Abstract
Endometrial carcinoma is one of the most common gynecological malignant tumors. Recent evidence has demonstrated that miR-21 is involved in the proliferation and invasion of endometrial carcinoma. This study aims to explore the effect of biological behavior of miR-21 on endometrial carcinoma its relationship with PTEN. First, Collected endometrial carcinoma and adjacent non-tumor issues, the relative expression levels of miR-21 and PTEN mRNA were quantitated by real-time polymerase chain reaction (RT-PCR). Next, endometrial carcinoma cell line Ishikawa were transfected with miR-21 inhibitor. After transfection, real-time PCR was used to detect the expression levels of miR-21. Then, the cells proliferation, the apoptotic rates and the invasion rates were detected by MTT method, flow cytometry, and Transwell assay. The expression levels of p-PTEN and PTEN proteins were detected by western blot. The results showed that miR-21 was significantly up-regulation and PTEN was down-regulation in endometrial carcinoma tissues (P < 0.05). miR-21 inhibitor were successfully transfected into Ishikawa cell. The cell proliferation activity, and the number invasion cells in the miR-21 inhibitor group was obviously lower than the miR-21 NC group and Normal group (P < 0.05). The apoptosis rate in the miR-21 inhibitor group was significantly higher than the miR-21 NC group and Normal group (P < 0.05). The expression levels of p-PTEN in miR-21 inhibitor groups were significantly higher than miR-21 NC group and Normal group (P < 0.05). Therefore, we concluded that miR-21 could promote cell proliferation and invasion ability, and inhibite cell apoptosis in endometrial carcinoma partially by regulating its target gene PTEN on post-transcriptional level. In brief, miR-21 may be a new early diagnosis mark and therapy target in endometrial carcinoma.
Keywords: Endometrial carcinoma, miR-21, PTEN, apoptosis, proliferation, invasion
Introduction
Endometrial cancer is one of the most common tumors in female reproductive system, and the fourth common malignant cancer in developed countries. The morbidity of endometrial cancer also ranks the seventh place of female cancer in the developing countries [1]. In recent years, the morbidity has been constantly on the rise with marked youth-oriented tendency. The main reason of treatment failure is distant metastasis and local recurrence. Hence, the pathogenesis of endometrial cancer and effective therapeutic target and early diagnosis mark has become the main subject in improving the prognosis of endometrial cancer [2].
MicroRNAs (miRNAs) were a group of small and single strand noncoding RNA gene products about 21~23 nt long that were very conservative in the evolution. They were usually processed by Dicer from precursors RNA with a characteristic hairpin secondary structure. microRNA-21 (miR-21) is an important member of the non-coding small RNA molecules that acts as a oncogene in the 17q23.2. miR-21 has been proved to be highly expressed in almost all solid tumors including endometrial cancer. Thus, it is regarded as a carcinogenic miRNA. A great number of studies have confirmed that the 3’ non-coding region of inhibitor gene mRNA of multiple tumors contains a site for miR-21 specific recognition and binding [4].
PTEN (phosphates and tension homology deleted on chromosome ten) is a tumor suppressor gene containing phosphatase activity. The mutation and inactivation of such gene is closely related to the development, progress and metastasis of most malignant tumor. The research shows that PTEN is one of the target proteins of miRNA-21, and is lowly expressed in gastric cancer, non-small-cell lung cancer, and colorectal cancer. The previous studies show that miR-21 affects the biological behavior of tumor cells by regulating the expression of PTEN gene, but its expression in endometrial cancer and the specific regulating effect have not yet been reported [5].
In this study, qRT-PCR was mainly applied to analyze the miR-21 and PTEN protein level of endometrial cancer tissue and adjacent non-tumor tissue. The miR-21 inhibitor was transferred into endometrial carcinoma cell and to observe the the effect of biological behavior of miR-21 on endometrial carcinoma.
Materials and methods
Clinical specimens, cell lines and cell cultures
18 fresh samples of endometrial carcinoma issues and adjacent non-tumor issues were collected from Shanghai dong-fang Hospital. None of the patients received radiochemotherapy before operating. All the specimens were confirmed by clinical and pathological diagnosis and stored in liquid nitrogen. The endometrial carcinoma Ishikawa cell lines were purchased from the Institute of Cryobiology, Chinese Academy of Sciences. The cells after resuscitation were cultured in high glucose DMEM medium, containing 10% fetal bovine serum and incubated at a constant incubator at 37°C, 5% CO2. The primers sequence of miR-21 was designed by PCR primer design software Primer Premier 5, and synthesized by Invitrogen company. has-mir-21: 5’-TCAACATCAGTCTG ATAAGCTA-3’, U6: 5’-TTCTCCGAACGTGTCACGT-3’.
Reagents and instruments
RPMU1640, fetal bovine serum and trypsase all were bought from Gibco (USA). LipofectamineTM 2000 and Trizol were bought from TAKARA Company, U.S. SYBR PrimeScriptTM miRNA RT-PCR Kit was purchased from Promega Company, U.S. The thiazolyl blue kit (MTT) was purchased from RiboBio Bio-chemistry Ltd. (Beijing). The propidiumiodide (PI) was bought from Sigma, U.S, as RNase A was from Fermentas (Canada). Lastly, the cell apoptosis kit was supplied by Hunan Clone times Biotechnology Co., Ltd. Rabbit anti-human p-PTEN, PTEN and mouse anti-human β-actin polyclonal antibody were purchased from BD, U.S.
Expression levels of mir-21 and PTEN mRNA in endometrial carcinoma were detected by RT-PCR
18 fresh samples of endometrial carcinoma and adjacent non-tumor issues were collected from Shanghai Dong-fang Hospital, and confirmed by pathology. While cutting, the central necrotic tissues should be avoided. Also, the distance of the adjacent tissues away from tumor tissues was 1 cm, and they ought to be cut into a size of 0.5 cm*0.5 cm*0.5 cm. The samples were then ground and the total RNA was extracted by Trizol. At the meantime, the quality and concentration of the total RNA extracted were analyzed as well as measured by Nanodrop 2000 Spectrophotometer. Following that, MMLV kit was utilized to reverse transfect such total RNA and synthesize cDNA. U6 was used as internal reference to undergo RT-PCR amplification. Three independent but repeated experiments were performed with 3 complex holes set for each time. The expression abundance of target gene was relatively quantified by 2-ΔΔCt, and the average value of the complex holes were obtained for later statistical analysis.
Expression of miR-21 in endometrial carcinoma cells after transfection with miR-21 inhibitor detected by RT-PCR
The mature miR-21 inhibitor and negative control were synthesized by Invitrogen company. They were then transfected using Lipo-2000 transfection kit (Invitrogen, USA). The samples were divided into miR-21 inhibitor group, negative control group, and normal group. After 48 hours of cell transfection, the RNA of each group was extracted utilizing TaqMan miRNA separation kit, while the changes in the expression of miR-21 in the each group was detected by qRT-PCR, respectively.
Effect of mir-21 on cell proliferation were detected by MTT
The cells of each groups in the logarithmic growth phase were digested and counted after 48 h of the transfection. Subsequently 3000 cells were inoculated into each pore of a 96-well plate, with 5 holes for each group. After adjusting cell density by microscope, the cells were placed into a 5% CO2 incubator at 37°C for a 5-day continuous culture. During each day, 20 μl of MTT with a concentration of 0.01 mol/L was added into the pores 4 hours prior to the end of daily culture. Next, 150 μl demithyl sulfoxide (DMSO) dissolved Formazan granules were added in and shaken in oscillator for 5 to 10 minutes. Subsequently, microplate reader was utilized to measure its optical density (OD) at 490 nm of its wavelength, which is OD490. Lastly, the daily proliferation folds of cells in each groups compared to the first day (OD490/fold) were calculated and a bar chart of cell proliferation was created using time as abscissa as well as OD490/fold as ordinates.
Effect of mir-21 on cell apoptosis were detected by flow cytometry
The cells of each groups in the logarithmic growth phase were digested and counted after 48 h of the transfection. Subsequently, the cells were gathered in 5 ml centrifugal tubes with 3 holes per group. After centrifugal action, the supernatant was discarded, whereas the cell precipitation was washed with PBS once and centrifuged again before resuspended. Next, the cell suspension was supplemented with Annexin V-APC for staining and properly hid away from light at room temperature for 10 to 15 minutes. Lastly, flow Cytometry was used to detect the cell apoptosis in each group.
Effect of mir-21 on cell migration were detected by transwell chamber invasion
The cells of each groups in the logarithmic growth phase were digested and counted after 48 h of the transfection. On the upper Transwell chamber that was evenly paved with Matrigel, 200 µL of non-serum culture medium that contained 5×104 cells was added. The Mixture was then incubated in a 5% CO2 incubator at 37°C for 24 hours. As the incubation finished, the cells were taken out and washed twice with PBS then fixed with 4% paraformaldehyde for 10 minutes. Afterwards, another 2 PBS washes were given before the cells were dyed with crystal violet for 15 minutes. Following another 2 PBS washes, the cells on the upper chamber were carefully wiped away with cotton bud. Eventually, pictures were taken in 8 random views under microscope for records.
Relative expression of p-PTEN, PTEN were detected by Western blot
The cells of each groups in the logarithmic growth phase were digested and counted after 48 h of the transfection. Afterwards, 150 μl of pre-cooling RIP A lysate that had been made into 1 mmol/L concentration by mixing with PMSF 1.5 μl was then supplemented, and the total protein was extracted. The process was performed on ice at 4°C centrifugal for 5 min at 10,000 r/min. After that, the supernatant was obtained to measure the protein concentration with BCA and denature at 99°C for 10 min. Subsequently, 50 μg protein was obtained for 10% SDSPAGE electrophoresis. The blots were electro transferred onto PVDF film and incubated in sealed suspension for 1 h. Following this, 1:100 diluted p-PTEN, PTEN primary antibody was added in to leave in overnight at 4°C. On the following day, the film was washed with TBST for 5 minutes and 3 times in a row. Lastly, 1:5000 HRP-marked secondary antibody and GAPDH were added in to incubate at 37°C for 2 h.
Statistical analysis
All statistical analyses were performed with Student’s t-test and represented as mean ± standard deviation (SD) unless noted otherwise. No sample was excluded from the analysis. The p values were designated as *P < 0.05, **P < 0.01, and n.s.-non-significant (P > 0.05).
Results
Relative expression of miR-21 and PTEN mRNA in tumor and adjacent tissues
The RT-PCR results show that the miR-21 expression in endometrial carcinoma was significantly higher than its corresponding adjacent tissue (Figure 1A). Whereas, the PTEN mRNA level in endometrial carcinoma tissue was significantly lower than that its corresponding adjacent tissue (Figure 1B). The expression levels of both endometrial carcinoma and adjacent tissues were tested by the non-parametric sum of ranks of paired samples, and there is a significant negative correlation between the two expressions (Figure 1C). All difference was statistically significant (P < 0.05).
Figure 1.
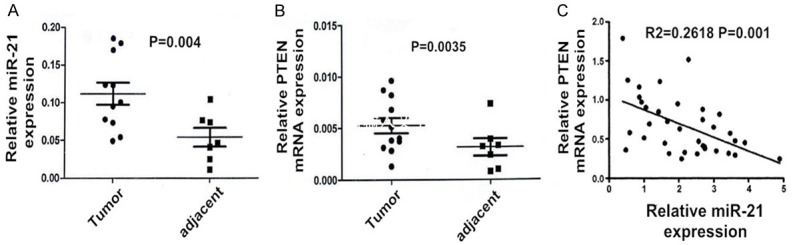
Real-time PCR for the relative expression level of miR-21 (A) and PTEN mRNA (B) in endometrial carcinoma tissues and the adjacent non-tumor tissues. There is a significant negative correlation between the two expressions (C).
miR-21 inhibitor was successfully transfected into endometrial carcinoma Ishikawa cells
After 48 h of miR-21 inhibitor transfection, the RT-PCR results show that the expression level of the miR-21 inhibitor group was obviously lower than the NC group and normal group (P < 0.05, Figure 2). The difference between the NC group and the mock group had no statistical significance (P > 0.05, Figure 2). In short, the miR-21 had successfully transfected into endometrial carcinoma Ishikawa cells.
Figure 2.
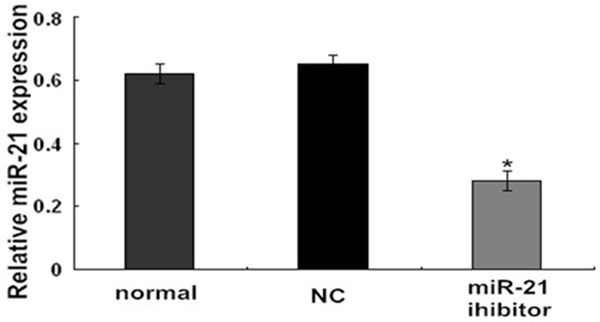
Effects on miR-21 expression after transfecting the endometrial carcinoma Ishikawa cells with a miR-21 inhibitor. *P < 0.05 versus the normal or NC group.
miR-21 promote cell proliferation
The MTT assay result demonstrated that the proliferation activity of cells in the miR-21 inhibitor transfection group was significantly lower than both the normal group (P < 0.05) and the NC group (P < 0.05, Figure 3). It was suggested the down-expression of miR-21 inhibited the proliferation ability of endometrial carcinoma Ishikawa cells.
Figure 3.
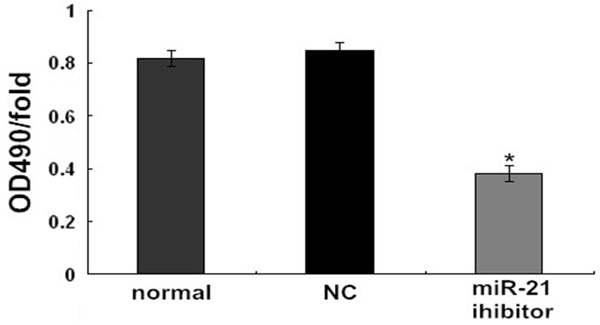
Effects of miR-21 on proliferation of endometrial carcinoma Ishikawa cell lines. *P < 0.05 versus the normal or NC group.
miR-21 inhibite cell apoptosis
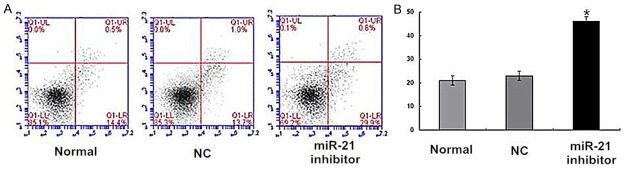
The flow cytometry results demonstrated that the apoptosis percentage of cells in the miR-21 inhibitor group was significantly higher than both the normal group (P < 0.05) and the NC group (P < 0.05, Figure 4). It was suggested that the down-expression of miR-21 promote cell apoptosis of endometrial carcinoma Ishikawa cells.
Figure 4.
The effects of miR-21 on apoptosis of endometrial carcinoma Ishikawa cell lines. Using flow cytometry. *P < 0.05 versus the normal or NC group.
miR-21 promote cell migration and invasion
The Transwell chamber invasion results showed that the number of cells passed through Transwell chamber was significantly decreased in miR-21 inhibitor group (P < 0.05, Figure 5). It was suggested that the down-expression of miR-21 had inhibited endometrial carcinoma Ishikawa cell migration and invasion.
Figure 5.
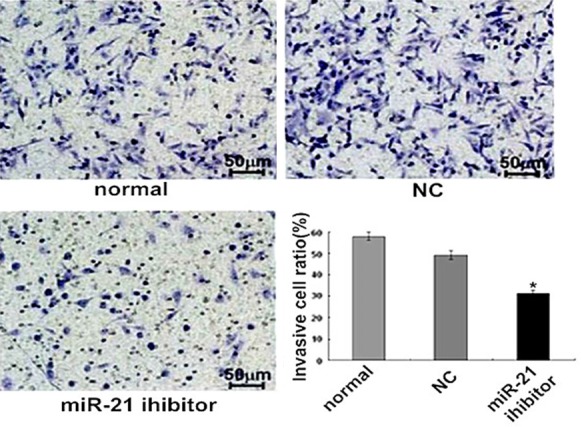
The effects of miR-21 on endometrial carcinoma Ishikawa cell lines migration detected using Transwell invasion chambers. *P < 0.05 versus the normal or NC group.
miR-21 downregulates the expressions of p-PTEN proteins
The western blot results showed that the expression of p-PTEN protein in the miR-21 inhibitor group was significantly higher than the normal group and NC group (P < 0.05, Figure 6). In addition, the PTEN protein expression had no significant difference (P > 0.05, Figure 6). It was suggested that the down-expression of miR-21 may upregulate the expressions of p-PTEN proteins directly or indirectly, but had no effect on the expression of PTEN proteins.
Figure 6.
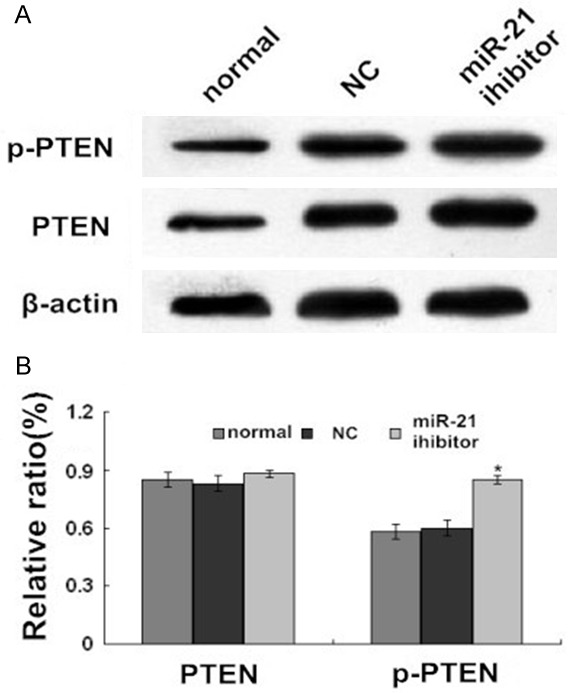
The expression levels of p-PTEN, PTEN in endometrial carcinoma Ishikawa cell lines. A. Protein blotting stripe. B. The relative content of p-PTEN, PTEN protein. *P < 0.05 versus the normal or NC group.
Discussion
microRNA (miRNA) combines with the 3’UTR region of target gene in the way of complementary pairing to degrade target gene mRNA or repress the translation of target gene mRNA, and negatively regulate the expression of target gene after transcription, thus affecting the proliferation, apoptosis, invasion and metastasis of tumor. The researches in recent year show that miRNA is closely related to the development and process of endometrial cancer, and participates in regulating the molecular mechanism of endometrial cancer process and regulating the expression of progestational hormone [4,6].
microRNA-34a (miR-34a) is an important member of the non-coding small RNA molecules that acts as a oncogene in the 17q23.2. miR-21 has been proved to be highly expressed in all solid tumors, including endometrial cancer, cervical cancer, gastric cancer, prostatic cancer, head and neck neoplasm, esophagus cancer, etc. A great number of studies have confirmed that the 3’ non-coding region of inhibitor gene mRNA of multiple tumors contains a site for miR-21 specific recognition and binding. Therefore, the inhibitor gene is the direct target molecule of miR-21. The high expression of miR-21 in tumor cells will markedly down-regulate the expression level of PTEN, TPM1, PDCD4 and other tumor suppressor gene, and this is probably because miR-21 is the molecular foundation of promoting tumor function [7-9]. A further study has shown that lowering the miR-21 level of tumor cell will up-regulate the expression of above-mentioned cells, and thus inhibit tumor cell proliferation, induce apoptosis and repress cell invasion and metastasis. Due to the difference of genetic background and tumor micro-environment in different tumor cells, miR-21 may act on different target genes in different tumors, affecting different cycles of tumor development and progress, and thus producing different effects. However, there are fewer reports on the expression of miR-21 in endometrial cancer and its regulation on target gene [10].
PTEN, the upstream signal molecule of PI2K/AKT pathway, is a cancer suppressor gene related to the cryobiology, including cell growth, apoptosis, proliferation, and adhesion, and plays a key role in the tumor development and process. Among all malignant tumors of gynecology, PTEN is closely related to endometrial carcinoma. The PTEN has a mutation rate of 23.5%~55%, and thus becomes a gene with the highest mutation rate in endometrial carcinoma, known as a house-keeping gene of endometrial cancer [11,12]. The deficiency of PTEN will weaken its regulation on cell growth as well as control on apoptosis, adhesion and migration, and as a result facilitates the development and prognosis of carcinogenesis [13]. In terms of cell adhesion dependent signal, PTEN affects cell adhesion and metastasis, and inhibits the infiltration and metastasis of tumor cell by regulating FAK and SHC phosphorylation based on its lipid phosphates’ activity. As PTEN can be used to act on PI3K/AKT signal transduction pathway, regulate cell growth and inhibit tumor together with PI3K/AKT pathway [14-16]. Hence, PTEN can be regarded as a new tumor suppressor gene as its unique mode of action can provide new target for the gene therapy of tumor and newly developed antitumor drugs.
In our paper, the relative expression level of miR-21 and PTEN in endometrial cancer as well as adjacent tissues were detected via qRT-PCR. The results showed that the expression of miR-21 in endometrial cancer tissue was significantly higher than that of adjacent non-tumor tissue. miR-21 inhibitor was successfully transfected into cells. The cell proliferation and invasion activity in the miR-21 inhibitor group was obviously lower than the miR-21 NC group and Normal group. And the apoptosis rate in the miR-21 inhibitor group was significantly higher than the miR-21 NC group and Normal group (P < 0.05). The expression of p-PTEN protein in miR-21 inhibitor group were significantly lower than miR-21 NC group and Normal group.
Above all, miR-21 is highly expressed in the endometrial cancer, and plays an oncogene effect in promoting the proliferation and invasion of endometrial and inhibiting apoptosis, and the partial mechanism may relate to the inhibition of PTEN on post-transcriptional level. In brief, miRNA-21 may become a new early diagnosis mark and therapy target in NSCLC.
Acknowledgements
This work was supported by Shanghai Dongfang Hospital laboratory.
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Li B, Lu W, Qu J, Zhang Y, Wan X. DICER1 regulates endometrial carcinoma invasion via histone acetylation and methylation. J Cancer. 2017;8:933–939. doi: 10.7150/jca.17435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuo K, Li M, Zhang X, Lu C, Wang S, Zhi K, He B. MiR-21 suppresses endothelial progenitor cell proliferation by activating the TGFβ signaling pathway via downregulation of WWP1. Int J Clin Exp Pathol. 2015;8:414–22. [PMC free article] [PubMed] [Google Scholar]
- 4.Abu Arab W. Video-assisted thoracoscopic surgery for non-small cell lung cancer. Minim Invasive Surg Oncol. 2017;1:1–11. [Google Scholar]
- 5.Bai J, Zhu X, Ma J, Wang W. miR-205 regulates A549 cells proliferation by targeting PTEN. Int J Clin Exp Pathol. 2015;8:1175–83. [PMC free article] [PubMed] [Google Scholar]
- 6.Shang Y, Wang LQ, Guo QY, Shi TL. MicroRNA-196a overexpression promotes cell proliferation and inhibits cell apoptosis through PTEN/Akt/FOXO1 pathway. Int J Clin Exp Pathol. 2015;8:2461–72. [PMC free article] [PubMed] [Google Scholar]
- 7.Mansoori B, Mohammadi A, Hashemzadeh S, Shirjang S, Baradaran A, Asadi M, Doustvandi MA, Baradaran B. Urtica dioica extract suppresses miR-21 and metastasis-related genes in breast cancer. Biomed Pharmacother. 2017;93:95–102. doi: 10.1016/j.biopha.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 8.Shi L, He Y, Bai B, Chen M. Effects of microRNA-21 inhibitor on apoptosis of type II alveolar epithelial cells in rats with hyperoxia-induced acute lung injury. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2017;29:244–248. doi: 10.3760/cma.j.issn.2095-4352.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Ohkawa K, Asakura T, Tsukada Y, Matsuura T. Antibody to human α-fetoprotein inhibits cell growth of human hepatocellular carcinoma cells by resuscitating the PTEN molecule: in vitro experiments. Int J Oncol. 2017;50:2180–2190. doi: 10.3892/ijo.2017.3982. [DOI] [PubMed] [Google Scholar]
- 10.Kong Q, Wang W, Li P. Regulator role of HPV E7 protein on miR-21 expression in cervical carcinoma cells and its functional implication. Int J Clin Exp Pathol. 2015;8:15808–13. [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai CY, Wu JC, Fang C, Chang AY. PTEN, a negative regulator of PI3K/Akt signaling, sustains brain stem cardiovascular regulation during mevinphos intoxication. Neuropharmacology. 2017;123:175–185. doi: 10.1016/j.neuropharm.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Moselhy J, Suman S, Alghamdi M, Chandarasekharan B, Das TP, Houda A, Ankem M, Damodaran C. Withaferin a inhibits prostate carcinogenesis in a PTEN-deficient mouse model of prostate cancer. Neoplasia. 2017;19:451–459. doi: 10.1016/j.neo.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shang Y, Wang LQ, Guo QY, Shi TL. MicroRNA-196a overexpression promotes cell proliferation and inhibits cell apoptosis through PTEN/Akt/FOXO1 pathway. Int J Clin Exp Pathol. 2015;8:2461–72. [PMC free article] [PubMed] [Google Scholar]
- 14.Schultz KA, Rednam SP, Kamihara J, Doros L, Achatz MI, Wasserman JD, Diller LR, Brugières L, Druker H, Schneider KA, McGee RB, Foulkes WD. PTEN, DICER1, FH, and their associated tumor susceptibility syndromes: clinical features, genetics, and surveillance recommendations in childhood. Clin Cancer Res. 2017;23:e76–e82. doi: 10.1158/1078-0432.CCR-17-0629. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi Y. Real-time intraoperative diagnosis of lung adenocarcinoma high risk histological features: a necessity for minimally invasive sublobar resection. Minim Invasive Surg Oncol. 2017;1:12–19. [Google Scholar]
- 16.Takahashi A, Matsuura M, Matoda M, Nomura H, Okamoto S, Kanao H, Kondo E, Omatsu K, Kato K, Utsugi K, Takeshima N. Clinicopathological features of early and late recurrence of endometrial carcinoma after surgical resection. Int J Gynecol Cancer. 2017;27:967–972. doi: 10.1097/IGC.0000000000000984. [DOI] [PubMed] [Google Scholar]