Abstract
Background: This study investigated the clinical implication of FAT2 in the progression, metastasis, and prognosis of gastric cancer. Methods: The expression of FAT2 in 436 clinicopathologically characterized gastric cancer cases and 92 control human non-tumor mucosa were analyzed by immunohistochemistry. Consequently, survival analysis was conducted to investigate the association of FAT2 expression and the development of gastric cancers. Results: FAT2 protein was found highly expressed in 90 of 92 (97.83%) control human non-tumor mucosa, while was highly expressed in 126 of 436 (28.90%) tumors samples and low in 310 of 436 (72.10%). The expression of FAT2 was associated with age, tumor size, depth of invasion, Lauren’s classification, lymph node and distant metastases, regional lymph node stage, TNM stage, and prognosis. In particular, for stage I, II, and III tumors patients the 5-year survival rate was lower in those with high expression of FAT2 than those with low expression. In stage IV tumors, the expression of FAT2 was not associated with the 5-year survival rate. Lauren’s classification and distant metastases, TNM stage, and expression of FAT2 were independent prognostic factors in the patients with gastric cancer, as revealed by Cox regression analysis. Conclusion: The expression of FAT2 in gastric cancer was significantly associated with lymph node and distant metastases, and poor prognosis. FAT2 was also associated with the collective invasion and influenced the prognosis of those patients.
Keywords: Gastric cancer, FAT2, metastases, prognosis
Introduction
Cancer is one of the leading causes of death in the world, and represents a tremendous burden on patients, families, and societies. As the second and third leading cancer in men and in women, respectively, stomach cancer mortality is over all the second leading in both sexes in China [1]. Though the development of surgery and adjuvant chemotherapies has improved, the prognosis for gastric cancer remains poor. A majority of patients with advanced cancer die of, not by the primary tumor, but complications caused by metastases. The transition from epithelial to migratory polarity may be a hallmark of cancer progression to an invasive and metastatic disease. Altered functions of polarity determinants lead to disrupted cell-cell adhesions, cytoskeleton rearrangements, and overall loss of epithelial homeostasis. Polarity proteins are engaged in diverse interactions and help cancer cells to adopt different invasion modes. Invading cancer cells can employ either the collective, mesenchymal, or amoeboid invasion modes, or actively switch between them, and gain intermediate phenotypes. Elucidation of the role of polarity proteins during these invasion modes and the associated transitions is a necessary step towards the understanding of metastasis [2].
FAT atypical cadherin 2 (FAT2) belongs to the human FAT gene family that consists of FAT1, FAT2, FAT3 and FAT4 genes, is the second identified human homolog of the Drosophila fat gene, which controls cell proliferation during Drosophila development [3-5]. The gene product most likely functions as a cell adhesion molecule, controlling cell proliferation and migration [6-10]. FAT2 mRNA was expressed in infant brain, cerebellum, gastric cancer, colorectal cancer [11], pancreatic cancer, ovarian cancer, esophageal cancer, skin squamous cell carcinoma, head, and neck cancer [12]. Depletion of FAT2 with shRNA promoted esophageal squamous cell carcinoma growth in vivo [13]. ΔNp63α promotes basal-like breast cancer (BLBC) and lung squamous cell carcinoma motility by inducing the expression of the atypical cadherin FAT2. FAT2 is highly expressed in breast cancer and lung squamous cell carcinoma and the elevated expression of FAT2 is correlated with poor patient outcome [14]. Given the limited number of cases reported, in this study we explored the role of FAT2 in gastric cancer invasion, metastasis, and prognosis.
Materials and methods
Archived gastric cancer samples and non-tumor mucosa
Gastric cancer tissues were collected from gastrectomy specimens from 436 patients from the Department of Surgery, Zhejiang Provincial People’s Hospital, Hangzhou, China, from January 1998 to January 2004. Tissues had been formalin-fixed, paraffin-embedded, and diagnosed clinically and histopathologically at the Departments of Gastrointestinal Surgery and Pathology. All these patients had follow-up records for > 5 years, with the follow-up deadline of December 2008. The survival time was calculated from the date of surgery to the follow-up deadline or the date of death, which was caused mainly by carcinoma recurrence or metastasis. As negative controls, ninety-two non-cancerous human gastric tissues were obtained from gastrectomy of adjacent gastric cancer margins, which were > 5 cm in size. Routine chemotherapy was given to the patients with advanced-stage disease after operation, but no radiation treatment was administered to any of the patients included in our study. The Review Board of Hospital Ethics Committee approved the study, and the informed consent from each participant was obtained before data collection.
Immunohistochemistry and evaluation of results
Immunohistochemical analyses were performed as previously described [15]. Sections were incubated with mouse anti-FAT2 (1:50; Santa Cruz, CA, USA) overnight at 4°C. The degree of immunostaining was reviewed and scored independently by two observers based on the proportion of positively stained tumour cells and the intensity of staining. Tumour cell proportion was scored as follows: 0 (≤5% positive tumour cells), 1 (6-25% positive tumour cells), 2 (26-50% positive tumour cells), and 3 (> 51% positive tumour cells). Staining intensity was graded accordingly to the following criteria: 0 (no staining), 1 (weak staining = light yellow), 2 (moderate staining = yellow brown), and 3 (strong staining = brown). Staining index was then calculated as the product of the proportion of positive tumour cells and the staining intensity score. Using this assessment, FAT2 expression in benign gastric epithelia and malignant lesions was evaluated by the staining index with the scores of 0, 1, 2, 3, 4, 6, or 9. A staining index score of ≥ 4 was defined as high FAT2 expression, and otherwise low expression.
Statistical analysis
All statistical analyses were performed using SPSS 19.0 software (Chicago, IL, USA). Upon the type of the data, Student’s t test, for continuous, or χ 2 or Fisher exact test, for categorical data, was employed for the statistical analyses. Survival curves were estimated using the Kaplan-Meier method, and the log-rank test was used to calculate differences between the curves. Multivariate analysis using the Cox proportional hazards regression model was performed to assess the prognostic values of the protein expression. Statistical significance was set at P < 0.05.
Results
Expression of FAT2 in gastric cancer and non-tumor mucosa
In the 92 control human non-tumor mucosa, FAT2 protein was found highly expressed in 90 (97.83%) samples. FAT2 was detected in 168 (38.53%) cases of human gastric cancer, of which 126 (28.90%) had high expression of FAT2 protein in the tumors. FAT2 was localized mainly in the cytoplasm or nucleus of cancer cells (Figure 1).
Figure 1.
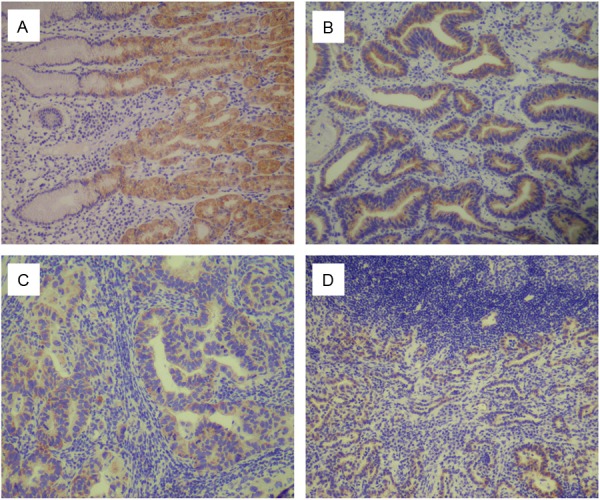
Immunohistochemical staining for FAT2 in gastric cancer lesions and noncancerous tissues. A: FAT2 was highly expressed in noncancerous tissues, magnification ×100. B: FAT2 was highly expressed in moderately differentiated adenocarcinoma, magnification ×100. C: FAT2 was highly expressed in moderately differentiated adenocarcinoma, magnification ×100. D: FAT2 was highly expressed in poorly differentiated adenocarcinoma, magnification ×100.
Relation between the expression of FAT2 and clinical features of gastric cancer
The expression of FAT2 was significantly correlated with age, tumor size, depth of invasion, Lauren’s classification, lymph node and distant metastases, regional lymph node stage and TNM stage (P < 0.05) (Table 1). The expression of FAT2 did not significantly correlate with sex, tumor location, differentiation, or histological classification (P > 0.05) (Table 1). The expression rate of FAT2 was gradually increased with the progression of tumor.
Table 1.
Relationship of FAT2 expression with pathological parameters of GC
| Clinical parameters | FAT2 | |||
|---|---|---|---|---|
|
| ||||
| Low | High | t/χ2 | P | |
| Age (yrs) | 58.24±12.08 | 61.04±12.04 | -2.194 | 0.029 |
| Gender | 1.298 | 0.255 | ||
| Male | 226 (72.7%) | 85 (27.3%) | ||
| Female | 84 (67.2%) | 41 (32.8%) | ||
| Location | 4.703 | 0.095 | ||
| Proximal | 38 (69.1%) | 17 (30.9%) | ||
| Middle | 107 (65.6%) | 56 (34.4%) | ||
| Distal | 165 (75.7%) | 53 (24.3%) | ||
| Size | 14.889 | < 0.01 | ||
| < 5 cm | 200 (78.1%) | 56 (21.9%) | ||
| ≥ 5 cm | 110 (61.1%) | 70 (38.9%) | ||
| Lauren classification | 92.260 | < 0.01 | ||
| Intestinal | 204 (91.5%) | 19 (8.5%) | ||
| Diffuse | 106 (49.8%) | 107 (50.2%) | ||
| Histology classification | 1.115 | 0.773 | ||
| Papillary adenocarcinoma | 11 (68.8%) | 5 (31.2%) | ||
| Tubular adenocarcinoma | 236 (72.4%) | 90 (27.6%) | ||
| Mucinous adenocarcinoma | 19 (65.5%) | 10 (34.5%) | ||
| Signet-ring cell carcinoma | 44 (67.7%) | 21 (32.3%) | ||
| Histologic differentiation | 5.155 | 0.161 | ||
| Well | 12 (92.3%) | 1 (7.7%) | ||
| Moderately | 95 (74.2%) | 33 (25.8%) | ||
| Poorly | 201 (68.6%) | 92 (31.4%) | ||
| Others | 2 (100.0%) | 0 (0.0%) | ||
| Invasion depth | 40.029 | < 0.01 | ||
| T1 | 52 (91.2%) | 5 (8.8%) | ||
| T2 | 93 (85.3%) | 16 (14.7%) | ||
| T3 | 154 (63.1%) | 90 (36.9%) | ||
| T4 | 11 (42.3%) | 15 (57.7%) | ||
| Lymphatic metastasis | 61.263 | < 0.01 | ||
| No | 154 (92.8%) | 12 (7.2%) | ||
| Yes | 156 (57.8%) | 114 (42.2%) | ||
| Regional lymph nodes | 87.333 | < 0.01 | ||
| PN0 | 154 (92.8%) | 12 (7.2%) | ||
| PN1 | 95 (69.9%) | 41 (30.1%) | ||
| PN2 | 51 (51.5%) | 48 (48.5%) | ||
| PN3 | 10 (28.6%) | 25 (71.4%) | ||
| Distant metastasis | 21.918 | < 0.01 | ||
| No | 282 (75.2%) | 93 (24.8%) | ||
| Yes | 28 (45.9%) | 33 (54.1%) | ||
| TNM Stages | 95.703 | < 0.01 | ||
| I | 86 (95.6%) | 4 (4.4%) | ||
| II | 95 (91.3%) | 9 (8.7%) | ||
| III | 103 (59.5%) | 70 (40.5%) | ||
| IV | 26 (37.7%) | 43 (62.3%) | ||
Correlation between FAT2 expression and patient prognosis
Cumulative 5-year survival rates for patients with low FAT2 expression were significantly higher than in patients with high FAT2 expression (Figure 1). We further analyzed the correlation between FAT2 expression and patient prognosis by Kaplan-Meier curves with univariate analyses (log-rank) according to TNM stage. In stage I, II, and III, the patients with high expression of FAT2 had significantly lower 5-year survival rate than those with low expression (P < 0.05) (Figures 2, 3 and 4). In stage IV, the expression of FAT2 was not correlated with the 5-year survival rate (P > 0.05) (Figure 5).
Figure 2.
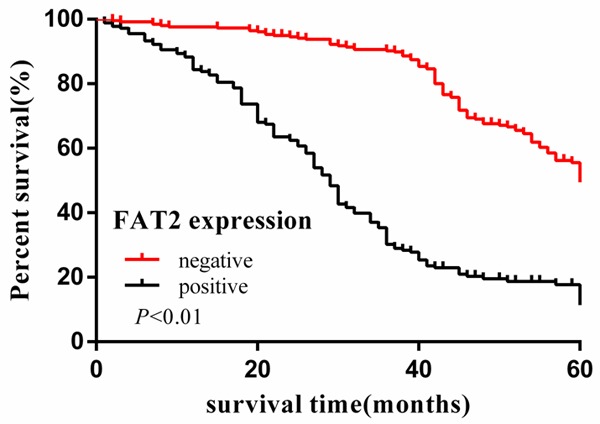
Kaplan-Meier curves with univariate analyses (log-rank) for patients with low FAT2 expression versus high FAT2 expression tumors. The cumulative 5-y survival rate was 52.6% in the low FAT2 protein expression group but was only 11.9% in the high expression group (P < 0.01).
Figure 3.
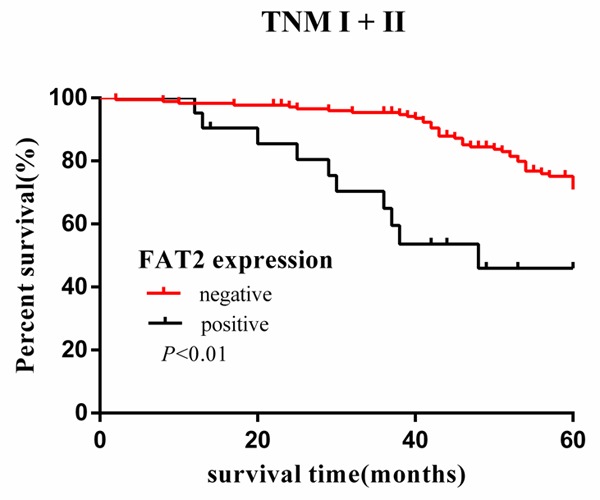
Kaplan-Meier curves with univariate analyses (log-rank) for patients with low FAT2 expression versus high FAT2 expression tumors in stage I and stage II. The cumulative 5-y survival rate was 75.7% in the low FAT2 protein expression group but was only 52.4% in the high expression group (P < 0.01).
Figure 4.
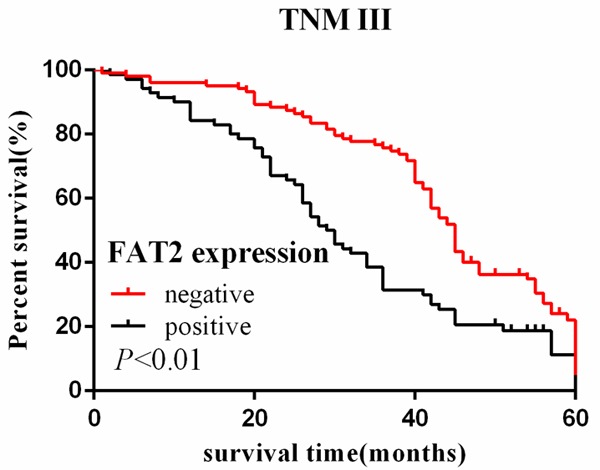
Kaplan-Meier curves with univariate analyses (log-rank) for patients with low FAT2 expression versus high FAT2 expression tumors in stage III. The cumulative 5-y survival rate was 23.3% in the low FAT2 protein expression group but was only 14.3% in the high expression group (P < 0.01).
Figure 5.
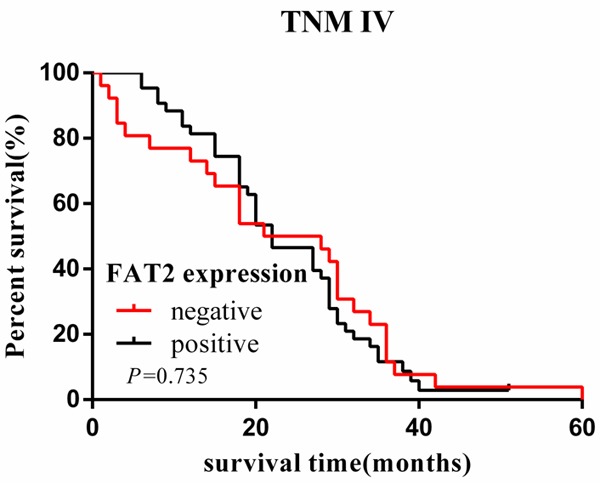
Kaplan-Meier curves with univariate analyses (log-rank) for patients with low FAT2 expression versus high FAT2 expression tumors in stage IV. The cumulative 5-y survival rate was 4.7% in the low FAT2 protein expression group but was only 0 in the high expression group (P = 0.735).
Multivariate analysis of clinicopathological parameters and prognosis
The factors with possible prognostic effects in gastric carcinoma were analyzed by Cox regression analysis (Table 2). The study revealed that Lauren’s classification (P = 0.0001), distant metastases (P = 0.026), TNM stage (P = 0.021), and expression of FAT2 (P = 0.0001) were independent prognostic factors in the patients with gastric cancer. However, age, sex, tumor location and size, histological classification, tumor differentiation, invasion depth, and regional lymph node stage did not play significant roles in prognosis.
Table 2.
Multivariate analysis as determined by Cox regression analysis
| 95% confidential interval | B | Hazard ratio | p | ||
|---|---|---|---|---|---|
|
|
|||||
| Lower | Upper | ||||
| Lauren classification | 1.655 | 3.208 | 0.835 | 2.304 | < 0.01 |
| Distant metastasis | 1.060 | 2.465 | 0.480 | 1.617 | 0.026 |
| TNM Stages | 1.066 | 2.208 | 0.428 | 1.534 | 0.021 |
| FAT2 expression | 1.399 | 2.443 | 0.615 | 1.849 | < 0.01 |
Discussion
Over the past decade, the majority studies have focused on FAT1 and FAT4, FAT1 was found to regulate cell migration and growth through specific protein-protein interactions of its cytoplasmic tail. In contrast, FAT4 has been shown to regulate the planar cell polarity pathway, the Hippo signaling pathway, the canonical Wnt signaling cascade, and the expression of YAP1, FAT2 and FAT3 are relatively less studied [16]. Wu et al reported complete coding sequence of FAT2 in 2000, which is closely related to FAT1 and was mapped to a region in human chromosome 5q33.1, encoding a 14.5-kbp mRNA transcript with an open reading frame of 4,349 amino acids [17]. The literature reported Fat2 may promote cell migration through mediating planar cell polarity and cell rotation. Viktorinova’ study suggest a feedback amplification mechanism between Fat2 localization and microtubule polarity involved in breaking symmetry and directing egg chamber rotation [18]. Fat2 recruits the WAVE regulatory complex (WRC) to transduce polarity information from a membrane receptor to a key actin regulator to control collective follicle cell migration during egg elongation [19]. FAT2 may govern planar cell polarity (PCP) and Hpo signaling in arachnoid cells [20]. Fat2 acts at an early stage to translate plus-end bias into coordinated actin-mediated collective cell migration [21].
FAT2 has played an important role in the molecular pathogenesis of (Esophageal squamous cell carcinoma) ESCC In vitro and in vivo. Human FAT2 is localized at immature adherens junctions in epidermal keratinocytes, and the knockdown of human FAT2 by siRNA inhibited the migration of the cultured HSC-1 human SCC cell line [22]. Furthermore, Lin et al reported that depletion of FAT2 with shRNA promoted ESCC growth in vivo, and 31/113 (27%) esophageal squamous cell carcinoma had mutations in FAT1, FAT2, FAT3 or FAT4 [13,14]. There are few studies about tumor, so, we analyzed the relationships between FAT2 expression and the clinicopathological characteristics of patients with gastric cancer, and explored the role of FAT2 in gastric cancer invasion, metastasis and prognosis. The current study revealed that positive expression of FAT2 correlated with age, tumor size, depth of invasion, Lauren’s classification, lymph node and distant metastases, regional lymph node stage and TNM stage. FAT2 was highly expressed in breast cancer and lung squamous cell carcinoma and the expression of FAT2 correlated with poor prognosis of patients [23]. Our results are consistent with the literature, the expression rate of FAT2 protein was about 30%. AJCC/UICC TNM staging system is the most commonly used scheme to predict prognosis in oncologic patients [24]. We also analyzed the relationship between expression of FAT2 and prognosis of patients according to TNM stage. In stage I, II, and III tumors, the 5-year survival rate in patients with high expression of FAT2 was significantly lower than that in patients with low expression.
However, the presence of mutations and alterations in protein expression strongly suggest that FAT2 may associate with invasiveness of cancer, but the precise role of FAT2 genes in cancer still remains inconclusive and needs further characterization. We will further investigate the specific mechanism of FAT2 in the development and progression of cancer, and FAT2 may be a new regulator of collective invasion and influencing the prognosis of patient.
Acknowledgements
This work was supported by Zhejiang Provincial Medical Science Research Foundation (2016KYA017 to YYW and 2016KYA163 to ZLY), National Natural Science Foundation of China (81602174 to HJW), Zhejiang Provincial Natural Science Foundation of China (LY16H160042 to HJW).
Disclosure of conflict of interest
None.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Gandalovičová A, Vomastek T, Rosel D, Brábek J. Cell polarity signaling in the plasticity of cancer cell invasiveness. Oncotarget. 2016;7:25022–25049. doi: 10.18632/oncotarget.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunne J, Hanby AM, Poulsom R, Jones TA, Sheer D, Chin WG, Da SM, Zhao Q, Beverley PC, Owen MJ. Molecular cloning and tissue expression of FAT, the human homologue of the Drosophila fat gene that is located on chromosome 4q34-q35 and encodes a putative adhesion molecule. Genomics. 1995;30:207–23. doi: 10.1006/geno.1995.9884. [DOI] [PubMed] [Google Scholar]
- 4.Wu Q, Maniatis T. Large exons encoding multiple ectodomains are a characteristic feature of protocadherin genes. Proc Natl Acad Sci U S A. 2000;97:3124–9. doi: 10.1073/pnas.060027397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoeng JC, Ivanov NV, Hodor P, Xia M, Wei N, Blevins R, Gerhold D, Borodovsky M, Liu Y. Identification of new human cadherin genes using a combination of protein motif search and gene finding methods. J Mol Biol. 2004;337:307–17. doi: 10.1016/j.jmb.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 6.Matsui S, Utani A, Takahashi K, Mukoyama Y, Miyachi Y, Matsuyoshi N. Human Fat2 is localized at immature adherens junctions in epidermal keratinocytes. J Dermatol Sci. 2007;48:233–6. doi: 10.1016/j.jdermsci.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Katoh Y, Katoh M. Comparative integromics on FAT1, FAT2, FAT3 and FAT4. Int J Mol Med. 2006;18:523–528. [PubMed] [Google Scholar]
- 8.Viktorinová I, König T, Schlichting K, Dahmann C. The cadherin Fat2 is required for planar cell polarity in the Drosophila ovary. Development. 2009;136:4123–4132. doi: 10.1242/dev.039099. [DOI] [PubMed] [Google Scholar]
- 9.Sharma P, McNeill H. Fat and Dachsous cadherins. Prog Mol Biol Transl Sci. 2013;116:215–235. doi: 10.1016/B978-0-12-394311-8.00010-8. [DOI] [PubMed] [Google Scholar]
- 10.Sadeqzadeh E, de Bock CE, Thorne RF. Sleeping giants: emerging roles for the fat cadherins in health and disease. Med Res Rev. 2014;34:190–221. doi: 10.1002/med.21286. [DOI] [PubMed] [Google Scholar]
- 11.Xie T, Cho YB, Wang K, Huang D, Hong HK, Choi YL, Ko YH, Nam DH, Jin J, Yang H, Fernandez J, Deng S, Rejto PA, Lee WY, Mao M. Patterns of somatic alterations between matched primary and metastatic colorectal tumors characterized by whole-genome sequencing. Genomics. 2014;104:234–241. doi: 10.1016/j.ygeno.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Katoh Y, Katoh M. Comparative integromics on FAT1, FAT2, FAT3 and FAT4. Int J Mol Med. 2006;18:523–8. [PubMed] [Google Scholar]
- 13.Lin DC, Hao JJ, Nagata Y, Xu L, Shang L, Meng X, Sato Y, Okuno Y, Varela AM, Ding LW, Garg M, Liu LZ, Yang H, Yin D, Shi ZZ, Jiang YY, Gu WY, Gong T, Zhang Y, Xu X, Kalid O, Shacham S, Ogawa S, Wang MR, Koeffler HP. Genomic and molecular characterization of esophageal squamous cell carcinoma. Nat Genet. 2014;46:467–73. doi: 10.1038/ng.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dang TT, Westcott JM, Maine EA, Kanchwala M, Xing C, Pearson GW. ΔNp63α induces the expression of FAT2 and Slug to promote tumor invasion. Oncotarget. 2016;7:28592–28611. doi: 10.18632/oncotarget.8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao ZS, Wang YY, Chu YQ, Ye ZY, Tao HQ. SPARC is associated with gastric cancer progression and poor survival of patients. Clin Cancer Res. 2010;16:260–268. doi: 10.1158/1078-0432.CCR-09-1247. [DOI] [PubMed] [Google Scholar]
- 16.Sadeqzadeh E, de Bock CE, Thorne RF. Sleeping giants: emerging roles for the fat cadherins in health and disease. Med Res Rev. 2014;34:190–221. doi: 10.1002/med.21286. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Liu J, Liang X, Chen J, Hong J, Li L, He Q, Cai X. History and progression of Fat cadherins in health and disease. Onco Targets Ther. 2016;9:7337–7343. doi: 10.2147/OTT.S111176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viktorinová I, Dahmann C. Microtubule polarity predicts direction of egg chamber rotation in Drosophila. Curr Biol. 2013;23:1472–1477. doi: 10.1016/j.cub.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Squarr AJ, Brinkmann K, Chen B, Steinbacher T, Ebnet K, Rosen MK, Bogdan S. Fat2 acts through the WAVE regulatory complex to drive collective cell migration during tissue rotation. J Cell Biol. 2016;212:591–603. doi: 10.1083/jcb.201508081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tate G, Kishimoto K, Mitsuya T. A novel mutation of the FAT2 gene in spinal meningioma. Oncol Lett. 2016;12:3393–3396. doi: 10.3892/ol.2016.5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen DY, Lipari KR, Dehghan Y, Streichan SJ, Bilder D. Symmetry breaking in an edgeless epithelium by fat2-regulated microtubule polarity. Cell Rep. 2016;15:1125–33. doi: 10.1016/j.celrep.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsui S, Utani A, Takahashi K, Mukoyama Y, Miyachi Y, Matsuyoshi N. Knockdown of Fat2 by siRNA inhibits the migration of human squamous carcinoma cells. J Dermatol Sci. 2008;51:207–210. doi: 10.1016/j.jdermsci.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Gao YB, Chen ZL, Li JG, Hu XD, Shi XJ, Sun ZM, Zhang F, Zhao ZR, Li ZT, Liu ZY, Zhao YD, Sun J, Zhou CC, Yao R, Wang SY, Wang P, Sun N, Zhang BH, Dong JS, Yu Y, Luo M, Feng XL, Shi SS, Zhou F, Tan FW, Qiu B, Li N, Shao K, Zhang LJ, Zhang LJ, Xue Q, Gao SG, He J. Genetic landscape of esophageal squamous cell carcinoma. Nat Genet. 2014;46:1097–102. doi: 10.1038/ng.3076. [DOI] [PubMed] [Google Scholar]
- 24.Sobin LH, Gospodarowicz MK, Wittekind C. International Union against Cancer (UICC) TNM classification of malignant tumors. 7th edition. New York: Wiley-Liss; 2010. [Google Scholar]


