Abstract
The aim of this study was to investigate the effect of dipeptidyl peptidase-4 (DPP-4) inhibitor on blood sugar level and cognitive ability in elderly patients with type 2 diabetes mellitus (T2DM) combined with post-stroke mild cognitive impairment (MCI). Thirty patients received DPP-4 inhibitor (study group), while another thirty received sulfonylurea (control group). Six months after treatment, markers regarding blood sugar were improved in both groups (all P<0.05) without intergroup differences (all P>0.05); scores regarding cognitive ability improved in the study group (both P<0.05) and were higher versus the control group (both P<0.01); the study group had higher Aβ1-42/Aβ1–40 value versus the pretreatment value (P<0.001), which differed from the control group (P<0.05); tumor necrosis factor-α and interleukin-6 concentrations decreased in both groups, while the study group had greater reductions; C-reactive protein value decreased after treatment in the study group (all P<0.05). Using DPP-4 inhibitor in elderly patients with T2DM combined with post-stroke MCI can lower blood sugar and improve cognitive ability. The mechanism may be associated with the improvement of Aβ gathering and reduction in inflammatory response.
Keywords: cognitive impairment, blood sugar, efficacy
Introduction
Incidence of type 2 diabetes mellitus (T2DM) has been rising each year due to trends in population aging. It has been estimated that by 2040, there will be 615 million people suffering from T2DM (1). Meanwhile, morbidity of cognitive impairment (CI) increases as patients age. Some studies have documented that the incidence rate of CI is 5–10% in patients over 65 years old and 20–50% in patients over 85 years, with a higher prevalence in women than in men (2). Stroke is believed to be the second biggest factor in inducing CI (3). There have been studies revealing that T2DM is one of the common risk factors in causing stoke and CI. Thus patients with T2DM are more likely to have cognitive dysfunction after a stroke (4–6).
Regarding the pathogenesis, it has been reported that T2DM and CI share common lesion characteristics including Aβ gathering, decreased ability in regulating protein phosphorylation, and participation of chronic inflammatory factors; moreover, insulin resistance and damage to insulin signal transmission are also common pathological bases in the occurrence of both diseases (7,8). Glucagon-like peptide-1 (GLP-1) is an endogenous incretin. It can promote the release of insulin from islet cell and keep the glucose content at a relatively stable level in the body (9). Some recent studies have demonstrated that GLP-1 can not only affect pancreatic islet function but also display neurotransmitter-like and neuron growth factor-like properties (10). GLP-1 agents, such as liraglutide and exenatide, have been demonstrated to be able to mitigate neurodegeneration in Alzheimer's disease (AD) and decrease memory and learning disabilities when used in the rat model of AD (11,12). However, some studies have found that GLP-1 gets readily hydrolyzed by dipeptidyl peptidase-4 (DPP-4) in the body, causing loss of activity and function. DPP-4 inhibitors including sitagliptin, vildagliptin, and linagliptin reduce sugar level mainly through inhibiting GLP-1 hydrolysis (13). Currently, studies regarding the improvement of cognitive ability by DPP-4 were primarily carried out among patients with AD, whereas studies performed in patients with T2DM combined with post-stroke mild cognitive impairment (MCI) were few (14,15). Therefore, in the present study, we used DPP-4 inhibitor in treating elderly patients with T2DM combined with post-stroke MCI and investigated its effect on patients' blood sugar level and cognitive ability.
Materials and methods
Patient characteristics
The present study was approved by the Ethics Committee of Heilongjiang Provincial Hospital, (Harbin, China) and informed consent was obtained from all individuals included in this study. Sixty patients treated in the department of neurology in Heilongjiang Provincial Hospital between January 2017 and June 2018 for T2DM combined with post-stroke CI were selected and randomized into a study group (treated with DPP-4 inhibitor) and a control group (treated with sulfonylurea) of 30 patients each. All patients were aged above 65 years.
Inclusion criteria were as follows: i) Patients who met the diagnostic criteria for T2DM (16); ii) patients who met the diagnostic criteria for post-stroke CI after assessment of cognitive ability and the condition was stabilized after treatment (17); iii) patients who met the following four criteria for MCI: a) patients with a score of ≥24 points in Mini-Mental State Examination (MMSE, 19 items, total score of 30 points); b) patients with a score of <26 points in Montreal Cognitive Assessment (MoCA) if they had over 12 years of education (one point was added to the MoCA score if patients did not have over 12 years of education); c) patients were reported by themselves or their family members to have hypomnesia; d) activity of daily living score <26 points (18); iv) patients had diabetes before stroke occurrence, and the antidiabetic drugs patients received were sulfonylurea and metformin instead of DPP-4 inhibitor.
Exclusion criteria were: i) Patients who were allergic to DPP inhibitor; ii) patients who had histories of craniocerebral trauma, epilepsy, and cerebrovascular disease; iii) patients who would not cooperate with cognitive testing; iv) patients who were taking glucocorticoid which would affect blood sugar level; v) patients who had cardiopulmonary insufficiency; vi) patients who had malignant tumors; vii) patients who had mental illness which would affect cognition.
Methods
According to a random number table, patients in the study were assigned to either a study group or a control group of 30 patients each. In the study group, patients received oral administration of 100 mg sitagliptin (Merck Sharp & Dohme, Ltd., registration number for imported medicine: H20090834), a DPP-4 inhibitor, one time per day and one pill each time; meanwhile, patients in the control group received sulfonylurea. If the blood sugar level was not lowered effectively, regular insulin would be administered temporarily to control the sugar content. Efficacy was evaluated at six months after treatment.
Outcome measures
Main outcome measures were: i) fasting blood glucose (FBG), 2-hour postprandial blood glucose (2hPG), and hemoglobin A1c (HbA1c) values before and six months after treatment; FBG and 2hPG were measured using Accu-Chek Performa blood glucose meter (Roche), while HbA1c was measured using DCA 2000 analyzer (Bayer); ii) MMSE and MoCA scores before and six months after treatment for cognitive evaluation.
Secondary outcome measures included C-reactive protein (CRP), tumor necrosis factor (TNF)-α, interleukin (IL)-6, and Aβ1–40 and Aβ1–42 values. Two tubes of venous blood (5 ml each) were collected from each patient at 8′clock in the morning before and six months after treatment. The blood samples were placed in sterile EDTA tubes and kept in a fridge at 4°C for 15 min. Afterward, samples were centrifuged at 1,500 × g at 4°C for 30 min to separate serum from plasma. The plasma was incubated with phosphate buffered saline containing 40 µl protease inhibitor at −80°C. Levels of CRP, TNF-α, and IL-6 in serum were measured using immunoturbidimetry, while values of Aβ1–40 and Aβ1–42 in plasma were measured using ELISA.
Statistical analysis
SPSS 17.0 software was applied for statistical analysis. Continuous variables are expressed as mean ± SD. t-test was performed if there were normal distribution and homogeneity of variance; comparison between two groups was conducted by independent-samples t-test; before versus after comparison within the group was performed by paired t-test; quartile was presented, and Wilcoxon rank-sum test was conducted if there were no normal distribution and homogeneity of variance. Pearson's Chi-squared test was performed for count data. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
There were no intergroup differences in sex, age, education background, body mass index, and comorbidities so that the results were comparable (all P>0.05, Table I).
Table I.
Patient characteristics and baseline data.
| Characteristics | Study group (n=30) | Control group (n=30) | χ2/t value | P-value |
|---|---|---|---|---|
| Sex (male:female) | 17:13 | 14:16 | 0.601 | 0.438 |
| Age (years) | 68.5±7.1 | 67.4±5.9 | 0.692 | 0.492 |
| Education (year) | 12.5±3.8 | 11.9±4.0 | 0.627 | 0.533 |
| Body mass index (kg/m2) | 25.71±3.76 | 25.59±4.28 | 0.110 | 0.913 |
| Diabetes duration | 8.17±3.05 | 8.97±2.65 | 0.534 | 0.128 |
| Stroke type | 0.268 | 0.605 | ||
| Ischemic stroke | 15 | 17 | ||
| Hemorrhagic stroke | 15 | 13 | ||
| Comorbidity | ||||
| Hyperlipemia | 0.067 | 0.759 | ||
| Yes | 17 | 16 | ||
| No | 13 | 14 | ||
| Hypertension | 0.635 | 0.426 | ||
| Yes | 20 | 17 | ||
| No | 10 | 13 | ||
| Coronary heart disease | 0.287 | 0.592 | ||
| Yes | 10 | 12 | ||
| No | 20 | 18 | ||
| Obesity | 0.635 | 0.426 | ||
| Yes | 10 | 13 | ||
| No | 20 | 17 | ||
| Hyperhomocysteinemia | 0.373 | 0.542 | ||
| Yes | 22 | 24 | ||
| No | 8 | 6 | ||
| Hyperuricemia | 0.693 | 0.405 | ||
| Yes | 19 | 22 | ||
| No | 11 | 8 |
Sugar blood content before and after treatment in the two groups
No intergroup differences were found in FBG, 2hPG, and HbA1c in the two groups before and after treatment (all P>0.05). Compared with the pretreatment values, these markers improved significantly six months after treatment (all P<0.05) (Table II).
Table II.
Sugar blood content before and after treatment in the two groups.
| Sugar blood content | Study group Before treatment | Control group Before treatment | t value | P-value | Study group After treatment | Control group After treatment | t value | P-value |
|---|---|---|---|---|---|---|---|---|
| FBG (mmol/l) | 9.51±1.24 | 9.29±1.32 | 0.666 | 0.508 | 7.19±1.81a | 7.17±1.77a | 0.050 | 0.960 |
| 2hPG (mmol/l) | 16.89±3.77 | 16.70±4.69 | 0.170 | 0.866 | 10.47±3.80a | 10.56±2.59a | 0.103 | 0.918 |
| HbA1c (%) | 8.56±1.25 | 8.86±3.17 | 0.471 | 0.640 | 6.97±1.25a | 7.35±2.48a | 0.749 | 0.457 |
FBG, fasting blood glucose; 2hPG, 2-hour postprandial blood glucose; HbA1c, hemoglobin A1c.
P<0.05 vs. before treatment.
Cognitive ability before and after treatment in the two groups
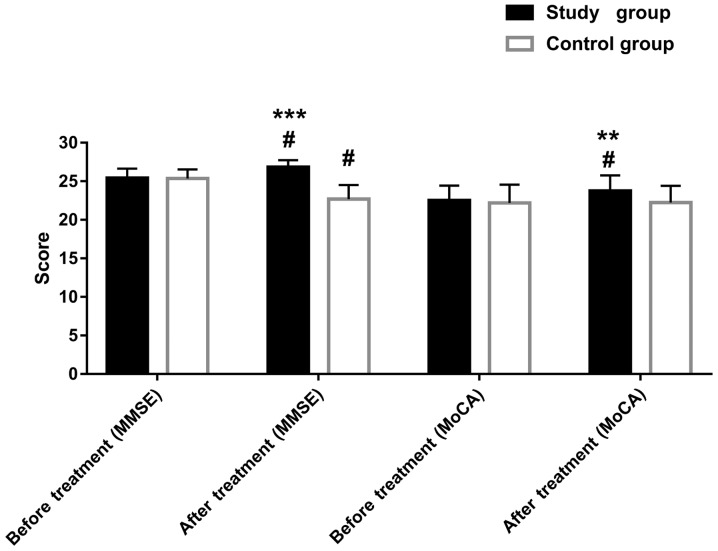
No intergroup differences were found in MMSE and MoCA scores in the two groups before treatment (both P>0.05). However, at six months after treatment, the MMSE and MoCA scores in the study group improved significantly compared with the pretreatment scores (both P<0.05). In the control group, the MMSE score was lower than that before the treatment (P<0.05). Differences were observed between the two groups regarding these scores after treatment (both P<0.01). (Table III and Fig. 1).
Table III.
Score of cognitive ability before and after treatment in the two groups.
| Scores | Study group Before treatment | Control group Before treatment | t value | P-value | Study group After treatment | Control group After treatment | t value | P-value |
|---|---|---|---|---|---|---|---|---|
| MMSE | 25.42±1.22 | 25.37±1.16 | 0.108 | 0.914 | 26.83±0.91a | 22.70±1.80a | 11.201 | <0.001 |
| MoCA | 22.50±1.94 | 22.20±2.35 | 0.538 | 0.592 | 23.73±2.03a | 22.23±2.18 | 2.759 | 0.008 |
MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment.
P<0.05 vs. before treatment within the same group.
Figure 1.
Score of cognitive ability before and after treatment in the two groups. MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment. **P<0.01, ***P<0.001 vs. the control group; #P<0.05 vs. before treatment.
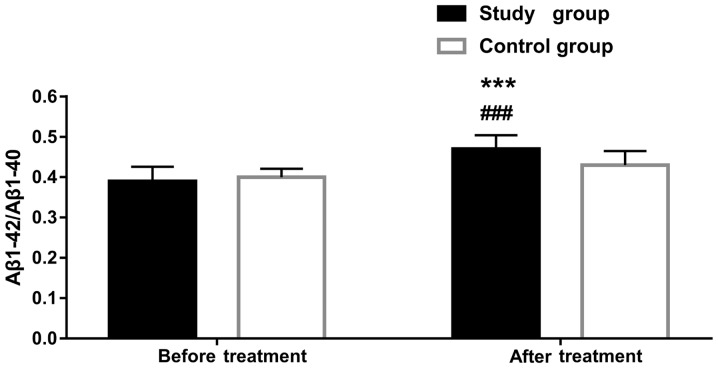
Aβ1-42, Aβ1-40, and Aβ1-42/Aβ1–40 levels in plasma before and after treatment in the two groups. There were no intergroup differences in Aβ1-42, Aβ1-40, and Aβ1-42/Aβ1–40 levels before treatment (all P>0.05). However, at six months after treatment, the value of Aβ1-42/Aβ1–40 improved significantly in the study group (P<0.001), whereas the levels of the other two markers were similar to those before treatment (both P>0.05). Aβ1-42/Aβ1–40 value differed between the two groups after treatment (P<0.05) (Table IV and Fig. 2).
Table IV.
Aβ1-42, Aβ1-40, and Aβ1-42/Aβ1–40 levels in plasma before and after treatment in the two groups.
| Markers | Study group Before treatment | Control group Before treatment | t value | P-value | Study group After treatment | Control group After treatment | t value | P-value |
|---|---|---|---|---|---|---|---|---|
| Aβ1–42 (ng/l) | 18.37±5.08 | 17.73±4.64 | 0.504 | 0.616 | 19.03±3.17 | 19.40±5.05 | 0.337 | 0.737 |
| Aβ1–40 (ng/l) | 46.50±11.19 | 43.80±9.98 | 0.987 | 0.328 | 41.03±9.18 | 44.97±10.83 | 1.517 | 0.135 |
| Aβ1-42/Aβ1–40 | 0.39±0.04 | 0.40±0.02 | 1.445 | 0.154 | 0.47±0.03a | 0.43±0.04 | 4.649 | <0.001 |
P<0.05 vs. before treatment within the same group.
Figure 2.
Aβ1-42/Aβ1–40 level in plasma before and after treatment in the two groups. ***P<0.001 vs. the control group; ###P<0.001 vs. before treatment.
CRP, TNF-α, and IL-6 levels before and after treatment in the two groups
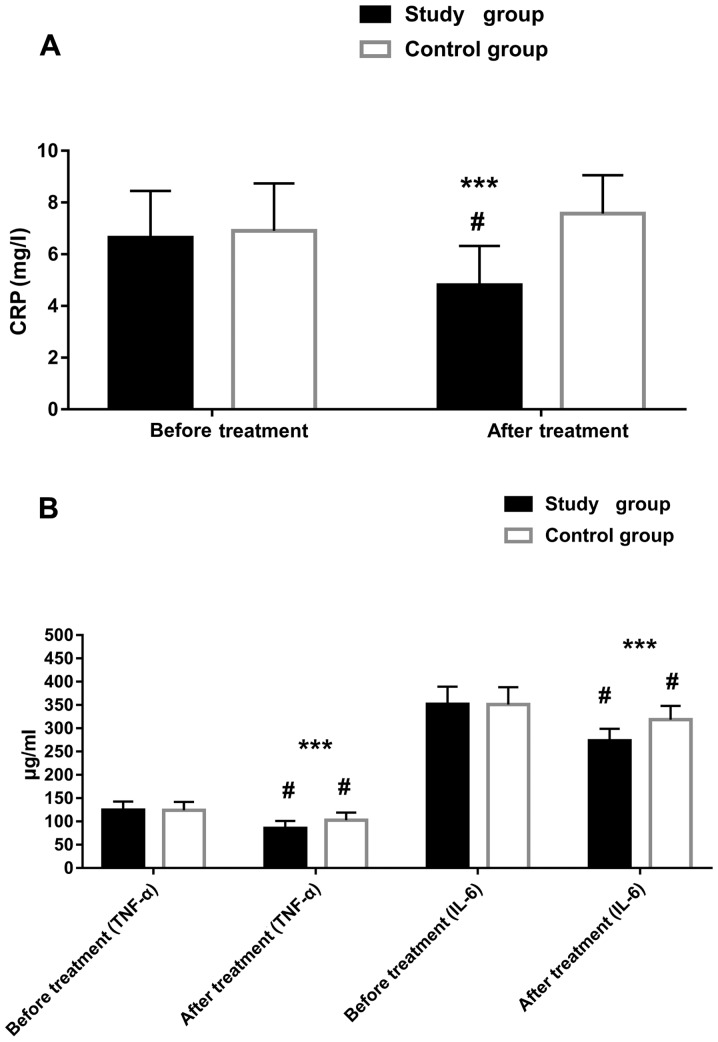
There were no intergroup differences in CRP, TNF-α, and IL-6 before treatment (all P>0.05). However, at six months after treatment, the levels of these markers improved greatly in the study group (all P<0.05). The control group had improved levels of TNF-α and IL-6 after treatment (both P<0.05). Intergroup differences were observed in the three markers after treatment, and the reductions in the study group were greater (all P<0.05) (Table V and Fig. 3).
Table V.
CRP, TNF-α, and IL-6 levels before and after treatment in the two groups.
| Factors | Study group Before treatment | Control group Before treatment | t value | P-value | Study group After treatment | Control group After treatment | t value | P-value |
|---|---|---|---|---|---|---|---|---|
| CRP (mg/l) | 6.64±1.81 | 6.90±1.84 | 0.917 | 0.363 | 4.80±1.52a | 7.57±1.48 | 7.153 | <0.001 |
| TNF-α (µg/ml) | 124.51±18.22 | 123.80±17.87 | 0.154 | 0.878 | 85.01±15.90a | 102.66±16.12a | 4.269 | <0.001 |
| IL-6 (µg/ml) | 351.37±37.86 | 351.00±37.32 | 0.038 | 0.969 | 273.27±25.64a | 318.34±29.73a | 6.287 | <0.001 |
CRP, C-reactive protein; TNF-α, tumor necrosis factor-α; IL-6 - interleukin-6.
P<0.05 vs. before treatment within the same group.
Figure 3.
CRP, TNF-α, and IL-6 levels before and after treatment in the two groups. (A) CRP level before and after treatment, (B) TNF-α and IL-6 levels before and after treatment. CRP, C-reactive protein; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6. ***P<0.001 vs. the control group; #P<0.05 vs. before treatment.
Discussion
Some clinical studies have displayed that elderly patients with T2DM are more likely to have CI, and the occurrence is often unnoticeable. The condition may be mild cognitive dysfunction in the beginning but turns to dementia as it progresses.
Pathogenesis of the disease may be associated with various factors, including Aβ gathering, chronic inflammation, and age, making CI more easily developed in elderly patients with T2DM; moreover, the occurrence of CI is closely correlated with glucose variability (19). Some researchers have reported that CI is more likely to occur in T2DM patients with long-term insulin resistance and hyperinsulinemia, and T2DM is regarded as a risk factor of CI. Furthermore, patients with T2DM and CI often have brain insulin resistance (20). Studies on stroke have indicated that in patients who had a stroke, the injury to neurovascular unit due to cerebra hypoxia-ischemia can impair cognitive ability (21,22). Other studies have exhibited that DPP-4 inhibitor not only has better blood sugar lowering effect versus other hypoglycemic agents but also attenuates CI induced by AD (23–26). In the present study, we compared the efficacy of DPP-4 inhibitor sitagliptin with that of sulfonylurea in the treatment and observed that the two agents performed similarly in lowering blood sugar content. However, in terms of improving cognitive ability, the study group given DPP-4 inhibitor exhibited better MMSE and MoCA scores than the control group, which aligns with the studies above.
It has been demonstrated that Aβ gathering, decreased ability in regulating protein phosphorylation, and the participation of chronic inflammatory factors are common lesion characteristics in both T2DM and CI (27). Regarding the mechanism of improving cognitive ability, we studied the Aβ gathering and chronic inflammatory factors. Some researchers have reported increased Aβ expressions in cerebral cortex and hippocampus of mice with diabetes, whereas using DPP-4 inhibitor decreases Aβ expression levels in these two areas (27). Another study has documented a reduction in Aβ expression in the cerebrospinal fluid of AD patients (28). However, it is not easy to perform a cerebrospinal fluid test during an early diagnosis of CI. Therefore, clinical studies were performed to find more easy-to-measure biomarkers in blood. Some studies on Aβ1–40 and Aβ1–42 have observed different expression changes including rising, no-change, and reduction in these two markers in plasma of patients with MCI (29–31). Moreover, the ratio of Aβ1–42 to Aβ1–40 has been found to decrease in patients with MCI, and the ratio reduces even much more in patients with AD (31). The high blood sugar content in T2DM can induce an immuno-inflammatory response. It has been revealed that levels of CRP, TNF-α, and IL-6 can all elevate in T2DM patients (32). Inflammatory factors also exist in patients with CI. Some studies have reported elevated levels of various inflammatory factors in CI patients, leading to neuroinflammation. Due to inflammatory response, microglia and astrocyte can be excessively activated and produce toxic substances that damage neurons, thereby causing neuron denaturalization and apoptosis (33). IL-6 is a type of inflammatory factor. Previous studies reported that IL-6 expression could begin to increase in plasma during the early stage of AD (34). However, another study reported no difference in IL-6 expression in serum between patients with MCI and normal people (35). CRP, a marker commonly used in clinic, is a protein synthesized in liver mediated by inflammatory factors such as IL-6 (36). In the present study, we compared the study group with the control group and found no intergroup differences in Aβ1–40 and Aβ1–42 in plasma before and after treatment in patients. After treatment, the study group had a significantly higher ratio of Aβ1–42 to Aβ1–40 than the control group. Moreover, no intergroup differences were observed in CRP, TNF-α, and IL-6 levels before treatment, whereas these values were much lower in the study group than in the control group after treatment. These results align with previous findings.
There were still some limitations in the study. The sample size was small, requiring a study with a larger sample size in the future. Also, the study period was relatively short, and the study was affected by several external factors including area of infarction or bleeding, the difference in the treatment plan for infarction and bleeding, and recovery duration. Therefore, a longer follow-up will be needed in future studies.
In conclusion, using DPP-4 inhibitor in treating elderly patients with T2DM combined with post-stroke MCI can decrease blood sugar level and improve cognitive ability. The mechanism may be associated with the improvement of Aβ gathering and reduction in the inflammatory response in the body.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- DPP-4
dipeptidyl peptidase-4
- T2DM
type 2 diabetes mellitus
- MCI
mild cognitive impairment
- CI
cognitive impairment
- GLP-1
glucagon-like peptide-1
- AD
Alzheimer's disease
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- FBG
fasting blood glucose
- 2hPG
2-hour postprandial blood glucose
- HbA1c
hemoglobin A1c
- CRP
C-reactive protein
- TNF
tumor necrosis factor
- IL
interleukin
- MoCA
Montreal Cognitive Assessment
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JX recorded and compared sugar blood content and wrote the manuscript. CW and CP conceived and designed the study. CP and HX were responsible for the collection and analysis of the experimental data. LX and XC interpreted the data and drafted the manuscript. XW and NW were responsible for the comparison of CRP, TNF-α and IL-6, and revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Heilongjiang Provincial Hospital (Harbin, China). Patients who participated in this research had complete clinical data. Signed written informed consents were obtained from the patients and/or guardians.
Patient consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Zaccardi F, Webb DR, Yates T, Davies MJ. Pathophysiology of type 1 and type 2 diabetes mellitus: A 90-year perspective. Postgrad Med J. 2016;92:63–69. doi: 10.1136/postgradmedj-2015-133281. [DOI] [PubMed] [Google Scholar]
- 2.Weuve J, Hebert LE, Scherr PA, Evans DA. Prevalence of Alzheimer disease in US states. Epidemiology. 2015;26:e4–e6. doi: 10.1097/EDE.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 3.Cumming TB, Marshall RS, Lazar RM. Stroke, cognitive deficits, and rehabilitation: still an incomplete picture. Int J Stroke. 2013;8:38–45. doi: 10.1111/j.1747-4949.2012.00972.x. [DOI] [PubMed] [Google Scholar]
- 4.Kosaraju J, Gali CC, Khatwal RB, Dubala A, Chinni S, Holsinger RM, Madhunapantula VS, Muthureddy Nataraj SK, Basavan D. Saxagliptin: A dipeptidyl peptidase-4 inhibitor ameliorates streptozotocin induced Alzheimer's disease. Neuropharmacology. 2013;72:291–300. doi: 10.1016/j.neuropharm.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Le Couteur DG, Wahl D, Naismith SL. Comorbidity and vascular cognitive impairment-no dementia (VCI-ND) Age Ageing. 2017;46:705–707. doi: 10.1093/ageing/afx080. [DOI] [PubMed] [Google Scholar]
- 6.Shi D, Chen X, Li Z. Diagnostic test accuracy of the Montreal Cognitive Assessment in the detection of post-stroke cognitive impairment under different stages and cutoffs: a systematic review and meta-analysis. Neurol Sci. 2018;39:705–716. doi: 10.1007/s10072-018-3254-0. [DOI] [PubMed] [Google Scholar]
- 7.Kosaraju J, Murthy V, Khatwal RB, Dubala A, Chinni S, Muthureddy Nataraj SK, Basavan D. Vildagliptin: An anti-diabetes agent ameliorates cognitive deficits and pathology observed in streptozotocin-induced Alzheimer's disease. J Pharm Pharmacol. 2013;65:1773–1784. doi: 10.1111/jphp.12148. [DOI] [PubMed] [Google Scholar]
- 8.Kornelius E, Lin CL, Chang HH, Li HH, Huang WN, Yang YS, Lu YL, Peng CH, Huang CN. DPP-4 Inhibitor linagliptin attenuates Aβ-induced cytotoxicity through activation of AMPK in neuronal cells. CNS Neurosci Ther. 2015;21:549–557. doi: 10.1111/cns.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graaf C, Donnelly D, Wootten D, Lau J, Sexton PM, Miller LJ, Ahn JM, Liao J, Fletcher MM, Yang D, et al. Glucagon-like peptide-1 and its class B G protein-coupled receptors: A long march to therapeutic successes. Pharmacol Rev. 2016;68:954–1013. doi: 10.1124/pr.115.011395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tramutola A, Arena A, Cini C, Butterfield DA, Barone E. Modulation of GLP-1 signaling as a novel therapeutic approach in the treatment of Alzheimer's disease pathology. Expert Rev Neurother. 2017;17:59–75. doi: 10.1080/14737175.2017.1246183. [DOI] [PubMed] [Google Scholar]
- 11.Hansen HH, Barkholt P, Fabricius K, Jelsing J, Terwel D, Pyke C, Knudsen LB, Vrang N. The GLP-1 receptor agonist liraglutide reduces pathology-specific tau phosphorylation and improves motor function in a transgenic hTauP301L mouse model of tauopathy. Brain Res. 2016;1634:158–170. doi: 10.1016/j.brainres.2015.12.052. [DOI] [PubMed] [Google Scholar]
- 12.Candeias EM, Sebastião IC, Cardoso SM, Correia SC, Carvalho CI, Plácido AI, Santos MS, Oliveira CR, Moreira PI, Duarte AI. Gut-brain connection: The neuroprotective effects of the anti-diabetic drug liraglutide. World J Diabetes. 2015;6:807–827. doi: 10.4239/wjd.v6.i6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Wang L, Jiang R, Xu Y, Zhao X, Li Y. Exendin-4 antagonizes Aβ1-42-induced attenuation of spatial learning and memory ability. Exp Ther Med. 2016;12:2885–2892. doi: 10.3892/etm.2016.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isik AT, Soysal P, Yay A, Usarel C. The effects of sitagliptin, a DPP-4 inhibitor, on cognitive functions in elderly diabetic patients with or without Alzheimer's disease. Diabetes Res Clin Pract. 2017;123:192–198. doi: 10.1016/j.diabres.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Yin Y, Gao D, Wang Y, Wang ZH, Wang X, Ye J, Wu D, Fang L, Pi G, Yang Y, et al. Tau accumulation induces synaptic impairment and memory deficit by calcineurin-mediated inactivation of nuclear CaMKIV/CREB signaling. Proc Natl Acad Sci USA. 2016;113:E3773–E3781. doi: 10.1073/pnas.1604519113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogedengbe S, Ezeani IU, Aihanuwa E. Comparison of clinical and biochemical variables in type 2 diabetes mellitus patients and their first-degree relatives with metabolic syndrome in Benin City, Nigeria: A cross sectional case controlled study. Endocr Regul. 2016;50:32–40. doi: 10.1515/enr-2016-0007. [DOI] [PubMed] [Google Scholar]
- 17.Blum S, Luchsinger JA, Manly JJ, Schupf N, Stern Y, Brown TR, DeCarli C, Small SA, Mayeux R, Brickman AM. Memory after silent stroke: hippocampus and infarcts both matter. Neurology. 2012;78:38–46. doi: 10.1212/WNL.0b013e31823ed0cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciesielska N, Sokołowski R, Mazur E, Podhorecka M, Polak-Szabela A, Kędziora-Kornatowska K. Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Psychiatr Pol. 2016;50:1039–1052. doi: 10.12740/PP/45368. (In Polish) [DOI] [PubMed] [Google Scholar]
- 19.Rizzo MR, Marfella R, Barbieri M, Boccardi V, Vestini F, Lettieri B, Canonico S, Paolisso G. Relationships between daily acute glucose fluctuations and cognitive performance among aged type 2 diabetic patients. Diabetes Care. 2010;33:2169–2174. doi: 10.2337/dc10-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson GS, Craft S. Modulation of memory by insulin and glucose: Neuropsychological observations in Alzheimer's disease. Eur J Pharmacol. 2004;490:97–113. doi: 10.1016/j.ejphar.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 21.Zheng G, Zheng Y, Xiong Z, Ye B, Tao J, Chen L. Effect of Baduanjin exercise on cognitive function in patients with post-stroke cognitive impairment: Study protocol for a randomised controlled trial. BMJ Open. 2018;8:e020954. doi: 10.1136/bmjopen-2017-020954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberg GA. Extracellular matrix inflammation in vascular cognitive impairment and dementia. Clin Sci (Lond) 2017;131:425–437. doi: 10.1042/CS20160604. [DOI] [PubMed] [Google Scholar]
- 23.Zhou JB, Bai L, Wang Y, Yang JK. The benefits and risks of DPP4-inhibitors vs. sulfonylureas for patients with type 2 diabetes: Accumulated evidence from randomised controlled trial. Int J Clin Pract. 2016;70:132–141. doi: 10.1111/ijcp.12761. [DOI] [PubMed] [Google Scholar]
- 24.Loh HH, Yee A, Loh HS, Sukor N, Kamaruddin NA. Comparative studies of dipeptidyl peptidase 4 inhibitor vs sulphonylurea among Muslim Type 2 diabetes patients who fast in the month of Ramadan: A systematic review and meta-analysis. Prim Care Diabetes. 2016;10:210–219. doi: 10.1016/j.pcd.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Bekiari E, Rizava C, Athanasiadou E, Papatheodorou K, Liakos A, Karagiannis T, Mainou M, Rika M, Boura P, Tsapas A. Systematic review and meta-analysis of vildagliptin for treatment of type 2 diabetes. Endocrine. 2016;52:458–480. doi: 10.1007/s12020-015-0841-1. [DOI] [PubMed] [Google Scholar]
- 26.Xi YD, Zhang DD, Ding J, Yu HL, Yuan LH, Ma WW, Han J, Xiao R. Genistein inhibits Aβ25-35-induced synaptic toxicity and regulates CaMKII/CREB pathway in SH-SY5Y cells. Cell Mol Neurobiol. 2016;36:1151–1159. doi: 10.1007/s10571-015-0311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kosaraju J, Holsinger RMD, Guo L, Tam KY. Linagliptin, a dipeptidyl peptidase-4 inhibitor, mitigates cognitive deficits and pathology in the 3×Tg-AD mouse model of Alzheimer's disease. Mol Neurobiol. 2017;54:6074–6084. doi: 10.1007/s12035-016-0125-7. [DOI] [PubMed] [Google Scholar]
- 28.de Leon MJ, Pirraglia E, Osorio RS, Glodzik L, Saint-Louis L, Kim HJ, Fortea J, Fossati S, Laska E, Siegel C, et al. Alzheimer's Disease Neuroimaging Initiative; National Alzheimer's Coordinating Center The nonlinear relationship between cerebrospinal fluid Aβ42 and tau in preclinical Alzheimer's disease. PLoS One. 2018;13:e0191240. doi: 10.1371/journal.pone.0191240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayeux R, Honig LS, Tang MX, Manly J, Stern Y, Schupf N, Mehta PD. Plasma A[beta]40 and A[beta]42 and Alzheimer's disease: Relation to age, mortality, and risk. Neurology. 2003;61:1185–1190. doi: 10.1212/01.WNL.0000091890.32140.8F. [DOI] [PubMed] [Google Scholar]
- 30.Rembach A, Faux NG, Watt AD, Pertile KK, Rumble RL, Trounson BO, Fowler CJ, Roberts BR, Perez KA, Li QX, et al. AIBL research group Changes in plasma amyloid beta in a longitudinal study of aging and Alzheimer's disease. Alzheimers Dement. 2014;10:53–61. doi: 10.1016/j.jalz.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Janelidze S, Stomrud E, Palmqvist S, Zetterberg H, van Westen D, Jeromin A, Song L, Hanlon D, Tan Hehir CA, Baker D, et al. Plasma β-amyloid in Alzheimer's disease and vascular disease. Sci Rep. 2016;6:26801. doi: 10.1038/srep26801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ebtehaj S, Gruppen EG, Parvizi M, Tietge UJF, Dullaart RPF. The anti-inflammatory function of HDL is impaired in type 2 diabetes: Role of hyperglycemia, paraoxonase-1 and low grade inflammation. Cardiovasc Diabetol. 2017;16:132. doi: 10.1186/s12933-017-0613-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prati F, Bartolini M, Simoni E, De Simone A, Pinto A, Andrisano V, Bolognesi ML. Quinones bearing non-steroidal anti-inflammatory fragments as multitarget ligands for Alzheimer's disease. Bioorg Med Chem Lett. 2013;23:6254–6258. doi: 10.1016/j.bmcl.2013.09.091. [DOI] [PubMed] [Google Scholar]
- 34.Mun MJ, Kim JH, Choi JY, Jang WC. Genetic polymorphisms of interleukin genes and the risk of Alzheimer's disease: An update meta-analysis. Meta Gene. 2016;8:1–10. doi: 10.1016/j.mgene.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim YS, Lee KJ, Kim H. Serum tumour necrosis factor-α and interleukin-6 levels in Alzheimer's disease and mild cognitive impairment. Psychogeriatrics. 2017;17:224–230. doi: 10.1111/psyg.12218. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Q, Qian G, Ding Z. Xuemaitong granules attenuate carotid atherosclerosis by decreasing the expression of CD14+CD16+ monocytes, IL-6, TNF-α, and hsCRP. Genet Mol Res. 2014;13:7519–7527. doi: 10.4238/2014.September.12.19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.