Abstract
Background
ESBL-producing Enterobacteriaceae (ESBL-E) are observed in many reservoirs. Pets might play an important role in the dissemination of ESBL-E to humans since they live closely together.
Objectives
To identify prevalence, risk factors, molecular characteristics, persistence and acquisition of ESBL-E in dogs and cats, and co-carriage in human–pet pairs belonging to the same household.
Methods
In a nationwide study, one person per household was randomly invited to complete a questionnaire and to submit a faecal sample. Dog and cat owners were invited to also submit a faecal sample from their pet. Repeated sampling after 1 and 6 months was performed in a subset. ESBL-E were obtained through selective culture and characterized by WGS. Logistic regression analyses and random forest models were performed to identify risk factors.
Results
The prevalence of ESBL-E carriage in these cohorts was 3.8% (95% CI: 2.7%–5.4%) for human participants (n=550), 10.7% (95% CI: 8.3%–13.7%) for dogs (n=555) and 1.4% (95% CI: 0.5%–3.8%) for cats (n=285). Among animals, blaCTX-M-1 was most abundant, followed by blaCTX-M-15. In dogs, persistence of carriage was 57.1% at 1 month and 42.9% at 6 months. Eating raw meat [OR: 8.8, 95% CI: 4.7–16.4; population attributable risk (PAR): 46.5%, 95% CI: 41.3%–49.3%] and dry food (OR: 0.2, 95% CI: 0.1–0.5; PAR: 56.5%, 95% CI: 33.2%–66.6%) were predictors for ESBL-E carriage in dogs. Human–dog co-carriage was demonstrated in five households. Human–cat co-carriage was not observed.
Conclusions
ESBL-E prevalence was higher in dogs than in humans and lowest in cats. The main risk factor for ESBL-E carriage was eating raw meat. Co-carriage in dogs and household members was uncommon.
Introduction
ESBL-producing Enterobacteriaceae (ESBL-E) have been observed in many reservoirs,1–5 including companion animals.6–8 In the Netherlands, approximately 18% of the households have a dog and 23% a cat.9 The prevalence and risk factors of ESBL-E in dogs and cats has been studied before, but often in a diseased population or in small sample sizes. In the Netherlands, the reported prevalence of ESBL-E was 45% and 55% in 20 healthy and diarrhoeic dogs, respectively, and 0% and 25% in 20 healthy and diarrhoeic cats, respectively.10 Pet contact was related to ESBL-E carriage in humans in a previous study,11 but little is known about transmission and co-carriage in humans and dogs belonging to the same household. In Sweden, identical ESBL-E strains were detected in dogs and humans in 2 out of 22 households studied.12 In addition, little is known about the persistence of ESBL-producing bacteria in dogs and cats. In one study, 84% of 38 dogs had ESBL-E carriage at least once in 6 months when tested monthly and faecal shedding of ESBL-E appeared to be highly dynamic over time.13
In this study we aimed to identify the prevalence, risk factors, molecular characteristics, persistence and acquisition of ESBL-E in dogs and cats, and co-carriage of these bacteria in human–pet pairs belonging to the same household.
Materials and methods
Study design, setting and data collection of the cross-sectional part of the study
We performed a monthly-repeated cross-sectional study among Dutch residents from November 2014 to November 2016. Each month during the study period, a random sample of ∼2000 residents, stratified by geographical region and degree of urbanization, including all age groups, was drawn from municipal registries covering the whole Dutch population (∼17 million inhabitants), as described earlier.14 One person per household was invited by regular mail to complete a web-based questionnaire; we enquired whether there was a dog or cat in the household and whether they were willing to participate with the dog or cat (or one of the dogs and cats, with the preference to participate with a dog). If they wanted to participate together with a dog or cat, additional questions about the dog or cat were immediately available to be filled out for the dog or cat (later on also called ‘pet’).
If the participant agreed to participate, faecal sample kits were provided to the participant’s home address, accompanied by a second questionnaire to ensure up-to-date information. The kit contained two pre-labelled sterile tubes for faecal sample collection and instructions regarding the collection and transport (one for the human participant and one for the pet). Transport was via regular mail in a special biological substance category B envelope.
Study design of the longitudinal part of the study
After 1 and 6 months from the first time of study participation, all phenotypically ESBL-E screen-positive participants and a random selection of non-carriers were invited to provide a faecal sample, accompanied by a third and fourth questionnaire to ensure up-to-date information. If the participant provided a faecal sample from the dog or cat during the cross-sectional part of the study, we asked them to do this again and in the same way as the first time, making longitudinal participation of the pets dependent on the longitudinal participation of the owners.
Questions regarding the pets included antimicrobial usage, hospitalization or visiting a veterinary clinic, having health complaints, predation, coprophagy (in the case of a dog only: ingestion of faeces, either their own or from other animals), feeding patterns, travelling abroad, staying in a kennel and having contact with other animals. In the second, third and fourth questionnaires, some of the questions from the first questionnaire were repeated to ensure up-to-date information about contact with animals, antimicrobial use in the 4 weeks prior to faecal sample collection and eating of raw or undercooked meat in the week prior to faecal sample collection. We sent reminders to the participants after 10 and 20 days in the case of no response.
ESBL-E detection
Samples were cultured overnight at 37°C on MacConkey agar with 1 mg/L cefotaxime (MacConkey+) (Oxoid, The Netherlands). In addition, 0.5 g of faecal material from each sample was cultured overnight at 37°C in 4.5 mL LB broth with 1 mg/L cefotaxime (LB+). When growth was observed on the MacConkey+ agar, up to five colonies with different morphologies were selected and recultured on MacConkey+ agar. The selective enrichment broths were discarded in these cases. In the absence of growth on the MacConkey+ plate, the LB+ enrichment broth was cultured on MacConkey+ agar. The isolates were stored in LB broth with 30% glycerol at −80°C until further use. Isolate species identification was performed using MALDI-TOF (Bruker, Germany). Only isolates belonging to Escherichia coli, Klebsiella pneumoniae and Enterobacter cloacae complex, which are the predominant ESBL-E causing infections in humans, were studied further.15
The isolates were first checked by specific PCR for the presence of genes encoding CTX-M-group-1, TEM and SHV ESBLs as these are the most common. Isolates negative in the PCR were tested for the presence of ESBL-encoding genes by the Check-MDR CT101 microarray (Check-Points, The Netherlands). ESBL-encoding genes were identified by specific PCRs and subsequent sequencing of the amplification products by conventional ABI sequencing technology (Thermo Fisher, USA and BaseClear, The Netherlands).
For WGS analysis, bacterial DNA was purified using the MO BIO UltraClean Microbial DNA Isolation Kit (QIAGEN, USA) and a library for sequencing with NextSeq (Illumina, USA) was prepared using the Nextera v2 kit (Illumina). Contigs were assembled with SPAdes genome assembler v3.10.1. Core-genome analysis was performed on the assembled contigs. Core-genome alignments were made using Parsnp v1.2.16 Recombination regions in the alignments were detected and corrected for using Gubbins.17 Phylogenetic trees were visualized with FigTree (http://tree.bio.ed.ac.uk/software/figtree/). The resistance genes, plasmids and multilocus STs were identified by ResFinder, PlasmidFinder and MLST of the Center for Genomic Epidemiology (DTU, Copenhagen, Denmark).18–20 Sequences of dog isolates were deposited in the European Nucleotide Archive short-read archive, under project number PRJEB31805. Sequences of human isolates were deposited at BioSample, with accession numbers SAMN11494421 to SAMN11494425.
Data analysis
The prevalence of ESBL-E carriage was calculated for dogs and cats.
The random forest algorithm was used to identify the relative importance of the putative factors in ESBL-E prediction. Random forest is a machine-learning algorithm that produces multiple decision trees based on bootstrapping and merges them together to get a more accurate and stable prediction. One thousand decision trees were grown to form the random forest and a random subset of variables was used at each split point, with 2 out of 25 variables used in each subset. To assess the importance of the variables, we evaluated the accuracy decrease (mean decrease of prediction accuracy by removing the variable in question from the model) and Gini decrease (mean decrease in the Gini index of node impurity; how each variable contributes to the homogeneity of the nodes and leaves in the resulting random forest). Higher decrease of accuracy and Gini index means higher variable importance.21 The AUC was calculated for the random forest model. An AUC value >0.7 is generally considered useful.22
A total of 25 putative risk factors were assessed by chi-squared or Fisher’s exact tests and a Benjamini and Hochberg correction for multiple testing was applied to P values in order to control for a false discovery rate of 0.15.23 All P values considered significant after Benjamini and Hochberg correction, as well as risk factors described in the literature, were included in univariate logistic regression analyses. Variables with a P value ≤0.10 in univariate analyses were selected for multivariable regression models built in a backward stepwise fashion. Variables showing a P value <0.05 and variables of which the covariates changed ≥10% remained in the model. Biologically plausible interactions between variables were assessed and if P values were <0.05 the interaction was added to the model.
The population attributable risk (PAR) among dogs, which is the proportion of ESBL-E carriers that would be prevented following elimination of the exposure, assuming the exposure is causal, was calculated. The adjusted ORs were used from the final multivariable logistic regression model for the variables significantly associated with ESBL-E carriage and the prevalence of exposure in the cases. Similarly, the 95% CIs were calculated based on the 95% CIs of the adjusted ORs.24
Missing values (2.5%–14%) in the dataset were imputed by chained equations, imputing and pooling 10 imputations.25 A sensitivity analysis was performed comparing the imputed data with complete case analyses.
To determine co-carriage of ESBL-E within households, we defined whether there was any association between human and pet co-carriage. Based on the observed prevalence of ESBL-E in humans and dogs, and assuming co-carriage in humans and dogs was uncorrelated, we determined the probability that both human and dog harboured ESBL-E (regardless of the ESBL genes) in a given household, as was done before.26 Likewise, within the households in which both human and dog were ESBL-E positive, we calculated the expected number of households sharing the same ESBL genes based on the variance in ESBL genes observed in humans and dogs. The expected values were compared with the observed values by binomial probability testing.
Analyses were performed in R v3.2.2 with the mice (v3.1.0) and randomForest packages (v4.6-14). Binomial probability testing was performed in Stata 13 (StataCorp LP, College Station, TX, USA).
Ethics
This study received ethics approval from the Medical Research Ethics Committee of the University Medical Center, Utrecht (WAG/om/14/012490). Informed consent was obtained from all participants. All participants gave consent and in the case of children, parents gave consent. All animal sampling was performed within the guidelines of the Dutch Animals Act (stb-2011-345) and the Animal Welfare Body Utrecht, meaning no additional license was required.
Results
In total, faecal samples from 555 dogs and 285 cats were received. Of the dogs, 265 (N=539; 49.2%) were male and 265 (N=526; 50.4%) had contact with other animals. There were 95 (N=478; 19.9%) and 372 (N=542; 68.6%) dogs who had been seen by a vet in the 4 weeks and the 12 months prior to faecal sample collection, respectively. In addition, 34 (N=476; 7.1%) and 74 (N=539; 13.7%) had received antimicrobials in the past 4 weeks and 6 months prior to faecal sample collection, respectively (Table 1).
Table 1.
Descriptive statistics, univariate and multivariate logistic regression and PAR of ESBL-E in dogs
| Variable | ESBL-E negative n=496 (89.4%) | ESBL-E positive n=59 (10.6%) | Univariate OR (95% CI) | Multivariate OR (95% CI) | PAR (95% CI) |
|---|---|---|---|---|---|
| Age, years | |||||
| 0–5 | 227 (47.2) | 26 (44.1) | |||
| >5 | 254 (52.8) | 33 (55.9) | |||
| Season | |||||
| autumn | 164 (33.4) | 18 (30.5) | ref. | ||
| spring | 130 (26.5) | 19 (32.2) | 1.3 (0.7–2.6) | ||
| summer | 93 (18.9) | 12 (20.3) | 1.2 (0.5–2.6) | ||
| winter | 104 (21.2) | 10 (16.9) | 0.9 (0.4–2.2) | ||
| Number of dogs in the household | |||||
| 1 | 377 (78.1) | 40 (67.8) | |||
| >1 | 106 (21.9) | 19 (32.2) | |||
| Gender | |||||
| female | 245 (51.0) | 29 (49.2) | |||
| male | 235 (49.0) | 30 (50.8) | 1.1 (0.6–1.9) | ||
| Dog breed | |||||
| crossbred | 166 (36.4) | 15 (26.8) | |||
| purebred | 290 (63.6) | 41 (73.2) | |||
| Contact with other dogs | |||||
| no | 236 (50.3) | 25 (43.9) | |||
| yes | 233 (49.7) | 32 (56.1) | |||
| Stayed in kennel in past 12 months | |||||
| no | 389 (81.4) | 51 (86.4) | |||
| yes | 89 (18.6) | 8 (13.6) | |||
| Abroad in the past 12 months | |||||
| no | 397 (82.4) | 44 (74.6) | |||
| yes | 85 (17.6) | 15 (25.4) | 1.6 (0.8–3.0) | ||
| Walking the dog in dog-walking area | |||||
| no | 363 (75.5) | 40 (67.8) | |||
| yes | 118 (24.5) | 19 (32.2) | |||
| Walking the dog in forest | |||||
| no | 193 (40.1) | 13 (22.0) | |||
| yes | 288 (59.9) | 46 (78.0) | 2.4 (1.2–4.5) | 2.2 (1.1–4.6) | 42.5 (7.1–61.0) |
| Walking the dog in area with livestock | |||||
| no | 437 (90.9) | 50 (84.7) | |||
| yes | 44 (9.1) | 9 (15.3) | |||
| Swimming | |||||
| no | 367 (76.3) | 38 (64.4) | |||
| yes | 114 (23.7) | 21 (35.6) | 1.8 (1.0–3.2) | ||
| Walking the dog without leash | |||||
| ≤50% of time | 278 (57.7) | 32 (54.2) | |||
| >50% of time | 204 (42.3) | 27 (45.8) | |||
| Coprophagy (eating stools) | |||||
| no | 341 (70.9) | 40 (67.8) | |||
| yes | 140 (29.1) | 19 (32.2) | |||
| Fed with dry feed (kibble) | |||||
| no | 26 (5.4) | 15 (25.4) | |||
| yes | 456 (94.6) | 44 (74.6) | 0.2 (0.1–0.3) | 0.2 (0.1–0.5) | 56.5 (33.2–66.6) |
| Fed with wet food | |||||
| no | 390 (80.9) | 46 (78.0) | |||
| yes | 92 (19.1) | 13 (22.0) | |||
| Fed with raw meat | |||||
| no | 438 (90.9) | 28 (47.5) | |||
| yes | 44 (9.1) | 31 (52.5) | 11.0 (6.1–20.1) | 8.8 (4.7–16.4) | 46.5 (41.3–49.3) |
| Hospitalized/consulted vet in the past 12 months | |||||
| no | 158 (32.7) | 12 (20.3) | |||
| yes | 325 (67.3) | 47 (79.7) | 1.9 (1.0–3.7) | ||
| Antimicrobial usage in the past 6 months | |||||
| no | 415 (86.3) | 50 (86.2) | |||
| yes | 66 (13.7) | 8 (13.8) | 1.0 (0.5–2.2) | ||
| Catch prey | |||||
| no | 377 (80.7) | 51 (87.9) | |||
| yes | 90 (19.3) | 7 (12.1) | |||
| Hospitalized/consulted vet in the past 4 weeks | |||||
| no | 348 (81.9) | 35 (66.0) | |||
| yes | 77 (18.1) | 18 (34.0) | 2.3 (1.2–4.3) | 2.0 (1.0–3.9) | 17.0 (0.3–25.3) |
| Stayed in kennel in past 4 weeks | |||||
| no | 396 (93.4) | 46 (86.8) | |||
| yes | 28 (6.6) | 7 (13.2) | |||
| Abroad in the past 4 weeks | |||||
| no | 410 (96.9) | 52 (98.1) | |||
| yes | 13 (3.1) | 1 (1.9) | 0.6 (0.1–4.8) | ||
| Antimicrobial usage in the past 8 weeks | |||||
| no | 393 (92.9) | 49 (92.5) | |||
| yes | 30 (7.1) | 4 (7.5) | 1.1 (0.4–3.2) |
For cats, 125 (N=282; 44.3%) were male, 165 (N=253; 65.2%) had contact with other animals and 25 (N=238; 10.5%) had visited a vet in the past 4 weeks. Antimicrobial use was reported in 12 (N=237; 5.1%) and 30 (N=275; 10.9%) in the 8 weeks or 6 months prior to sample collection, respectively (Table S1, available as Supplementary data at JAC Online).
Prevalence of ESBL-E carriage
ESBL-E were detected in samples of 59 dogs (10.6%, 95% CI: 8.3%–13.6%) and 4 cats (1.4%, 95% CI: 0.5%–3.8%). ESBL-E carriers were distributed equally over the country, without discernible differences in the degree of urbanization (Table S2). The number of ESBL-E per gram of faeces was variable and did not have a normal distribution. The median was 6.1 × 104 (IQR: 9.9 × 103–3.3 × 105).
Risk factors in dogs
In random forest analyses, ‘eating of raw meat’ was the best predictor for ESBL-E carriage in dogs, followed by the absence of ‘eating dry feed’ (Figure S1). The AUC of the random forest model was 0.78, meaning the random forest is useful for prediction (>0.70). Risk factors for carriage with ESBL-E and the associated PAR for dogs were ‘eating raw meat’ (OR: 8.8, 95% CI: 4.7–16.4; PAR: 46.5, 95% CI: 41.3–49.3), ‘walking the dog in the forest’ (OR: 2.2, 95% CI: 1.1–4.6; PAR: 42.5, 95% CI: 7.1–61.0) and ‘hospitalized or visited a vet in the past 4 weeks’ (OR: 2.0, 95% CI: 1.0–3.9; PAR: 17.0, 95% CI: 0.3–25.3). ‘Eating dry feed’ appeared protective for ESBL-E carriage in dogs (OR: 0.2, 95% CI: 0.1–0.5; PAR: 56.5, 95% CI: 33.2–66.6). Complete case analysis did not give significantly different results compared with the analysis on the imputed data (data not shown).
Risk factors could not be determined for cats.
Co-carriage in humans and pets
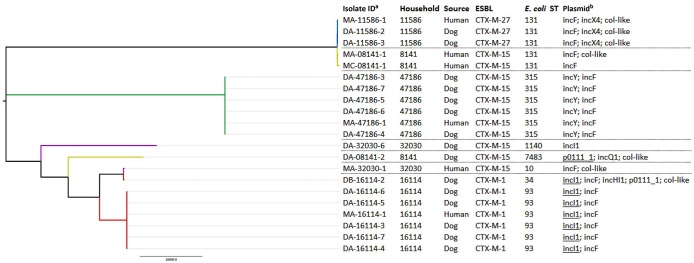
There were 550 pairs of faecal samples from human participants and dogs and 282 pairs from human participants and cats (for five dogs and three cats, a faecal sample from the owner was missing). The prevalence of ESBL-E carriage in these cohorts was 3.8% (95% CI: 2.7%–5.4%) for human participants, 10.7% (95% CI: 8.3%–13.7%) for dogs and 1.4% (95% CI: 0.5%–3.8%) for cats. There was no co-carriage found between cat owners and cats. In seven households, both the human participant and the dog were ESBL-E positive, of which five harboured the same ESBL gene. The isolates from the five human participant–dog pairs carrying the same ESBL gene were subjected to WGS. The core-genome cluster analysis is shown in Figure 1. In three human participant–dog pairs, no distinction can be made between the human participant and the dog isolates, based on the core genome. Additionally, if multiple isolates were selected from one dog sample, no distinction could be made between isolates, indicating low within-sample diversity.
Figure 1.
Core-genome cluster analysis of isolates from dogs and owners with matching ESBLs. Each household is represented by a colour. aDA/MA, initial measurement; DB/MB, second sample 1 month after selection of participants; MC, third sample 6 months after selection of participants. bAll plasmids identified. Underlined plasmids harboured both the PCR-based replicon typing reference gene and ESBL gene on the same contig. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Given the 550 households and the observed ESBL-E prevalence in humans and dogs, the expected prevalence of both being ESBL-E positive (regardless of harbouring the same ESBL gene) within a household in a one-to-one relationship based on chance was 0.4%. This corresponds to 2 out of 550 households, which is less than the observed 7 households in which both were ESBL-E positive (P value=0.013). Within these seven households, there were five in which the human and the dog carried identical ESBL genes. By taking the variance of the ESBL genes into account, the chance of harbouring the same ESBL gene in cases where both were ESBL-E positive was 0.19%. Therefore, 1.3 out of 7 households were expected to be harbouring identical ESBL genes (P value=0.004).
ESBL gene diversity in dogs and cats and comparison with humans
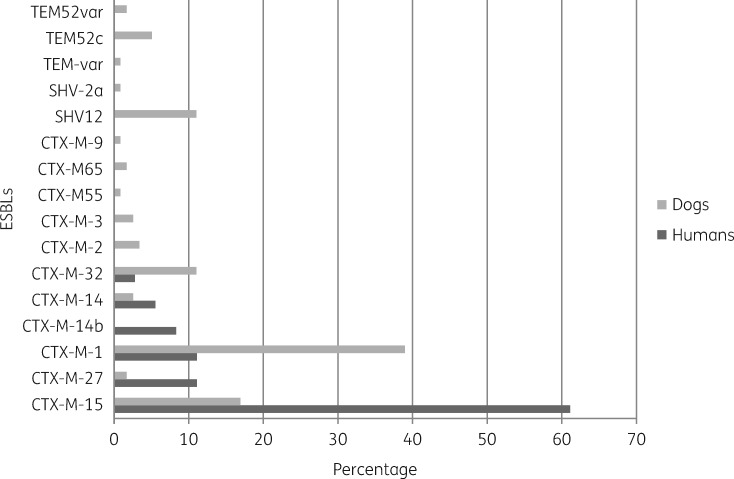
In faecal samples of 28 (47%) of 59 ESBL-E-positive dogs, multiple ESBL allele variants (maximum of three) were detected and there were 90 different isolates in total. Potential duplicates within a sample were excluded (e.g. multiple blaCTX-M-1 genes within one sample were counted as one). The most frequently observed ESBL gene was blaCTX-M-1 (n=32; 35.6%; 95% CI: 25.9%–46.4%), followed by blaCTX-M-15 (n=19; 21.1%; 95% CI: 13.4%–31.2%), blaCTX-M-32 (n=10; 11.1%; 95% CI: 5.7%–19.9%), blaSHV-12 (n=8; 8.9%; 95% CI: 4.2%–17.3%) and blaTEM-52 (n=7; 7.8%; 95% CI: 3.5%–15.9%) (Figure 2). Among 24 isolates from colonized dog owners, blaCTX-M-15 (n=15; 62.5%; 95% CI: 40.8%–80.4%) was most prevalent and the distribution of ESBL genes differed compared with dog isolates (Figure 2). ESBL gene distribution was comparable between dog owners and non-dog owners (Figure S2). All four ESBL-E from cats harboured blaCTX-M-1.
Figure 2.
Distribution of ESBL types in participating dog owners and dogs at baseline. The distribution of ESBL genes in isolates from dog owners differed compared with those from dogs.
Longitudinal results for dogs and cats
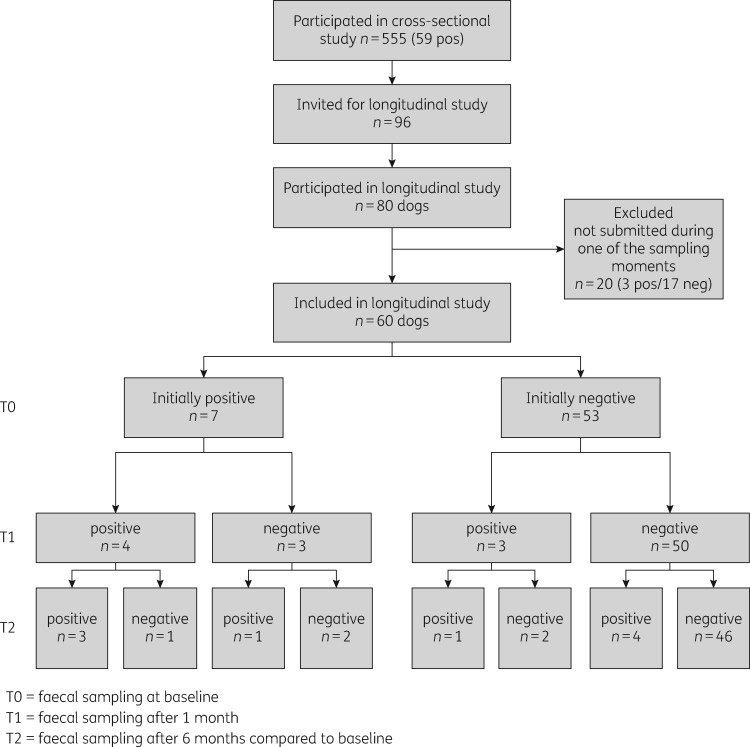
We received samples from 80 dogs and 38 cats after 1 month and from 61 dogs and 28 cats after 6 months (60 dogs had samples at all three timepoints). The prevalence of ESBL-E carriage was comparable at each timepoint [being 11.7% (N=7), 11.7% (N=7) and 15.0% (N=9), respectively], as was the predominance of blaCTX-M-1 (Table 2). Persistence of carriage was 57.1% at 1 month and 42.9% at 6 months. For those that had lost carriage at 1 month, ESBL-E carriage was detected in 13.2% at 6 months (Figure 3).
Table 2.
ESBLs in dogs from the longitudinal part
| Timepoint | Dog ID |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2661 | 3154 | 7183 | 8141 | 9709 | 11027 | 13664 | 15110 | 15218 | 15371 | 15710 | 16114 | 25440 | 27659 | 30208 | 38556 | 49409 | |
| Baseline | ND | CTX-M-65 | NDa | CTX-M-15b | — | CTX-M-1 | — | CTX-M-1 | — | CTX-M-1 | — | CTX-M-1 | — | CTX-M-55 | CTX-M-1 | CTX-M-1 | CTX-M-15 |
| TEM-52c | SHV-12 | CTX-M-15 | CTX-M-15 | CTX-M-15 | |||||||||||||
| CTX-M-14 | X | CTX-M-1 | — | — | CTX-M-1 | SHV-12 | CTX-M-1 | — | CTX-M-1 | ND | CTX-M-1 | — | — | — | CTX-M-2 | CTX-M-1 | |
| 1 month | CTX-M-15 | CTX-M-32 | CTX-M-32 | ||||||||||||||
| SHV-12 | |||||||||||||||||
| ND | — | CTX-M-1 | — | CTX-M-1 | CTX-M-1 | — | CTX-M-1 | CTX-M-1 | X | CTX-M-1 | — | CTX-M-1 | — | CTX-M-1 | X | CTX-M-14 | |
| 6 months | CTX-M-32 | SHV-12 | TEM-52c | ||||||||||||||
| TEM-52c | |||||||||||||||||
ND, not determined; —, sample negative in culture; X, sample not submitted by participant.
Sample positive in culture, no ESBL gene was observed.
Human sample also positive for ESBL.
Figure 3.
Flow chart of the ESBL status of dogs at baseline and after 1 and 6 months. pos, positive; neg, negative.
Although the number of positive samples at each timepoint was more or less the same, the specific dogs or ESBL genes involved were not. If dogs were positive at consecutive samplings, in most cases other ESBL-E or another combination of different ESBL-E were observed, indicating transient carriage. Since this is an observational study and we only screened for the ESBL gene and not the E. coli ST or plasmid, it cannot be determined whether recurring ESBL genes (mainly blaCTX-M-1) either persisted, were present due to recurring uptake, or appeared completely independent from prior presence.
Discussion
In this nationwide cross-sectional and longitudinal study of ESBL-E carriage in 555 dogs and 285 cats, the observed prevalence was 10.6% and 1.4%, respectively. The prevalence of ESBL-E in dogs was higher than the observed prevalence of 4.5% in humans from our previous study14 collected in the same households at the same time, indicating that dogs are a larger reservoir for ESBL-E when looking at the general population of humans and dogs. A study performed in the UK (2008–09) observed an ESBL-E prevalence of 1.9% in vet-visiting dogs, which is considerably lower than the observed prevalence in healthy dogs in the current study.27
Random forest analysis indicated that being fed with raw meat was most predictive for ESBL-E carriage. In a study of 73 healthy dogs from the UK, ESBL production was only observed in one E. coli isolate, but the authors proved that being fed with raw meat was a risk factor for antimicrobial resistance in general.28 A study in dogs visiting a veterinarian also indicated that eating raw poultry meat was a risk factor for ESBL carriage.27 Also, in 36 cats, raw pet feed was associated with ESBL carriage.29 ESBL has been detected in raw meat as well.30–32 The fact that being fed with dry feed (kibble) is a protective factor in our study is probably due to the inverse association between being fed with dry feed and being fed with raw meat. The observed risk factors ‘walking the dog in the forest’ and ‘walking the dog in an area with livestock’ are probably related to exposure to other animals or their faecal material. Although we did not identify coprophagic behaviour as a risk factor in our analyses, a previous study in 78 dogs and 22 cats attending a veterinary hospital did for resistance to ampicillin, amoxicillin/clavulanic acid, cephamycin, ciprofloxacin, streptomycin and trimethoprim/sulfamethoxazole.33 Being hospitalized or having consulted a vet in the 4 weeks prior to sampling was not observed as a risk factor before. We did not observe antimicrobial usage of the animals as a risk factor, as has been observed previously.27 We did not find discernible differences in the prevalence of ESBL-E between the degrees of urbanization. This might be explained by the fact that the observed risk factors are not related to the degree of urbanization (e.g. being fed with raw meat).
We presented that from the seven dogs initially positive, 57.1% were still positive after 1 month and 42.9% after 6 months. Furthermore, we observed an acquisition rate of 13.2% after 6 months in dogs initially negative. Another Dutch study screened for ESBL-E and AmpC in faecal samples from 38 dogs monthly for 6 months; 32 dogs (84%) had at least one faecal sample positive for ESBL/AmpC during the study period.13 That study also indicated that the faecal carriage of ESBLs in dogs had a transient character. However, by comparing both studies, it should be noted that in the present study AmpC-type β-lactamases were not included in the calculations.
In 5 out of 550 households, both the human and dog samples were positive for the same ESBL gene, which was more than expected based on chance. Although co-carriage in human–dog pairs was demonstrated, having a dog was not observed as a risk factor in our previous study14 and there was no significant difference in ESBL-E prevalence between dog owners and people not owning a dog. In three out of the five pairs, the human and dog isolates could not be distinguished based on core-genome and plasmid content. In total, 59 dogs had a positive faecal sample, indicating humans within these households were exposed. This means that in only 5% of the exposed households we have observed a match in ESBL-E carriage between humans and dogs, which is relatively low. This finding is also in line with a pooled analysis on molecular relatedness of ESBL/AmpC-producing E. coli in humans, animals, food and the environment, in which some overlap was observed in various reservoirs, but most reservoirs had a distinct distribution in gene frequency.30 The co-carriage rate might be different in other (high prevalence) countries and although data on ESBLs in relation to co-carriage with owners are scarce, a study conducted in Brazil, screening 134 human–dog pairs, demonstrated that there were 42 pairs in which MDR E. coli strains were isolated from both and in 9.5% (4/42) of the pairs identical PFGE profiles were detected, denoting the sharing of strains.34
Although we collected 285 faecal samples from cats, we were not able to do risk factor analyses due to the low number of ESBL-E-positive cats. A limitation is that pets were only included in the longitudinal study if a household member was also included. In addition, since WGS was only performed in dogs in which we observed co-carriage within the household, we were not able to calculate the chance of co-carriage given the chance of sharing the combination of the same ESBL genes, STs and plasmids. However, the more characteristics are added to the calculation, the higher the diversity and therefore the lower the chance of co-carriage within households will be.
In conclusion, the prevalence of ESBL-E in healthy dogs from the community is high, while it is low in cats (10.6% and 1.4%, respectively). Being fed with raw meat is the main risk factor in dogs. The acquisition rate of ESBL-E in dogs was 13.2% after 6 months. Furthermore, we indicated there is evidence of co-carriage in dogs and humans belonging to the same household, and this occurred more often than expected based on chance only, suggesting either clonal transmission of ESBL-E between humans and pets within households, or exposure to the same source.
Supplementary Material
Acknowledgements
We would like to thank all participants for their contribution in submitting samples and filling in the questionnaires. We would also like to thank Linda van der Graaf-van Bloois for her technical assistance in the WGS analysis. In addition, we thank L. J. L. Muller, J. P. M. Vlooswijk, G. M. A. Riemens-van Zetten, M. R. C. Rogers, R. Zuidema and L.W. Pisa for technical assistance and/or help with logistics.
Funding
This work was supported by the Dutch public private partnership 1Health4Food (1H4F) and by the Dutch Ministry of Health, Welfare, and Sport. ESBL Attribution (ESBLAT) consortium project number TKI AF-12067.
Transparency declarations
None to declare.
References
- 1. Blaak H, de Kruijf P, Hamidjaja R. et al. Prevalence and characteristics of ESBL-producing E. coli in Dutch recreational waters influenced by wastewater treatment plants. Vet Microbiol 2014; 171: 448–59. [DOI] [PubMed] [Google Scholar]
- 2. Overdevest I, Willemsen I, Rijnsburger M. et al. Extended-spectrum β-lactamase genes of Escherichia coli in chicken meat and humans, the Netherlands. Emerg Infect Dis 2011; 17: 1216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dohmen W, Bonten MJM, Bos MEH. et al. Carriage of extended-spectrum β-lactamases in pig farmers is associated with occurrence in pigs. Clin Microbiol Infect 2015; 21: 917–23. [DOI] [PubMed] [Google Scholar]
- 4. Reuland EA, Naiemi N, Kaiser AM. et al. Prevalence and risk factors for carriage of ESBL-producing Enterobacteriaceae in Amsterdam. J Antimicrob Chemother 2016; 71: 1076–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Platteel TN, Hall MAL, Stuart JWC. et al. Predicting carriage with extended-spectrum β-lactamase-producing bacteria at hospital admission: a cross-sectional study. Clin Microbiol Infect 2015; 21: 141–6. [DOI] [PubMed] [Google Scholar]
- 6. Pomba C, Rantala M, Greko C. et al. Public health risk of antimicrobial resistance transfer from companion animals. J Antimicrob Chemother 2017; 72: 957–68. [DOI] [PubMed] [Google Scholar]
- 7. Poirel L, Nordmann P, Ducroz S. et al. Extended-spectrum β-lactamase CTX-M-15-producing Klebsiella pneumoniae of sequence type ST274 in companion animals. Antimicrob Agents Chemother 2013; 57: 2372–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dierikx CM, van Duijkeren E, Schoormans AH. et al. Occurrence and characteristics of extended-spectrum-β-lactamase- and AmpC-producing clinical isolates derived from companion animals and horses. J Antimicrob Chemother 2012; 67: 1368–74. [DOI] [PubMed] [Google Scholar]
- 9.HAS Hogeschool and Utrecht University Faculty of Veterinary Medicine. Feiten & Cijfers Gezelschapsdierensector 2015. 2015. https://www.rijksoverheid.nl/documenten/rapporten/2015/11/03/feiten-cijfers-gezelschapsdierensector-2015.
- 10. Hordijk J, Schoormans A, Kwakernaak M. et al. High prevalence of fecal carriage of extended spectrum β-lactamase/AmpC-producing Enterobacteriaceae in cats and dogs. Front Microbiol 2013; 4: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meyer E, Gastmeier P, Kola A. et al. Pet animals and foreign travel are risk factors for colonisation with extended-spectrum β-lactamase-producing Escherichia coli. Infection 2012; 40: 685–7. [DOI] [PubMed] [Google Scholar]
- 12. Ljungquist O, Ljungquist D, Myrenås M. et al. Evidence of household transfer of ESBL-/pAmpC-producing Enterobacteriaceae between humans and dogs—a pilot study. Infect Ecol Epidemiol 2016; 6: 31514.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baede VO, Wagenaar JA, Broens EM. et al. Longitudinal study of extended-spectrum-β-lactamase- and AmpC-producing Enterobacteriaceae in household dogs. Antimicrob Agents Chemother 2015; 59: 3117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van den Bunt G, van Pelt W, Hidalgo L. et al. Prevalence, risk factors and genetic characterisation of extended-spectrum β-lactamase and carbapenemase-producing Enterobacteriaceae (ESBL-E and CPE): a community-based cross-sectional study, the Netherlands, 2014 to 2016. Euro Surveill 2019; 24: pii=1800594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Voets GM, Platteel TN, Fluit AC. et al. Population distribution of β-lactamase conferring resistance to third-generation cephalosporins in human clinical Enterobacteriaceae in the Netherlands. PLoS One 2012; 7: e52102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Treangen TJ, Ondov BD, Koren S. et al. The harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol 2014; 15: 524.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Croucher NJ, Page AJ, Connor TR. et al. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res 2015; 43: e15.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zankari E, Hasman H, Cosentino S. et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 2012; 67: 2640–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carattoli A, Zankari E, Garciá-Fernández A. et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 2014; 58: 3895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Larsen MV, Cosentino S, Rasmussen S. et al. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol 2012; 50: 1355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Breiman L. Random forests. Mach Learn 2001; 45: 5–32. [Google Scholar]
- 22. Steyerberg E. Clinical Prediction Model: A Practical Approach to Development, Validation, and Updating. Springer, 2009. [Google Scholar]
- 23. Benjamini Y, Hochberg Y.. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 1995; 57: 289–300. [Google Scholar]
- 24. Rockhill B, Newman B, Weinberg C.. Use and misuse of population attributable fractions. Am J Public Health 1998; 88: 15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Buuren S, Groothuis-Oudshoorn K.. mice: multivariate imputation by chained equations in R. J Stat Softw 2011; 45: 1–67. [Google Scholar]
- 26. Liakopoulos A, van den Bunt G, Geurts Y. et al. High prevalence of intra-familial co-colonization by extended-spectrum cephalosporin resistant Enterobacteriaceae in preschool children and their parents in Dutch households. Front Microbiol 2018; 9: 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wedley AL, Dawson S, Maddox TW. et al. Carriage of antimicrobial resistant Escherichia coli in dogs: prevalence, associated risk factors and molecular characteristics. Vet Microbiol 2017; 199: 23–30. [DOI] [PubMed] [Google Scholar]
- 28. Schmidt VM, Pinchbeck GL, Nuttall T. et al. Antimicrobial resistance risk factors and characterisation of faecal E. coli isolated from healthy Labrador retrievers in the United Kingdom. Prev Vet Med 2015; 119: 31–40. [DOI] [PubMed] [Google Scholar]
- 29. Baede VO, Broens EM, Spaninks MP. et al. Raw pet food as a risk factor for shedding of extended-spectrum β-lactamase-producing Enterobacteriaceae in household cats. PLoS One 2017; 12: e0187239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dorado-García A, Smid JH, van Pelt W. et al. Molecular relatedness of ESBL/AmpC-producing Escherichia coli from humans, animals, food and the environment: a pooled analysis. J Antimicrob Chemother 2018; 73: 339–47. [DOI] [PubMed] [Google Scholar]
- 31. Leverstein-van Hall MA, Dierikx CM, Cohen Stuart J. et al. Dutch patients, retail chicken meat and poultry share the same ESBL genes, plasmids and strains. Clin Microbiol Infect 2011; 17: 873–80. [DOI] [PubMed] [Google Scholar]
- 32. Cohen Stuart J, van den Munckhof T, Voets G. et al. Comparison of ESBL contamination in organic and conventional retail chicken meat. Int J Food Microbiol 2012; 154: 212–4. [DOI] [PubMed] [Google Scholar]
- 33. Leite-Martins LR, Mahú MIM, Costa AL. et al. Prevalence of antimicrobial resistance in enteric Escherichia coli from domestic pets and assessment of associated risk markers using a generalized linear mixed model. Prev Vet Med 2014; 117: 28–39. [DOI] [PubMed] [Google Scholar]
- 34. Carvalho AC, Barbosa AV, Arais LR. et al. Resistance patterns, ESBL genes, and genetic relatedness of Escherichia coli from dogs and owners. Braz J Microbiol 2016; 47: 150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.