Abstract
The aim of this study was to investigate the association between piezo type mechanosensitive ion channel component 2 (PIEZO2)-promoter methylation with and its clinical value for laryngeal squamous cell carcinoma (LSCC). Quantitative methylation-specific polymerase chain reaction technology was applied to measure PIEZO2 promoter methylation levels from 99 LSCC patients. Inclusive in the analysis were 133 (117 LSSC and 16 normal) samples from The Cancer Genome Atlas (TCGA). Our results showed significantly higher levels of PIEZO2 promoter methylation in LSCC than normal tissues (our cohort: P = 2.94E-21; TCGA cohort: P = 1.07E-19). In addition, PIEZO2 methylation was significantly associated with gender, differentiation, tumor (T) stage, lymph node metastasis, and clinical stage. The areas under the receiver characteristic curves (AUCs) based on our cohort and TCGA cohort were 0.917 and 0.978, respectively. Meanwhile, our study confirmed that PIEZO2 promoter hypermethylation could independently predict a poorer overall survival of LSCC patients (hazard ratio = 6.671; 95% confidence interval = 2.087-21.324). In conclusion, our study revealed that PIEZO2 promoter hypermethylation was a risk factor and might be involved in progression and metastasis, as well as serve as a potential clinical biomarker of LSCC.
Keywords: LSCC, PIEZO2, methylation, metastasis, diagnosis, prognosis
Introduction
Laryngeal cancer is one of the most common head and neck malignancies that accounts for 2.1% of new cases worldwide [1]. Laryngeal squamous cell carcinoma (LSCC) is the main histological subtype of laryngeal cancer. According to epidemiologic data from the National Central Cancer Registry of China, 26,400 newly diagnosed and 14,500 deaths due to LSCC were projected to occur in China in 2015 [2]. The incidence and mortality of LSCC has a male: female ratio of approximately 4:1 [3]. Currently, the main treatment strategies for LSCC are total or partial laryngectomy and postoperative radiotherapy, which are associated with serious impairment of laryngeal function and low quality of life, especially in advanced stage disease (stage III or IV) patients [4]. Despite recent advances in therapeutic strategies, the 5-year survival rate of LSCC remains unsatisfactory [5]. However, due to lack of specific symptoms in the early stages, the majority of patients are diagnosed at an advanced stage. Therefore, precise prediction at the molecular level and identification of effective early biomarkers for LSCC are urgently needed for individual diagnosis and therapy.
As in other types of cancer, LSCC progression is a multistep process involving intricate interactions between multiple factors, including environmental influence (cigarette smoking, alcohol consumption, and exposure to chemical pollutants), genetic susceptibility, and epigenetic modifications [6,7]. Accumulating evidence reveals the importance of epigenetic modifications in the biological processes of many human cancers [8-10]. DNA methylation is an important epigenetic modification, and aberrant promoter methylation at cytosine-phosphate-guanine (CpG) island leads to transcriptional inactivation of tumor suppressor genes (TSGs) in cancer initiation, progress, invasion, and metastasis [11-13]. Moreover, because aberrant methylation is a relatively early molecular change in carcinogenesis [14], aberrant methylation of TSGs has been proposed as diagnostic and prognostic biomarkers for a wide range of cancers [15-17]. Therefore, the identification of DNA methylation biomarkers may have great potential for early diagnosis and prognosis of LSCC.
Piezo proteins (Piezo1 and Piezo2) are important mechano-gated ion channels identified in 2010 through siRNA knockdown of endogenous mechanically activated (MA) currents in the neuronal cell line N2A [18]. Mutations in Piezo channels have been related to numerous diseases in humans [19,20]. Piezo-type mechanosensitive ion channel component 2 (PIEZO2/FAM38B), located on chromosome 18p11.21, encodes Piezo2, which plays important roles in a variety of physiological and pathological processes, such as recognition of endothelial and visceral pain [21], proprioception and touch sensation [22], airway stretch [23], and mechano-transduction [24]. A previous study showed that PIEZO2 was a candidate biomarker for visceral hypersensitivity in irritable bowel syndrome [25]. Moreover, PIEZO2 was a regulator of tumor angiogenesis and hyperpermeability in glioma, and was involved in cellular processes such as proliferation, differentiation, and migration [26]. However, the association of PIEZO2 with other cancers, especially LSCC, remains uninvestigated.
The aim of the present study was to investigate the association between PIEZO2 promoter methylation and LSCC, as well as its potential diagnostic and prognostic value for LSCC. Furthermore, we extracted available data from The Cancer Genome Atlas (TCGA) database to verify our findings.
Materials and methods
Patient characteristics and tissue specimen collection
All the tumors and their corresponding normal tissues were collected from 99 LSCC patients who underwent surgery at the Department of Otolaryngology-Head and Neck Surgery at Ningbo Lihuili Hospital between June 2010 and August 2016. Before surgery and tissue collection, all patients were informed and signed the consent forms. All specimens were obtained fresh and immediately stored in liquid nitrogen at -80°C. In addition, two pathologists performed histological diagnosis of tumor and paired normal tissues. None of the patients underwent chemotherapy or radiotherapy before surgery. None of the patients had a history of hereditary cancer. Overall survival (OS) data were recorded from all patients after surgery. During follow-up, seven patients were lost and 37 died. All experiments were approved by the Ethical Committee of Ningbo Lihuili Hospital.
DNA extraction and bisulfite conversion
Genomic DNA was isolated using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) as recommended by the manufacturer. The concentration and quality of DNA were measured using an ultramicronucleic acid ultraviolet tester (NANODROP 1000, Wilmington, USA). The extracted DNA was then bisulfite-converted using the ZYMO EZ DNA Methylation-Gold Kit according to the manufacturer’s protocol (Zymo Research, Orange, CA, USA). The bisulfite-converted DNA was stored in Tris-ethylenediaminetetraacetic acid (TE) buffer for subsequent methylation analysis.
Quantitative methylation-specific polymerase chain reaction (qMSP) analysis
The methylation level of PIEZO2 promoter (chr18:11148892-11149007) in 99 paired LSCC and normal tissues were determined using qMSP. The qMSP primers for PIEZO2 were designed with MethPrimer (www.urogene.org/methprimer/) [27]. The primers for the amplified sequences of PIEZO2 were: forward, 5’-GGAGTTAGGCGGGAGTATAGTAC-3’, and reverse, 5’-TTCTTCAAAAATAACTAATACCGAA-3’. ACTB was simultaneously amplified as internal control [28] and methylated DNA from a healthy person (Zymo Research, Orange, CA, USA) served as positive methylation control. Each reaction mixture (10 μl) contained 1 μl bisulfite-modified DNA, 0.5 μl forward primer, 0.5 μl reverse primer, 5 μl SYBR Green I Master (Roche, Basel, Switzerland), and 3 μl DNase/RNase free water (Roche, Basel, Switzerland). The PCR amplification was performed in 384-well plates using the Roche LightCycler 480II instrument (Roche, Basel, Switzerland). The PCR reaction was conducted using the following condition: 95°C for 10 min, amplification for 45 cycles at 95°C for 20 s, 60°C for 30 s, and 72°C for 30 s. A melting curve step was performed at 95°C for 15 s, 1 min at 60°C, and then increasing temperature at 0.11°C per second for up to 95°C to measure fluorescence signal. The percentage of methylated reference (PMR) of PIEZO2 promoter was calculated using the following formula: 2-[ΔCt (Samples)-ΔCt (Positive control)], in which ΔCt = Ct (PIEZO2) - Ct (ACTB).
TCGA cohort
DNA methylation profiles (Illumina Human Methylation 450K) and details of clinical information of 133 samples (117 LSCC and 16 normal tissues) were downloaded from the TCGA website (https://cancergenome.nih.gov/). Two Illumina Human Methylation 450K BeadChip probes (cg12951849 on chr18:11148933 and cg03602280 on chr18:11148915) were in the qMSP amplification fragment (chr18:11148892-11149007), and their relative methylation levels were calculated using the formula: bead_M/(bead_M + bead_U). High correlation was observed between methylation levels of these two CpG sites (r = 0.884, P<0.001, data not shown). The average value of the ratios of the two sites was calculated and used for subsequent analysis.
Statistical analyses
All statistical analysis was performed using SPSS v18.0 (SPSS Inc., Chicago, IL, USA). Independent or paired Student’s t-tests were applied for comparisons of PIEZO2 methylation between different groups. Receiver operating characteristic (ROC) analysis was used to evaluate the diagnostic value of PIEZO2 methylation for LSCC [29]. Kaplan-Meier method and log-rank test were applied to the survival data of the LSCC patients classified into two groups according to the median value of PIEZO2 methylation level. Univariate and multivariate Cox proportional hazard models were used to test the prognostic value of PIEZO2 methylation for LSCC patients. A two-tailed P value of less than 0.05 was considered statistically significant. All figures were drawn using GraphPad Prism 6 software (GraphPad, San Diego, CA).
Results
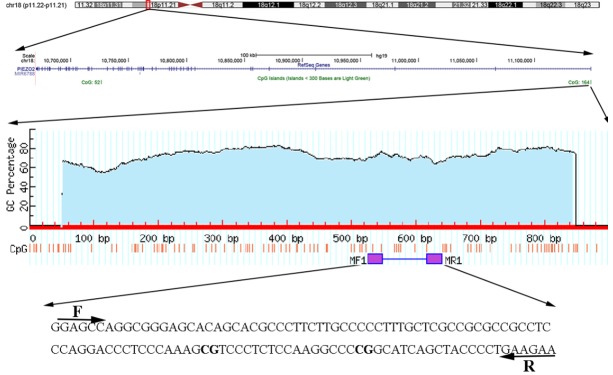
In this study, we recruited 99 LSCC patients to investigate the association of PIEZO2 promoter methylation with LSCC by qMSP method. The amplified fragment and two mapped CpG probes (cg12951849 and cg03602280) of Illumina Human Methylation 450K in the PIEZO2 promoter region are shown in Figure 1 using the University of California Santa Cruz (UCSC) Genome Browser (http://genome.ucsc.edu/). In our study cohort, the results showed that PIEZO2 promoter methylation levels were significantly higher in LSCC than paired non-tumor tissues (Figure 2A, P = 2.94E-21). To validate our findings, we downloaded the methylation profiles of 117 LSCC and 16 normal tissues from the TCGA data portal for further analysis. In the TCGA cohort, we also observed higher hypermethylated PIEZO2 promoter in tumors than normal tissues (Figure 2B, P = 1.07E-19).
Figure 1.
The quantitative methylation-specific PCR (qMSP) amplification fragment and two available CpG probes (cg12951849 and cg03602280) in PIEZO2 promoter of Illumina Human Methylation 450K.
Figure 2.
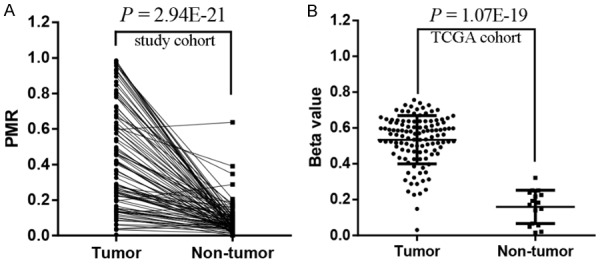
Analysis of PIEZO2 promoter methylation in LSCC patients. A: Our study cohort: P = 2.94E-21; B: TCGA cohort: P = 1.07E-19.
Subsequently, we examined the correlation between PIEZO2 promoter methylation status and clinicopathological characteristics, including gender, age, smoking behavior, drinking history, histological classification, tumor (T) classification, lymph node metastasis and clinical stage of LSCC patients. In our study cohort, PIEZO2 promoter methylation levels were significantly related to histological classification (P = 0.036), T classification (P = 0.007), lymph node metastasis (P = 0.041), and clinical stage (P = 0.006, Table 1). In the TCGA cohort, PIEZO2 promoter methylation levels were significantly associated with age (P = 0.037) and histological grade (P = 0.046, Table 2). However, no statistically significant correlation was found with other clinicopathological characteristics.
Table 1.
The association of PIEZO2 promoter methylation with clinicopathological characteristics of LSCC patients in our study cohort
| Characteristics | N | Mean ± SD | P value |
|---|---|---|---|
| Gender | |||
| Female | 4 | 0.35±0.26 | 0.559 |
| Male | 95 | 0.44±0.30 | |
| Age | |||
| <60 y | 50 | 0.44±0.33 | 0.95 |
| ≥60 y | 49 | 0.44±0.26 | |
| Smoking behavior | |||
| No | 19 | 0.38±0.29 | 0.314 |
| Yes | 80 | 0.45±0.29 | |
| Histological classification | |||
| Well and Moderately | 85 | 0.41±0.28 | 0.036* |
| Poorly | 14 | 0.59±0.33 | |
| T classification | |||
| T1+2 | 58 | 0.37±0.26 | 0.007* |
| T3+4 | 41 | 0.54±0.31 | |
| Lymph metastasis | |||
| No | 64 | 0.39±0.28 | 0.041* |
| Yes | 35 | 0.52±0.31 | |
| Clinical stage | |||
| Stage I+II | 45 | 0.35±0.25 | 0.006* |
| Stage III+IV | 54 | 0.51±0.31 |
The difference of PIEZO2 promoter methylation between these groups was significant.
Table 2.
The association of PIEZO2 promoter methylation with clinicopathological characteristics of LSCC patients in TCGA cohort
| Characteristics | N | Mean ± SD | P value |
|---|---|---|---|
| Gender | |||
| Female | 20 | 0.48±0.16 | 0.037* |
| Male | 97 | 0.55±0.13 | |
| Age | |||
| <60 y | 40 | 0.53±0.16 | 0.886 |
| ≥60 y | 77 | 0.54±0.12 | |
| Smoking behavior | |||
| No | 37 | 0.56±0.13 | 0.197 |
| Yes | 80 | 0.52±0.14 | |
| Alcohol history | |||
| No | 39 | 0.53±0.15 | 0.923 |
| Yes | 76 | 0.54±0.13 | |
| Histologic grade | |||
| G1+2 | 80 | 0.52±0.15 | 0.046* |
| G3+4 | 33 | 0.57±0.10 | |
| T classification | |||
| T1+2 | 21 | 0.56±0.11 | 0.531 |
| T3+4 | 81 | 0.54±0.13 | |
| Lymph metastasis | |||
| No | 41 | 0.53±0.13 | 0.727 |
| Yes | 55 | 0.54±0.13 | |
| Clinical stage | |||
| Stage I+II | 14 | 0.57±0.11 | 0.425 |
| Stage III+IV | 88 | 0.54±0.13 |
The difference of PIEZO2 promoter methylation between these groups was significant.
The ROC curve was used to evaluate the potential diagnostic value of PIEZO2 promoter methylation. A large area under the ROC curve (AUC) indicates high diagnostic accuracy. The maximum Youden index was used as a cut-off point. In our study cohort, the AUC was 0.917 at a cut-off value of 0.128 (Figure 3A). The sensitivity and specificity were 0.879 and 0.869, respectively. The positive predictive value (PPV) and negative predictive value (NPV) were 0.87 and 0.878, respectively. In the TCGA cohort, we revealed an AUC of 0.978 at a cut-off value of 0.251 (Figure 3B). The sensitivity and specificity were 0.957 and 0.938, respectively, while the PPV and NPV were 0.997 and 0.75, respectively.
Figure 3.
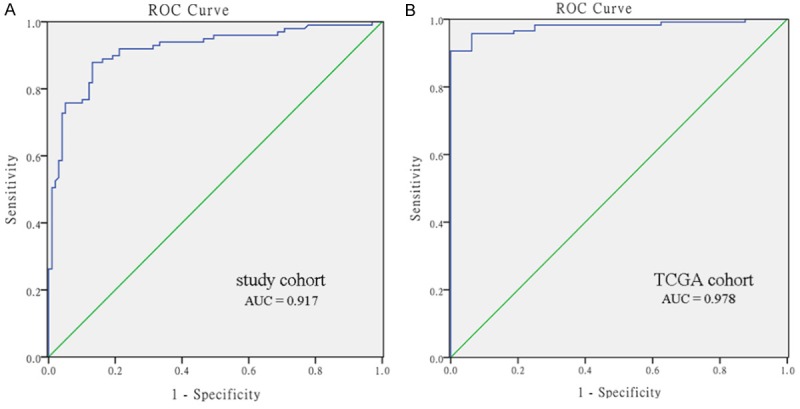
Receiver operating characteristic (ROC) curve. In our study cohort, the area under the curve (AUC) was 0.917. In TCGA cohort, the AUC was 0.978.
We constructed survival curves to investigate whether PIEZO2 promoter methylation could be a prognostic biomarker for LSCC (Figure 4). The median methylation level was used as a cut-off point. Kaplan-Meier analysis and log-rank test confirmed that hypermethylated PIEZO2 was significantly associated with poor outcome of LSCC patients (log-rank P = 0.01). Univariate Cox proportional hazards analysis also revealed a significantly increased risk of death for LSCC patients with hypermethylated PIEZO2 (hazard ratio (HR) = 7.129; 95% confidence interval (CI) = 2.480-20.492; P = 0.001). Subsequently, a multivariate Cox proportional hazard analysis was performed by adjusting for age, smoking behavior, histological differentiation, T classification, lymphatic metastasis, and clinical stage. The results indicated PIEZO2 promoter methylation was an independent predictive biomarker of OS of LSCC patients (HR = 6.671; 95% CI = 2.087-21.324; P = 0.001, Table 3).
Figure 4.
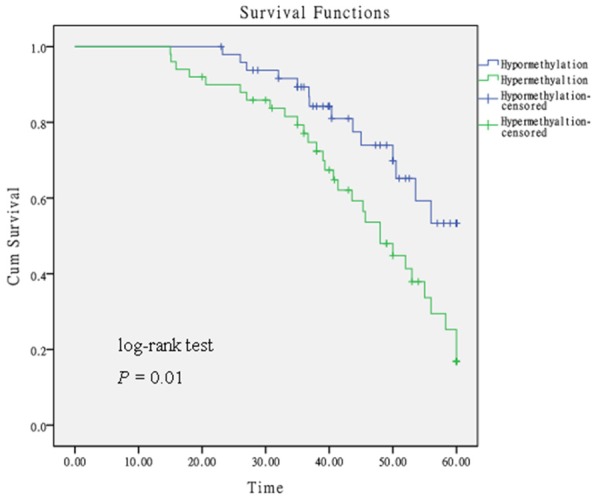
The Kaplan-Meier survival curve. Kaplan-Meier survival analysis of overall survival in 99 LSCC patients stratified according to PIEZO2 methylation status. The median methylation level was used as a cut-off point.
Table 3.
Multivariate Cox proportional hazards analysis of the 99 LSCC patients
| Characteristics | N | P value | HR | 95% CI |
|---|---|---|---|---|
| Age | 99 | 0.279 | 0.98 | 0.944-1.017 |
| Smoking behavior | ||||
| No (Ref) | 19 | - | 1 | - |
| Yes | 80 | 0.668 | 1.196 | 0.527-2.714 |
| Differentiation | ||||
| Well and Moderately (Ref) | 85 | - | 1 | - |
| Poorly | 14 | 0.419 | 0.685 | 0.274-1.715 |
| T classification | ||||
| T1+2 (Ref) | 58 | - | 1 | - |
| T3+4 | 41 | 0.156 | 1.861 | 0.788-4.392 |
| Lymphatic metastasis | ||||
| No (Ref) | 64 | - | 1 | - |
| Yes | 35 | 0.179 | 1.897 | 0.745-4.831 |
| Clinical stage | ||||
| Stage I+II (Ref) | 45 | - | 1 | - |
| Stage III+IV | 54 | 0.055 | 3.125 | 0.100-1.024 |
| PIEZO2 Methylation | 99 | 0.001 | 6.671 | 2.087-21.324 |
N: number; Ref: reference category; HR: hazard ratio; CI: confidence interval.
Discussion
LSCC is one of the most common head and neck malignancies with a poor prognosis in the event of recurrent or metastatic disease. Currently, due to lack of efficient biomarkers, biopsy via laryngoscope is still the gold standard for diagnosis of LSCC [30]. Since the approach is limited for its invasiveness and the results are always influenced by the operator’s experience, there is a pressing need for reliable biomarkers that might aid in the early diagnosis and risk stratification of LSCC patients for primary treatment and subsequent surveillance.
DNA methylation is one of the most important epigenetic silencing mechanisms of tumor suppressor genes (TSGs) that relates to cancer onset and procession [31]. Moreover, aberrant methylation is a relatively early molecular change during the onset of cancer [14]. Furthermore, for development of convenient detection methods, methylation biomarker has been reported to have great potential for cancer early screening and diagnosis [32]. Piezo2 is an important mechanical gated ion channel that plays a vital role in regulating mechanosensory transduction in mammalian cells [18]. Mutations in PIEZO2 have been reported to cause a variety of human diseases [22]. Recent studies demonstrated that PIEZO2 caused tumor growth inhibition, reduced vascular density, and vascular hyperpermeability through the Wnt/β-catenin signaling pathway [26]. In the present study, qMSP technology was applied to measure DNA methylation levels of PIEZO2 promoter from 99 LSCC and paired normal tissues. The data of 133 samples from TCGA were also downloaded to confirm our findings.
Our results showed that the methylation levels of PIEZO2 promoter were significantly higher in LSCC than corresponding normal tissues, which was consistent with analysis of the TCGA cohort, suggesting that PIEZO2 promoter hypermethylation was a risk factor for LSCC. The incidence and mortality of LSCC has a gender bias [3]. Based on the TCGA cohort, we observed a significant upward trend of PIEZO2 promoter methylation level in male compared to female patients, which provides a clue to suggest that the epigenetic mechanism of aberrant DNA methylation might increase the susceptibility of males to LSCC more than females. T stage, lymphatic metastasis and clinical stage are important prognostic factors for cancer patients [33,34]. Interestingly, in our study cohort, hypermethylation of PIEZO2 promoter was more prevalent in advanced T stage, lymph node metastasis, as well as advanced clinical stages, which suggests that aberrant methylation of PIEZO2 promoter might be involved in the progression and metastasis of LSCC. Moreover, both in our study and TCGA cohort, poorly differentiated LSCC tissues were more likely to have an increased PIEZO2 promoter methylation level, suggesting the potential to distinguish different stages of differentiation of LSCC.
The early screening of LSCC depends on the presence of clinical symptoms and imaging examinations, such as laryngoscopy, computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) [30]. However, because of nonspecific symptoms in the early stages of LSCC, especially for supraglottic LSCC, and lack of effective diagnostic biomarkers, a low early diagnostic rate brings challenges for treatment. Accumulating evidence has revealed that abnormal methylation can occur early in carcinogenesis, which has the potential to provide early detection of cancer, particularly in people with inherited risk factors [35,36]. In the present study, we constructed ROC curve and used the AUC to determine the diagnostic value of PIEZO2 methylation for LSCC. The AUC was close to 1.0, which signifies a near perfect risk prediction [37]. The AUC for our study and TCGA cohort were 0.917 and 0.978, respectively. Compared to the diagnostic accuracy of conventional cancer-related biomarkers such as carcinoembryonic antigen (CEA), tissue polypeptide antigen (TPA), squamous cell carcinoma antigen (SCCA), and cytokeratin 19-fragments (CYFRA21-1) [38,39], PIEZO2 methylation had a higher AUC, suggesting that testing for PIEZO2 methylation might be a potential tool for the diagnosis of LSCC.
Tumor node metastasis (TNM) staging classification is still a vital tool in the prediction of cancer prognosis [40,41]. However, the latest edition of the TNM classification couldn’t absolutely satisfy clinical application, due to the heterogeneous molecular mechanisms and clinical behavior of LSCC. Previous studies have shown that epigenetic biomarkers were precise prognostic markers for cancer [42,43]. In the present study, log-rank test revealed the association of PIEZO2 hypermethylation with poor overall survival, which was comparable to our univariate Cox proportional hazards analysis results. In addition, a multivariate Cox proportional hazard analysis was performed to confirm that PIEZO2 methylation was an independent unfavorable factor for LSCC outcomes. All these findings suggested that hypermethylation of PIEZO2 could be a potential biomarker for prognosis of LSCC. However, due to the relatively small sample size, rigorous clinical studies with larger sample sizes will be essential to corroborate our findings.
In conclusion, PIEZO2 promoter hypermethylation was associated with the risk and progression of LSCC. These findings provide clues for further studies on the role of PIEZO2 in LSCC. Additionally, PIEZO2 hypermethylation is a potential biomarker for the early diagnosis and prognosis of LSCC patients.
Acknowledgements
This research was supported by grants from the Zhejiang Provincial Natural Science Foundation of China (No. LY14H160003), the Scientific Innovation Team Project of Ningbo (No. 2012B82019), the Ningbo Social Developmental Key Research Project (No. 2012C5015), the Ningbo Natural Science Foundation (No. 2017A610236), the Medical and Health Research Project of Zhejiang Province (No. 2012ZDA042), and the Medical and Health Training Project of Zhejiang Province (No. 2014PYA017).
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 4.Kada S, Hirano S, Tateya I, Kitamura M, Ishikawa S, Kanda T, Asato R, Tanaka S, Ito J. Ten years single institutional experience of treatment for advanced laryngeal cancer in Kyoto University. Acta Otolaryngol Suppl. 2010:68–73. doi: 10.3109/00016489.2010.492237. [DOI] [PubMed] [Google Scholar]
- 5.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 6.Menvielle G, Fayosse A, Radoi L, Guida F, Sanchez M, Carton M, Cyr D, Schmaus A, Cenee S, Fevotte J, Delafosse P, Stücker I, Luce D ICARE study group. The joint effect of asbestos exposure, tobacco smoking and alcohol drinking on laryngeal cancer risk: evidence from the French population-based case-control study, ICARE. Occup Environ Med. 2016;73:28–33. doi: 10.1136/oemed-2015-102954. [DOI] [PubMed] [Google Scholar]
- 7.Muscat JE, Wynder EL. Tobacco, alcohol, asbestos, and occupational risk factors for laryngeal cancer. Cancer. 1992;69:2244–2251. doi: 10.1002/1097-0142(19920501)69:9<2244::aid-cncr2820690906>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 8.Jin L, Vu T, Yuan G, Datta PK. STRAP promotes stemness of human colorectal cancer via epigenetic regulation of the NOTCH pathway. Cancer Res. 2017;77:5464–5478. doi: 10.1158/0008-5472.CAN-17-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin B, Zhou X, Lin S, Wang X, Zhang M, Cao B, Dong Y, Yang S, Wang JM, Guo M, Huang J. Epigenetic silencing of PRSS3 provides growth and metastasis advantage for human hepatocellular carcinoma. J Mol Med (Berl) 2017;95:1237–1249. doi: 10.1007/s00109-017-1578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JL, Lee YS, Song MJ, Hong SH, Ahn JH, Seo EH, Shin SP, Lee SJ, Johnson BH, Stampfer MR, Kim HP, Kim SY, Lee YS. Epigenetic regulation of RNA polymerase III transcription in early breast tumorigenesis. Oncogene. 2017;36:6793–6804. doi: 10.1038/onc.2017.285. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Chen C, Bi X, Zhou C, Huang T, Ni C, Yang P, Chen S, Ye M, Duan S. DNA methylation of CMTM3, SSTR2, and MDFI genes in colorectal cancer. Gene. 2017;630:1–7. doi: 10.1016/j.gene.2017.07.082. [DOI] [PubMed] [Google Scholar]
- 12.Shen Z, Chen X, Li Q, Zhou C, Xu Y, Yu R, Ye H, Li J, Duan S. Elevated methylation of CMTM3 promoter in the male laryngeal squamous cell carcinoma patients. Clin Biochem. 2016;49:1278–1282. doi: 10.1016/j.clinbiochem.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Zhou C, Li J, Li Q. CDKN2A methylation in esophageal cancer: a meta-analysis. Oncotarget. 2017;8:50071–50083. doi: 10.18632/oncotarget.18412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trujillo KA, Jones AC, Griffith JK, Bisoffi M. Markers of field cancerization: proposed clinical applications in prostate biopsies. Prostate Cancer. 2012;2012:302894. doi: 10.1155/2012/302894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao B, Zhou X, Yang W, Ma J, Zhou W, Fan D, Hong L. The role of cell-free DNA in predicting colorectal cancer prognosis. Expert Rev Gastroenterol Hepatol. 2018;12:39–48. doi: 10.1080/17474124.2017.1372191. [DOI] [PubMed] [Google Scholar]
- 16.Shen S, Wang G, Shi Q, Zhang R, Zhao Y, Wei Y, Chen F, Christiani DC. Seven-CpG-based prognostic signature coupled with gene expression predicts survival of oral squamous cell carcinoma. Clin Epigenetics. 2017;9:88. doi: 10.1186/s13148-017-0392-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song L, Yu H, Jia J, Li Y. A systematic review of the performance of the SEPT9 gene methylation assay in colorectal cancer screening, monitoring, diagnosis and prognosis. Cancer Biomark. 2017;18:425–432. doi: 10.3233/CBM-160321. [DOI] [PubMed] [Google Scholar]
- 18.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bae C, Gnanasambandam R, Nicolai C, Sachs F, Gottlieb PA. Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1. Proc Natl Acad Sci U S A. 2013;110:E1162–1168. doi: 10.1073/pnas.1219777110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lukacs V, Mathur J, Mao R, Bayrak-Toydemir P, Procter M, Cahalan SM, Kim HJ, Bandell M, Longo N, Day RW, Stevenson DA, Patapoutian A, Krock BL. Impaired PIEZO1 function in patients with a novel autosomal recessive congenital lymphatic dysplasia. Nat Commun. 2015;6:8329. doi: 10.1038/ncomms9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrari LF, Bogen O, Green P, Levine JD. Contribution of Piezo2 to endothelium-dependent pain. Mol Pain. 2015;11:65. doi: 10.1186/s12990-015-0068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahmud AA, Nahid NA, Nassif C, Sayeed MS, Ahmed MU, Parveen M, Khalil MI, Islam MM, Nahar Z, Rypens F, Hamdan FF, Rouleau GA, Hasnat A, Michaud JL. Loss of the proprioception and touch sensation channel PIEZO2 in siblings with a progressive form of contractures. Clin Genet. 2017;91:470–475. doi: 10.1111/cge.12850. [DOI] [PubMed] [Google Scholar]
- 23.Nonomura K, Woo SH, Chang RB, Gillich A, Qiu Z, Francisco AG, Ranade SS, Liberles SD, Patapoutian A. Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature. 2017;541:176–181. doi: 10.1038/nature20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schrenk-Siemens K, Wende H, Prato V, Song K, Rostock C, Loewer A, Utikal J, Lewin GR, Lechner SG, Siemens J. PIEZO2 is required for mechanotransduction in human stem cellderived touch receptors. Nat Neurosci. 2015;18:10–16. doi: 10.1038/nn.3894. [DOI] [PubMed] [Google Scholar]
- 25.Bai T, Li Y, Xia J, Jiang Y, Zhang L, Wang H, Qian W, Song J, Hou X. Piezo2: a candidate biomarker for visceral hypersensitivity in irritable bowel syndrome? J Neurogastroenterol Motil. 2017;23:453–463. doi: 10.5056/jnm16114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang H, Liu C, Zhou RM, Yao J, Li XM, Shen Y, Cheng H, Yuan J, Yan B, Jiang Q. Piezo2 protein: a novel regulator of tumor angiogenesis and hyperpermeability. Oncotarget. 2016;7:44630–44643. doi: 10.18632/oncotarget.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18:1427–1431. doi: 10.1093/bioinformatics/18.11.1427. [DOI] [PubMed] [Google Scholar]
- 28.Eads CA, Lord RV, Kurumboor SK, Wickramasinghe K, Skinner ML, Long TI, Peters JH, DeMeester TR, Danenberg KD, Danenberg PV, Laird PW, Skinner KA. Fields of aberrant CpG island hypermethylation in Barrett’s esophagus and associated adenocarcinoma. Cancer Res. 2000;60:5021–5026. [PubMed] [Google Scholar]
- 29.Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207-212. [DOI] [PubMed] [Google Scholar]
- 30.Jovanovic MB. [Diagnosis of laryngeal carcinoma] . Med Pregl. 2008;61:591–595. doi: 10.2298/mpns0812591j. [DOI] [PubMed] [Google Scholar]
- 31.Wang N, Sui F, Ma J, Su X, Liu J, Yao D, Shi B, Hou P, Yang Q. Site-specific hypermethylation of RUNX3 predicts poor prognosis in gastric cancer. Arch Med Res. 2016;47:285–292. doi: 10.1016/j.arcmed.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Arantes LM, de Carvalho AC, Melendez ME, Carvalho AL, Goloni-Bertollo EM. Methylation as a biomarker for head and neck cancer. Oral Oncol. 2014;50:587–592. doi: 10.1016/j.oraloncology.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 33.Feng M, Wang W, Fan Z, Fu B, Li J, Zhang S, Lang J. Tumor volume is an independent prognostic indicator of local control in nasopharyngeal carcinoma patients treated with intensitymodulated radiotherapy. Radiat Oncol. 2013;8:208. doi: 10.1186/1748-717X-8-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sham JS, Choy D. Prognostic factors of nasopharyngeal carcinoma: a review of 759 patients. Br J Radiol. 1990;63:51–58. doi: 10.1259/0007-1285-63-745-51. [DOI] [PubMed] [Google Scholar]
- 35.Sloane MA, Wong JW, Perera D, Nunez AC, Pimanda JE, Hawkins NJ, Sieber OM, Bourke MJ, Hesson LB, Ward RL. Epigenetic inactivation of the candidate tumor suppressor USP44 is a frequent and early event in colorectal neoplasia. Epigenetics. 2014;9:1092–1100. doi: 10.4161/epi.29222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walters RJ, Williamson EJ, English DR, Young JP, Rosty C, Clendenning M, Walsh MD, Parry S, Ahnen DJ, Baron JA, Win AK, Giles GG, Hopper JL, Jenkins MA, Buchanan DD. Association between hypermethylation of DNA repetitive elements in white blood cell DNA and earlyonset colorectal cancer. Epigenetics. 2013;8:748–755. doi: 10.4161/epi.25178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones CM, Athanasiou T. Summary receiver operating characteristic curve analysis techniques in the evaluation of diagnostic tests. Ann Thorac Surg. 2005;79:16–20. doi: 10.1016/j.athoracsur.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 38.Barak V, Meirovitz A, Leibovici V, Rachmut J, Peretz T, Eliashar R, Gross M. The diagnostic and prognostic value of tumor markers (CEA, SCC, CYFRA 21-1, TPS) in head and neck cancer patients. Anticancer Res. 2015;35:5519–5524. [PubMed] [Google Scholar]
- 39.Eleftheriadou A, Chalastras T, Ferekidou E, Kyriou L, Yiotakis I, Pappas Z, Ferekidis E, Kandiloros D. Clinical effectiveness of tumor markers in squamous cell carcinoma of the larynx. Anticancer Res. 2006;26:2493–2497. [PubMed] [Google Scholar]
- 40.Chen K, Chen H, Yang F, Sui X, Li X, Wang J. Validation of the eighth edition of the TNM staging system for lung cancer in 2043 surgically treated patients with non-small-cell lung cancer. Clin Lung Cancer. 2017;18:e457–e466. doi: 10.1016/j.cllc.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Schlitter AM, Jesinghaus M, Jager C, Konukiewitz B, Muckenhuber A, Demir IE, Bahra M, Denkert C, Friess H, Kloeppel G, Ceyhan GO, Weichert W. pT but not pN stage of the 8th TNM classification significantly improves prognostication in pancreatic ductal adenocarcinoma. Eur J Cancer. 2017;84:121–129. doi: 10.1016/j.ejca.2017.06.034. [DOI] [PubMed] [Google Scholar]
- 42.Chen X, Yang Y, Liu J, Li B, Xu Y, Li C, Xu Q, Liu G, Chen Y, Ying J, Duan S. NDRG4 hypermethylation is a potential biomarker for diagnosis and prognosis of gastric cancer in Chinese population. Oncotarget. 2017;8:8105–8119. doi: 10.18632/oncotarget.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sailer V, Gevensleben H, Dietrich J, Goltz D, Kristiansen G, Bootz F, Dietrich D. Clinical performance validation of PITX2 DNA methylation as prognostic biomarker in patients with head and neck squamous cell carcinoma. PLoS One. 2017;12:e0179412. doi: 10.1371/journal.pone.0179412. [DOI] [PMC free article] [PubMed] [Google Scholar]