Abstract
Dact3 is a negative regulator of Wnt/β-catenin signaling. c-Myb promotes tumor cell invasion through Wnt/β-catenin pathway. However, the detailed mechanism by which Dact3 and c-Myb modulate the progression of non-small cell lung cancer (NSCLC) remains unclear. In this study, the expressions of Dact3 and c-Myb in 254 surgically resected NSCLC samples were detected by immunohistochemistry. We transfected Dact3 cDNA to A549 and H157 cells or siRNA-Dact3 to SPC cells and examined above effects on the activity of Wnt/β-catenin signaling by Western blot and luciferase activity assay, in addition to cell biological behavior by Transwell and MTT assay. Dact3 expression was reduced in NSCLC tissue. Reduced Dact3 expression was correlated with lymph node metastasis and poor prognosis of NSCLC (P<0.05). In addition, Dact3 expression was negatively correlated with the c-Myb expression (R = -0.626, P<0.05). Dact3 transfection resulted in c-Myb reduced expression in NSCLC cells, as well as decreased activity of Wnt/β-catenin signaling and reduced cell invasive and proliferative capacity. siRNA-Dact3 transfection had the opposite effect. Our results indicate that Dact3 may inhibit the malignant phenotype of NSCLC through downregulation of c-Myb.
Keywords: Dact3, c-Myb, c-Myc, β-catenin, NSCLC
Introduction
It is common knowledge that lung cancer is a global problem, which is a serious threat to human health [1]. The rate of early diagnosis is very low so that the postoperative survival time of patients with lung cancer is short. Therefore, it is necessary for us to identify a new indicator for the treatment of lung cancer.
Wnt signaling is a conserved intracellular process that takes part in development and cancer [2,3]. It is divided into two types: β-catenin-dependent (canonical) and β-catenin-independent (noncanonical) Wnt pathways. Dishevelled (Dvl) is a scaffold protein in Wnt signaling pathway [4]. As a direct binding partner of Dvl, Dact (Dapper antagonist of beta-catenin, including Dact1, Dact2, and Dact3) plays an important role in regulating intercellular signaling pathways [5,6]. Previous studies indicated that the Dact had negative effect on Wnt signaling [7]. Dact1 and Dact2 antagonize Wnt signaling in some human cancers [8,9]. The third Dact family member, Dact3, is an epigenetic regulator of Wnt/β-catenin signaling in colorectal cancer [10]. However, up to now nothing is known about the effect of Dact3 on Wnt/β-catenin signaling in NSCLC.
Transcription factor c-Myb plays an important role in cell cycle regulation and cell proliferation [11]. Myb contributes to cancer cell self-renewal through Wnt signaling [12]. c-Myb overexpression resulted in malignant transformation through regulating genes involved in tumourigenesis [13,14]. However, its clinical significance in NSCLC remains unclear. In this study, we aimed to detect the expressions of Dact3 and c-Myb in NSCLC tissues and analyse their correlations with clinicopathological factors. In addition, we further identify their involved mechanisms in NSCLC cell lines.
Materials and methods
Patients and specimens
There are 254 surgically resected samples of NSCLC patients in the First Affiliated Hospital of China Medical University between 2007 and 2010. The patients had not received chemotherapy or radiotherapy before surgery. Tumor types were diagnosed independently by three well experienced pathologists. The pTNM standards come from the International Union against Cancer (seventh edition) and 2004 WHO histological classification for lung cancer. This study was conducted according to the guidelines of the institutional review boards at the First Affiliated Hospital of China Medical University, we have obtained internal review board approval and/or patients informed consent for this study. Clinicopathological factors of the patients are shown in Table 1.
Table 1.
The correlation of Dact3 and c-Myb expressions with clinicopathological factors
| Clinicopathological feature | Dact3 expression | c-Myb expression | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Normal | Reduced | P | Positive | Negative | P | |
| Total | 78 | 176 | 191 | 63 | ||
| Age (years) | ||||||
| <60 | 28 | 81 | 0.133 | 86 | 23 | 0.236 |
| ≥60 | 50 | 95 | 105 | 40 | ||
| Gender | ||||||
| Male | 33 | 76 | 0.897 | 84 | 25 | 0.550 |
| Female | 45 | 100 | 107 | 38 | ||
| Histology | ||||||
| Squamous cell carcinoma | 35 | 94 | 0.124 | 102 | 27 | 0.181 |
| Adenocarcinoma | 40 | 68 | 75 | 33 | ||
| Others | 3 | 14 | 14 | 3 | ||
| TNM stage | ||||||
| I | 41 | 63 | 0.012 | 69 | 35 | 0.007 |
| II+III | 37 | 113 | 122 | 28 | ||
| Lymphatic metastasis | ||||||
| No | 61 | 110 | 0.014 | 122 | 49 | 0.041 |
| Yes | 17 | 66 | 69 | 14 | ||
| Differentiation | ||||||
| Well | 26 | 64 | 0.641 | 65 | 25 | 0.416 |
| Moderate or poor | 52 | 112 | 126 | 38 | ||
Immunohistochemical staining and evaluation
The steps of immunohistochemistry staining were the same as the prevous study [15]. The primary antibodies used were as follows: Dact3 (1: 300; Sigma-Aldrich, St. Louis, MO), c-Myb (1:50; Abcam, Cambridge, MA, USA), c-Myc (1:100; Santa Cruz Biotechnology, Dallas, TX) and β-catenin (1:100; Santa Cruz).
The percentage of positively immunohistochemical staining cells was calculated by counting 400 tumor cells. For Dact3, the scoring criteria were defined as follows: the proportion of positively staining cells (0 = absent; 1 = 1-25%; 2 = 26-50%; 3 = 51-75%; 4 = more than 75%); the staining intensity (0 = negative; 1 = weak; 2 = moderate; 3 = strong). The product of above two scores was the final score. Positive expression of Dact3 was identified as the score no less than 3; negative expression was identified as the score less than 3. For c-Myb, the immunohistochemical results showed that positive staining only localized in the nucleus of tumor cell. For c-Myc, the positive staining localized in the nucleus or nucleus/cytoplasm of tumor cell. For β-catenin, the scoring criteria were the same as the previous report [16].
Cell culture and transfection
We purchased H157 (from lung squamous cell carcinoma), A549 and SPC (from lung adenocarcinoma) cell lines from the American Type Culture Collection (Manassas, USA). Cells were cultured in the medium of RPMI 1640 (Invitrogen, Carlsbad, USA) including 10% fetal bovine serum. Dact3 plasmids were purchased from OriGene (Rockville, USA). siRNA-Dact3 (5’-GCGUGUGUGUACAGCUGAUTT-3’ and 5’-AUCACCUGCAGGCACAGCGTT-3’) and scramble siRNA (5’-UCCUCGGAGACUGUCACGUUT-3’ and 5’-ACGUGACACGUUCCGUGATTT-3’) were purchased from Shanghai GenePharma Co., Ltd (Shanghai, China). Lipofectamine 2000 (Invitrogen) was used for transfection.
Western blotting
We used RIPA lysis buffer to extract total protein. Nuclear and cytoplasmic extracts were prepared by using Nuclear and Cytoplasmic Protein Extraction kit (Beyotime Biotech, China). The steps of Western blot were the same as previous report [10]. The primary antibodies are as follows: Dact3 (Mouse monoclonal, 1:1000; Sigma-Aldrich), c-Myb (Mouse monoclonal, 1:2000; Abcam), c-Myc (Mouse monoclonal, 1:300; Santa Cruz), β-catenin (Mouse monoclonal, 1:300; Santa Cruz), LaminB1 (Mouse monoclonal, 1:500, Santa Cruz), β-actin (Mouse monoclonal, 1:3000, Sigma), α-tubulin (Mouse monoclonal, 1:500, Santa Cruz).
Transwell assay
We used polycarbonate membrane (8 μm pore) in 24-well plate and added Matrigel (BD Bioscience, San Jose, CA) to the upper surface of the membrane. Cells (3 × 105 cells in 100 μl serum-free medium) were cultured in the upper chamber, and the lower chamber was filled with medium containing 10% FBS (fetal bovine serum). After incubation for 20 h, we stained cells with hematoxylin (Sigma) and selected 10 fields (400 × magnification) randomly by microscope to get the number of invaded cells.
MTT assay
We plated cells (different treatment) in 96-well plates (3000 cells in each well) and cultured them in medium containing 10% FBS for 4 days. For demonstrating the survival status of cells, we added 20 μl MTT (thiazolyl blue, 5 mg/ml) solution to each well and incubated it for 4 h at 37°C. Then it was replaced by the resultant MTT formazan solubilized in DMSO (150 μl). We obtained the results by the way of spectrophotometer (490 nm) under microplate reader (Bio-Rad, Hercules, CA).
Dual-luciferase assay
Cells were plated in 24-well plates for 24 h and then transfected with TOPFlash or FOPFlash plasmid (Addgene, Cambridge, USA). We used Dual-Luciferase Assay System (Promega, Madison, WI) to examine the expression of reporter gene after incubation for 30 h at 37°C. The activity of Tcf-mediated gene transcription was determined from the ratio of TOPFlash to FOPFlash luciferase activity normalized to Renilla luciferase activity from the control plasmid pRL-TK. All experiments were performed by three times. Dact3 cDNA was co-transfected with TOPFlash or FOPFlash plasmid for analyzing the effect of Dact3 on Wnt/β-catenin signaling.
Statistical analysis
SPSS 17.0 was performed for this study. We applied Chi-squared test to evaluate the correlation with clinicopathological characteristics. The Spearman correlation test was applied to assay the correlations among the expression of Dact3, c-Myb, c-Myc and β-catenin. Kaplan-Meier analysis was selected to compare survival time among various NSLSC patients. A two-tailed P<0.05 was thought to be statistically significant.
Results
Dact3 expression is negatively correlated with c-Myb expression in NSCLC tissues
Dact3 was expressed in the cytoplasm of bronchial epithelial cells in normal lung tissues, while it was significantly reduced in NSCLC samples (69.3%, 176 cases, Table 1; the other 78 cases were nuclear expression and cytoplasmic expression, data not shown) (Figure 1A-C). In the 104 cases with TNM stage I, Dact3 expression was reduced in 63 samples (60.6%). In the 150 cases with TNM stage II-III, reduced expression of Dact3 was shown in 113 samples (75.3%). The ratio of Dact3 reduced expression was significantly higher in stage II-III than in stage I (P<0.05). Dact3 reduced expression was also associated with lymph node metastasis of NSCLC (P<0.05, Table 1). Then we detected the expression of c-Myb in normal lung and NSCLC tissues. We found that c-Myb was expressed mainly in the nucleus of NSCLC tumor cell (Table 2, 191 cases, 191/254 = 75.2%; the other 63 cases were mainly in cytoplasm, data not shown), while loss expression was shown in bronchial epithelial cells of normal lung tissues (Figure 1D-F). Positive expression of c-Myb was correlated with high pathologic TNM stage and lymph node metastasis of NSCLC (P<0.05, Table 1).
Figure 1.
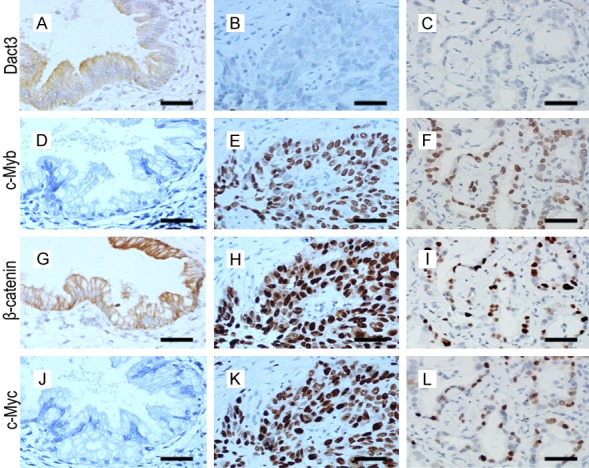
Immunostaining of Dact3, c-Myb, β-catenin and c-Myc in normal bronchial epithelial cells (A, D, G, J), lung squamous cell carcinoma (B, E, H, K) and lung adenocarcinoma (C, F, I, L). Original magnification × 400; scale bar, 20 μm.
Table 2.
Relationship among Dact3, c-Myb, c-Myc and β-catenin expressions in NSCLC
| Dact3 | ||||
|---|---|---|---|---|
|
|
||||
| Normal | Reduced | Correlation coefficient | P | |
| c-Myb | ||||
| Positive | 27 | 164 | -0.626 | 0.000 |
| Negative | 51 | 12 | ||
| c-Myc | ||||
| Positive | 43 | 128 | -0.173 | 0.006 |
| Negative | 35 | 48 | ||
| β-catenin | ||||
| Nuclear positive | 7 | 45 | -0.190 | 0.002 |
| Nuclear negative | 71 | 131 | ||
Above results showed that Dact3 expression was reduced in NSCLC. In contrast, c-Myb expression was positive in NSCLC tissues, mainly located in nucleus. Dact3 was negatively correlated with c-Myb in NSCLC tissues (R = -0.626, P = 0.000, Table 2).
We used the Kaplan-Meier analysis to examine the differences of postoperative survival among the patients with different Dact3 and c-Myb expressions. Dact3 reduced expression was correlated with shorter postoperative survival (50.516±2.953 vs 64.033±4.344 months, P<0.05, Figure 2A); positive c-Myb expression was correlated with shorter postoperative survival (50.219±2.799 vs 68.152±4.862 months, P<0.05, Figure 2B). The patients with reduced Dact3 and positive c-Myb expressions had significantly shorter postoperative survival time than others (49.959±3.042 vs 63.247±4.094 months, P<0.05, Figure 2C). Above results showed a negative relationship between Dact3 and c-Myb in the progression of NSCLC.
Figure 2.
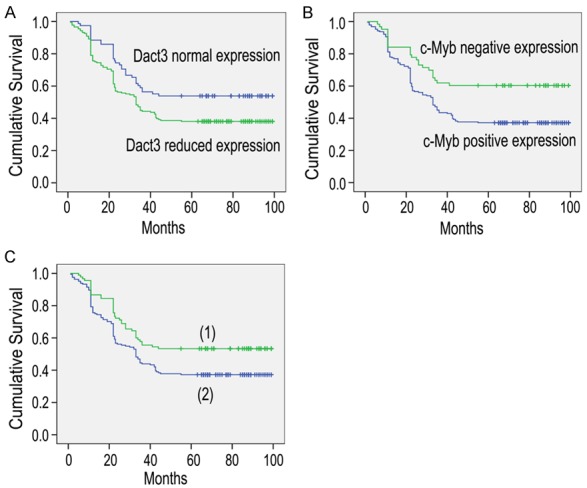
Postoperative survival among NSCLC patients with different expressions of Dact3 and c-Myb (A-C). (1) the patients without reduced Dact3 and positive c-Myb expressions; (2) the patients with reduced Dact3 and positive c-Myb expressions.
Dact3 inhibits c-Myb-induced activation of Wnt/β-catenin signaling in NSCLC cell
The target genes of c-Myb contain c-Myc, which is associated with Wnt/β-catenin signaling [17,18]. The expressions of β-catenin and c-Myc were detected by immunohistochemistry in NSCLC tissues (Figure 1G-L).
Statistical analysis showed that Dact3 reduced expression were negatively associated with nuclear positive expression of β-catenin (R = -0.190, P = 0.002), as well as c-Myc positive expression (R = -0.173, P = 0.006) and c-Myb positive expression (R = -0.626, P = 0.000, Table 2); positive c-Myb expression was significantly associated with β-catenin nuclear accumulation as well as positive c-Myc expression (Table 3), suggesting that Dact3 might inhibit Wnt/β-catenin signaling through negatively regulation of c-Myb.
Table 3.
Relationship among c-Myb, Dact3, c-Myc and β-catenin expressions in NSCLC
| c-Myb | ||||
|---|---|---|---|---|
|
|
||||
| Positive | Negative | Correlation coefficient | P | |
| Dact3 | ||||
| Normal | 27 | 51 | -0.626 | 0.000 |
| Reduced | 164 | 12 | ||
| c-Myc | ||||
| Positive | 135 | 36 | 0.125 | 0.047 |
| Negative | 56 | 27 | ||
| β-catenin | ||||
| Nuclear positive | 46 | 6 | 0.156 | 0.013 |
| Nuclear negative | 145 | 57 | ||
Dact3 transfection significantly upregulated the level of Dact3 (Figure 3), which resulted in downregulation of c-Myb expression in both A549 and H157 cells (with low expression of Dact3). In addition, β-catenin nuclear translocation and c-Myc expression were negatively regulated by Dact3 (Figure 3). The detection of β-catenin promoter luciferase reporter also confirmed that Dact3 inhibitory effect on canonical Wnt/β-catenin signaling (Figure 4B, 4C). siRNA-Dact3 transfection had the opposite effect in SPC cell line (with high expression of Dact3) (Figure 5).
Figure 3.
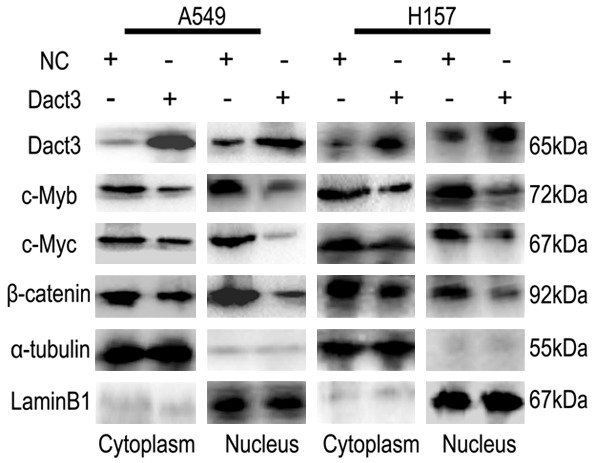
The effect of Dact3 transfection on β-catenin nuclear translocation and the expressions of c-Myb and c-Myc in A549 and H157 cells. α-tubulin: cytoplasm control. Lamin B1: nuclear control.
Figure 4.
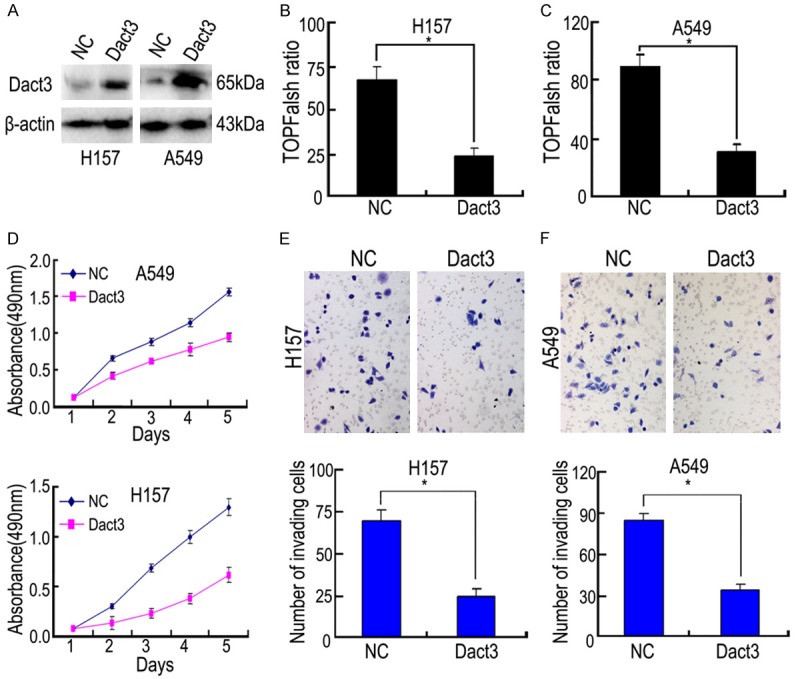
The effect of Dact3 transfection on the canonical Wnt signaling activity (B, C), cell proliferation (D) and cell invasion (E, F) in H157 and A549. (A) Dact3 protein level in H157 and A549 with Dact3 transfection; β-actin: reference control. The graph in (E, F) shows the number of invading cells. *, P<0.05 compared with negative control (NC). Error bars, S.D.
Figure 5.
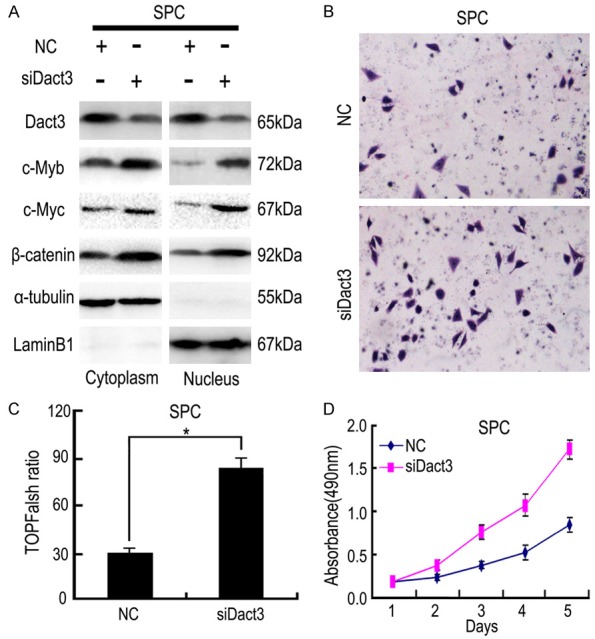
The effect of Dact3-siRNA transfection on β-catenin nuclear translocation and the expressions of c-Myb and c-Myc (A), cell invasion (B), canonical Wnt signaling activity (C) and cell proliferation (D) in SPC cells. α-tubulin: cytoplasm control. LaminB1: nuclear control. *, P<0.05 compared with negative control (NC). Error bars, S.D.
The effect of Dact3 in the proliferation and invasiveness of NSCLC cells
We performed MTT and Transwell to further test the above-mentioned effects of Dact3 in the biological behavior of NSCLC cells. In both A549 and H157 cells, Dact3 transfection significantly inhibited cell proliferation and invasion compared to the control group (Figure 4D-F). In SPC cells, siRNA-Dact3 transfection had the opposite effect (Figure 5). Above results suggested that Dact3 might inhibit cell proliferation and invasion through its downregulation of c-Myb.
Discussion
Dact takes part in various types of signaling pathways during embryo development and tumorigenesis [10,19]. It functions as an antagonist of Wnt signaling [8,20]. As a member of Dact family, Dact3 negatively regulate Wnt/β-catenin signaling in colorectal cancer [8]. In this work, we found that Dact3 expression was significantly reduced in NSCLC tissues, compared with normal bronchial epithelium. In addition, its effect on biological behavior of NSCLC cells indicated that Dact3 might inhibit the progression of NSCLC. However, the mechanism by which Dact3 is involved in NSCLC progression is unclear.
Abnormal activation of Wnt/β-catenin pathway is important for cancer progression [21]. There are many signal factors in Wnt/β-catenin signaling. The activation of Wnt/β-catenin pathway results in β-catenin nuclear translocation so as to induce transcription of TCF (T-cell specific transcription factor) target genes, including c-Myc [22]. c-Myb promotes cell invasion through the Wnt/β-catenin pathway in breast cancer [23]. In addition, c-Myb bound to the c-Myc promoter and upregulated its expression [18]. According to our results, positive c-Myb expression was significantly associated with β-catenin nuclear accumulation as well as positive c-Myc expression, which is correlated with the poor prognosis of NSCLC.
Our statistical analysis showed a strong negative correlation between Dact3 and c-Myb expression in NSCLC tissues. Dact3 transfection inhibited c-Myb expression of NSCLC cells, as well as c-Myc expression and β-catenin nuclear translocation. siRNA-Dact3 transfection had the opposite effect. These results were in accordance with previous reports [8,18].
Epigenetic regulation is important for tumor progression. Promoter hypermethylation could cause transcriptional gene silence of Dact1 in gastric cancer [24]. Dact2 is frequently silenced by promoter region hypermethylation in human lung cancer [25]. Repression of Dact3 in colon cancer does not require DNA methylation but instead involves bivalent histone modifications [10]. Thus Dact3 reduction in NSCLC is likely to be due to epigenetic regulation.
In conclusion, Dact3 and c-Myb are closely associated with the progression of NSCLC. Dact3 may influence the progression of NSCLC through downregulation of c-Myb in Wnt/β-catenin pathway.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81602022 to Huanyu Zhao, No. 81171650 and 81672082 to Guangping Wu).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Pramanik KC, Fofaria NM, Gupta P, Ranjan A, Kim SH, Srivastava SK. Inhibition of β-catenin signaling suppresses pancreatic tumor growth by disrupting nuclear β-catenin/TCF-1 complex: critical role of STAT-3. Oncotarget. 2015;6:11561–11574. doi: 10.18632/oncotarget.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bone RA, Bailey CS, Wiedermann G, Ferjentsik Z, Appleton PL, Murray PJ, Maroto M, Dale JK. Spatiotemporal oscillations of Notch1, Dll1 and NICD are coordinated across the mouse PSM. Development. 2014;141:4806–4816. doi: 10.1242/dev.115535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wharton KA Jr. Runnin’ with the Dvl: proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev Biol. 2003;253:1–17. doi: 10.1006/dbio.2002.0869. [DOI] [PubMed] [Google Scholar]
- 5.Cheyette BN, Waxman JS, Miller JR, Takemaru K, Sheldahl LC, Khlebtsova N, Fox EP, Earnest T, Moon RT. Dapper, a Dishevelled-associated antagonist of beta-catenin and JNK signaling, is required for notochord formation. Dev Cell. 2002;2:449–461. doi: 10.1016/s1534-5807(02)00140-5. [DOI] [PubMed] [Google Scholar]
- 6.Schubert FR, Sobreira DR, Janousek RG, Alvares LE, Dietrich S. Dact genes are chordate specific regulators at the intersection of Wnt and Tgf-β signaling pathways. BMC Evol Biol. 2014;14:157. doi: 10.1186/1471-2148-14-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao X, Wen J, Zhang L, Li X, Ning Y, Meng A, Chen YG. Dapper1 is a nucleocytoplasmic shuttling protein that negatively modulates Wnt signaling in the nucleus. J Biol Chem. 2008;283:35679–35688. doi: 10.1074/jbc.M804088200. [DOI] [PubMed] [Google Scholar]
- 8.Yang ZQ, Zhao Y, Liu Y, Zhang JY, Zhang S, Jiang GY, Zhang PX, Yang LH, Liu D, Li QC, Wang EH. Downregulation of HDPR1 is associated with poor prognosis and affects expression levels of p120-catenin and beta-catenin in nonsmall cell lung cancer. Mol Carcinog. 2010;49:508–519. doi: 10.1002/mc.20622. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Dong Y, Zhang Y, Wang X, Xu L, Yang S, Li X, Dong H, Xu L, Su L, Ng SS, Chang Z, Sung JJ, Zhang X, Yu J. DACT2 is a functional tumor suppressor through inhibiting Wnt/β-catenin pathway and associated with poor survival in colon cancer. Oncogene. 2015;34:2575–2585. doi: 10.1038/onc.2014.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang X, Tan J, Li J, Kivimäe S, Yang X, Zhuang L, Lee PL, Chan MT, Stanton LW, Liu ET, Cheyette BN, Yu Q. DACT3 is an epigenetic regulator of Wnt/beta-catenin signaling in colorectal cancer and is a therapeutic target of histone modifications. Cancer Cell. 2008;13:529–541. doi: 10.1016/j.ccr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim KH, Seol HJ, Kim EH, Rheey J, Jin HJ, Lee Y, Joo KM, Lee J, Nam DH. Wnt/beta-catenin signaling is a key downstream mediator of MET signaling in glioblastoma stem cells. Neuro Oncol. 2013;15:161–171. doi: 10.1093/neuonc/nos299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.9intestinal stem cell genes and self-renewal. Stem Cells. 2011;29:2042–2050. doi: 10.1002/stem.761. [DOI] [PubMed] [Google Scholar]
- 13.Cai W, Li Q, Yang Z, Miao X, Wen Y, Huang S, Ouyang J. Expression of p53 upregulated modulator of apoptosis (PUMA) and C-myb in gallbladder adenocarcinoma and their pathological significance. Clin Transl Oncol. 2013;15:818–824. doi: 10.1007/s12094-013-1010-8. [DOI] [PubMed] [Google Scholar]
- 14.Lu H, Wang Y, Huang Y, Shi H, Xue Q, Yang S, He S, Wang H. Expression and prognostic role of c-Myb as a novel cell cycle protein in esophageal squamous cell carcinoma. Clin Transl Oncol. 2013;15:796–801. doi: 10.1007/s12094-013-1009-1. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Wang Y, Zhang Y, Miao Y, Zhao Y, Zhang PX, Jiang GY, Zhang JY, Han Y, Lin XY, Yang LH, Li QC, Zhao C, Wang EH. Abnormal expression of p120-catenin, E-cadherin, and small GTPases is significantly associated with malignant phenotype of human lung cancer. Lung Cancer. 2009;63:375–382. doi: 10.1016/j.lungcan.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Xu HT, Wang L, Lin D, Liu Y, Liu N, Yuan XM, Wang EH. Abnormal β-Catenin and reduced Axin expression are associated with poor differentiation and progression in non-small cell lung cancer. Am J Clin Pathol. 2006;125:534–541. doi: 10.1309/0MDY-02KH-EW1F-6RT6. [DOI] [PubMed] [Google Scholar]
- 17.Berge T, Matre V, Brendeford EM, Saether T, Lüscher B, Gabrielsen OS. Revisiting a selection of target genes for the hematopoietic transcription factor c-Myb using chromatin immunoprecipitation and c-Myb knockdown. Blood Cells Mol Dis. 2007;39:278–286. doi: 10.1016/j.bcmd.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Ciznadija D, Tothill R, Waterman ML, Zhao L, Huynh D, Yu RM, Ernst M, Ishii S, Mantamadiotis T, Gonda TJ, Ramsay RG, Malaterre J. Intestinal adenoma formation and MYC activation are regulated by cooperation between MYB and Wnt signaling. Cell Death Differ. 2009;16:1530–1538. doi: 10.1038/cdd.2009.94. [DOI] [PubMed] [Google Scholar]
- 19.Lee WC, Hough MT, Liu W, Ekiert R, Lindström NO, Hohenstein P, Davies JA. Dact2 is expressed in the developing ureteric bud/collecting duct system of the kidney and controls morphogenetic behaviour of collecting duct cells. Am J Physiol Renal Physiol. 2010;299:F740–751. doi: 10.1152/ajprenal.00148.2010. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Yang Y, Liu X, Herman JG, Brock MV, Licchesi JD, Yue W, Pei X, Guo M. Epigenetic regulation of the Wnt signaling inhibitor DACT2 in human hepatocellular carcinoma. Epigenetics. 2013;8:373–382. doi: 10.4161/epi.24113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong Z, Li M, Wang W, Mo P, Yu L, Liu K, Ren W, Li W, Zhang H, Xu J, Yu C. Steroid receptor coactivator 1 promotes human hepatocellular carcinoma progression by enhancing Wnt/β-Catenin signaling. J Biol Chem. 2015;290:18596–18608. doi: 10.1074/jbc.M115.640490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Jin K, van Pelt GW, van Dam H, Yu X, Mesker WE, Ten Dijke P, Zhou F, Zhang L. c-Myb enhances breast cancer invasion and metastasis through the Wnt/β-catenin/Axin2 pathway. Cancer Res. 2016;76:3364–3375. doi: 10.1158/0008-5472.CAN-15-2302. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Kang W, Go MY, Tong JH, Li L, Zhang N, Tao Q, Li X, To KF, Sung JJ, Yu J. Dapper homolog 1 is a novel tumor suppressor in gastric cancer through inhibiting the nuclear factor-κB signaling pathway. Mol Med. 2012;18:1402–1411. doi: 10.2119/molmed.2012.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia Y, Yang Y, Brock MV, Zhan Q, Herman JG, Guo M. Epigenetic regulation of DACT2, a key component of the Wnt signalling pathway in human lung cancer. J Pathol. 2013;230:194–204. doi: 10.1002/path.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]


