Abstract
Objectives
Streptococcus agalactiae [group B streptococci (GBS)] have been considered uniformly susceptible to penicillin. However, increasing reports from Asia and North America are documenting penicillin-non-susceptible GBS (PRGBS) with mutations in pbp genes. Here we report, to the best of our knowledge, the first two PRGBS isolates recovered in Europe (AC-13238-1 and AC-13238-2), isolated from the same patient.
Methods
Two different colony morphologies of GBS were noted from a surgical abscess drainage sample. Both were serotyped and antimicrobial susceptibility testing was performed by different methodologies. High-throughput sequencing was done to compare the isolates at the genomic level, to identify their capsular type and ST, to evaluate mutations in the pbp genes and to compare the isolates with the genomes of other PRGBS isolates sharing the same serotype and ST.
Results
Isolates AC-13238-1 and AC-13238-2 presented MICs above the EUCAST and CLSI breakpoints for penicillin susceptibility. Both shared the capsular type Ia operon and ST23. Genomic analysis uncovered differences between the two isolates in seven genes, including altered pbp genes. Deduced amino acid sequences revealed critical substitutions in PBP2X in both isolates. Comparison with serotype Ia clonal complex 23 PRGBS from the USA reinforced the similarity between AC-13238-1 and AC-13238-2, and their divergence from the US strains.
Conclusions
Our results support the in-host evolution of β-lactam-resistant GBS, with two PRGBS variants being isolated from one patient.
Introduction
Streptococcus agalactiae [group B streptococci (GBS)] are widely recognized pathogens of the neonatal period, increasingly associated with invasive disease in adults, particularly among the elderly and those with underlying conditions.1 While GBS have been considered universally susceptible to β-lactams, with penicillin being the first-line drug for both prevention and treatment of GBS infections,2 penicillin-non-susceptible GBS (PRGBS) clinical isolates have been reported for over a decade. The first description from Japan included isolates recovered as early as 1995 that were non-susceptible to penicillin and cephalosporins through the acquisition of point mutations in pbp genes, leading to the production of proteins with low-affinity for β-lactam binding.3 Allelic exchange experiments revealed the critical role of the acquisition of two substitutions (V405A and/or Q557E) adjacent to the conserved active-site motifs in the transpeptidase domain of PBP2X,3 but numerous substitutions have been found among PRGBS in PBP1A, PBP2A and PBP2B.3–11 While multiple studies from Japan7,8,12 initially suggested a geographically confined distribution of PRGBS, more recent reports from other countries including Canada,4,9 Korea5 and the USA6 support a wider dissemination of these isolates. In Canada, two studies have demonstrated the acquisition of PBP mutations by β-lactam-susceptible GBS after prolonged administration of penicillin resulting in PRGBS.4,9 To date there have been no reported cases of PRGBS in Europe, even though PBP mutations were found among GBS with reduced cephalosporin susceptibility in Italy.13
Materials and methods
Serotyping and antimicrobial susceptibility testing
Capsular serotyping was done by slide agglutination with IMMULEX STREP-B Kit (Statens Serum Institut, Copenhagen, Denmark).
Antimicrobial susceptibility testing of both isolates was performed by broth microdilution. Penicillin MIC was also determined with Etest strips (bioMérieux, Marcy-l’Étoile, France), according to the guidelines of CLSI14 and EUCAST.15 Additional testing with the automated systems WalkAway (Beckman Coulter, Brea, CA, USA) and VITEK 2 (bioMérieux) was performed. Finally, a test for PRGBS was done by standard disc diffusion with ceftibuten, oxacillin and ceftizoxime discs, as described by Kimura et al.16
High-throughput sequencing
The two GBS isolates (AC-13238-1, ERR3464558 and AC-13238-2, ERR3464559) were sequenced using the NextSeq system (Illumina, San Diego, CA, USA) and analysed by both mapping and de novo assembly approaches. The draft genomes and detailed methods can be found at https://doi.org/10.5281/zenodo.3479195.
Amino acid sequences were deduced from the pbp1a, pbp1b, pbp2a, pbp2b and pbp2x genes and compared with the penicillin-susceptible reference strain 2603V/R (GenBank accession number NC_004116) using Geneious software 8.9.1 (Biomatters, Ltd, Auckland, New Zealand).
Results and discussion
A >75-year-old male patient presented at the emergency room with progressive right hip pain. Medical history included a cement-free hip prosthesis on both sides, implanted 20 years ago. The soft parts of the right hip and the right upper leg showed no signs of inflammation besides tenderness on palpation, but blood testing revealed leucocytosis and reduced kidney function. Sonography of the right upper leg showed an echo-poor intramuscular formation in M. vastus lateralis and an effusion in the right hip joint. Sonographically directed puncture of the echo-poor formation showed a macroscopically sanious fluid. Surgical drainage of the abscess in the right upper leg was sent for culture. Linezolid treatment was started intra-operatively. Post-operatively the patient went to the ICU because of his critical condition.
After observation of Gram-positive streptococci and reduction of infection parameters, treatment was de-escalated to benzylpenicillin. Following improvement of the general condition of the patient, kidney function and infection parameters, the patient was transferred to standard care, with termination of antibiotic treatment after 8 days. The patient was discharged from hospital 12 days post-operation, with restored kidney function and no infection parameters.
Two different colony morphologies were evident upon culture, one type being more haemolytic and pigmented. Both were confirmed as GBS and named isolates AC-13238-1 and AC-13238-2.
After agglutination with specific sera, AC-13238-1 was considered non-typeable and AC-13238-2 was serotype Ia. Both isolates were susceptible to erythromycin, clindamycin, levofloxacin, linezolid and vancomycin, and resistant to tetracycline (Table S1, available as Supplementary data at JAC Online). They presented, however, different MICs of penicillin depending on the method used to test susceptibility (Table 1). AC-13238-1 and AC-13238-2 were non-susceptible to penicillin according to broth microdilution and Etest (presenting MICs of 0.5 and 1 mg/L, respectively), following the CLSI or EUCAST methods and interpretative criteria. Both isolates presented reduced diameters of growth-inhibitory zones with oxacillin, ceftizoxime and ceftibuten discs. Additional antimicrobial susceptibility testing with commercial automated systems revealed lower MICs by up to two dilutions with WalkAway and failed to produce results in VITEK 2. This is in agreement with a study from Japan in which MICs determined by broth microdilution and VITEK 2 were significantly different, with VITEK 2 failing to detect almost half the cases of PRGBS,17 raising the possibility of missed cases of PRGBS in laboratories in which automated systems are routinely used. MICs of other β-lactams were also elevated (Table S1).
Table 1.
MICs, zone diameters and amino acid substitutions in PBPs found in PRGBS isolates AC-13238-1 and AC-13238-2
| Isolate | PEN MIC (mg/L)a |
Disc diffusion (mm) |
Amino acid substitutionsc |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| microdilution | Etestb | WalkAway | VITEK 2 | PEN | OXA | CTB | CTX | PBP2X | PBP1A | PBP2B | |
| AC-13238-1 | 0.5 | 0.5 | 0.12 | UD | 27 | 7 | 0 | 16 | I377V, F395L, V405A, H438Y, V510I | A521V, del719–722, N723S, V726A | V80A |
| AC-13238-2 | 1 | 1 | 0.5 | UD | 23 | 0 | 0 | 15 | I377V, V405A, H438Y, V510I, Q557E | T526I, del719–722, N723S, V726A | V80A |
PEN, penicillin; OXA, oxacillin; CTB, ceftibuten; CTX, cefotaxime; UD, undetermined.
Each MIC value was obtained consistently across three independent assays.
Same PEN MIC determined by Etest according to EUCAST and CLSI guidelines.
The amino acid positions are those of the corresponding genes in strain 2603V/R (GenBank accession number NC_004116). Substitutions strongly associated with PRGBS are shown in bold.
The genomes of AC-13238-1 and AC-13238-2 were 1 970 769 bp and 1 970 815 bp long, respectively. Genomic analysis confirmed that both contained the genes encoding the Eps surface protein, pilus island 2a, the type Ia capsular polysaccharide (CPS) loci and shared the same MLST-based ST23. AC-13238-1 and AC-13238-2 differed from each other in seven genes (Table S2). Among these, a single nucleotide deletion at position 302 of the neuC gene of AC-13238-1 resulted in a premature stop codon in the CPS locus. This gene is involved in the synthesis of sialic acid, present in all GBS serotypes. Loss of sialylation was shown to significantly reduce the amount of surface-associated CPS produced by GBS,18 so this mutation potentially impacted capsule production, explaining the observed non-reactivity of this isolate with the type Ia-specific sera. Additional differences included two non-synonymous mutations in each of the pbp1a and pbp2x genes.
Comparison of the deduced amino acid sequence of the pbp genes of AC-13238-1 and AC-13238-2 with those of the susceptible strain 2603V/R revealed, in each isolate, the presence of five amino acid substitutions, four amino acid substitutions and one amino acid substitution in the pbp2x, pbp1a and pbp2b genes, respectively, relative to the reference (Table 1). Of note, both isolates shared the V405A substitution and AC-13238-2 also carried the Q557E substitution in PBP2X. These are considered key substitutions due to their proximity to the transpeptidase domain active site motifs 402SSN404 and 552KSG554, which, by allelic exchange experiments, were shown to contribute to a significant increase in β-lactam MIC.3 Other substitutions in PBP2X (I377V, H438Y and V510I in both isolates and F395L in AC-13238-1) were all previously described among both β-lactam-resistant and -susceptible isolates,6,8,10,11,13,19 suggesting that these do not contribute to the increased MICs. In PBP1A the isolates shared an N723S (already detected among non-susceptible GBS in Japan),11 a V726A substitution (not previously described) and a deletion of four amino acid residues (719–722), which was shown to be present in various GBS isolates regardless of penicillin susceptibility.11 Additionally, the isolates each had one different substitution in PBP1A, not previously described: AC-13238-1 and AC-13238-2 carried an A521V and a T526I substitution, respectively, the latter in the same site as the T526A substitution, reported in a PRGBS isolated from a patient with a prosthetic hip joint infection after prolonged low-dose penicillin therapy.4 Finally, both isolates carried a V80A substitution in PBP2B, also previously detected in PRGBS isolates from Japan.11
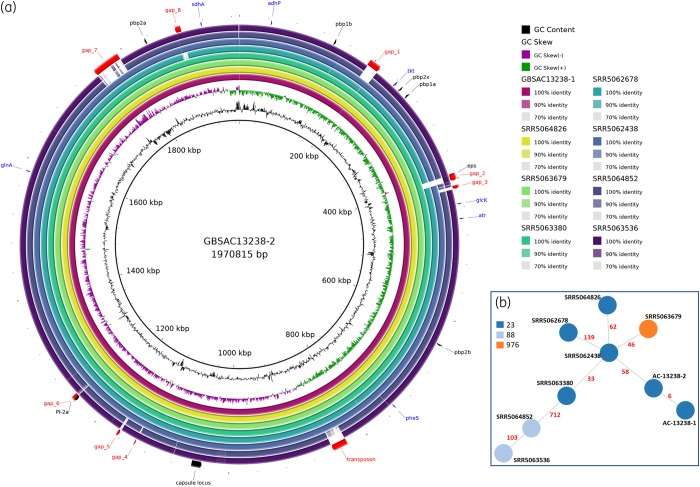
In Japan, most PRGBS isolates share the capsular serotype VI and belong to clonal complex (CC) 1,8,20 in agreement with a clonal expansion model of particularly resistant lineages. In the USA, however, decreased β-lactam susceptibility has been found across multiple serotypes and clones,6 including serotype Ia/CC23, suggesting that the stepwise accumulation of mutations in the pbp genes is occurring independently of the genetic background. We compared our two isolates with the genomes of seven serotype Ia isolates identified in the USA carrying PBP mutations associated with decreased β-lactam susceptibility,6 using the genome of AC-13238-2 as the reference (Figure 1). Significant additional genetic information was found in our isolates relative to some of the Ia/CC23 isolates from the USA (Figure 1a). Comparison of the core genome, consisting of 1664 genes, showed a distance of six alleles between AC-13238-1 and AC-13238-2, and minimum and maximum distances of these to the US serotype Ia/CC23 isolates of 58 and 724 alleles, respectively (Figure 1b). These results indicate that our isolates are very similar to each other, but quite different from the other serotype Ia/CC23 PRGBS described to date. Taken together, genomic analyses support the hypothesis of in-host evolution of PRGBS, with two variants of the same strain being isolated from one surgical sample. Even though the patient recovered completely from the infection, it is not possible to state that the altered PBPs had no effect on the penicillin treatment course since the patient was given linezolid (to which both isolates were susceptible) intra-operatively and the antimicrobial therapy was de-escalated to benzylpenicillin only after isolation of GBS.
Figure 1.
Genomic comparison of AC-13238-1 and AC-13238-2 with the genomes of seven serotype Ia/CC23 isolates identified in the USA carrying PBP mutations associated with decreased β-lactam susceptibility. (a) Whole-genome comparison. The innermost black ring shows the AC-13238-2 genome length (kb), used as the reference. The second and third innermost rings show GC content and GC skew, respectively. The eight outermost rings represent AC-13238-1 and the seven serotype Ia/CC23 isolates, shaded according to their respective percentage nucleotide identity to the query sequence (AC-13238-2), determined by BLAST. Several loci are represented: red, regions present in AC-13238-1 and AC-13238-2, but not in all other Ia/CC23 isolates (gaps); black, regions used to characterize the isolates (PBPs, capsular locus, epsilon surface protein gene and pilus operon); and blue, MLST genes. (b) Core-genome minimum spanning tree of serotype Ia/CC23 PRGBS (1664 genes). Colours were attributed to nodes (strains) according to their ST. Numbers of different alleles between strains are shown in red.
PRGBS are reported at an increasing rate, but the clinical significance of in vitro penicillin non-susceptibility of PRGBS is still not fully understood. While studies reporting the acquisition of PBP mutations by GBS isolates recovered from patients undergoing β-lactam therapy have not been associated with treatment failure,4,7,9 continued surveillance is critical as PRGBS may impact future therapeutic strategies for the prevention and management of GBS infections.
Supplementary Material
Acknowledgements
We thank Catarina Inês Mendes for technical support.
Funding
This work was partly supported by the ONEIDA project (LISBOA-01-0145-FEDER-016417) co-funded by FEEI–Fundos Europeus Estruturais e de Investimento from Programa Operacional Regional Lisboa 2020 and by national funds from Fundação para a Ciência e a Tecnologia (FCT) and UID/BIM/50005/2019, project funded by Fundação para a Ciência e a Tecnologia/Ministério da Ciência, Tecnologia e Ensino Superior (MCTES) through Fundos do Orçamento de Estado. E.R.M. was supported by Fundação para a Ciência e a Tecnologia (DL 57/2016/CP1451/CT0009).
Transparency declarations
M.v.d.L. has received research grants administered through his university and received honoraria for serving on the speaker’s bureaus of Pfizer and Merck Sharp and Dohme. J.M.-C. has received research grants administered through his university and received honoraria for serving on the speaker’s bureaus of Pfizer and Merck Sharp and Dohme. M.R. has received honoraria for serving on the speaker’s bureau of Pfizer and for consulting for GlaxoSmithKline and Merck Sharp and Dohme. All other authors: none to declare.
References
- 1. Raabe VN, Shane AL.. Group B Streptococcus (Streptococcus agalactiae). Microbiol Spectr 2019; 7: doi:10.1128/microbiolspec.GPP3-0007-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Verani JR, McGee L, Schrag SJ.. Prevention of perinatal group B streptococcal disease: revised guidelines from CDC, 2010. MMWR Recomm Rep 2010; 59: 1–36. [PubMed] [Google Scholar]
- 3. Kimura K, Suzuki S, Wachino J. et al. First molecular characterization of group B streptococci with reduced penicillin susceptibility. Antimicrob Agents Chemother 2008; 52: 2890–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gaudreau C, Lecours R, Ismail J. et al. Prosthetic hip joint infection with a Streptococcus agalactiae isolate not susceptible to penicillin G and ceftriaxone. J Antimicrob Chemother 2010; 65: 594–5. [DOI] [PubMed] [Google Scholar]
- 5. Yi A, Kim C-K, Kimura K. et al. First case in Korea of group B streptococcus with reduced penicillin susceptibility harboring amino acid substitutions in penicillin-binding protein 2X. Ann Lab Med 2019; 39: 414–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Metcalf BJ, Chochua S, Gertz RE. et al. Short-read whole genome sequencing for determination of antimicrobial resistance mechanisms and capsular serotypes of current invasive Streptococcus agalactiae recovered in the USA. Clin Microbiol Infect 2017; 23: 574.e7–14. [DOI] [PubMed] [Google Scholar]
- 7. Nagano N, Kimura K, Nagano Y. et al. Molecular characterization of group B streptococci with reduced penicillin susceptibility recurrently isolated from a sacral decubitus ulcer. J Antimicrob Chemother 2009; 64: 1326–8. [DOI] [PubMed] [Google Scholar]
- 8. Nagano N, Nagano Y, Toyama M. et al. Nosocomial spread of multidrug-resistant group B streptococci with reduced penicillin susceptibility belonging to clonal complex 1. J Antimicrob Chemother 2012; 67: 849–56. [DOI] [PubMed] [Google Scholar]
- 9. Longtin J, Vermeiren C, Shahinas D. et al. Novel mutations in a patient isolate of Streptococcus agalactiae with reduced penicillin susceptibility emerging after long-term oral suppressive therapy. Antimicrob Agents Chemother 2011; 55: 2983–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seki T, Kimura K, Reid ME. et al. High isolation rate of MDR group B streptococci with reduced penicillin susceptibility in Japan. J Antimicrob Chemother 2015; 70: 2725–8. [DOI] [PubMed] [Google Scholar]
- 11. Nagano N, Nagano Y, Kimura K. et al. Genetic heterogeneity in pbp genes among clinically isolated group B streptococci with reduced penicillin susceptibility. Antimicrob Agents Chemother 2008; 52: 4258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nagano N, Nagano Y, Toyama M. et al. Penicillin-susceptible group B streptococcal clinical isolates with reduced cephalosporin susceptibility. J Clin Microbiol 2014; 52: 3406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Piccinelli G, Carlentini G, Gargiulo F. et al. Analysis of point mutations in the pbp2x, pbp2b, and pbp1a genes of Streptococcus agalactiae and their relation with a reduced susceptibility to cephalosporins. Microb Drug Resist 2017; 23: 1019–24. [DOI] [PubMed] [Google Scholar]
- 14.CLSI. Performance Standards for Antimicrobial Susceptibility Testing—Twenty-Eighth Edition: M100 2018.
- 15.EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 9.0, 2019. http://www.eucast.org/clinical_breakpoints/.
- 16. Kimura K, Wachino J, Kurokawa H. et al. Practical disk diffusion test for detecting group B streptococcus with reduced penicillin susceptibility. J Clin Microbiol 2009; 47: 4154–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kimura K, Nagano N, Nagano Y. et al. Ability of the VITEK® 2 system to detect group B streptococci with reduced penicillin susceptibility (PRGBS). J Antimicrob Chemother 2013; 68: 1442–4. [DOI] [PubMed] [Google Scholar]
- 18. Chaffin DO, Mentele LM, Rubens CE.. Sialylation of group B streptococcal capsular polysaccharide is mediated by cpsK and is required for optimal capsule polymerization and expression. J Bacteriol 2005; 187: 4615–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Banno H, Kimura K, Seki T. et al. High isolation rate and multidrug resistance tendency of penicillin-susceptible group B Streptococcus with reduced ceftibuten susceptibility in Japan. Eur J Clin Microbiol Infect Dis 2018; 37: 1511–9. [DOI] [PubMed] [Google Scholar]
- 20. Kimura K, Nagano N, Nagano Y. et al. Predominance of sequence type 1 group with serotype VI among group B streptococci with reduced penicillin susceptibility identified in Japan. J Antimicrob Chemother 2011; 66: 2460–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.