 3 months were no significantly different from those in 1 month (P>0.05). In 6 months these two muscle fibers went through a significant decrease when compared with 1 month (P<0.05). Similarly the percentages of type IIb and type IIx muscle fibers in 3 months were no significantly different from those in 1 month (P>0.05). On the contrary, type IIb and type IIx muscle fibersincreased significantly in 6 months when compared with those in 1 month (P<0.05). After 6 months, the percentages of type I and type IIa muscle fibers increased asthe month increased (P<0.05), except for the non-significant change between 6 and 12 months of type I muscle fiber (P>0.05). Simultaneously, the percentages of type IIb muscle fibers and type IIx muscle fibers dropped significantly as the month increased (P<0.05), except for the changes of the non-significant change between 18 and 24 months of type IIx muscle fiber (P>0.05). The data areshowed in the Tables 4, 5, 6, 7 and 8 below.
Table 4.
Changes of muscle types during aging process
| Muscle fibers | Type I | Type IIa | Type IIb | Type IIx |
|---|---|---|---|---|
| 1 month | 3.31%±0.96% | 12.27%±1.91% | 59.69%±1.95% | 24.73%±1.22% |
| 3 months | 2.96%±0.64% | 11.40%±1.50% | 60.32%±3.19% | 25.33%±1.96% |
| 6 months | 2.35%±0.15% | 7.03%±1.60% | 63.75%±2.67% | 26.89%±2.03% |
| 12 months | 2.66%±0.59% | 14.99%±1.09% | 60.24%±2.49% | 22.12%±2.08% |
| 18 months | 4.80%±1.12% | 18.67%±1.66% | 57.89%±1.49% | 18.63%±1.65% |
| 24 months | 6.16%±0.76% | 22.40%±3.27% | 53.71%±3.57% | 17.73%±2.31% |
Table 5.
P value of Type I muscle comparison between different time points
| I | 3 | 6 | 12 | 18 | 24 |
|---|---|---|---|---|---|
| 1 | 0.347 | 0.006* | 0.089 | 0.005* | 0.000* |
| 3 | 0.010* | 0.297 | 0.000* | 0.000* | |
| 6 | 0.125 | 0.000* | 0.000* | ||
| 12 | 0.000* | 0.000* | |||
| 18 | 0.006* |
P<0.05.
Table 6.
P value of Type IIa muscle comparison between different time points
| IIa | 3 | 6 | 12 | 18 | 24 |
|---|---|---|---|---|---|
| 1 | 0.273 | 0.000* | 0.002* | 0.000* | 0.000* |
| 3 | 0.000* | 0.000* | 0.000* | 0.000* | |
| 6 | 0.000* | 0.000* | 0.000* | ||
| 12 | 0.000* | 0.000* | |||
| 18 | 0.007* |
P<0.05.
Table 7.
P value of Type IIb muscle comparison between different time points
| IIb | 3 | 6 | 12 | 18 | 24 |
|---|---|---|---|---|---|
| 1 | 0.602 | 0.001* | 0.059 | 0.034* | 0.000* |
| 3 | 0.018* | 0.095 | 0.043* | 0.000* | |
| 6 | 0.007* | 0.000* | 0.000* | ||
| 12 | 0.022* | 0.000* | |||
| 18 | 0.003* |
P<0.05.
Table 8.
P value of Type IIx muscle comparison between different time points
| IIx | 3 | 6 | 12 | 18 | 24 |
|---|---|---|---|---|---|
| 1 | 0.424 | 0.011* | 0.004* | 0.000* | 0.000* |
| 3 | 0.096 | 0.002* | 0.000* | 0.000* | |
| 6 | 0.000* | 0.000* | 0.000* | ||
| 12 | 0.000* | 0.000* | |||
| 18 | 0.332 |
P<0.05.
Results showed that type I and type IIa muscle fibers were negatively related to CMAP amplitudes. (P<0.05), Pearson correlation: -0.408 and -0.408). Meanwhile type IIb muscle fibers were positively related to CMAP amplitudes (P<0.05, Pearson correlation: 0.588). The data areshowed in the Figure 2 below.
Figure 2.
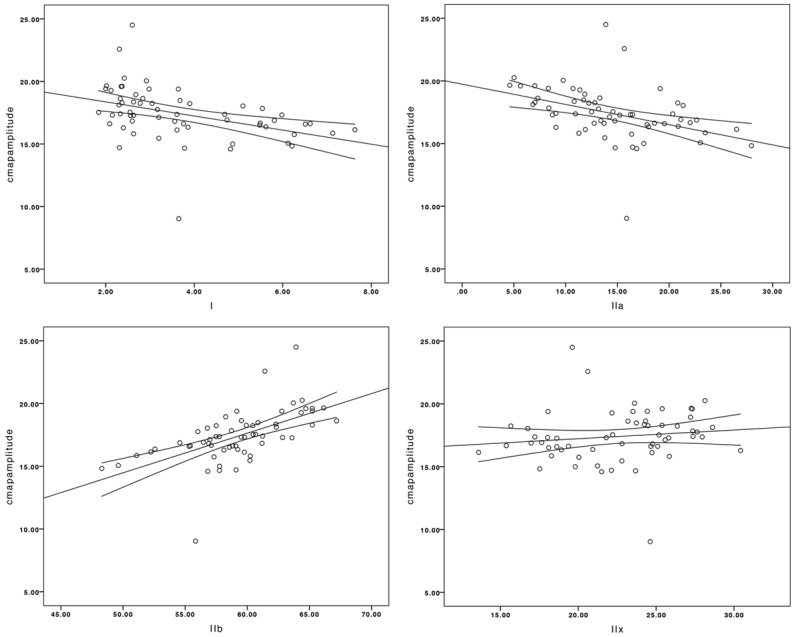
The changes of type I and type IIa muscle fibers were negatively related to the shifts of CMAP amplitudes. (P<0.05, Pearson correlation: -0.408 and -0.408). The changes of type IIb muscle fibers were positively related to the shifts of CMAP amplitudes (P<0.05, Pearson correlation: 0.588). The changes of type IIx fiber muscle were not related to the shifts of CMAP amplitudes (P>0.05).
Discussion
This study aimed to investigate the age-related changes on different muscle fibers and peripheral nerves. There were no significant changes on CMAP amplitudes of peroneal nerves within 3 months. At 6 months, CMAP amplitudes increased significantly than that in 1 month. After that CMAP amplitudes showed a decreasing trend with aging. Buschbacher et al. also found decrease in CMAP amplitude of peroneal nerve in older age subjects as compared to the younger individuals [7]. In addition, Huang et al. found that the subjects with older age had smaller amplitudes compared to the younger age group [8]. Hennessey et al. also found similar decrease in CMAP amplitude of the median nerve in the elderly [9]. However, CMAP latencies of peroneal nerves showed different results. CMAP latencies showed significant increasesince6 months when compared with that in 1 month.
Noteworthy, although a variety of experiments existed in terms of the comparison between the elderly and younger individuals, there was no studyinvestigating change during the process of aging.In this study, CMAP was compared among the three different periods (neonate, youth and elderly). It was defined that the neonate phase within the first 3 months, youth phase 3-6 months, and elderly phase 6-24 months.
According to the results, CMAP underwent a growing process at the beginning. Initially, peripheral nerve function stood still in the neonate phase. In youth phase, the peripheral nerve function developed. The peripheral nerve functionwas lost partially after the youth phase. CMAP latencies showed no significant difference within first 3 months, and the reason might be the hyposensitivity of CMAP latencies or relatively small sample sizes of the study. CMAP measurement can assess the function of peripheral nerve by stimulating the nerve and recording the activities of the target muscle. The changes of CMAP in this study demonstrated that aginghad definite effects on peripheral nerve functions. Till youth, peripheral nerves developed. With aging, the reason why amplitude showed a decreasing trend after youth is probably the decrease of the muscle mass and motor unit size. Besides, decrease in the number of nerve fibers might lead to the same consequence as well [10-12].
In this study, the data were summarized into 6age groups to simplify the presentation. The analysis of age effects on muscle fibers and NCS was performed (Figure 3). The transformations of different muscle fibers were also assessed under the whole process of aging.
Figure 3.
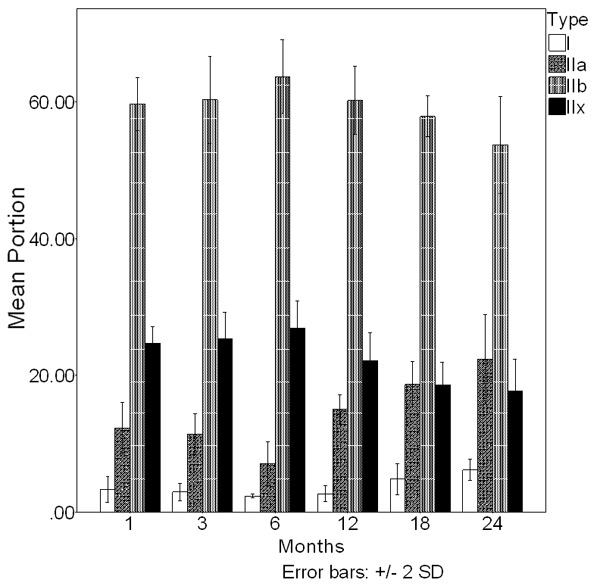
Before 6 months the percentages of type I muscle fibers and type IIa muscle fibers gone through a decrease compared with the beginning of experiment (P<0.05). And the percentages of type IIx muscle fibers and type IIb muscle fibers have increased compared with the beginning of experiment (P<0.05). After 6 months, the percentages of type I muscle fibers and type IIx muscle fibers have increased significantly than 6 months before (P<0.05). Simultaneously, the percentages of type IIa muscle fibers and type IIb muscle fibers dropping significantly than before (P<0.05).
The percentages of type I and type IIa muscle fibers in 3 months were not significantly different from those in 1 month (P>0.05). In 6 months these two muscle fibers went through a significant decrease compared with those in 1 month (P<0.05). Similarly, the percentages of type IIb and type IIx muscle fibers in 3 months were not significantly different from those in 1 month (P>0.05). In 6 months, type IIb and type IIx muscle fibers increased significantly compared with those in 1 month (P<0.05). After 6months, the percentages of type I and type IIa muscle fibers increased significantly than before (P<0.05), except for type I in 12 months (P>0.05). Simultaneously, the percentages of type IIb muscle fibers and type IIx muscle fibers dropped significantly after 6 months (P<0.05), except for type IIx in 24 months. Kevin et al. [13] also found there was a shiftin the proportion of different muscle fibers with aging in healthy people. After 20 years old, Type I muscle fiber would decrease consecutively. And Type IIx muscle fiber would increase. They concluded that older muscles required a longer period in translating adaptive responses at the gene-transcript level into changes in the expression of MHC protein isoforms. Xin Feng et al. [14] showed that aging had similar effects on monkeys compared with humans, which focused on the vastus lateralis muscle of monkey. Thus, there was a fast-to-slow transformation of muscle fibers during aging.
In addition, some researches in regard to involuntary muscle showed that Type IIb and IIx decreased consistent with selective degeneration of peripheral nerves in the older rats, mice and humans [15-17]. Some researches confirmed the myofiber changes by immunohistochemistry. Several studies revealed that the etiopathogenesis of myofiber changes mainly due to the degeneration of nuclei in muscles. Extensive DNA damage occurring with aging caused the transformation of different muscle fibers and the decrease of myofiber mass [18,19]. Transformation of type II muscle fibers caused by aging (23%) was more severe than Type I (57%) [20]. According to Mathieu-Costello’s study [21], aging would cause the disproportion between capillary surface per fiber surface and fiber mitochondrial volume in muscles. Nevertheless, muscle fiber mitochondrial volume deteriorates always occurred predominantly in slow fibers. The reason may be that type I and type IIa muscle fibers were oxidative, whereas type IIx and type IIb fibers were primarily glycolytic, hence type I and IIa muscle fibers have more oxidative capacity than type IIb and IIx [20]. In addition, the study of Gorza [5] identified that type I can be transformed into type IIx muscle fiber as illustrated: I↔II/IIa ↔ IIa ↔ IIa/X ↔ IIa ↔ IIb/x ↔ IIb. Type IIa used to be defined as another relatively slow-twitch fiber [5,20].
Others believed that the proportion of type I and IIa fibers increased, possibly reflecting reinnervation of neighboring denervated type IIb and/or IIx fibers by the most abundant type I and IIa fibers. Indeed, there was an increase in DNA damage of nuclei from older animals by oxidative stress [22]. That was to say the effects of denervation and reinnervationwere fulfilled in the whole process of aging.
The changes of MHC protein expression, the damage of nuclei, and the apoptosis of myofibers may all decrease muscle strength and functions. In this study, the effects of NCS in the process of musculoskeletal degenerationwere highlighted. In this study, with the process of aging, changes of CMAP amplitude were significantly related to the transition of I, IIa, IIb muscle fiber phenotypes (P<0.05, Pearson correlation: -0.408, -0.408 and 0.588). Thus, it can behypothesized that peripheral nerves played a key role in the aging of skeletal muscles. And the exception of Type IIx fiber muscle may due to the small sample size of this study. According to the results, it can be concluded that at the beginning of lifetime, the function of peripheral nerves and the quantity of musculoskeletal fibers developed. That is the reason why the amplitude of CMAP increased and the slow-to-fast muscle fiber shift existed in the first 12 months. After youth, either the musculoskeletal functions or peripheral nerves degenerated. Consequently, the amplitude of CMAP decreased, the latencies of CMAP increased, and the majority of muscle fiber tended to be slow switch fibers.
Conclusions
In conclusion, the degeneration of NCS possibly contributes to the changes of myofibers to a significant degree. Further investigation of the factors that can cause fiber type transitions during aging may be continued especially in the developing period before youth. Thus, proper factors may be available inmanagements of muscle atrophy.
Acknowledgements
The study was funded by the National Natural Science Foundation of China (NO. 81401851) and the Fundamental Research Funds for the Central Universities (NO. 10247201756).
Disclosure of conflict of interest
None.
References
- 1.Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol (1985) 1991;71:644–650. doi: 10.1152/jappl.1991.71.2.644. [DOI] [PubMed] [Google Scholar]
- 2.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 3.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 4.Ohnuki Y, Umeki D, Cai W, Kawai N, Mototani Y, Shiozawa K, Jin HL, Fujita T, Tanaka E, Saeki Y, Okumura S. Role of masseter muscle beta (2)-adrenergic signaling in regulation of muscle activity, myosin heavy chain transition, and hypertrophy. J Pharmacol Sci. 2013;123:36–46. doi: 10.1254/jphs.12271fp. [DOI] [PubMed] [Google Scholar]
- 5.Gorza L. Identification of a novel type 2 fiber population in mammalian skeletal muscle by combined use of histochemical myosin ATPase and anti-myosin monoclonal antibodies. J Histochem Cytochem. 1990;38:257–265. doi: 10.1177/38.2.2137154. [DOI] [PubMed] [Google Scholar]
- 6.Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- 7.Young A, Stokes M, Crowe M. The size and strength of the quadriceps muscles of old and young men. Clin Physiol. 1985;5:145–154. doi: 10.1111/j.1475-097x.1985.tb00590.x. [DOI] [PubMed] [Google Scholar]
- 8.Huang CR, Chang WN, Chang HW, Tsai NW, Lu CH. Effects of age, gender, height, and weight on late responses and nerve conduction study parameters. Acta Neurol Taiwan. 2009;18:242–249. [PubMed] [Google Scholar]
- 9.Hennessey WJ, Falco FJ, Braddom RL. Median and ulnar nerve conduction studies: normative data for young adults. Arch Phys Med Rehabil. 1994;75:259–264. doi: 10.1016/0003-9993(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs JM, Love S. Qualitative and quantitative morphology of human sural nerve at different ages. Brain. 1985;108:897–924. doi: 10.1093/brain/108.4.897. [DOI] [PubMed] [Google Scholar]
- 11.Tohgi H, Tsukagoshi H, Toyokura Y. Quantitative changes with age in normal sural nerves. Acta Neuropathol. 1977;38:213–220. doi: 10.1007/BF00688067. [DOI] [PubMed] [Google Scholar]
- 12.Vital A, Vital C, Rigal B, Decamps A, Emeriau JP, Galley P. Morphological study of the aging human peripheral nerve. Clin Neuropathol. 1990;9:10–15. [PubMed] [Google Scholar]
- 13.Leeuwenburgh C, Gurley CM, Strotman BA, Dupont-Versteegden EE. Age-related differences in apoptosis with disuse atrophy in soleus muscle. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1288–1296. doi: 10.1152/ajpregu.00576.2004. [DOI] [PubMed] [Google Scholar]
- 14.Feng X, Zhang T, Xu Z, Choi SJ, Qian J, Furdui CM, Register TC, Delbono O. Myosin heavy chain isoform expression in the Vastus Lateralis muscle of aging African green vervet monkeys. Exp Gerontol. 2012;47:601–607. doi: 10.1016/j.exger.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greising SM, Mantilla CB, Gorman BA, Ermilov LG, Sieck GC. Diaphragm muscle sarcopenia in aging mice. Exp Gerontol. 2013;48:881–887. doi: 10.1016/j.exger.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol. 2013;45:2191–2199. doi: 10.1016/j.biocel.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Rowan SL, Rygiel K, Purves-Smith FM, Solbak NM, Turnbull DM, Hepple RT. Denervation causes fiber atrophy and myosin heavy chain co-expression in senescent skeletal muscle. PLoS One. 2012;7:e29082. doi: 10.1371/journal.pone.0029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radak Z, Naito H, Kaneko T, Tahara S, Nakamoto H, Takahashi R, Cardozo-Pelaez F, Goto S. Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle. Pflugers Arch. 2002;445:273–278. doi: 10.1007/s00424-002-0918-6. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, Kewitt K, Walter CA, Richardson A. Does oxidative damage to DNA increase with age? Proc Natl Acad Sci U S A. 2001;98:10469–10474. doi: 10.1073/pnas.171202698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Pessin JE. Mechanisms for fibertype specificity of skeletal muscle atrophy. Curr Opin Clin Nutr Metab Care. 2013;16:243–250. doi: 10.1097/MCO.0b013e328360272d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathieu-Costello O, Ju Y, Trejo-Morales M, Cui L. Greater capillary-fiber interface per fiber mitochondrial volume in skeletal muscles of old rats. J Appl Physiol (1985) 2005;99:281–289. doi: 10.1152/japplphysiol.00750.2004. [DOI] [PubMed] [Google Scholar]
- 22.Baker SJ, Reddy EP. Modulation of life and death by the TNF receptor superfamily. Oncogene. 1998;17:3261–3270. doi: 10.1038/sj.onc.1202568. [DOI] [PubMed] [Google Scholar]


