Abstract
It has been well established that silymarin has hepatoprotective and anti-fibrotic effects. But the mechanisms are poorly understood. In recent years, the role of Ly6Chi monocytes in liver fibrosis has been well demonstrated. Thus, in present study we aimed to investigate whether silymarin can relieve liver fibrosis by reducing Ly6Chi monocytes infiltration. The mouse model of liver fibrosis was established by injected with carbon tetrachloride (CCl4) via intraperitoneal repeatedly. Mice in silymarin group received silymarin treatment by gavage. Silymarin significantly reduced liver inflammation and fibrosis of the mice induced by CCl4 injection, as revealed by liver histological and pathological analysis. Mice administrated by silymarin exhibited less infiltration of Ly6Chi monocytes. But there was no difference on other tested leukocyte subsets between CCl4 group and silymarin group. Meanwhile, further study found that silymarin significantly reduced CCl4-induced increased expression of tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β1 and monocyte chemoattractant protein 1 (MCP-1), which was in line with the decreased numbers of intrahepatic Ly6Chi monocytes. In conclusion, our study showed that the anti-inflammatory and anti-fibrotic effects of silymarin could be contributed to the prevention of Ly6Chi monocytes infiltration into the injured livers, which will give us a better understanding on the cellular mechanism of hepatoprotective and anti-fibrotic effect for silymarin.
Keywords: Silymarin, liver fibrosis, Ly6Chi monocytes, monocyte chemoattractant protein-1, transforming growth factor-β1
Introduction
Liver fibrosis is caused by imbalances between liver inflammation and repair owing to many chronic liver diseases, for example viral infection, toxic damage, metabolic disorders and alcohol abuse, characterized by excessive deposition of extracellular matrix (ECM) [1,2]. Fibrous deposition in liver, especially collagen-I, can protect hepatocytes against various toxic stimuli. However, dysregulated and excessive fibrous deposition can lead to liver structural damage, liver malfunction and liver cirrhosis [3]. Up until now there was no effective drug to treat liver fibrosis during clinical practice [4]. For the sake of better therapeutic targets, pathophysiological mechanism of liver fibrosis has been further studied in recent years. Massive studies have shown that innate immunity, especially macrophages, plays a key role in liver fibrosis [2,5-7].
Liver macrophages can promote liver fibrosis through multiple pathways, for example, releasing proinflammatory factors, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6, to in induce hepatocyte necrosis, and pro-fibrogenic cytokines, such as transforming growth factor (TGF)-β1, to directly activate hepatic stellate cells (HSC) [1,6,8-12]. There are two major sources of macrophages in the liver: one is the long-lived, self-renewing resident Kupffer cells (KFs) which are seeded from embryonic progenitors. In the steady state, KCs maintain without the contribution of circulating bone marrow-derived monocytes [13]. The second is the migration of monocytes from bone marrow and peripheral blood under pathological conditions [14-16]. Following injury, the monocytes in the peripheral blood are largely chemotactic into the liver and activated into macrophages which released proinflammatory and pro-fibrogenic cytokines to aggravate liver injury and fibrosis [15,16]. These findings suggest that inhibition of monocytes infiltration may be a novel target for the treatment of liver fibrosis [17].
Further researches revealed that monocytes are mainly divided into two major subsets according to cell surface molecules Ly6C: classical monocytes (Ly6Chi monocytes) and nonclassical monocytes (Ly6Clo monocytes) [18,19]. Classical monocytes express high levels of CCR2 and Ly6C but low levels of CX3CR1, with a proinflammatory and pro-fibrotic effect. On the other hand, nonclassical monocytes express high levels of CX3CR1 and low levels of CCR2 and Ly6C with anti-inflammatory and anti-fibrosis effect [18,19]. High expression of CCR2 receptor of Ly6Chi monocytes can combine with chemoattractant protein 1 (MCP-1), which is elevated in acute and chronic liver diseases, resulting in chemotaxis of Ly6Chi monocytes into the liver [9,20-22]. Compared to wild mice, MCP-1-/- and CCR2-/- mice exhibited less liver fibrosis in both murine models of toxic (carbon tetrachloride (CCl4)) and metabolic (methioninecholine-deficient diet) liver fibrosis [23]. Similar phenomenon was observed in mice administrated with pharmacological inhibition of MCP-1 [24]. Thus, the strategy to decrease infiltration of Ly6Chi monocytes through MCP-1/CCR2 axis has become an important target for the treatment of liver fibrosis [17].
Silybum marianum (L.Gaertn) is a medicinal plant of the genus compositae. Silymarin is a compound which contains the total medicinal components of silybum marianum, mainly containing silybin, isosilybin, silydianin and silychristin [25]. As a traditional protecting-liver drug, silymarin has specific efficacy and extremely low toxicity, widely used in the treatment of liver disease [25,26]. Many clinical and animal studies have confirmed that silymarin has the function of protecting liver cells and relieving liver fibrosis [27,28]. But the underlying mechanism remains obscure. Therefore, in the present study we investigated whether silymarin can alleviate CCl4-induced liver fibrosis by inhibiting the infiltration of Ly6Chi monocytes.
Materials and method
Mice
A total of 30 male C57BL/6 mice weighing 22-25 g were bought from Beijing Vital River Experimental Animals Technology (Beijing, China). The mice were housed to laboratory conditions (23°C, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) for 1 week prior to experimentation.
Experimental protocol
Mice model of liver fibrosis was established according to the method as previously described [29]. Mice were randomly assigned into three groups: the control group in which mice were injected with olive oil and orally given sodium carboxymethylcellulose (CMC-Na) as control; the CCl4 group in which mice were injected intraperitoneally with 0.6 ml/kg dose of CCl4 (CCl4: olive oil = 1:4, 3 μl/g CCl4 oil) twice weekly for 4 weeks, and without silymarin treatment; the silymarin group in which mice were injected intraperitoneally with CCl4 as mice in the CCl4 group, but treated with silymarin. Silymarin was dissolved in 0.5% sodium CMC-Na and given once daily by gavage at 100 mg/kg/d. This dose of silymarin was the optimal dose proved by the previous studies [27,28]. For the control group and CCl4 group, mice were administrated orally with the same amount of CMC-Na aqueous solution.
Within 48 hours of the last drug administration, mice were anaesthetized with chloral hydrate and sacrificed for tissue collection. Liver tissues were removed and washed with phosphate buffered solution (PBS). A small portion of liver tissues was isolated and put on ice for flow cytometry. Part of liver tissues was fixed in 10% formalin for hematoxylin-eosin (HE) staining, Masson staining and immunohistochemistry (IHC) study. The remaining was frozen with liquid nitrogen for real-time PCR.
HE and Masson staining
After 48 h of formalin fixation, mice liver tissues were embedded in paraffin, and cut to 3-μm-thick slices, which were stained with HE staining and Masson’s trichrome staining according to standard protocols. The sections were scanned and analyzed by a pathologist who was blinded to the different treatments in the experiment.
Immunohistochemical staining
After xylene dewaxing and gradient ethanol hydration, 3-μm-thick paraffin sections were immersed in 3% H2O2 for 15 min to block endogenous peroxidase, and boiling in ethylenediaminetetraacetic acid (EDTA)-alkaline solution for antigen retrieval. Then sample sections were incubated with various primary antibody: α-SMA (ab5694, Abcam, USA), collagen-I (ab34710, Abcam, USA), F4/80 (ab111101, Abcam, USA), CD45 (ab10558, Abcam, USA), CD11b (ab13357, Abcam, USA), TGF-β1 (ab92486, Abcam, USA) and MCP-1 (ab25124, Abcam, USA) overnight at 4°C. After incubation with primary antibody, sample sections would be flushed with PBS, then go on incubating with secondary antibody (Life Technologies, Carlsbad, USA) at 37°C for 25 min. Finally, generally diaminobezidin (DAB) stained, hematoxylin slightly stained and neutral balata fixed.
Absolute counts of CD45+ cells (leucocytes), F4/80+ cells (macrophages) and CD11b+ cells (monocytes) per high-power field (hpf) of stained liver sections were manually assessed in 5 different fields per mouse in a blinded fashion by experienced pathologists.
Pictures of Masson staining, α-SMA and collagen-I immunohistochemical staining were converted to pixels by Image-ProPlus software. Positive staining area of Masson staining (blue), α-SMA and collagen-I immunohistochemical staining (brown) per hpf of stained liver sections were assessed in 5 different fields per mouse in a blinded fashion by experienced pathologists. The extent of liver fibrosis was assessed by the percentage between pixels in the positive staining area and pixels in the whole image.
Flow cytometry
Flow cytometry for analyzing intrahepatic leucocytes was performed as described previously [29]. Briefly. After PBS washing twice, liver sample was cut into small pieces of 3-4 mm3. Five pieces were immediately put in a disposable disaggregator Medicon with 1 ml PBS and processed in the Medimachine System for 1 min. Disaggregated cells were removed and pressed through 70 µm cell strainers to obtain single cell suspensions. Single cell suspensions were incubated immediately monoclonal antibodies for 20 min in the dark. Related antibodies were as follows: CD45 (557235, BD Pharmingen, USA), CD11b (557397, BD Pharmingen, USA), Gr1/Ly6C (560595, BD Pharmingen, USA), Ly6G (551460, BD Pharmingen, USA) and F4/80 (25-4801-82, eBioscience, USA), NK1.1 (557391, BD Pharmingen, USA), CD3 (13-0032-80, eBioscience, USA), CD11c (557400, BD Pharmingen, USA) and CD19 (550992, BD Pharmingen, USA). At last, flow-cytometric analysis was performed on a FACS Aria II (BD Bioscience, USA).
Real-time gene expression analysis
Total RNA in frozen liver tissues was extracted using TRIzol reagent (Life Technologies, USA), subsequently converted to cDNA by the PrimeScript RT Master Mix kit (Takara, China). Quantitative real-time PCR was performed on a Step-One Plus (Applied Biosystems) using SYBR Premix ExTaq kit (Takara, China). All primers and PCR product sizes of this study are listed in Table 1. β-actin was used as an internal control [30].
Table 1.
Sequences of Primers Used for real time PCR [30]
| Gene | Direction | Primer sequence (5’-3’) |
|---|---|---|
| TGF-β1 | Forward | GTGGAAATCAACGGGATCAG |
| Reverse | ACTTCCAACCCAGGTCCTTC | |
| MCP-1 | Forward | ATTGGGATCATCTTGCTGGT |
| Reverse | CCTGCTGTTCACAGTTGCC | |
| IL-1β | Forward | GGTCAAAGGTTTGGAAGCAG |
| Reverse | TGTGAAATGCCACCTTTTGA | |
| IL-6 | Forward | CATTTCCACGATTTCCCAGA |
| Reverse | TCCCTCTGTGATCTGGGAAG | |
| TNF-α | Forward | AGGGTCTGGGCCATAGAACT |
| Reverse | CCACCACGCTCTTCTGTCTAC | |
| β-actin | Forward | GGCTGTATTCCCCTCCATCG |
| Reverse | CCAGTTGGTAACAATGCCATGT |
Statistical analysis
All data were expressed as the mean ± standard error of the mean (SEM). Statistical analysis was performed using one-way analysis of variance (ANOVA) test by SPSS 22.0 software. P<0.05 was considered to be statistically significant.
Results
Silymarin inhibited CCl4-caused liver inflammation
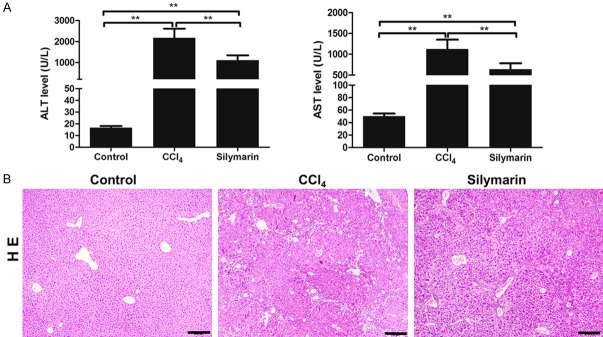
The hepatoprotective effect of silymarin was evaluated mainly by liver function and HE staining. As shown in Figure 1, the mice in control group exhibited intact liver tissue, no necrosis of liver cells and normal level ALT and AST. Afterrepeated CCl4 injection intraperitoneally, the liver tissues of mice in the CCl4 group showed obviously liver cells steatosis, necrosis and leukocytes infiltration which were significantly relieved by silymarin administration. Besides, silymarin also markedly reduced increased ALT and AST induced by CCl4 injection (P<0.01, P<0.01) (Figure 1B). These results suggested that silymarin can significantly reduce CCl4-induced liver inflammation in vivo.
Figure 1.
Silymarin attenuated CCl4-caused liver inflammation. A: ALT and AST levels of mice in each group (n=10 per group). B: Hematoxylin and eosin staining of the liver tissues. All data are expressed as the mean ± SEM. **P<0.01. Original magnification: ×100; bar =50 μm.
Silymarin reduced HSCs activation induced by CCl4 and alleviated liver fibrosis
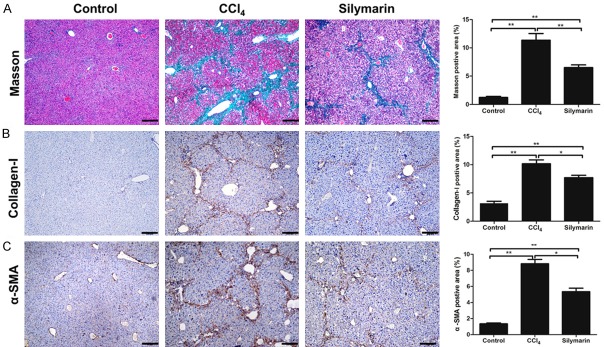
The anti-fibrotic effect of silymarin was evaluated mainly by Masson staining, collagen-I and α-SMA immunohistochemistry. Masson staining was used to observe the collagen fibers of liver tissues. Through analyzing Masson staining of liver tissue in different groups, it was found that liver fibrosis had become apparent after repeated CCl4 injection intraperitoneally, and total collagen fibers remarkably increased. However, the liver of mice administrated with silymarin exhibited significantly decreased collagen deposition (P<0.05) (Figure 2A), which was consistent with that of collagen-I immunohistochemistry (Figure 2B). In addition, the protein abundance of α-SMA, the marker of activated HSCs, was detected by immunohistochemistry during liver fibrosis. The results showed that α-SMA expression was significantly elevated in the fibrotic liver (P<0.01), but was significantly reduced by silymarin (P<0.05)(Figure 2C). These results suggested that silymarin can reduce HSCs activation induced by CCl4 and alleviate liver fibrosis.
Figure 2.
Silymarin reduced CCl4-caused liver fibrosis in mice. A: Masson staining of the liver tissues and statistical analyses of positive area (n=10 per group). B: Collagen-I staining of the liver tissues and statistical analyses of positive area (n=10 per group). C: α-SMA staining and of the liver tissues and statistical analyses of positive area (n=10 per group). All data are expressed as the mean ± SEM. *P<0.05, **P<0.01. Original magnification: ×100; bar =50 μm.
Silymarin reduced Ly6Chi monocytes infiltration during liver fibrosis
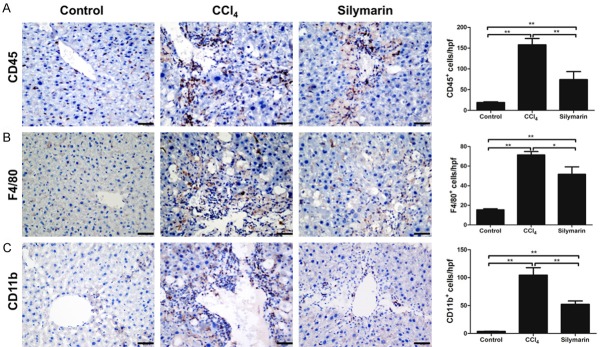
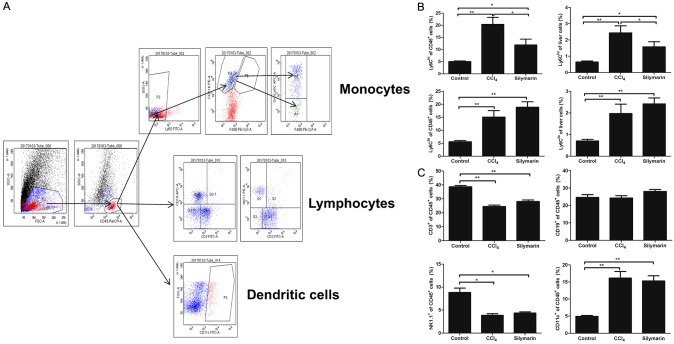
Inhibitory effect of silymarin for Ly6Chi monocytes infiltration was assessed by immunohistochemistry and flow cytometry. Through CD45, CD11b and F4/80 immunohistological staining, we found that there were massive influx of CD45+ leucocytes (P<0.01), especially F4/80+ macrophages and CD11b+ monocytes after challenged with CCl4 for 4 weeks (P<0.01, P<0.01), which were significantly reduced after silymarin treatment (P<0.01, P<0.01, P<0.05)(Figure 3). Flow cytometry data revealed that both proportion of Ly6Chi monocytes (CD45+Ly6G-CD11b+F4/80+Ly6Chi) subset and Ly6Clo monocyte (CD45+Ly6G-CD11b+F4/80+Ly6Clo) increased significantly in the liver tissues of CCl4 group as compared with the control group (Figure 4B). And the livers of mice administrated with silymarin had a significantly lower proportion of Ly6Chi monocyte infiltration than that of CCl4 group. However, there was no difference on the proportion of Ly6Clo monocyte between silymarin group and CCl4 group (Figure 4B). We also did not observe any differences in terms of B cells, T cells, natural killer (NK) cells and dendritic cells (DC) (Figure 4C). These results demonstrated that silymarin can reduce Ly6Chi monocytes infiltration, but have no effect on other leucocyte subpopulations in vivo during liver fibrosis.
Figure 3.
Silymarin reduced infiltrations of leukocytes, monocytes and macrophages in liver fibrosis. A: Immunohistochemistry staining of CD45+ leukocytes and statistical analyses of positive cells (n=10 per group). B: Immunohistochemistry staining of F4/80+ macrophages and statistical analyses of positive cells (n=10 per group). C: Immunohistochemistry staining of CD11b+ monocytes and statistical analyses of positive cells (n=10 per group). All data are expressed as the mean ± SEM. *P<0.05, **P<0.01. Original magnification: ×400; Bar =200 μm.
Figure 4.
Silymarin reduced infiltrations of Ly6Chi monocytes in liver fibrosis. A: Gating strategy of Ly6Chi monocytes, Ly6Clo monocytes, B cell, T cell, NK cell, and DC cell for flow cytometric analysis. B: Proportions of Ly6Chi monocytes and Ly6Clo monocytes monocytes in liver leukocytes and liver total cells (n=10 per group). C: Proportions of B cells, T cells, NK cells, and DC cells in liver leukocytes (n=10 per group). All data are expressed as the mean ± SEM. *P<0.05, **P<0.01.
Silymarin reduced expressions of Ly6Chi monocytes associated pro-inflammatory and pro-fibrogenic cytokines
Ly6Chi monocytes are able to promote liver fibrosis by releasing pro-inflammatory and pro-fibrogenic cytokines [15,16]. Thus, we measured Ly6Chi monocytes associated pro-inflammatory and pro-fibrogenic cytokines in different groups by real-time PCR. As shown in the Figure 5, the intrahepatic mRNA expressions of TNF-α and TGF-β1 significantly increased after CCl4 administration (P<0.01, P<0.01). Mice in silymarin group had significant lower intrahepatic expressions of TNF-α and TGF-β1 as compared with CCl4 group (P<0.01, P<0.05), which was in line with the reduced numbers of intrahepatic Ly6Chi monocytes.
Figure 5.
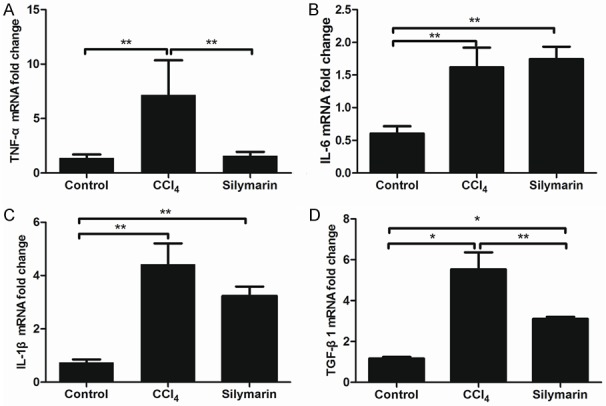
Silymarin Inhibited Gr1hi monocyte associated pro-inflammatory and pro-fibrogenic cytokines (n=10 per group). All data are expressed as the mean ± SEM. *P<0.05, **P<0.01.
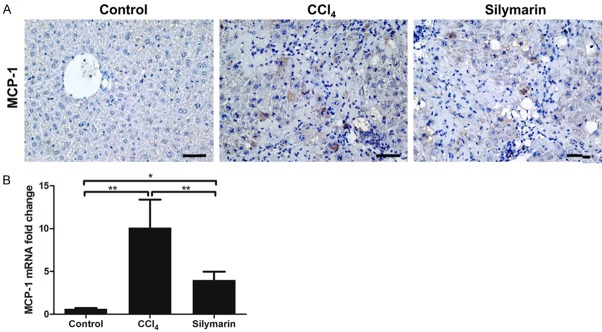
Silymarin inhibited the expression of chemokine MCP-1 in liver fibrosis
MCP-1 is considered to be the prime monocytes chemotactic factor [9,21,22]. Through analyzing immunohistological staining and real time PCR for MCP-1, we found that mice in CCl4 group had a significantly increased expression of MCP-1 after challenged with CCl4 for 4 weeks, which was in line with increased numbers of intrahepatic Ly6Chi monocytes. Meanwhile, silymarin was able to reduce increased expression of MCP-1 induced CCl4 injection (P<0.01) (Figure 6). These findings may partly explain why silymarin can reduce Ly6Chi monocytes infiltration during liver fibrosis.
Figure 6.
Silymarin inhibited the expression of chemokine MCP-1. A: Immunohistochemical staining of MCP-1 in the liver tissues. B: Hepatic mRNA expression of MCP-1 (n=10 per group). All data are expressed as the mean ± SEM. *P<0.05, **P<0.01. Original magnification: ×400; Bar =200 μm.
Discussion
In recent years, there have been many studies on the role of macrophages, especially monocytes-derived macrophages in liver fibrosis [2,5,17]. In the CCl4-induced mice model of liver fibrosis, CCR2-/- and CCR2-/-CCR6-/- mice, compared to wild-type mice, exhibited decreased infiltration of Ly6Chi monocytes and milder liver fibrosis. Such protection would disappear after the adoptive transfer of wild-type Ly6Chi monocytes, which suggested that targeting Ly6Chi monocyte infiltration may be a key strategy for liver fibrosis treatment [9,30]. In current study, we established mouse model of liver fibrosis by repeated CCl4 injections intraperitoneally, then confirmed that Ly6Chi monocytes markedly increased in liver fibrosis.
As a traditional hepatoprotective drug, silymarin is widely used in the treatment of liver fibrosis, acute and chronic hepatitis, which has been proven to have a good curative effect with minimal drug toxicity [26]. Clichici et al revealed that silymarin administered in CCl4-induced fibrosis model is capable of reducing liver inflammation and fibrosis [28]. Younis et al have found that nano-formulations of silymarin, as nanoparticles, improved its ability to resolve cholestasis-induced liver fibrosis [31]. However, little is known about its mechanism of anti-fibrosis. A latest study showed that silibinin, one of the active components of silymarin, can inhibited MCP-1 secretion in cancer-associated fibroblasts (CAFs), which, in turn, reduced immune cells recruitment [32]. Therefore, we raised such a question: is anti-fibrotic effect of silymarin achieved by antagonizing the infiltration of Ly6Chi monocytes? In the present study, we confirmed that silymarin has a notable anti-fibrosis effects by Masson staining, α-SMA and collagen-I immunohistochemistry, which was consistent with the results of other researchers [27,28,31]. Meanwhile, flow cytometry and immunohistochemical analysis showed a significant amount of leukocytes infiltration during liver fibrosis. We found that silymarin significantly inhibits the infiltration of Ly6Chi monocytes, but has little effect on other leucocytes subpopulations, indicating that anti-fibrosis effect of silymarin may be achieved by inhibiting Ly6Chi monocytes infiltration.
In acute and chronic liver injury, monocytes can promote liver injury and fibrosis by a variety of approaches, for example releasing TNF-α and TGF-β1 [15,16]. TNF-α is mainly secreted by monocytes and macrophages in the acute and chronic liver injury [33], and may trigger the production of many other pro-inflammatory cytokines and induce hepatocytes death through the recruitment of neutrophils [34,35]. Bannwart et al revealed that silibinin can inhibit TNF-α production of monocytes from preeclamptic pregnant women in vitro [36]. Zaulet et al revealed that silymarin can protect hepatocytes against structural and ultrastructural injuries induced by Bisphenol A (BPA) by reducing TNF-α secretion [37]. These studies suggested that silymarin is able to inhibit TNF-α secretion and protect hepatocytes. In our present study, it was also found that TNF-α secretion significantly decreased in CCl4-injected mice treated with silymarin, which may explain why silymarin can protect hepatocytes in acute and chronic liver inflammation. TGF-β1 is the strongest cytokine which has been found so far [38,39]. Similar to TNF-α, monocytes and macrophages are prime sources of TGF-β1 in acute and chronic liver injury [40]. In the present study, the result of real-time PCR showed that silymarin is able to reduce increased mRNA expression of TGF-β1 induced by CCl4 injection, which was in line with the decreased numbers of intrahepatic Ly6Chi monocytes. These findings may partly explain why silymarin can alleviate liver fibrosis.
MCP-1/CCR2 axis is the key point of monocytes chemotaxis [41,42]. Compared to wild-type mice, both MCP-1-/- and CCR2-/- mice exhibited less Ly6Chi monocytes infiltration and milder liver fibrosis [23]. Furthermore, pharmacological inhibition for MCP-1 may be capable of limiting chronic liver injury and fibrosis in vivo [24]. These studies suggested the importance of MCP-1 for the infiltration of Ly6Chi monocytes. Chang et al confirmed that silymarin can inhibit MCP-1 expression of human mesangial cells stimulated with TNF-α and IL-1β [43]. Besides, silibinin also can inhibit MCP-1 secretion in CAFs [32]. In our study, it was also proven that silymarin can reduce the expression of MCP-1 in liver fibrosis, which partly explained why silymarin can reduce the infiltration of monocytes. However, the mechanism by which silymarin reduces MCP-1 secretion deserves further investigation.
In conclusion, we found that silymarin has inhibitory effect on liver fibrosis, which may be associated with reduction of the Ly6Chi monocytes infiltration by inhibiting MCP-1 secretion. These results suggest that silymarin is a promising candidate in the prevention and treatment of liver fibrosis.
Acknowledgements
The study was supported from the National Natural Science Foundation of China (81672025 and 81702011), Medical Science and Technology Development Foundation of Nanjing (ZDX16004 and YKK16118), Jiangsu Provincial Medical Innovation Team (CXTDA2017005), Jiangsu Science and Technology Development Plan (BE2017605), Natural Science Foundation of Jiangsu Province for Young Scholar (BK20160121) and Nanjing Medical Science and Technique Development Foundation (QRX17121).
Disclosure of conflict of interest
None.
References
- 1.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14:181–194. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- 3.Bourbonnais E, Raymond VA, Ethier C, Nguyen BN, El-Leil MS, Meloche S, Bilodeau M. Liver fibrosis protects mice from acute hepatocellular injury. Gastroenterology. 2012;142:130–139. doi: 10.1053/j.gastro.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 4.Altamirano-Barrera A, Barranco-Fragoso B, Mendez-Sanchez N. Management strategies for liver fibrosis. Ann Hepatol. 2017;16:48–56. doi: 10.5604/16652681.1226814. [DOI] [PubMed] [Google Scholar]
- 5.Adhyatmika A, Putri KS, Beljaars L, Melgert BN. The elusive antifibrotic macrophage. Front Med. 2015;2:81. doi: 10.3389/fmed.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ju C, Tacke F. Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell Mol Immunol. 2016;13:316–327. doi: 10.1038/cmi.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson MW, Harmon C, O’Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol. 2016;13:267–276. doi: 10.1038/cmi.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen SR, Quaranta V, Linford A, Emeagi P, Rainer C, Santos A, Ireland L, Sakai T, Sakai K, Kim YS, Engle D, Campbell F, Palmer D, Ko JH, Tuveson DA, Hirsch E, Mielgo A, Schmid MC. Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat Cell Biol. 2016;18:549–560. doi: 10.1038/ncb3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, Merad M, Luedde T, Trautwein C, Tacke F. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50:261–274. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- 10.Pradere JP, Kluwe J, De Minicis S, Jiao JJ, Gwak GY, Dapito DH, Jang MK, Guenther ND, Mederacke I, Friedman R, Dragomir AC, Aloman C, Schwabe RF. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology. 2013;58:1461–1473. doi: 10.1002/hep.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liaskou E, Zimmermann HW, Li KK, Oo YH, Suresh S, Stamataki Z, Qureshi O, Lalor PF, Shaw J, Syn WK, Curbishley SM, Adams DH. Monocyte subsets in human liver disease show distinct phenotypic and functional characteristics. Hepatology. 2013;57:385–398. doi: 10.1002/hep.26016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmermann HW, Seidler S, Nattermann J, Gassler N, Hellerbrand C, Zernecke A, Tischendorf JJ, Luedde T, Weiskirchen R, Trautwein C, Tacke F. Functional contribution of elevated circulating and hepatic non-classical CD14-CD16 monocytes to inflammation and human liver fibrosis. PLoS One. 2010;5:e11049. doi: 10.1371/journal.pone.0011049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–57. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melino M, Gadd VL, Alexander KA, Beattie L, Lineburg KE, Martinez M, Teal B, Le Texier L, Irvine KM, Miller GC, Boyle GM, Hill GR, Clouston AD, Powell EE, MacDonald KP. Spatiotemporal characterization of the cellular and molecular contributors to liver fibrosis in a murine hepatotoxic-injury model. Am J Pathol. 2016;186:524–538. doi: 10.1016/j.ajpath.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 15.Beattie L, Sawtell A, Mann J, Frame TC, Teal B, de Labastida Rivera F, Brown N, Walwyn-Brown K, Moore JW, MacDonald S, Lim EK, Dalton JE, Engwerda CR, MacDonald KP, Kaye PM. Bone marrow-derived and resident liver macrophages display unique transcriptomic signatures but similar biological functions. J Hepatol. 2016;65:758–768. doi: 10.1016/j.jhep.2016.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott CL, Zheng F, De Baetselier P, Martens L, Saeys Y, De Prijck S, Lippens S, Abels C, Schoonooghe S, Raes G, Devoogdt N, Lambrecht BN, Beschin A, Guilliams M. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat Commun. 2016;7:10321–10330. doi: 10.1038/ncomms10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brempelis KJ, Crispe IN. Infiltrating monocytes in liver injury and repair. Clin Transl Immunology. 2016;5:e113. doi: 10.1038/cti.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 19.Sunderkötter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 20.Seki E, de Minicis S, Inokuchi S, Taura K, Miyai K, van Rooijen N, Schwabe RF, Brenner DA. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009;50:185–197. doi: 10.1002/hep.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imamura M, Ogawa T, Sasaguri Y, Chayama K, Ueno H. Suppression of macrophage infiltration inhibits activation of hepatic stellate cells and liver fibrogenesis in rats. Gastroenterology. 2005;128:138–146. doi: 10.1053/j.gastro.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell C, Couton D, Couty JP, Anson M, Crain AM, Bizet V, Rénia L, Pol S, Mallet V, Gilgenkrantz H. Dual role of CCR2 in the constitution and the resolution of liver fibrosis in mice. Am J Pathol. 2009;174:1766–1775. doi: 10.2353/ajpath.2009.080632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehling J, Bartneck M, Wei X, Gremse F, Fech V, Möckel D, Baeck C, Hittatiya K, Eulberg D, Luedde T, Kiessling F, Trautwein C, Lammers T, Tacke F. CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis. Gut. 2014;63:1960–1971. doi: 10.1136/gutjnl-2013-306294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baeck C, Wehr A, Karlmark KR, Heymann F, Vucur M, Gassler N, Huss S, Klussmann S, Eulberg D, Luedde T, Trautwein C, Tacke F. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut. 2012;61:416–426. doi: 10.1136/gutjnl-2011-300304. [DOI] [PubMed] [Google Scholar]
- 25.Neha , Jaggi AS, Singh N. Silymarin and its role in chronic diseases. Adv Exp Med Biol. 2016;929:25–44. doi: 10.1007/978-3-319-41342-6_2. [DOI] [PubMed] [Google Scholar]
- 26.Federico A, Dallio M, Loguercio C. Silymarin/Silybin and chronic liver disease: a marriage of many years. Molecules. 2017;22:85–92. doi: 10.3390/molecules22020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sokar SS, El-Sayad ME, Ghoneim ME, Shebl AM. Combination of sitagliptin and silymarin ameliorates liver fibrosis induced by carbon tetrachloride in rats. Biomed Pharmacother. 2017;89:98–107. doi: 10.1016/j.biopha.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Clichici S, Olteanu D, Filip A, Nagy AL, Oros A, Mircea PA. Beneficial effects of silymarin after the discontinuation of CCl4-induced liver fibrosis. J Med Food. 2016;19:789–797. doi: 10.1089/jmf.2015.0104. [DOI] [PubMed] [Google Scholar]
- 29.Huang R, Liu Y, Xiong Y, Wu H, Wang G, Sun Z, Chen J, Yan X, Pan Z, Xia J, Zhang Z, Wang J, Wu C. Curcumin protects against liver fibrosis by attenuating infiltration of Gr1hi monocytes through inhibition of monocyte chemoattractant protein-1. Discov Med. 2016;21:447–457. [PubMed] [Google Scholar]
- 30.Miura K, Yang L, van Rooijen N, Ohnishi H, Seki E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1310–1321. doi: 10.1152/ajpgi.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Younis N, Shaheen MA, Abdallah MH. Silymarin-loaded eudragit((R)) RS100 nanoparticles improved the ability of silymarin to resolve hepatic fibrosis in bile duct ligated rats. Biomed Pharmacother. 2016;81:93–103. doi: 10.1016/j.biopha.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 32.Ting H, Deep G, Kumar S, Jain AK, Agarwal C, Agarwal R. Beneficial effects of the naturally occurring flavonoid silibinin on the prostate cancer microenvironment: role of monocyte chemotactic protein-1 and immune cell recruitment. Carcinogenesis. 2016;37:589–599. doi: 10.1093/carcin/bgw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engele M, Stössel E, Castiglione K, Schwerdtner N, Wagner M, Bölcskei P, Röllinghoff M, Stenger S. Induction of TNF in human alveolar macrophages as a potential evasion mechanism of virulent mycobacterium tuberculosis. J Immunol. 2002;168:1328–1337. doi: 10.4049/jimmunol.168.3.1328. [DOI] [PubMed] [Google Scholar]
- 34.Ito H, Ando K, Ishikawa T, Saito K, Takemura M, Imawari M, Moriwaki H, Seishima M. Role of TNF-alpha produced by nonantigen-specific cells in a fulminant hepatitis mouse model. J Immunol. 2009;182:391–397. doi: 10.4049/jimmunol.182.1.391. [DOI] [PubMed] [Google Scholar]
- 35.Zimmermann HW, Seidler S, Gassler N, Nattermann J, Luedde T, Trautwein C, Tacke F. Interleukin-8 is activated in patients with chronic liver diseases and associated with hepatic macrophage accumulation in human liver fibrosis. PLoS One. 2011;6:e21381. doi: 10.1371/journal.pone.0021381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bannwart CF, Peraçoli JC, Nakaira-Takahagi E, Peraçoli MT. Inhibitory effect of silibinin on tumour necrosis factor-alpha and hydrogen peroxide production by human monocytes. Nat Prod Res. 2010;24:1747–1757. doi: 10.1080/14786410903314492. [DOI] [PubMed] [Google Scholar]
- 37.Zaulet M, Kevorkian SEM, Dinescu S, Cotoraci C, Suciu M, Herman H, Buburuzan L, Badulescu L, Ardelean A, Hermenean A. Protective effects of silymarin against bisphenol A-induced hepatotoxicity in mouse liver. Exp Ther Med. 2017;13:821–828. doi: 10.3892/etm.2017.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 39.Inagaki Y, Higashiyama R, Higashi K. Novel anti-fibrotic modalities for liver fibrosis: molecular targeting and regenerative medicine in fibrosis therapy. J Gastroenterol Hepatol. 2012;27(Suppl 2):85–88. doi: 10.1111/j.1440-1746.2011.07006.x. [DOI] [PubMed] [Google Scholar]
- 40.Heymann F, Hammerich L, Storch D, Bartneck M, Huss S, Rüsseler V, Gassler N, Lira SA, Luedde T, Trautwein C, Tacke F. Hepatic macrophage migration and differentiation critical for liver fibrosis is mediated by the chemokine receptor C-C motif chemokine receptor 8 in mice. Hepatology. 2012;55:898–909. doi: 10.1002/hep.24764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xuan W, Qu Q, Zheng B, Xiong S, Fan GH. The chemotaxis of M1 and M2 macrophages is regulated by different chemokines. J Leukoc Biol. 2015;97:61–69. doi: 10.1189/jlb.1A0314-170R. [DOI] [PubMed] [Google Scholar]
- 42.Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology. 2014;147:577–594. doi: 10.1053/j.gastro.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 43.Chang JW, Kim CS, Kim SB, Park SK, Park JS, Lee SK. Proinflammatory cytokine-induced NFkappaB activation in human mesangial cells is mediated through intracellular calcium but not ROS: effects of silymarin. Nephron Exp Nerhrol. 2006;103:e156–165. doi: 10.1159/000092906. [DOI] [PubMed] [Google Scholar]