Abstract
Sepsis commonly progresses to disseminated intravascular coagulation and induces the activation of heparanase (HPA) and the shedding of endothelial glycocalyx constituents, including syndecan-1 (SDC-1) and heparan sulphate (HS). However, the degradation of glycocalyx and its association with coagulation disorders remains undetermined. The present study aimed to evaluate the effect of unfractionated heparin (UFH) and N-acetylheparin (NAH), which is a non-anticoagulant heparin derivative, on endothelial glycocalyx and coagulation function in a lipopolysaccharide (LPS)-induced sepsis rat model, and to compare the differences observed in coagulation function between UFH and NAH. Experimental rats were randomly assigned to four groups: Control; LPS; UFH + LPS; and NAH + LPS. Rats were administered UFH or NAH and subsequently, ~1 min later, administered LPS (10 mg/kg; intravenous). The blood and lung tissues of rats were collected 0.5, 2 and 6 h after LPS injection, and were used for subsequent analysis. The results demonstrated that HPA activity and SDC-1 and HS levels increased, and this increase was associated with inflammatory cytokines and coagulation/fibrinolysis markers in the sepsis rat model. Histopathological examination was performed, and the lung injury score and lung wet/dry ratio indicated that UFH and NAH also significantly improved lung tissue injury. The results of the ELISA analysis demonstrated that UFH and NAH treatment: i) significantly decreased the levels of inflammatory cytokines including tumor necrosis factor-α and interleukin-6; ii) inhibited HPA activity and protected the integrity of the glycocalyx, which was identified by decreased HS and SDC-1 levels; and iii) decreased the levels of prothrombin fragment 1+2, thrombin-antithrombin complex, and plasminogen activator inhibitor-1 and increased the levels of fibrinogen and antithrombin-III. Preconditioning with UFH decreased the plasma activated partial thromboplastin time. These results indicated that UFH and NAH may alleviate sepsis-induced coagulopathy, and this effect may have been due to an inhibition of HPA activity and decrease in the shedding of the endothelial glycocalyx.
Keywords: sepsis, unfractionated heparin, N-acetylheparin, glycocalyx, coagulation
Introduction
Sepsis, or septic shock, is a systemic response to infection and causes excessive microvascular coagulation that leads to disseminated intravascular coagulation (DIC). DIC is closely associated with the development of multiple organ dysfunction (1). Sepsis is caused by infectious agents that are induced by lipopolysaccharides (LPS), viruses, bacteria and a number of other infections. LPS injections have commonly been used to generate experimental sepsis models (2). During sepsis, LPS causes an inflammatory response that results in the release of a large number of inflammatory cytokines, including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) (3). The inflammatory responses may lead to the activation of the coagulation system, the downregulation of anticoagulant proteins and the inhibition of the fibrinolytic system (4).
Coagulation abnormalities may occur in 50–70% of patients with sepsis (4,5). Coagulation activation is one of the most important characteristics in the pathophysiological of sepsis, and thrombin generation serves a key role in the activation of coagulation. Prothrombin fragment 1+2 (F1+2) is the earliest molecular marker for prothrombin activation. In the present study, F1+2 and thrombin-antithrombin complex (TAT) were selected to detect the early stages of coagulation (6). The coagulation system is stimulated during sepsis, and the corresponding anticoagulation system is activated, which is followed by the profibrinolytic and antifibrinolytic systems (4,5). Antithrombin (AT), which is also known as AT-III, is a physiological inhibitor of serine proteases that also exerts an inhibitory effect on other coagulation factors and serine proteases (7). AT mitigates vascular leakage by inhibiting neutrophil activation during acute lung injury (7). The initiation of fibrinolysis is mediated by plasminogen activators and is regulated by plasminogen activator inhibitor-1 (PAI-1). PAI-1, which indicates poor patient prognosis in sepsis-induced DIC, mediates the inhibition of endogenous fibrinolysis during sepsis (8).
Endothelial activation and dysfunction are important features of sepsis (3). The endothelium responds to LPS or cytokines by exhibiting structural changes and functional changes, including leukocyte infiltration, vasodilation, increased vascular permeability and plasma protein exudation and platelet adhesion (9). The endothelial glycocalyx is a complex network of cell-bound proteoglycans that are located on the surface of endothelial cells and this network is one of the earliest sites that is associated with sepsis (10,11). The endothelial glycocalyx contains anchor protein syndecan-1 (SDC-1), heparan sulfate (HS) and other glycosaminoglycans (GAGs), and is an anti-adhesive and anticoagulant layer that protects vascular endothelial cells and maintains vascular integrity (12). Heparanase (HPA) is an endo-β-D-glucuronidase that degrades HS side chains at specific intra-chain sites. Nadir et al (13) demonstrated that HPA increased coagulation activity via the stimulation of tissue factor (TF) expression in endothelial and cancer cells. Schmidt et al (14) demonstrated that pulmonary endothelial glycocalyx serves an important role in regulating neutrophils adhesion. However, the modes of activation of HPA and glycocalyx degradation products, and their association with coagulation, remain largely undetermined.
Unfractionated heparin (UFH) is a glycosaminoglycan that is largely used as anti-thrombotic and anticoagulant drug since its identification over 100 years ago. The anti-inflammatory properties and anticancer activity of UFH have been studied extensively, it has been previously indicated that UFH inhibited the activation of nuclear factor-κB (NF-κB) induced by LPS (15). The efficacy and safety of heparin use in patients with sepsis remains controversial, and these patients have high risk of hemorrhage (16). NAH, a non-anticoagulant heparin derivative, binds histones, prevents histone-mediated cytotoxicity in vitro and has been demonstrated to improve mortality in LPS/CLP induced sepsis mouse models (17). A previous study demonstrated that heparin, as the competitive antagonist, inhibited the activity of HPA, an endogenous HS-specific glucuronidase, and prevented LPS-induced endothelial glycocalyx loss (14).
The present study aimed to explore the association between the products of glycocalyx degradation and coagulation in a sepsis rat model. Secondly, the present study aimed to evaluate the effect of UFH and NAH, a non-anticoagulant heparin derivative, on endothelial glycocalyx and coagulation function in an LPS-induced sepsis rat model, and to compare the differences in coagulation function between UFH and NAH.
Materials and methods
Animals
Male Sprague-Dawley rats (6–8 weeks; weight, 180–220 g) were obtained from the Model Animal Research Center of Nanjing University. All animals were housed in standard conditions (22±2°C; 50±10% relative humidity; 12:12 h light: Dark cycle). The rats had ad libitum access to food and water. The rats acclimated to the environment for 3–5 days prior to the experiment.
The animal care and experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, and the protocol was approved by the Institutional Animal Care and Use Committee of Binzhou Medical University Hospital.
Reagents and antibodies
LPS (Escherichia coli LPS 055:B5), UFH and NAH were purchased from Sigma-Aldrich; Merck KGaA. ELISA kits for rat TNF-α (cat. no. CSB-E11987r), IL-6 (cat. no. CSB-E04640r), F1+2 (cat. no. CSB-E13264r), TAT (cat. no. CSB-E08432r), AT (cat. no. CSB-E13885r) and PAI-1 (cat. no. CSB-E07948r) were purchased from Cusabio Technology LLC. SDC-1 (cat. no. E02S0301, Shanghai BlueGene Biotech Co., Ltd.) level and Heparin sulfate (HS; cat. no. DG94646Q; Beijing Donggeboye Biological Technology Co., Ltd.) were determined using an ELISA according to the manufacturer's protocol. Fluorescence decay-resistant medium (cat. no. S2100) was purchased from Beijing Solarbio Science & Technology Co., Ltd.. Mouse Anti-Heparan Sulfate (10E4 epitope) antibody (cat. no. 370255-1) was purchased from United States Biological. The anti-SDC-1 antibody (cat. no. ab128936) was purchased from Abcam. The goat polyclonal thrombomodulin (BDCA-3) antibody (cat. no. AF3894) was purchased from R&D Systems, Inc. Rhodamine-conjugated anti-rat IgG (cat. no. ZF-0318) was purchased from ZSGB-BIO (Beijing, China) and fluorescein isothiocyanate (FITC)-conjugated anti-goat IgG (cat. no. TA130029) was purchased from OriGene Technologies, Inc.
Animal model of sepsis
Experimental rats were randomly allocated into the control, LPS, UFH + LPS and NAH + LPS groups. The rats in the LPS group received an intravenous (i.v.) injection of 10 mg/kg ml LPS. The dose of LPS in rats was performed as previously described (18) to establish the model of sepsis. The rats in the UFH + LPS group and NAH + LPS group received a tail intravenous injection of 100 U/kg body weight UFH (19) or 1 mg/kg body weight NAH (14) diluted in 1 ml normal saline (NS), while the control rats received vehicle NS only. UFH or NAH were administered concomitantly and this was followed ~1 min later by the addition of LPS (10 mg/kg; 1 ml/kg of body weight; i.v.). The rats in the control group were injected with NS instead of LPS in the same manner. All rats remained alive after 0.5, 2 and 6 h of LPS stimulation. Following this, the rats were sacrificed by cervical dislocation following anesthesia by peritoneal injection of 1% sodium pentobarbital (40 mg/kg body weight). Plasma and lung tissue samples were then harvested.
ELISA for TNF-α, IL-6, SDC-1, HS, F1+2, TAT, AT and PAI-1
Blood was collected from the harvested abdominal aorta and stored in an anticoagulant tube and centrifuged at 4°C for 20 min at 900 × g. Collected plasma samples were stored at −80°C. TNF-α, IL-6, SDC-1, HS, F1+2, TAT, AT and PAI-1 were evaluated with the corresponding ELISA kits in accordance with the manufacturer's protocol.
HPA activity assay
Plasma samples were collected at 0.5, 2 and 6 h after LPS administration and HPA activity was measured. The blood was collected from the harvested abdominal aorta and stored in an anticoagulant tube and centrifuged at 4°C for 20 min at 900 × g. Collected plasma samples were stored at −80°C. The HPA activities were measured using an Heparan Degrading Enzyme Assay kit (cat. no. MK412; Takara Bio, Inc.) according to the manufacturer's protocol.
Blood coagulation tests
Blood was collected and the plasma was separated as aforementioned. The prothrombin time (PT), activated partial thromboplastin time (APTT) and levels of fibrinogen (FIB) were measured using a coagulometer (CS-5100; Sysmex Corporation). According to the International Society of Thrombosis and Hemostasis (ISTH) scoring system, the PT, APTT, FIB, AT and TAT have been used to diagnostic screening of coagulation function for DIC (20).
Pulmonary histologic examination
The lower lobe, collected from the right lung, was fixed in 4% paraformaldehyde for 48 h at 4°C and embedded in paraffin. Sections (5 µm) were stained with hematoxylin for 5 min at room temperature and then with eosin for 3 min at room temperature. Tissue sections were observed under an optical microscope. Hematoxylin and eosin staining was performed according to the standard method used to assess lung injury. The lung injury scores (LIS) were calculated as previously described by Aeffner et al (21). ALI was scored as follows: i) Alveolar congestion; ii) hemorrhage; iii) infiltration or aggregation of neutrophils in the airspace or vessel wall; and iv) thickness of alveolar wall/hyaline membrane formation. Each item was scored on a 5-point scale as follows: 0, minimal damage; 1, mild damage; 2, moderate damage; 3, severe damage; and 4, maximal damage. In each stained sample, six high-magnification fields were selected randomly, then graded for average LIS. Repeated-measures data were statistically analyzed using a repeated-measures analysis of variance (ANOVA).
Measurement of lung wet/dry (W/D) weight ratio
To assess the magnitude of lung tissue edema, the lung W/D weight ratio was calculated. A period of 6 h after LPS administration, rats were euthanized and left lungs were excised using blunt dissection, weighed to obtain the ‘wet’ weight. They were subsequently placed in an oven at 60°C for 48 h to acquire the ‘dry’ weight.
Immunofluorescence
The lung tissues were fixed using 4% paraformaldehyde for 48 h at 4°C, dehydrated with alcohol (100% for 20 min, 95% for 10 min, 85% for 10 min, 75% for 10 min and 50% for 10 min) and embedded with paraffin and cut into 4 µm sections. The sections were then incubated with mouse anti-heparan sulfate (10E4 epitope; cat. no. 370255-1; 1:200) or anti-SDC-1 antibody (cat. no. ab128936; 1:463) overnight at 4°C. Sequentially, the sections were incubated for 1 h at 37°C, washed 3 times with PBS and incubated with rhodamine-conjugated anti-rat IgG (cat. no. ZF-0318; 1:200) for 1 h at room temperature. The sections were then washed 3 times with PBS for 10 min each time and incubated with secondary antibody goat polyclonal against BDCA-3 (cat. no. AF3894; 1:200) overnight at 4°C. Sequentially, sections were washed again and incubated with FITC-conjugated anti-goat IgG (cat. no. TA130029; 1:200) for 1 h at room temperature. The sections were washed again and incubated with DAPI (1 mg/ml, 1:300) for 8 min at room temperature. Finally, sections were washed and blocked with fluorescence decay-resistant medium for 1 min at room temperature. Sections were observed using a fluorescence microscope (×200; Olympus BX53; Olympus Corporation). Data were quantified using Image J 1.37v (National Institutes of Health), and the results were statistically analyzed using SPSS 25.0 (IBM Corp).
Statistical analysis
The data are expressed as the mean ± standard deviation. The LIS, lung W/D weight ratio, IL-6, HPA, HS, SDC-1, TAT and PAI-1 data were normally distributed, so statistical comparisons were determined using a one-way ANOVA, followed by the Student-Newman-Keuls (SNK) test for multiple group comparison. The TNF-α, F1+2 and AT data were nonnormally distributed, so statistical comparisons were determined using a Kruskal-Wallis test along with Bonferroni correction. The associations between inflammation parameters, glycocalyx degradation products, and coagulation parameters were determined using a Spearman rank correlation test. Statistical analyses was conducted using SPSS v.25.0 (IBM Corp). P<0.05 was considered to indicate a statistically significant difference.
Results
Preconditioning with heparin attenuated LPS-induced lung injury
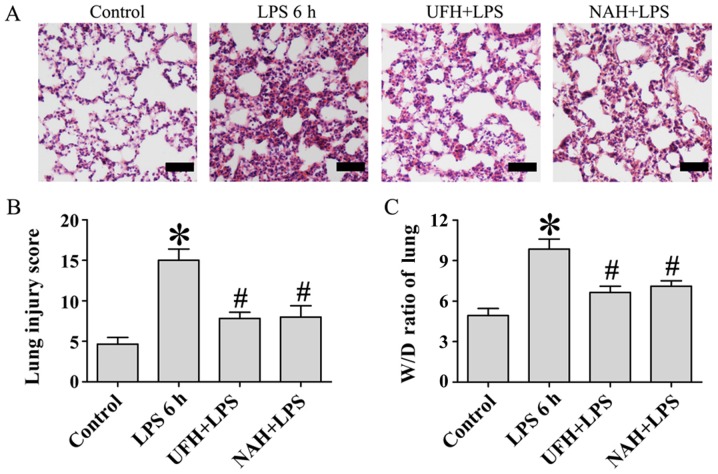
The primary organ affected during sepsis is the lung (14). Previous studies have demonstrated that UFH and NAH may decrease the severity of sepsis-associated acute lung injury and lethality in LPS-induced mice (17,19,22). Therefore, pulmonary histopathology was examined using hematoxylin and eosin staining and lung injury score to assess the protective effect of UFH and NAH (Fig. 1A and B). The lung tissues in the LPS group were characterized by alveolar capillary barrier integrity, alveolar epithelial injury, interstitial infiltration by neutrophils, alveolar edema, and fibrin deposition. However, LPS-induced histopathological changes were significantly alleviated by preconditioning with UFH and NAH following LPS stimulation, which was indicated by decreased neutrophil infiltration and protein leakage and the improved integrity of alveolar capillary barrier.
Figure 1.
Effect of heparin on histopathologic changes and lung W/D ratio in LPS-induced lung injury. Sprague-Dawley male rats were injected i.v. with UFH (100 U/kg) or NAH (1 mg/kg) and subsequently treated with LPS (10 mg/kg; 1 ml/kg of body weight; i.v.). Rats in the control group were injected i.v. with an equal volume of NS. A total of 6 h following LPS injection, the lung histopathologic changes were observed using (A) hematoxylin and eosin staining (n=6 rats/group; magnification, ×200; scale bar, 50 µm). (B) Lung tissues from each experimental group were processed and the lung injury score was used for histological evaluation. (C) The lung W/D ratios in each treatment group were quantified. Data are presented as mean ± standard deviation from 3 independent experiments. *P<0.05 vs. the control group; #P<0.05 vs. the LPS 6 h group. Statistical comparisons were determined using a one-way analysis of variance followed by the Student-Newman-Keuls test for multiple group comparisons. W/D, wet/dry; LPS, lipopolysaccharide; UFH, unfractionated heparin; NAH, N-acetylheparin; NS, normal saline; i.v., intravenous.
Pulmonary edema is one of the primary features of acute respiratory distress syndrome (ARDS) (14). In the present study, lung W/D weight ratios were used to independently evaluate LPS-induced changes in pulmonary vascular permeability to water. A period of 6 h after LPS injection, the lung W/D ratio was significantly decreased compared with the control group. Preconditioning with UFH or NAH inhibited this increase in the lung W/D ratio compared with the LPS group (Fig. 1C). These results demonstrated that UFH and NAH demonstrated a protective effect on LPS-induced acute lung injury in rats.
Plasma inflammatory cytokines, HPA activity, glycocalyx components and coagulation/fibrinolysis markers in rats with LPS-induced sepsis
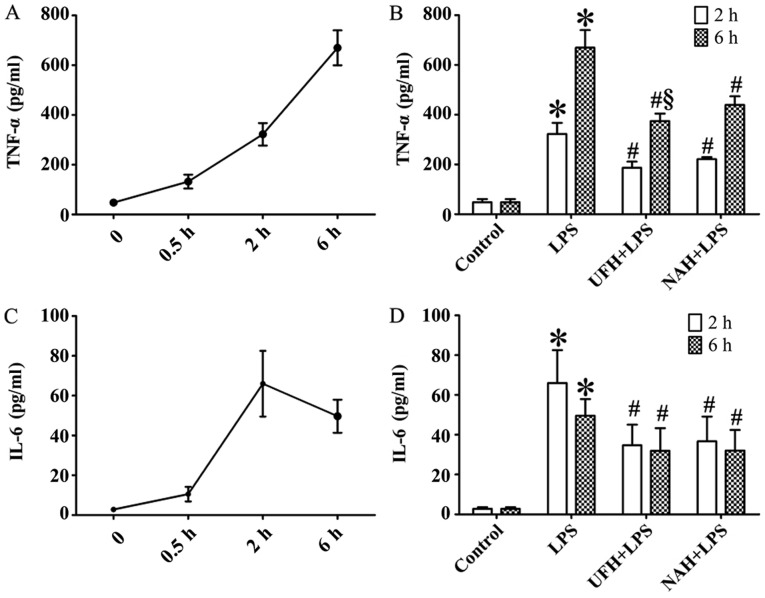
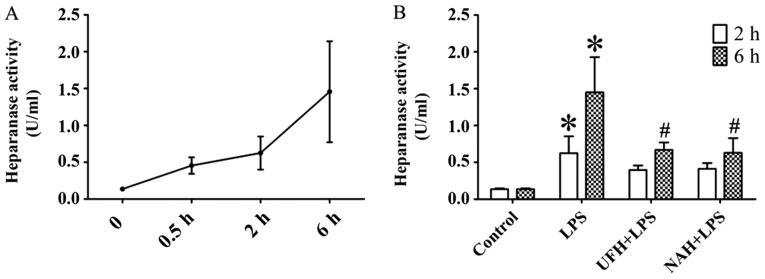
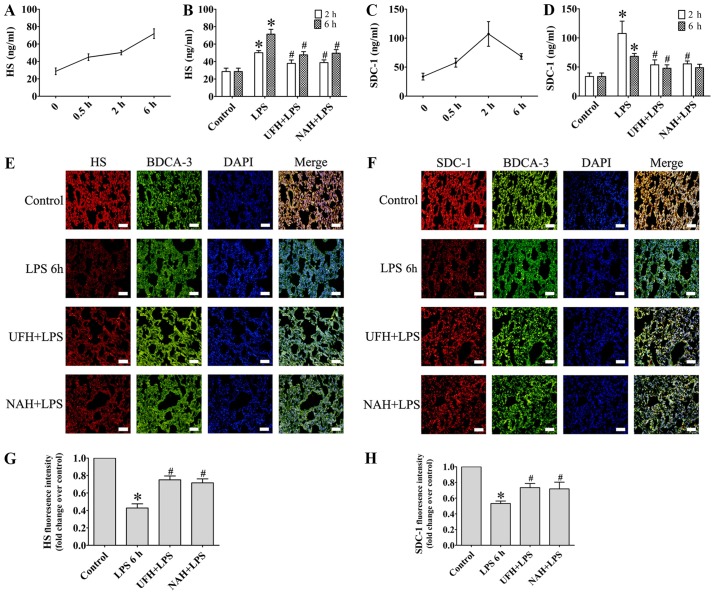
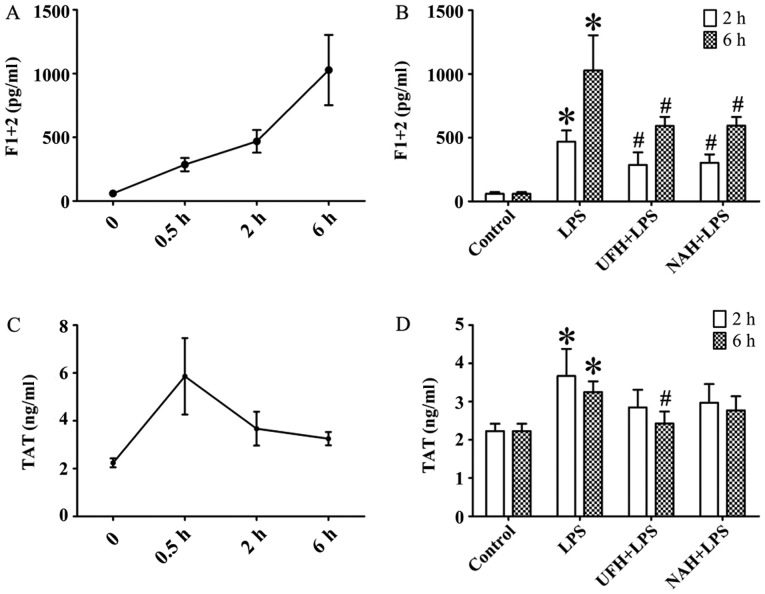
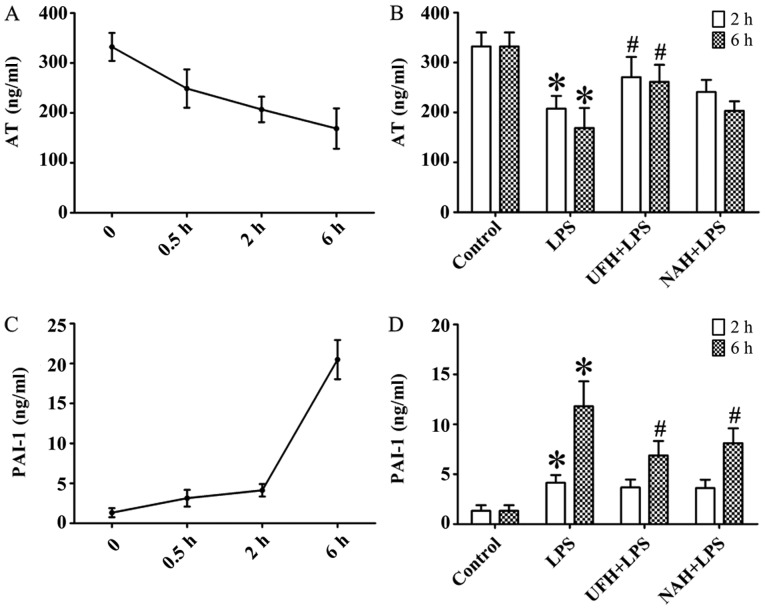
In the LPS group, the concentration of TNF-α was increased significantly from 2 to 6 h. The IL-6 level markedly increased from 0.5 to 2 h and then decreased slowly (Fig. 2A and C). HPA activity (Fig. 3A) and HS levels (Fig. 4A) increased slowly from 0.5 to 2 h, and then increased markedly from 2 to 6 h. The plasma level of SDC-1 (Fig. 4C) increased sharply at 2 h and subsequently decreased slowly. The F1+2 level increased significantly from 2 to 6 h (Fig. 5A). Additionally, TAT level increased markedly at 0.5 h and subsequently decreased significantly and progressively decreased from 2 to 6 h (Fig. 5C). The level of AT gradually decreased, and PAI level gradually increased from 0.5 to 6 h (Fig. 6A and C). In the present study, glycocalyx components (HS and SDC-1) and HPA activity were positively correlated with inflammatory cytokines (TNF-α and IL-6) and coagulation/fibrinolysis markers (F1+2 and PAI-1), and were negatively correlated with AT anticoagulant protein (Table I).
Figure 2.
Effect of heparin on inflammatory cytokines in plasma of rats with LPS-induced sepsis. (A) TNF-α and (C) IL-6 were assessed using an ELISA at 0.5, 2 and 6 h after LPS injection, respectively. Effect of UFH or NAH on levels of (B) TNF-α and (D) IL-6 in plasma at 2 and 6 h after LPS injection. Data are presented as mean ± standard deviation. There were 6 animals in each group at 0.5, 2 and 6 h. *P<0.05 vs. the control group; #P<0.05 vs. the LPS group; §P<0.05 vs. the NAH + LPS group. The TNF-α data were analyzed using a Kruskal-Wallis test followed by a pairwise comparisons test. The IL-6 data were analyzed using a one-way analysis of variance followed by a Student-Newman-Keuls test for multiple group comparisons. LPS, lipopolysaccharide; TNF-α, tumor necrosis factor-α; IL, interleukin; UFH, unfractionated heparin; NAH, N-acetylheparin.
Figure 3.
Effect of heparin on heparanase activity in rats with LPS-induced sepsis. (A) Heparanase activity was determined using an ELISA at 0.5, 2 and 6 h after LPS injection. (B) Effect of UFH or NAH on heparanase activity in plasma at 2 and 6 h after LPS injection. Data are presented as mean ± standard deviation. There were 6 animals in each group at 0.5, 2 and 6 h. *P<0.05 vs. the control group; #P<0.05 vs. the LPS group. The HPA data were analyzed using a one-way analysis of variance, followed by a Student-Newman-Keuls test for multiple group comparisons. LPS, lipopolysaccharide; UFH, unfractionated heparin; NAH, N-acetylheparin.
Figure 4.
Effect of heparin on endothelial glycocalyx degradation products in an LPS-induced sepsis rat model. (A) HS and (C) SDC-1 in plasma was determined using an ELISA at 0.5, 2 and 6 h after LPS injection. Effect of UFH or NAH on (B) HS and (D) SDC-1 in plasma at 2 and 6 h after LPS injection. The distribution of (E) HS (red) and (F) SDC-1 (red) in rat lungs was assessed using staining with specific antibodies. Endothelial cell marker of pulmonary vascular was assessed using BDCA-3 (green) staining (magnification, ×200; scale bar, 50 µm). (G) Fluorescence intensity analyses of the images presented in (E). (H) Fluorescence intensity analyses of the images presented in (F). Data are presented as mean ± standard deviation for 3 independent experiments. *P<0.05 vs. the control group; #P<0.05 vs. the LPS group. The HS and SDC-1 data were determined using a one-way analysis of variance followed by a Student-Newman-Keuls test for multiple group comparisons. LPS, lipopolysaccharide; HS, heparan sulphate; SDC-1, syndecan-1; UFH, unfractionated heparin; NAH, N-acetylheparin; BDCA-3, thrombomodulin.
Figure 5.
Effect of heparin on activated coagulation parameters in an LPS-induced sepsis rat model. (A) F1+2 and (C) TAT were determined using an ELISA at 0.5, 2 and 6 h after LPS injection, respectively. Effect of UFH or NAH on (B) F1+2 and (D) TAT in the plasma at 2 and 6 h after LPS injection. Data are presented as mean ± standard deviation. There were 6 animals in each group at 0.5, 2 and 6 h. *P<0.05 vs. the control group; #P<0.05 vs. the LPS group. The F1+2 data were analyzed using a Kruskal-Wallis test along with Bonferroni correction. The TAT data were analyzed using a one-way analysis of variance followed by a Student-Newman-Keuls test for multiple group comparisons. LPS, lipopolysaccharide; F1+2, prothrombin fragment 1+2; TAT, thrombin-antithrombin complex; UFH, unfractionated heparin; NAH, N-acetylheparin.
Figure 6.
Effect of heparin on anti-coagulation and fibrinolysis parameters in an LPS-induced sepsis rat model. (A) AT and (C) PAI-1 were determined using an ELISA at 0.5, 2 and 6 h after LPS injection, respectively. Effect of UFH or NAH on (B) AT and (D) PAI-1 in plasma at 2 and 6 h after LPS injection. Data are presented as mean ± standard deviation. There were 6 animals in each group at 0.5, 2 and 6 h, respectively. *P<0.05 vs. the control group; #P<0.05 vs. the LPS group. The AT data were analyzed using a Kruskal-Wallis test along with Bonferroni correction. The PAI-1 data were analyzed using a one-way analysis of variance followed by a Student-Newman-Keuls test for multiple group comparisons. LPS, lipopolysaccharide; AT, antithrombin; PAI-1, plasminogen activator inhibitor-1; UFH, unfractionated heparin; NAH, N-acetylheparin.
Table I.
Correlations between glycocalyx components and inflammatory cytokines and coagulation/fibrinolysis markers.
| HPA | HS | SDC-1 | ||||
|---|---|---|---|---|---|---|
| rho | P-value | rho | P-value | rho | P-value | |
| Inflammatory cytokines | ||||||
| TNF-α | 0.94 | <0.001 | 0.95 | <0.001 | 0.47 | 0.066 |
| IL-6 | 0.83 | <0.001 | 0.47 | 0.066 | 0.72 | 0.002 |
| Coagulation/fibrinolysis markers | ||||||
| F1+2 | 0.92 | <0.001 | 0.88 | <0.001 | 0.45 | 0.08 |
| TAT | 0.238 | 0.374 | 0.28 | 0.284 | 0.61 | 0.012 |
| AT | −0.847 | <0.001 | −0.78 | <0.001 | −0.447 | 0.083 |
| PAI-1 | 0.918 | <0.001 | 0.79 | <0.001 | 0.294 | 0.269 |
HPA, heparanase; HS, heparan sulfate; SDC-1, syndecan-1; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; F1+2, prothrombin fragment 1+2; TAT, thrombin-antithrombin complex; AT, antithrombin; PAI-1, plasminogen activator inhibitor-1. Correlations between HPA, HS, SDC-1 and TNF-α, IL-6, F1+2, TAT, AT-III and PAI-1 were assessed by using the Spearman rank test.
Effect of heparin on plasma of inflammatory cytokines in rats with LPS-induced sepsis
Preconditioning rats with UFH or NAH decreased LPS-induced TNF-α and IL-6 expression levels after 2 and 6 h. The effect of UFH on TNF-α levels was improved compared with that of NAH (Fig. 2B and D).
Effect of heparin on HPA activity and endothelial glycocalyx integrity in an LPS-induced sepsis rat model
Compared with the control group, HPA activity in the LPS group was significantly increased, whereas preconditioning with UFH or NAH inhibited HPA activity compared with the LPS group (Fig. 3B). The integrity of endothelial glycocalyx was indicated by plasma HS and SDC-1 levels, and the expression of HS and SDC-1 in the pulmonary vascular endothelial cells. Preconditioning with UFH or NAH significantly suppressed the shedding of HS and SDC-1 and decreased the plasma levels of HS and SDC-1 in rats with sepsis. No statistically significant difference was observed between the UFH + LPS and NAH + LPS groups (Fig. 4B and D).
Effect of heparin on coagulation/fibrinolysis markers in rats with LPS-induced sepsis
The levels of F1+2 and TAT were markedly increased in the LPS group compared with the control group. UFH and NAH treatment also decreased LPS-induced F1+2 levels in the plasma at 2 and 6 h. UFH treatment decreased LPS-induced TAT deposition in the plasma of sepsis rat models at 6 h. Preconditioning with NAH decreased TAT level compared with the LPS group. However, the effect was not statistically significant. No statistically significant difference was observed between the UFH + LPS and NAH + LPS groups (Fig. 5B and D). Preconditioning with UFH increased the AT level compared with the LPS group and NAH + LPS group. Preconditioning with NAH increased the AT level compared with the LPS group. However, the effect was not statistically significant. Preconditioning with UFH or NAH decreased the PAI-1 level compared with the LPS group at 6 h after LPS injection, and there was no statistically significant difference observed between the UFH + LPS and NAH + LPS groups (Fig. 6B and D).
Effect of heparin on APTT, PT and FIB level in an LPS-induced sepsis rat model
The APTT was 20.1 sec (mean) in the control animals and was significantly increased to 60.9 sec (mean) 6 h after the LPS injection. Preconditioning with UFH significantly inhibited the LPS-induced prolongation of the APTT to 49.6 sec (mean) at 6 h. Preconditioning with NAH inhibited the LPS-induced prolongation of the APPT to 55.6 sec (mean) at 6 h. However, the effect was not statistically significant (Table II).
Table II.
Effect of UFH or NAH on APTT, PT and FIB level in LPS-induced sepsis rat model.
| Group | APTT, sec | PT, sec | FIB levels, g/l |
|---|---|---|---|
| Control | 20.5±1.4 | 15.6±0.4 | 2.5±0.11 |
| LPS 6h | 60.9±5.8a | 18.3±1.9 | 1.3±0.23a |
| UFH + LPS | 49.6±4.6b,c | 18.3±1.0 | 1.9±0.34b |
| NAH + LPS | 55.6±5.9 | 18.7±1.2 | 1.7±0.24b |
Data are presented as mean ± standard deviation. There were 6 animals in each group.
P<0.05 vs. the control group
P<0.05 vs. the LPS 6 h group
P<0.05 vs. the NAH + LPS group. A Kruskal-Wallis test along with Bonferroni correction was used for data that were nonnormally distributed. LPS, lipopolysaccharide; UFH, Unfractionated heparin; NAH, N-acetylheparin; APTT, activated partial thromboplastin time; PT, prothrombin time; FIB, fibrinogen.
The PT was 15.8 sec (mean) in the control animals and was significantly increased to 18.3 sec (mean) 6 h following the LPS injection. Preconditioning with UFH or NAH did not affect the PT (Table II).
LPS injection decreased the plasma fibrinogen level, which decreased to 54% of the normal level after 6 h. Preconditioning with UFH or NAH significantly inhibited the LPS-induced decrease in plasma fibrinogen level. However, the effect was not statistically significant between the two groups (Table II).
Discussion
Sepsis is a systemic inflammatory response syndrome that is caused by infectious agents. A large number of mediators can lead to the activation of the coagulation system and the inhibition of anticoagulant mechanisms and fibrinolysis. Additionally, the coagulation system may in turn affect the inflammatory response (3,4). In the present study, in rats with LPS-induced sepsis, plasma inflammation cytokines indicated a positive correlation with coagulation parameters. Previous studies have demonstrated that heparin decreased the levels of the inflammatory cytokines TNF-α, IL-6, IL-8 and IL-1β in an LPS-induced sepsis model (15,19,22). Similar results were indicated in the present study, which demonstrated that preconditioning with NAH or UFH inhibited the LPS-induced cytokines of TNF-α and IL-6. The results also indicated that UFH was more effective compared with NAH, and this may be due to an increased level of coagulation activation, contributing to an increase in the level of inflammation.
The glycocalyx is a negatively charged network that is composed of core proteins and side chains that modulate the microvascular environment, coagulation, thrombosis and vascular permeability (23). Edema and increased vascular permeability are the major characteristics of ARDS, which is a common manifestation of sepsis-associated organ dysfunction (24,25). The results of the present study demonstrated that UFH and NAH significantly alleviated lung edema and decreased the shedding of HS and SDC-1. Wildhagen et al (17) and Li et al (19) demonstrated that UFH and NAH may alleviate LPS induced lung pathological changes and lung edema through downregulating the NF-κB signaling pathway and binding histones. These results were consistent with those of previous studies (17,19), which demonstrated that UFH and NAH attenuated LPS-induced acute lung injury in rats. HPA is an endo-β-glucuronidase that is involved in the cleavage of heparan sulfate side chains, and serves an important role in inflammation and mediates acute pulmonary and renal injuries during sepsis (14,26). Heparan sulfates are the most common endothelial cell surface GAG, comprising 50–90% of the GAG pool. Schmidt et al (14) revealed that HPA inhibition prevented LPS-associated glycocalyx shedding and neutrophil adhesion and attenuated sepsis-induced acute lung injury, and these data support the results of the present study. Therefore, UFH and NAH may decrease shedding of HS due to the inhibition of HPA activity. Syndecans serve important regulatory roles in a number of biological processes, including inflammation, calcium metabolism and wound healing, and the shedding of syndecans is regulated by matrix metalloproteinases (MMPs) (27). Circulating levels of SDC-1 have been indicated to represent the extent of endothelial damage and glycocalyx degradation. A marked correlation was observed between the levels of IL-6 and SDC-1 in the sepsis and surgery groups of a previous study (28). In the present study, glycocalyx degradation products in plasma revealed a positive correlation with inflammatory factors, and the protective effects of UFH and NAH were observed on SDC-1 shedding. This protective effect may be due to the anti-inflammatory effects of UFH or NAH and the subsequent inhibition MMPs activity. However, the specific mechanisms governing this are yet to be determined.
During sepsis, pathogenic agents and inflammatory mediators regulate coagulation through at least 3 simultaneous pathways: The activation of pro-coagulation pathways, the downregulation of physiological anticoagulant production and the inhibition of fibrinolysis (29). F1+2 and TAT are regarded as the sensitive indicators of thrombin generation (6). Consistent with previous studies, an immediate increase in F1+2, TAT, APTT, PT and PAI-1, and a decrease in levels of AT and FIB were observed in rat plasma following LPS administration in the present study. Additionally, preconditioning with UFH was indicated to decrease the levels of F1+2, TAT and APTT and increase the levels of FIB and AT in plasma. UFH has been indicated to exert an anticoagulant effect by binding to the lysine site on AT, which is also modulated by platelets, fibrin, thrombin, factors Xa, IXa, XIa and XIIa and TF (30,31). Tipoe (8) demonstrated that high levels of PAI-1 may predict an adverse outcome in severe sepsis, and suppressed fibrinolysis has been suggested to be one the most important predictors of multiple organ dysfunction during DIC. The results of the present study identified that preconditioning UFH attenuated the level of PAI-1. However, NAH does not contain a binding site for AT, but may improve the coagulopathy in LPS-induced sepsis. This result may be attributed to NAH inhibiting of HPA activity and decreasing the loss of glycocalyx. Sieve et al (12) indicated that HPA upregulated the expression of blood coagulation initiator TF in endothelial and tumor cells, resulting in increased cell surface coagulation activity. Vascular endothelial glycocalyx includes anticoagulant heparan sulfate, which is a small subpopulation (0.5–10%) of HS and contains a specific pentasaccharide motif with high affinity for plasma AT. Molecules of heparan sulfate combined with AT have been demonstrated to prevent microvascular thrombosis and contribute to the maintenance of microvascular patency (32). Vink et al (33) indicated that the specific disruption of glycocalyx promoted thrombin generation and platelet adhesion within a short time period. Chappell et al (34) indicated that protection of the endothelial glycocalyx decreased platelet adhesion in an ischemia/reperfusion pig model. Ikeda et al (35) revealed that SDC-1 levels were associated with the severity of illness and mortality and with DIC development in sepsis. These results support the suggestion that glycocalyx damage may aggravate coagulation disorder, and the inhibition of glycocalyx shedding may further improve coagulation.
No specific pharmacotherapy is available for septic coagulopathy, the efficacy of heparin for sepsis has not been well established, and the use of heparin for sepsis may increase hemorrhage. The present study indicated that NAH protected the vascular endothelial glycocalyx and concomitantly improved partial coagulation disorders. These results suggest that the inhibition of glycocalyx shedding may be used for improving coagulation disorders in sepsis. However, the present study lacks direct evidence to identify the effect of the destruction of glycocalyx induced by HPA on coagulation function. Additionally, the present study observed the sepsis model over a short time period of 6 h. Therefore, future studies should investigate the effects of glycocalyx components on the coagulation/fibrinolytic system, and this will aid in the determination of the pathogenesis of coagulation dysfunction in sepsis.
In conclusion, the results of the present study demonstrated that UFH and NAH alleviated coagulopathy and glycocalyx loss, and that glycocalyx component (HS and SDC-1) levels were significantly correlated with coagulation/fibrinolysis markers in LPS-induced sepsis. These results indicated that NAH treatment may alleviate coagulation disorders through the inhibition of HPA activity and protection of the integrity of the glycocalyx. It may be hypothesized that the degradation of endothelial glycocalyx may be one of the important causes of coagulation dysfunction in sepsis.
Acknowledgements
Not applicable.
Funding
This work has received funding from the Medicine and Health Science Technology Development Plan of Shandong Province (grant no. 2018WS549), National Natural Science Foundation of China (grant no. 81670078), Taishan Scholar Project of Shandong Province, Key Clinical Specialty Project of Shandong Province, and Department of Clinical Laboratory, Yantai Affiliated Hospital of Binzhou Medical University.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
XH performed the majority of the experiments and prepared the manuscript. SH, XL and TW performed the ELISAs. HX and JL performed the immunofluorescence analyses. GK and HH analyzed the data. BX performed the blood coagulation tests and participated in experimental design. DH and XW designed the experiments. WZ analyzed and interpreted the data and participated in language editing. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The animal care and experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, and the protocol was approved by the Institutional Animal Care and Use Committee of Binzhou Medical University Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 2.Berthelsen LO, Kristensen AT, Tranholm M. Animal models of DIC and their relevance to human DIC: A systematic review. Thromb Res. 2011;128:103–116. doi: 10.1016/j.thromres.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Schouten M, Wiersinga WJ, Levi M, van der Poll T. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol. 2008;83:536–545. doi: 10.1189/jlb.0607373. [DOI] [PubMed] [Google Scholar]
- 4.Levi M, van der Poll T. Coagulation and sepsis. Thromb Res. 2017;149:38–44. doi: 10.1016/j.thromres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Simmons J, Pittet JF. The coagulopathy of acute sepsis. Curr Opin Anaesthesiol. 2015;28:227–236. doi: 10.1097/ACO.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu XL, Wang XZ, Liu XX, Hao D, Jaladat Y, Lu F, Sun T, Lv CJ. Low-dose heparin as treatment for early disseminated intravascular coagulation during sepsis: A prospective clinical study. Exp Ther Med. 2014;7:604–608. doi: 10.3892/etm.2013.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rehberg S, Yamamoto Y, Sousse LE, Jonkam C, Zhu Y, Traber LD, Cox RA, Prough DS, Traber DL, Enkhbaatar P. Antithrombin attenuates vascular leakage via inhibiting neutrophil activation in acute lung injury. Crit Care Med. 2013;41:e439–e446. doi: 10.1097/CCM.0b013e318298ad3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tipoe TL, Wu WK, Chung L, Gong M, Dong M, Liu T, Roever L, Ho J, Wong MC, Chan MT, et al. Plasminogen activator inhibitor 1 for predicting sepsis severity and mortality outcomes: A systematic review and meta-analysis. Front Immunol. 2018;9:1218. doi: 10.3389/fimmu.2018.01218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ince C, Mayeux PR, Nguyen T, Gomez H, Kellum JA, Ospina-Tascón GA, Hernandez G, Murray P, De Backer D, ADQI XIV Workgroup The endothelium in sepsis. Shock. 2016;45:259–270. doi: 10.1097/SHK.0000000000000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin L, Koczera P, Zechendorf E, Schuerholz T. The endothelial glycocalyx: New diagnostic and therapeutic approaches in sepsis. Biomed Res Int. 2016;2016:3758278. doi: 10.1155/2016/3758278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchimido R, Schmidt EP, Shapiro NI. The glycocalyx: A novel diagnostic and therapeutic target in sepsis. Crit Care. 2019;23:16. doi: 10.1186/s13054-018-2292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sieve I, Münster-Kühnel AK, Hilfiker-Kleiner D. Regulation and function of endothelial glycocalyx layer in vascular diseases. Vascul Pharmacol. 2018;100:26–33. doi: 10.1016/j.vph.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Nadir Y, Brenner B, Zetser A, Ilan N, Shafat I, Zcharia E, Goldshmidt O, Vlodavsky I. Heparanase induces tissue factor expression in vascular endothelial and cancer cells. J Thromb Haemost. 2006;4:2443–2451. doi: 10.1111/j.1538-7836.2006.02212.x. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, Zemans RL, Bowman JC, Koyanagi DE, Yunt ZX, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18:1217–1223. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Ma X. The role of heparin in sepsis: Much more than just an anticoagulant. Br J Haematol. 2017;179:389–398. doi: 10.1111/bjh.14885. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, Chi C, Guo L, Wang X, Guo L, Sun J, Sun B, Liu S, Chang X, Li E. Heparin therapy reduces 28-day mortality in adult severe sepsis patients: A systematic review and meta-analysis. Crit Care. 2014;18:563. doi: 10.1186/s13054-014-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wildhagen KC, García de Frutos P, Reutelingsperger CP, Schrijver R, Aresté C, Ortega-Gómez A, Deckers NM, Hemker HC, Soehnlein O, Nicolaes GA. Nonanticoagulant heparin prevents histone-mediated cytotoxicity in vitro and improves survival in sepsis. Blood. 2014;123:1098–1101. doi: 10.1182/blood-2013-07-514984. [DOI] [PubMed] [Google Scholar]
- 18.Fodor RŞ, Georgescu AM, Cioc AD, Grigorescu BL, Cotoi OS, Fodor P, Copotoiu SM, Azamfirei L. Time- and dose-dependent severity of lung injury in a rat model of sepsis. Rom J Morphol Embryol. 2015;56:1329–1337. [PubMed] [Google Scholar]
- 19.Li X, Li Z, Zheng Z, Liu Y, Ma X. Unfractionated heparin ameliorates lipopolysaccharide-induced lung inflammation by downregulating nuclear factor-κB signaling pathway. Inflammation Dec. 2013;36:1201–1208. doi: 10.1007/s10753-013-9656-5. [DOI] [PubMed] [Google Scholar]
- 20.Taylor FB, Jr, Toh CH, Hoots WK, Wada H, Levi M, Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH) Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327–1330. doi: 10.1055/s-0037-1616068. [DOI] [PubMed] [Google Scholar]
- 21.Aeffner F, Bolon B, Davis IC. Mouse models of acute respiratory distress syndrome: A review of analytical approaches, pathologic features, and common measurements. Toxicol Pathol. 2015;43:1074–1092. doi: 10.1177/0192623315598399. [DOI] [PubMed] [Google Scholar]
- 22.Zhao D, Ding R, Liu Y, Yin X, Zhang Z, Ma X. Unfractionated heparin protects the protein C system against lipopolysaccharide-induced damage in vivo and in vitro. Exp Ther Med. 2017;14:5515–5522. doi: 10.3892/etm.2017.5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rizzo AN, Dudek SM. Endothelial glycocalyx repair: Building a wall to protect the lung during sepsis. Am J Respir Cell Mol Biol. 2017;56:687–688. doi: 10.1165/rcmb.2017-0065ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang X, Kong G, Li Y, Zhu W, Xu H, Zhang X, Li J, Wang L, Zhang Z, Wu Y, et al. Decitabine and 5-azacitidine both alleviate LPS induced ARDS through anti-inflammatory/antioxidant activity and protection of glycocalyx and inhibition of MAPK pathways in mice. Biomed Pharmacother. 2016;84:447–453. doi: 10.1016/j.biopha.2016.09.072. [DOI] [PubMed] [Google Scholar]
- 25.Confalonieri M, Salton F, Fabiano F. Acute respiratory distress syndrome. Eur Respir Rev. 2017;26 doi: 10.1183/16000617.0116-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen S, He Y, Hu Z, Lu S, Yin X, Ma X, Lv C, Jin G. Heparanase mediates intestinal inflammation and injury in a mouse model of sepsis. J Histochem Cytochem. 2017;65:241–249. doi: 10.1369/0022155417692536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Afratis NA, Nikitovic D, Multhaupt HA, Theocharis AD, Couchman JR, Karamanos NK. Syndecans-key regulators of cell signaling and biological functions. FEBS J. 2017;284:27–41. doi: 10.1111/febs.13940. [DOI] [PubMed] [Google Scholar]
- 28.Steppan J, Hofer S, Funke B, Brenner T, Henrich M, Martin E, Weitz J, Hofmann U, Weigand MA. Sepsis and major abdominal surgery lead to flaking of the endothelial glycocalix. J Surg Res. 2011;165:136–141. doi: 10.1016/j.jss.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 29.Tsao CM, Ho ST, Wu CC. Coagulation abnormalities in sepsis. Acta Anaesthesiol Taiwan. 2015;53:16–22. doi: 10.1016/j.aat.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Kakkar VV, Bentley PG, Scully MF, MacGregor IR, Jones NA, Webb PJ. Antithrombin III and heparin. Lancet. 1980;1:103–104. doi: 10.1016/S0140-6736(80)90538-3. [DOI] [PubMed] [Google Scholar]
- 31.Onishi A, St Ange K, Dordick JS, Linhardt RJ. Heparin and anticoagulation. Front Biosci (Landmark Ed) 2016;21:1372–1392. doi: 10.2741/4462. [DOI] [PubMed] [Google Scholar]
- 32.Shworak NW, Kobayashi T, de Agostini A, Smits NC. Anticoagulant heparan sulfate to not clot-or not? Prog Mol Biol Transl Sci. 2010;93:153–178. doi: 10.1016/S1877-1173(10)93008-1. [DOI] [PubMed] [Google Scholar]
- 33.Vink H, Constantinescu AA, Spaan JA. Oxidized lipoproteins degrade the endothelial surface layer: Implications for platelet-endothelial cell adhesion. Circulation. 2000;101:1500–1502. doi: 10.1161/01.CIR.101.13.1500. [DOI] [PubMed] [Google Scholar]
- 34.Chappell D, Brettner F, Doerfler N, Jacob M, Rehm M, Bruegger D, Conzen P, Jacob B, Becker BF. Protection of glycocalyx decreases platelet adhesion after ischaemia/reperfusion: An animal study. Eur J Anaesthesiol. 2014;31:474–481. doi: 10.1097/EJA.0000000000000085. [DOI] [PubMed] [Google Scholar]
- 35.Ikeda M, Matsumoto H, Ogura H, Hirose T, Shimizu K, Yamamoto K, Maruyama I, Shimazu T. Circulating syndecan-1 predicts the development of disseminated intravascular coagulation in patients with sepsis. J Crit Care. 2018;43:48–53. doi: 10.1016/j.jcrc.2017.07.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.