Abstract
Objective: The aim of study was to investigate the expression of microRNA-27b in different prostate tissues and its anti-tumor effects in prostate cancer. Methods: Measuring the expression of microRNA-27b, evaluating the PI3K protein expression in 28 benign prostatic hyperplasia and 63 prostate cancer tissues, analyzing the correlation between miRNA-27b and PI3K, and miRNA-27b’s correlation with Gleason Grading and clinical stages were analyzed. We divided the prostate cancer patients into two groups: low group and high group, comparing the overall survival and progression free survival. In the cell experiment, the PC3 was divided into three groups: NC group, BL group and miRNA group. The cells of difference groups were measuring the cell proliferation, apoptosis and cycle and evaluating PI3K, AKT and P21 protein expressions of difference groups. Results: The microRNA-27b expression of prostate cancer significantly increased Compared with benign prostatic hyperplasia (P<0.05). The PI3K protein expression of prostate cancer tissues were significantly enhanced compared with benign prostatic hyperplasia. The PI3K protein expression was positive correlation with miRNA-27b in cancer tissues. Furthermore, the microRNA-27b expression was significantly correlated with the Gleason Grading and clinical stages in prostate cancer (P<0.05, respectively). The patients with higher miR-27b expression level had both poorer overall survival and progression free survival. In cell experiment, the cell proliferation of miRNA group was significantly lower than NC group (P<0.05); the cell apoptosis and G1 phase of miRNA group were significantly difference compared with NC group (P<0.05, respectively); Compared with NC group, PI3K, AKT and P21 protein expressions were significantly down-regulation in miRNA group (P<0.05, respectively). Conclusions: miR-27b was up-regulated in prostate cancer tissue compared with benign prostatic hyperplasia tissues, and its expression level was correlated with a variety of important clinical pathological parameters. In the treatment of prostate cancer, miR-27b inhibition had effects to suppress prostate cancer proliferation by regulation PI3K/AKT/P21 signaling pathway. Moreover; miR-27b may serve as a promising biomarker for predicting the prognosis of prostate cancer.
Keywords: Biomarker, miR-27b, prostate cancer, prognosis
Introduction
Prostate cancer is a common malignant tumor in the male reproductive system, and is the second highest in the western countries [1]. The overall incidence of prostate cancer is not high in China; however, there is a significant growth trend in recent years [2]. At present, the most effective measures for the treatment of prostate cancer are surgical treatment or hormone therapy, but with the progress of the disease, the patient is easy to relapse or metastasis, seriously affected the prognosis and quality of life of patients. Therefore, the early diagnosis of prostate cancer and positive treatment, can significantly improve the quality of life of patients.
Recent studies have indicated that the microRNA genes involved in the non-coding small molecules play an important role in the regulation of gene expression, which is closely related to the progression of the tumor. MiRNAs (microRNAs) are a class of endogenous, non coding protein single chain small molecules RNA [3-5]. The size of miRNAs are about 20~25 nt, which accounts for about 1% of the whole genome. Study found that miRNA expression was prevalent in all eukaryotic cells, was involved in the growth of the organism development, signal transduction, tissue differentiation, diseases process, and also decided to many other physiological and behavioral changes [6-8].
This study intends to through the detection microRNA-27b (miR-27b) expression in different prostate tissues, and miRNA-27b and tumor pathological grade, clinical stage and prognosis of the correlation, for the diagnosis and treatment of prostate cancer provides a certain reference value.
Materials and methods
Patients and tissue samples
The relevant operations of the 63 prostatic cancer and 28 benign prostatic hyperplasia patients included in this study were approved by the ethics committee of Westchina second university hospital. All patients signed informed consent. Collecting cervical cancer and hyperplasia tissues from 2009 to 2015 at the Department of laboratory Medicine, westchina second university hospital. The tissue samples were immediately placed in liquid nitrogen and stored in the refrigerator at -80 until analysis.
RNA isolation and real-time PCR
Followed by the manufacturer’s instructions, extracting total RNA by TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and reversed transcribed into cDNA by PrimeScript reverse transcription-PCR (RT-PCR) kit (TaKaRa, Dalian, China). Using SYBR Premix Ex TaqTM II kit (TaKaRa) to RT-PCR detection on 7500 Real-Time PCR systems (Applied Biosystems, Carlsbad, CA, USA). The PCR cycling was that 95°C for 30 s; 95°C for 5 s, 40 cycles; and 60°C for 34 s. The U6 small nuclear RNA was used as internal controls and the delta-Ct method was exploited to determine relative expression level of miR-27b and PI3K. PI3K: F: 5’-CAAAGCCGAGAACCTATTGCGAG-3’; R: 5’-GTTTGACTTCGCCATCTACCAC-3’.
Immunohistochemistry (IHC)
Taking the tissues from the patients and fixing with formalin solution, Paraffin embedded, sliced. We evaluated the PI3K protein expression in difference tissues by Immunohistochemistry SP methods.
Cell culture
PC3 cells were divided into 3 groups: NC group were treated with nothing; BL group was translation with empty carrier and miRNA group were translation with si-miRNA-27b. All PC3 cell were cultured in DMEM and RPMI 1640 medium, respectively as previously described (20). The cell culture medium was supplemented with 10% FBS, and incubated in humidified atmosphere of 5% CO2 at 37°C. Fresh cell culture mediums were replaced every 2-3 days and cells were passaged until reached 100% confluence.
Cell transfection
Transfection of NC, BL and miRNA-27b inhibitor into PC3 cells was performed using Lipofectamine 2000 reagent (Invitrogen) according to manufacturer’s instructions. 48 hours later, the following experiments were carried out.
MTT
PC3 cell proliferation was measured by MTT. 200 μl cells at the concentration of 1×104/ml were seeded into 96-well plates. After incubation for 24 hours, 20 μl MTT (5 mg/ml, Sigma, USA) was added, the plates were incubated for 4 hours at 37°C and 150 μl DMSO was added. 10 minutes after incubation at room temperature, optical densities (OD) at 490 nm were measured by Bio-Rad iMark plate reader.
Apoptosis analysis assay
FITC apoptosis detection kit (Vazyme, US) was performed to detect cell apoptosis levels. 48 h after transfection, PC3 cells were washed with cold PBS, re-suspended in binding buffer with Annexin V-FITC/propidium iodide (PI) and incubated for 15 min at room temperature without light. Flow cytometry (FCM) (Becton Dickinson, New Jersey, USA) was used to detect the apoptotic rate.
Cell cycle analysis
Cells were collected by 0.25% trypsin, fixed in 70% ethanol at 4°C overnight, washed with cold PBS for 3 times. Afterwards, cells were re-suspended with RNase A (Sigma, USA) at 37°C for 30 min, incubated with PI (0.05 mg/mL) for 30 min at 4°C in the dark. Flow cytometer (BD Biosciences, USA) was used for cell accession. Data analysis was carried out by CellQuest (BD Biosciences, USA).
Western blot
48 h after transfection, Log-phase PC3 cells were collected for western blot analysis. Total cellular protein was extracted using RIPA Buffer and the BCA protein quantitative kit (Thermo, USA) was performed for protein concentration detection. Proteins were resolved using SDS-PAGE analysis and transferred to a polyvinylidene fluoride (PVDF) membrane, and then the membrane was blocked at room temperature for 1 h in PBS solution supplemented with 5% fat-free milk. Subsequently, the membrane were incubated with a primary antibody (anti-GAPDH 1:1000; anti-P2 1:1000; anti-PI3K 1:1000; anti-AKT 1:1000) at room temperature for 2 h, then washed three times with TBST solution, followed by incubating with a secondary antibody (1:5000) at room temperature for 1 h. The protein bands was imaged and then analyzed.
Statistical analysis
The data were all analyzed by SPSS 21.0 statistics software (SPSS Inc., Chicago, IL, USA). MiR-27b expression was comparative analysis in two tissues by using two-sample Student’s t-test. The correlation between miR-27b expression level and clinical pathological parameters were analyzed by chi square test. Using Kaplan-Meier method to analysis overall survival (OS) and Progression-free Survival (PFS) curves, and using long-rank test to evaluate their prognostic effect. Using ANOVA analysis to evaluated the impact of patients’ survival and clinical covariates. The factors, were significantly differences in the ANOVA analysis, were investigated the independent effects of these variables in next step. P<0.05 was considered to indicate a significant statistical difference.
Results
The miR-27b gene expression level in prostate cancer samples and normal tissues
We had measured the miR-27b gene and PI3K gene and protein expression levels of prostatic cancer and BPH tissue by RT-PCR or IHC methods. The PI3K protein expressions of prostatic cancer were stronger than BPH tissues (shown in Figure 1A). These results confirmed that the miR-27b level was significantly up-regulated in prostatic cancer tissues than BPH tissues (P<0.01) (the data was shown in Figure 1B). MiR-27b expression was positive correlation with PI3K gene expression in prostatic cancer (the data was shown in Figure 1C).
Figure 1.
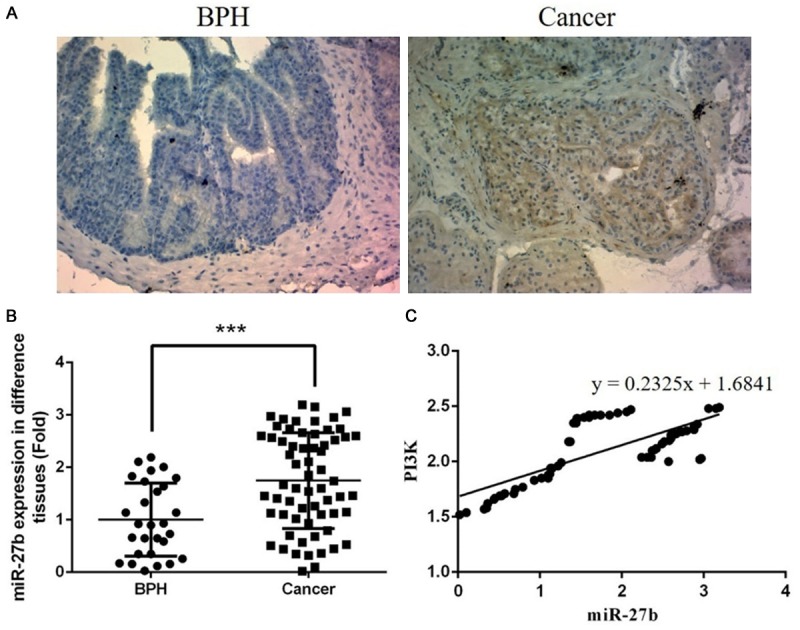
Clinical data of prostatic cancer tissues and BPH tissues. A. The PI3K protein in BPH and prostatic cancer tissues (×200); B. miRNA-27b gene expression of prostatic cancer and BPH tissues. ***: P<0.05, compared with BPH. C. Correlation between miR-27b and PI3K. The miR-27b was positive correlation with PI3K (r=0.2325).
Correlation analysis between the expression of miR-27b and Gleason score
There were significantly differences among the four groups (BPH, G1, G2 and G3) (P<0.05, respectively). The data was shown in Table 1.
Table 1.
miR-27b expression in difference pathological classification
| Pathological classification | n | miR-27b expression (fold) |
|---|---|---|
| BPH | 28 | 1.00±0.70 |
| G1 | 33 | 1.84±0.14* |
| G2 | 26 | 2.29±0.15*,** |
| G3 | 4 | 3.03±0.02*,**,*** |
G1: Gleason is less than 7; G2: Gleason is 7; G3: Gleason is more than 7.
P<0.05, compared with BPH;
P<0.05, compared with G1;
P<0.05, compared with G2.
Correlation analysis between the expression of miR-27b and clinical stages
There were significantly differences among the four groups (BPH, T1, T2 and T3) (P<0.05, respectively). The data was shown in Table 2.
Table 2.
miR-27b expression in difference clinical stages
| Clinical stages | n | miR-27b expression (fold) |
|---|---|---|
| BPH | 28 | 1.00±0.70 |
| T1 | 36 | 1.81±0.16* |
| T2 | 24 | 2.25±0.13*,** |
| T3-T4 | 3 | 3.02±0.02*,**,*** |
P<0.05, compared with BPH;
P<0.05, compared with T1;
P<0.05, compared with T2.
miR-27b expression was an independent prognosis factor for prostatic cancer
In multivariable analysis, we found low miR-27b expression (OS, relative risk [RR] 3.66, 95% confidence interval [CI] 2.78-4.29, P<0.05; PFS, [RR] 6.17, 95% [CI] 1.26-3.10, P=0.008) was independent prognostic factors of unfavorable survival in human prostatic cancer. (The data was shown in Figures 2 and 3).
Figure 2.
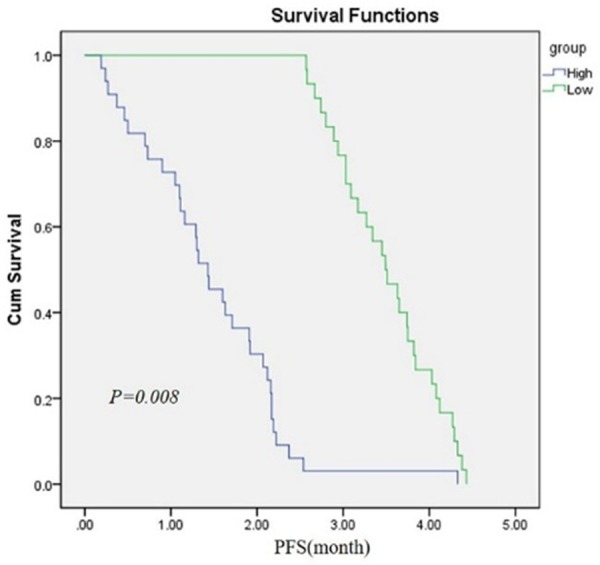
Kaplan-Meier survival curves for prostate cancer patients according to miR-27b expression. The patients with low expression level of miR-27b had a poorer Progression free survival (PFS) (P<0.05).
Figure 3.
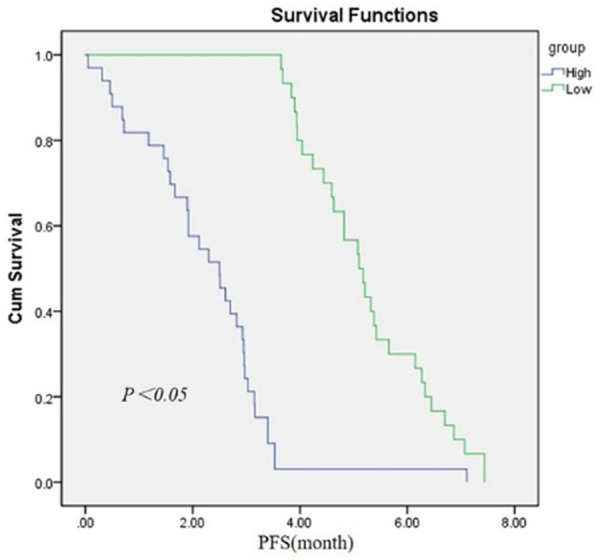
Kaplan-Meier survival curves for prostate cancer patients according to miR-27b expression. The patients with low expression level of miR-27b had a poorer overall survival rate (P<0.05).
MTT assay
Compared with NC group, the cell proliferation of miRNA group was significantly reduced (P<0.05); however, there were no significantly difference between NC and BL groups (P>0.05). The data was shown in Figure 4.
Figure 4.
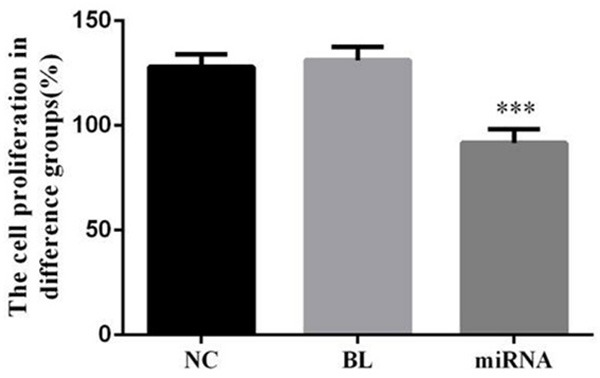
The cell proliferation in difference groups. ***: P<0.05, Compared with NC group.
Cell apoptosis in difference groups
Compared with NC group, the cell apoptosis of miRNA group was significantly increased (P<0.05); however, there were no significantly difference between NC and BL groups (P>0.05). The data was shown in Figure 5.
Figure 5.
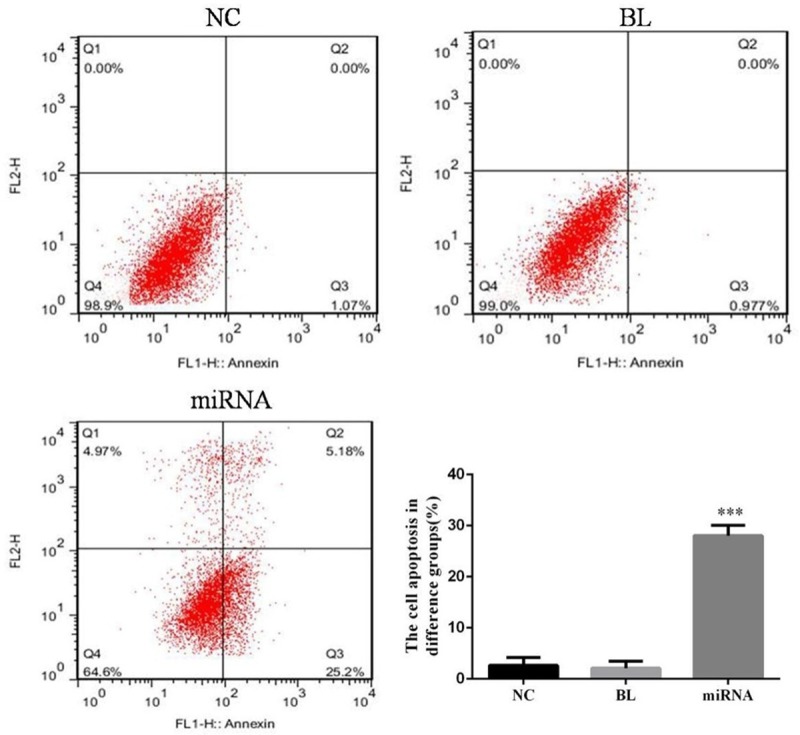
The cell apoptosis in difference groups. ***: P<0.05, Compared with NC group.
Cell cycle analysis
The G1 phase of miRNA group was significantly up-regulation compared with NC group (P<0.05), There were no significantly difference between NC and BL groups (P>0.05). The data was shown in Figure 6.
Figure 6.
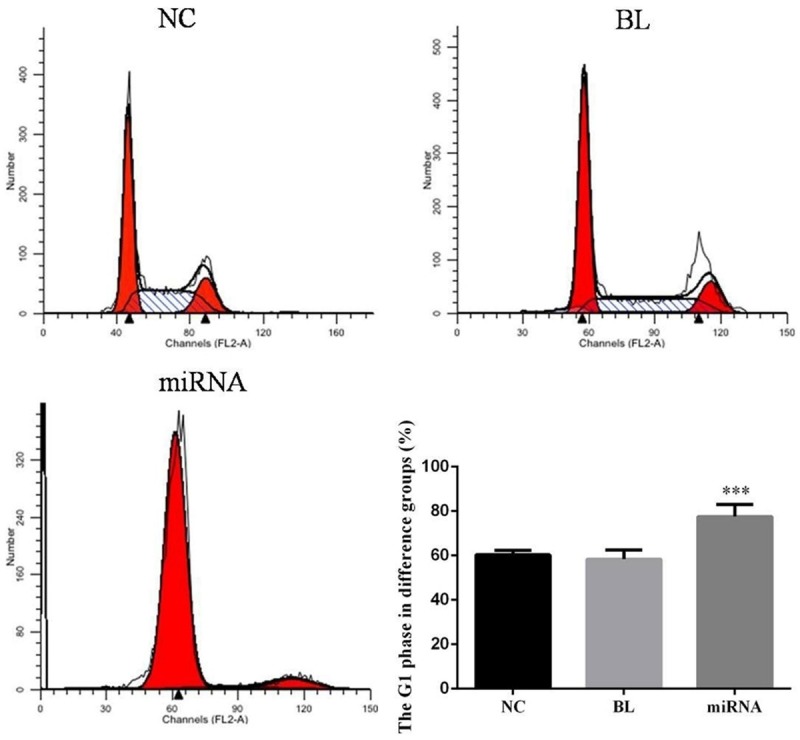
The cell cycle in difference groups. ***: P<0.05, Compared with NC group.
WB assay
Compared with NC group, the relative PI3K and AKT proteins expressions of miRNA group were significantly reduced (P<0.05, respectively); the P21 protein expression of miRNA group was significantly increased (P<0.05). However, there were no significantly differences between NC group and BL group in PI3K, AKT and P21 proteins expression (P>0.05, respectively). The data was shown in Figure 7.
Figure 7.
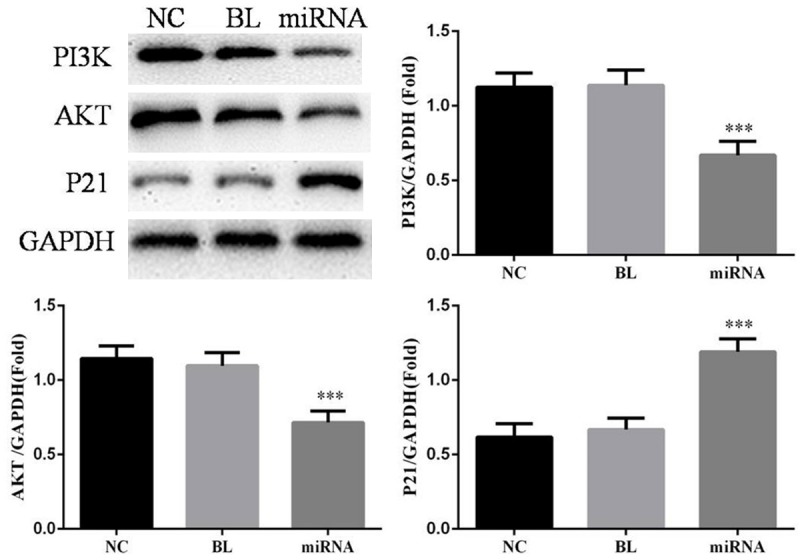
The relative protein expressions in difference groups. ***: P<0.05, Compared with NC group.
Discussion
Early signs of prostate cancer are similar to that of patients with benign prostatic hyperplasia, which often appear to have an invasion or metastasis, resulting in treatment failure or poor quality of life. Depend on this results, early diagnosis is very important for the treatment of prostate cancer. At this stage, PSA, AMACR and p63 are often used as markers for the diagnosis of prostate cancer.
Prostate specific antigen (PSA) screening has been widely used in the diagnosis of prostate cancer, however, only 6.2% of patients because of the increase in PSA and final diagnosis, the average PSA of the part of the patient is 46.1 g/L [9]. Patients often due to delay in the development of the disease to the advanced stage, and then lead to the treatment of prostate cancer and poor prognosis. α-Methylacyl-CoA recemase (AMACR) can be encoded by P504S gene. It was found that AMACR/P504S is highly expressed in prostate cancer, and it can be used as a relatively specific tumor marker [10], but it has appeared in many malignant tumors, such as lung cancer, lymphatic cancer and so on. It can be seen that AMACR could not only be used for the diagnosis of prostate cancer, and other biomarkers can play a role in the diagnosis. P63 and P53, homology gene, had been proved to be a new type of basal cell markers; their advantages are that they can identify the integrity of the cell [11-13]. But for early invasive prostate cancer patients, the diagnosis is not significant. It can be known that although there are some diagnostic values in the diagnosis of prostate cancer, the specificity is not high. So it is very important to search for new biomarkers for early diagnosis of prostate cancer.
MicroRNAs are a class of post transcriptional regulation of gene expression of non-coding RNAs, existing research has shown that miRNAs in a certain extent is important in the regulation of cell biology behavior role, including control of cell differentiation, cell cycle, cell growth and immune response [14]. This indicates that miRNAs is closely related to the progression of the disease, especially the tumor. By reverse transcription and real-time quantitative expression analysis of prostate tissue, miRNA was stable in the presence of plasma, serum, formaldehyde fixed tissue, and even found consistent with the level of blood vessels [15]. Therefore, miRNA is not only suitable for the detection of prostate cancer as a non-invasive biomarker, but also may be used for staging of prostate cancer, observation of prognosis and monitoring of treatment response. Recent studies have found that miRNAs plays a regulatory role in the development and metastasis of tumors, including prostate cancer.
Research reported that miRNA could be used with existing biomarkers and techniques to increase the predictive effect of cancer. Recent progress and metastasis of prostate cancer cells in the presence of miR-141 in the presence of and in the presence of PSA were found to be associated with the presence of an anti-male and castration resistant prostate cancer cells. According to the regression model and probability analysis, the ratio of miR-141 level to predict the clinical outcome is more than 8.3 folds [16]. Gade et al constructed two points graph which are described the relationship between miRNA and miRNA expression, by using 98 hormone sensitive prostate cancer samples [17]. This may provide a biochemical indicator for predicting recurrence of prostate cancer after radical prostatectomy. Barnadas et al [18] recently discovered in an array are increased or 8p2 NKX31 gene deletion 8q24.3 miR-151 African American prostate cancer patients, 67% of malignant outcome (in radiotherapy or peeling after androgen PSA levels and/or recurrent), only 17% had a good prognosis (prostate cancer root governing skill undetectable serum PSA over 9 years), suggesting that in these patients, miRNA can index as the detection of recurrence.
MiR-27b is located on chromosome 9q22.32. Research shows that miR-27b expression in breast cancer increased significantly, and the related with the prognosis, the in vivo and in vitro experiments confirmed that inhibiting mir-27b promotes Nischarin (A novel protein that interacts with integrin subunit) expression, and epidermal growth factor (EGF), tumor necrosis factor alpha (TNF-α) can through the expression of Akt/NF kappa B (a transcription factor protein family) signal pathway up-regulated miR-27b expression [19]. In glioma, miR-27b expression was significantly increased, and inhibiting mir-27b slow glioma cell growth and invasion process, and induce the apoptosis of tumor cells and down regulate the expression of signal transduction and transcription activation factor 3 (STAT3), C-myc (as a member of the myc gene family) and cyclin-D1 [20]. While in colorectal cancer, the expression of mir-27b had lower. Over expression of mir-27b can inhibit tumor proliferation, follow-up study found that mir-27b can target to the effect on vascular endothelial growth factor C (VEGF-C) 3’-UTR (3’-Untranslated regions and 3’-non translated region), and inhibition of tumor angiogenesis [21-23].
PI3K/AKT signaling pathway was an important role in cell apoptosis and proliferation, this signaling pathway was activation state in most cancer cells [24,25]. P21, a key member of cyclin-dependent kinase inhibitor (CDKI), is belonged to cytokine negative regulation factor, and regulates G1 phase transition to S phase [26,27]. In some relative studies were shown that regulation PI3K/AKT/P21 signaling pathway had effects to suppress the cell proliferation and improve cell apoptosis [28-30].
In this study, through the study of the relationship between the expression of miR-27b and the prognosis of prostate cancer in, and mir-27b and prostate cancer clinical pathological factors and correlation, we found expression of mir-27b and prostate cancer Gleason score (P<0.05), and decreased gradually with the increase of Gleason score and clinical grading, suggesting that mir-27b on prostate cancer cell differentiation may have an important role. In the prognosis of prostate cancer patients, we found that there were significantly differences between miR-27b low and high groups in PFS and OS (P<0.05, respectively). All of those suggested that miR-27b was helpful to evaluate the degree of malignancy and prognosis of patients with prostate cancer, which could be considered as an important basis for diagnosis of prostate cancer.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Wong MC, Goggins WB, Wang HH, Fung FD, Leung C, Wong SY, Ng CF, Sung JJ. Global incidence and mortality for prostate cancer: analysis of temporal patterns and trends in 36 countries. Eur Urol. 2016;70:862–874. doi: 10.1016/j.eururo.2016.05.043. [DOI] [PubMed] [Google Scholar]
- 3.Yang JY, Sun YW, Liu DJ, Zhang JF, Li J, Hua R. MicroRNAs in stool samples as potential screening biomarkers for pancreatic ductal adenocarcinoma cancer. Am J Cancer Res. 2014;4:663–673. [PMC free article] [PubMed] [Google Scholar]
- 4.Adem BF, Bastos NR, Dias F, Teixeira AL, Medeiros R. miRNAs: mediators of ErbB family targeted therapy resistance. Pharmacogenomics. 2016;17:1175–1187. doi: 10.2217/pgs-2016-0038. [DOI] [PubMed] [Google Scholar]
- 5.Navickas R, Gal D, Laucevičius A, Taparauskaitė A, Zdanytė M, Holvoet P. Identifying circulating MicroRNAs as biomarkers of cardiovascular disease: a systematic review. Cardiovasc Res. 2016;111:322–37. doi: 10.1093/cvr/cvw174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song SJ, Poliseno L, Song MS, Ala U, Webster K, Ng C, Beringer G, Brikbak NJ, Yuan X, Cantley LC, Richardson AL, Pandolfi PP. MicroRNA-antagonism regulates breast cancer stemness and metastasis via TET-family-dependent chromatin remodeling. Cell. 2013;154:311–324. doi: 10.1016/j.cell.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurozumi A, Goto Y, Okato A, Ichikawa T, Seki N. Aberrantly expressed microRNAs in bladder cancer and renal cell carcinoma. J Hum Genet. 2017;62:49–56. doi: 10.1038/jhg.2016.84. [DOI] [PubMed] [Google Scholar]
- 8.Mukohyama J, Shimono Y, Yamashita K, Sumi Y, Mukohara T, Minami H, Kakeji Y. Effect of xenotransplantation site on microRNA expression of human colon cancer stem cells. Aniticancer Res. 2016;36:3679–3786. [PubMed] [Google Scholar]
- 9.Peyromaure M, Debré B, Mao K, Zhang G, Wang Y, Sun Z, Xu D, Jiang J, Sun Y. Management of prostate cancer in China: a multicenter report of 6 institutions. J Urol. 2005;174:1794–1797. doi: 10.1097/01.ju.0000176817.46279.93. [DOI] [PubMed] [Google Scholar]
- 10.Luo J, Zha S, Gage WR, Dunn TA, Hicks JL, Bennett CJ, Ewing CM, Platz EA, Ferdinandusse S, Wanders RJ, Trent JM, Isaacs WB, De Marzo AM. Alpha-methylacyl-CoA racemase: a new molecular marker for prostate cancer. Cancer Res. 2002;62:2220–2228. [PubMed] [Google Scholar]
- 11.Shah RB, Zhou M, LeBlanc M, Snyder M, Rubin MA. Comparison of the basal cell-specific markers, 34betaE12 and p63, in the diagnosis of prostate cancer. Am J Surg Pathol. 2002;26:1161–1168. doi: 10.1097/00000478-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Xu-Monette ZY, Zhang S, Li X, Manyam GC, Wang XX, Xia Y, Visco C, Tzankov A, Zhang L, Montes-Moreno S, Dybkaer K, Chiu A, Orazi A, Zu Y, Bhagat G, Richards KL, Hsi ED, Choi WW, van Krieken JH, Huh J, Ponzoni M, Ferreri AJ, Zhao X, Møller MB, Parsons BM, Winter JN, Piris MA, Medeiros LJ, Young KH. p63 expression confers significantly better survival outcomes in high-risk diffuse large B-cell lymphoma and demonstrates p53-like and p53-independent tumor suppressor function. Aging (Albany NY) 2016;8:345–365. doi: 10.18632/aging.100898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monteiro LS, Delgado ML, Ricardo S, do Amaral B, Salazar F, Pacheco JJ, Lopes CA, Bousbaa H, Warnakulasuryia S. Prognostic significance of CD44v6, p63, podoplanin and MMP-9 in oral squamous cell carcinomas. Oral Dis. 2016;22:303–312. doi: 10.1111/odi.12442. [DOI] [PubMed] [Google Scholar]
- 14.Ambros V. MicroRNAs: genetically sensitized worms reveal new secrets. Curr Biol. 2010;20:R598–600. doi: 10.1016/j.cub.2010.05.054. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natt Acad Sci U S A. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzales JC, Fink LM, Goodman OB Jr, Symanowski JT, Vogelzang NJ, Ward DC. Comparison of circulating microRNA 141 to circulating tumor cells, lactate dehydrogenase, and prostate-specific antigen for determining treatment response in patients with metastatic prostate cancer. Clin Genitourin Cancer. 2011;9:39–45. doi: 10.1016/j.clgc.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Gade S, Porzelius C, Fälth M, Brase JC, Wuttig D, Kuner R, Binder H, Sültmann H, Beissbarth T. Graph based fusion of miRNA and mRNA expression data improves clinical outcome prediction inprostate cancer. BMC Bioinformatics. 2011;12:488. doi: 10.1186/1471-2105-12-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnabas N, Xu L, Savera A, Hou Z, Barrack ER. Chromosome 8 markers of metastatic prostate cancer in African American men: gain of the MIR151 gene and loss of the NKX3-1 gene. Prostate. 2011;71:857–871. doi: 10.1002/pros.21302. [DOI] [PubMed] [Google Scholar]
- 19.Jin L, Wessely O, Marcusson EG, Ivan C, Calin GA, Alahari SK. Prooncogenic factors miR-23b and miR-27b are regulated by Her2/Neu, EGF, and TNF-α in breast cancer. Cancer Res. 2013;73:2884–2896. doi: 10.1158/0008-5472.CAN-12-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L, Li H, Han L, Zhang K, Wang G, Wang Y, Liu Y, Zheng Y, Jiang T, Pu P, Jiang C, Kang C. Expression and function of miR-27b in human glioma. Oncol Rep. 2011;26:1617–1621. doi: 10.3892/or.2011.1458. [DOI] [PubMed] [Google Scholar]
- 21.Ye J, Wu X, Wu D, Wu P, Ni C, Zhang Z, Chen Z, Qiu F, Xu J, Huang J. miRNA-27b targets vascular endothelial growth factor C to inhibit tumor progression and angiogenesis incolorectal cancer. PLoS One. 2013;8:e60687. doi: 10.1371/journal.pone.0060687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subramaniam KS, Omar IS, Kwong SC, Mohamed Z, Woo YL, Mat Adenan NA, Chung I. Cancer-associated fibroblasts promote endometrial cancer growth via activation of interleukin-6/STAT-3/c-Myc pathway. Am J Cancer Res. 2016;6:200–213. [PMC free article] [PubMed] [Google Scholar]
- 23.Jhaveri K, Teplinsky E, Silvera D, Valeta-Magara A, Arju R, Giashuddin S, Sarfraz Y, Alexander M, Darvishian F, Levine PH, Hashmi S, Zolfaghari L, Hoffman HJ, Singh B, Goldberg JD, Hochman T, Formenti S, Esteva FJ, Moran MS, Schneider RJ. Hyperactivated mTOR and JAK2/STAT3 pathways: molecular drivers and potential therapeutic targets of inflammatory and invasive ductal breast cancers after neoadjuvant chemotherapy. Clin Breast Cancer. 2016;16:113–122. doi: 10.1016/j.clbc.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis. 2004;9:667–676. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- 25.Xie Q, Wen H, Zhang Q, Zhou W, Lin X, Xie D, Liu Y. Inhibiting PI3K-AKT signaling pathway is involved in antiumor effects of ginsenoside Rg3 in lung cancer cell. Biomed Phamacother. 2016;85:16–21. doi: 10.1016/j.biopha.2016.11.096. [DOI] [PubMed] [Google Scholar]
- 26.Morelli MB, Amantini C, Nabissi M, Cardinali C, Santoni M, Bernardini G, Santoni A, Santoni G. Axitinib induces senescence-associated cell death and necrosis in glioma cell lines: the proteasome inhibitor, bortezomib, potentiates axitinib-induced cytotoxicity in a p21 (Waf/Cip1) dependent manner. Oncotarget. 2017;8:3380–3395. doi: 10.18632/oncotarget.13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shtutman M, Chang BD, Schools GP, Broude EV. Cellular model of p-21-induced senescence. Methods Mol Biol. 2017;1534:31–39. doi: 10.1007/978-1-4939-6670-7_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forte D, Salvestrini V, Corradi G, Rossi L, Catani L, Lemoli RM, Cavo M, Curti A. The tissue inhibitor of metalloproteinases-1 (TIMP-1) promotes survival and migration of acute myeloid leukemia cells through CD63/PI3K/Akt/p21 signaling. Oncotarget. 2017;8:2261–2274. doi: 10.18632/oncotarget.13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Jiang J, Zhao M, Luo X, Liang Z, Zhen Y, Fu Q, Deng X, Lin X, Li L, Luo R, Liu Z, Fang W. microRNA-374a suppresses colon cancer progression by directly reducing CCND1 to inactivate the PI3K/AKT pathway. Oncotarget. 2016;7:41306–41319. doi: 10.18632/oncotarget.9320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prudnikova TY, Villamar-Cruz O, Rawat SJ, Cai KQ, Chernoff J. Effects of p21-activated kinase 1 inhibition on 11q13-amplified ovarian cancer cells. Oncogene. 2016;35:2178–2185. doi: 10.1038/onc.2015.278. [DOI] [PMC free article] [PubMed] [Google Scholar]


