Abstract
Epicardial fat, a local visceral fat depot surrounded by visceral pericardial sac, surrounds the coronary arteries for most of their course and may contribute to the development of coronary atherosclerosis by local production of inflammatory cytokines. Some studies on non-invasive measurement of epicardial fat mass have shown that epicardial fat mass is associated with the increased incidence of coronary artery disease (CAD), onset and progression of coronary plaque, mainly including major adverse cardiovascular events, myocardial ischemia, and atrial fibrillation. In the present study the correlation of adiponectin, chemerin, and vascular endothelial growth factor (VEGF) with the epicardial fat volume in patients with coronary artery disease was explored, and the risk factors for vascular remodeling of CAD patients were analyzed. A total of 50 CAD patients, treated in Chongzuo People's Hospital from August 2017 to September 2018, were enrolled as the observation group, and further 50 healthy individuals, who underwent physical examinations in the hospital at the same period, were enrolled as the control group. RT-qPCR was adopted to detect the expression levels of adiponectin, chemerin and VEGF in the two groups, a 64-slice dual-source CT to detect epicardial fat volume, and Pearson's correlation to analyze adiponectin, chemerin, VEGF and epicardial fat volume. Logistic regression analysis was performed to analyze the risk factors for vascular remodeling in CAD patients, and a receiver operating characteristic (ROC) curve analysis was used to analyze the value of indexes with multifactorial significance in vascular remodeling. The observation group showed obviously larger epicardial fat volume than the control group (P<0.001), lower adiponectin expression than the control group (P<0.001), and higher chemerin and VEGF expression than the control group (P<0.001). In the observation group, adiponectin expression decreased with the increase of epicardial fat volume (P<0.001), while the expression of chemerin and VEGF increased with the increase of epicardial fat volume (P<0.001). Remodeling occurred in 27 of the 50 patients. ROC curve analysis showed that the areas under the curves of adiponectin, chemerin, VEGF and epicardial fat volume were 0.697, 0.652, 0.696 and 0.689, respectively. Epicardial fat volume, adiponectin, chemerin and VEGF are independent risk factors for vascular remodeling and the expression of adiponectin, chemerin and VEGF can reflect epicardial fat volume.
Keywords: adiponectin, chemerin, vascular endothelial growth factor, epicardial fat volume, coronary artery disease
Introduction
Coronary artery disease (CAD) is a common clinical cardiovascular disease (1). The American Heart Association has reported that in 2016 the number of CAD patients ≥20 years of age had reached 15.5 million and the incidence increased with age (2). At present, the pathogenesis of CAD is not completely clear, however it is certain that hypertension, diabetes, obesity and dyslipidemia are risk factors for the onset of CAD (3,4). As people's living standards and dietary habits change, unhealthy diets lead to an increasing number of obese patients. A study has shown that visceral obesity patients develop CAD more rapidly and suffer a higher incidence of acute myocardial infarction (5). In addition, the risk of developing CAD is not the same for individuals with the same body fat, which is mainly caused by the different distribution of body fat (6).
Epicardial fat is a kind of adipose tissue, which promotes remodeling of coronary artery. It has been reported that the development of CAD is closely related to epicardial fat and its volume change is an important index for the severity of CAD (7). At present, the gold standard for epicardial fat volume detection is magnetic resonance imaging (MRI), multislice computed tomography (MSCT) and other imaging methods (8). MRI and MSCT have a relatively long detection time and high cost, while serological detection is less expensive and requires shorter time than MRI and MSCT (9). Therefore, it is critical to find serum indexes to reflect the changes in the epicardial fat volume of patients. Adiponectin is a protein secreted by adipocyte, which can regulate glucose metabolism, improve insulin resistance and fight atherosclerosis, and is expressed obviously lower in the serum of CAD patients (10). Chemerin belongs to the adipocyte-factor family, which has the function of leukocyte chemotaxis and can promote the development of inflammatory response in injured tissues by recruiting inflammatory cells of chemerin receptors (11). Vascular endothelial growth factor (VEGF) is a vascular endothelial cell mitogen with high specificity, which plays an important regulatory role in angiogenesis and is highly expressed in the serum of CAD patients (12). However, it is unclear whether adiponectin, chemerin and VEGF can be adopted as observation indexes for changes in the epicardial fat volume.
The present study explored the correlation of adiponectin, chemerin, and VEGF with epicardial fat volume of CAD patients and its potential clinical value to provide a reference for clinicians in diagnosis and treatment.
Subjects and methods
Clinical data
A total of 50 CAD patients, treated in Chongzuo People's Hospital (Chongzuo, China) from August 2017 to September 2018, were enrolled as the observation group, and additional 50 healthy subjects, who underwent physical examination in the hospital at the same period, were enrolled as the control group. Subjects in the control group exhibited normal values in all clinical laboratory detection tests and were without combined congenital organ dysfunction. This study was conducted with the approval of the medical Ethics Committee of Chongzuo People's Hospital. Inclusion criteria: All patients included met the criteria of the 2012 USA Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease (13). The patients and their families understood the purpose of the study, and an informed consent was signed. Patients were diagnosed with CAD by imaging and their clinical data were complete. Exclusion criteria: Patients with angina pectoris, silent myocardial ischemia, heart failure, arrhythmia, and sudden death; patients with abnormal thyroid function, infectious diseases or tumors; patients with recent trauma or history of surgery; pregnant women; patients with hepatic and kidney function obstacle.
Reagents and instruments
EasyPure Blood RNA kit, TransScript II Green Two-Step qRT-PCR SuperMix (both from Beijing TransGen Biotech Co., Ltd.; ER101-01 and AQ301-01, respectively), adiponectin, chemerin and VEGF primers, and related sequences (all designed and synthesized by Shanghai GenePharma Co., Ltd.), PCR instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.; ABI 7500) and 64-slice dual-source CT instrument (Siemens AG; SOMATOM Definition Flash).
Methods
Collection of peripheral blood and RT-qPCR detection
The study participants fasted from 8 p.m. on the day before the test. Peripheral venous blood (5 ml) was collected from both groups in the morning. Following standing for 30 min, the blood was centrifuged at 1,500 × g, at 25°C for 10 min. The supernatant was collected for PCR detection, and the total RNA of the collected serum was extracted using the EasyPure Blood RNA kit. Total RNA purity, concentration and integrity of the extracted total RNA were detected via ultraviolet spectrophotometry and agarose gel electrophoresis. Reverse transcription was performed by 5X TransScript® II All-in-One SuperMix for qPCR and gDNA Remover kit (both from Beijing TransGen Biotech Co., Ltd.) in strict accordance with the manufacturer's instructions. The reaction system consisted of 1 µg of total RNA, 4 µg of 5X TransScript® II All-in-One SuperMix, 1 µg of gDNA Remover, and RNase-free Water (added for a total of 20 µl). The reaction conditions were as follows: Incubation at 50°C for 15 min, and at 85°C for 5 sec. Then, PCR amplification was carried out. PCR reaction system: 1 µl of cDNA, 0.4 µl of upstream primers and 0.4 µl of downstream primers, 10 µl of 2X TransScript® Tip Green qPCR SuperMix, Passive Reference Dye (50X) and Nuclease-free Water (added for a total volume of 20 µl) (all from Beijing TransGen Biotech Co., Ltd.). PCR reaction conditions: Initial denaturation at 94°C for 30 sec, denaturation at 94°C for 5 sec and annealing and extending at 60°C for 30 sec for a total of 40 cycles (14). The upstream and downstream primers of adiponectin were 5′-GCATTCAGTGTGGGATTGGAG-3′ and 5′-AGACTGTGATGTGGTAGGCAAAG-3′, respectively; the upstream and downstream primers of chemerin were 5′-AAACCCGAGTGCAAAGTCAG-3′ and 5′-CCGCAGAACTTGGGTCTCTAT-3′, respectively; the upstream and downstream primers of VEGF were 5′-GAAGGTGAAGGTCGGAGTC-3′ and 5′-TGGTTCCCGAAACGCTGAG-3′, respectively, and the upstream and downstream primers of GAPDH were 5′-GAAGGTGAAGGTCGGAGTC-3′ and 5′-GAAGATGGTGATGGGATTTC-3′, respectively. Three multiple pores were set for each sample, and the experiment was carried out 3 times. GAPDH was the internal reference, and 2−ΔΔCq (14) was used to analyze the data.
Epicardial fat volume detection
Participants in both groups were subjected to 64-slice dual-source CT detection, and their epicardial fat volume was detected by Volumer software (GE Healthcare). Contrast agent was injected intravenously at 5–10 min before detection, and nitroglycerin and metoprolol were administered orally to dilate vessels and control the heart rate. Tissues with −250 to −30 HU in CT were set as adipose tissues. The cardiac fiber membrane identifiable from the lower apical edge in the left ventricular at the origin of the left pulmonary artery of newly transverse sinus midpoint was selected (namely the cardiac membrane boundary), and it was selected at intervals of 0.5–1.0 cm layer by layer. Finally the fat volume was calculated using the Volumer software.
Vascular remodeling detection
Contrast examination was performed on the left and right coronary arteries of all participants. According to the data obtained from the 64-slice dual-source CT detection, the vascular remodeling index of the observation group was calculated: Vascular remodeling index = (external elastic membranous area at lesions)/(vascular area at reference site). At vascular remodeling index ≥1 the patient developed remodeling.
Observation indexes
Main observation indexes: The two groups were compared in expression of adiponectin, chemerin and VEGF in serum, and in epicardial fat volume, and the correlation of adiponectin, chemerin, and VEGF with epicardial fat volume in the observation group was analyzed. Secondary observation indexes: The two groups were compared in clinical data, and vascular remodeling of patients in the observation group was observed and analyzed; risk factors for vascular remodeling in CAD patients were analyzed, and receiver operating characteristic (ROC) curve analysis was adopted to analyze the value of indexes with multivariate significance in vascular remodeling.
Statistical analysis
Collected data were analyzed by SPSS 20.0 software (IBM Corp.), relevant graphs were created using GraphPad Prism 7 software (GraphPad Software, Inc.), and the distribution of data was analyzed using the Kolmogorov-Smirnov test. Enumeration data were expressed as n (%), and analyzed using Chi-square (χ2) test. Measurement data were expressed as the mean ± standard deviation (mean ± SD). Data in normal distribution were subjected to t-test and were expressed by a t value. Comparisons between groups were performed using the independent samples t-test. Multivariate logistic regression was adopted to analyze the risk factors for vascular remodeling. ROC analysis was adopted to map the areas under the independent risk factor curves. Pearson's correlation analysis was used to analyze the correlation of adiponectin, chemerin, and VEGF with epicardial fat volume in the observation group. P<0.05 was considered to indicate a statistically significant difference.
Results
Clinical data analysis
The clinical data of the two groups were collected for analysis. There was no statistical significance between the two groups in sex, age, body mass index (BMI), family history of CAD, smoking history, alcohol abuse history and place of residence (all P>0.05) (Table I).
Table I.
Comparison of baseline data between the two groups [n (%), mean ± SD].
| Factors | Observation group (n=50) | Control group (n=50) | t/χ2 | P-value |
|---|---|---|---|---|
| Sex | 1.099 | 0.295 | ||
| Male | 35 (70.00) | 30 (60.00) | ||
| Female | 15 (30.00) | 20 (40.00) | ||
| Age (years) | 60.1±8.4 | 59.7±9.2 | 0.227 | 0.821 |
| BMI (kg/m2) | 25.74±2.84 | 24.79±2.25 | ||
| Family history of CAD | 3.241 | 0.072 | ||
| Yes | 20 (40.00) | 29 (58.00) | ||
| No | 30 (60.00) | 21 (42.00) | ||
| Smoking history | 0.713 | 0.398 | ||
| Yes | 35 (70.00) | 31 (62.00) | ||
| No | 15 (30.00) | 19 (38.00) | ||
| Alcohol abuse history | 0.749 | 0.102 | ||
| Yes | 5 (10.00) | 6 (12.00) | ||
| No | 45 (90.00) | 44 (88.00) | ||
| Place of residence | 0.694 | 0.405 | ||
| City | 30 (60.00) | 34 (68.00) | ||
| Country | 20 (40.00) | 16 (32.00) |
BMI, body mass index; CAD, coronary artery disease.
Epicardial fat volume of the two groups
Epicardial fat volume of the two groups was detected, and it was found that the observation group showed obviously larger epicardial fat volume than the control group (189.22±29.08 vs. 132.48±23.42 cm3) (P<0.001) (Fig. 1).
Figure 1.
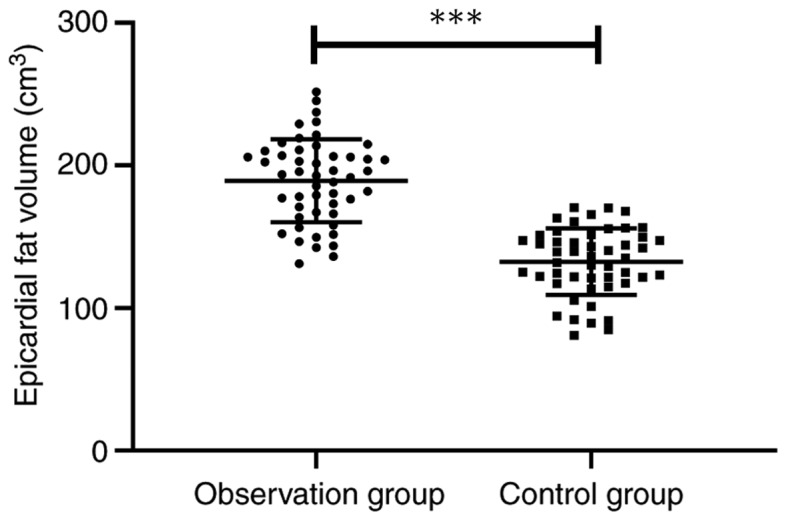
Comparison of epicardial fat volume between the observation and control group. In the observation group (189.22±29.08 cm3) the epicardial fat volume was obviously larger than that of the control group (132.49±23.42 cm3) (t=10.748, ***P<0.001).
Expression of adiponectin, chemerin and VEGF in the two groups
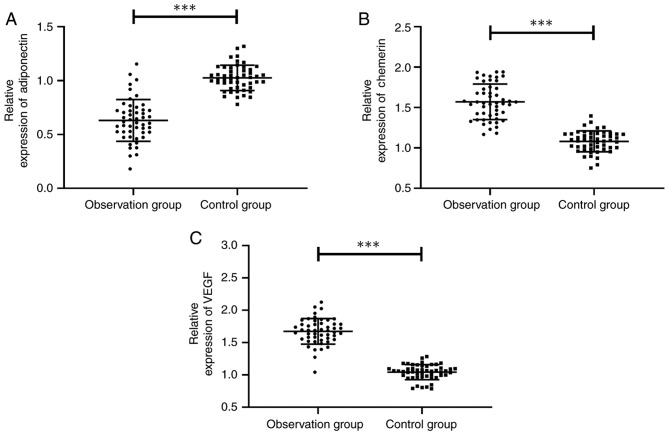
The expression of adiponectin, chemerin and VEGF in the two groups was compared, and it was found that the observation group showed obviously lower adiponectin expression than the control group, with a significant difference (P<0.001), and obviously higher expression of chemerin and VEGF than the control group, with a significant difference (both P<0.001) (Fig. 2).
Figure 2.
Comparison of the expression of adiponectin, chemerin and VEGF between the observation and control group. (A) Comparison of adiponectin expression between the observation group (0.631±0.194) and the control group (1.026±0.117) (t=12.315, P<0.001). (B) Comparison of chemerin expresion between the observation group (1.571±0.220) and the control group (1.082±0.129) (t=13.576, P<0.001). (C) Comparison of VEGF expression between the observation group (1.674±0.198) and the control group (1.043±0.116) (t=19.432, P<0.001). ***P<0.001. VEGF, vascular endothelial growth factor..
Correlation of adiponectin, chemerin and VEGF with epicardial fat volume in the observation group
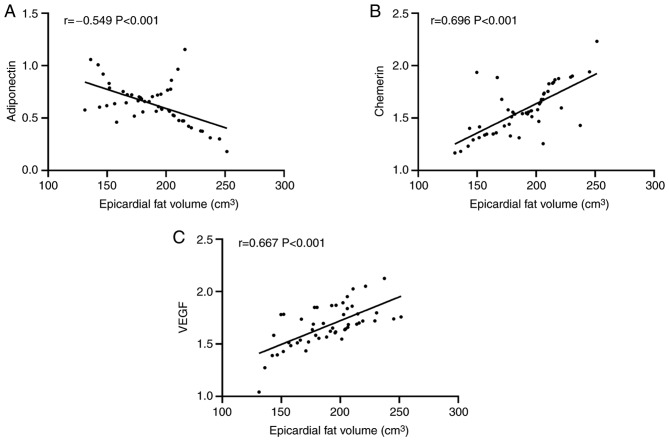
Pearson's correlation analysis showed that in the observation group, the adiponectin expression gradually decreased with the increase of epicardial fat volume, presenting a negative correlation (P<0.001), and the expression levels of chemerin and VEGF gradually increased with the increase of epicardial fat volume presenting a positive correlation (both P<0.001) (Fig. 3).
Figure 3.
Correlation of the expression of adiponectin, chemerin, and VEGF with epicardial fat volume in the observation group. Epicardial fat volume was (A) negatively correlated with adiponectin (r=−0.549, P<0.001), and positively correlated with (B) chemerin (r=0.696, P<0.001) and (C) VEGF (r=0.667, P<0.001). VEGF, vascular endothelial growth factor.
Analysis of risk factors for vascular remodeling in the observation group
Statistical analysis of vascular remodeling in the observation group showed that 27 of the 50 patients developed remodeling, and the patients were divided into a remodeling group (n=27) and a non-remodeling group (n=23). Clinical data of patients were collected for univariate analysis, and it was found that adiponectin, chemerin, VEGF and epicardial fat volume were risk factors for vascular remodeling (all P<0.05). Subsequently, remodeling of patients was used as the independent variable and factors with significance in univariate analysis were taken as covariate, and assignment was performed. Binary logistic regression was performed and forward logistic regression was adopted to analyze the results. It was found that epicardial fat volume (OR: 6.945, 95% CI: 1.385–34.825), adiponectin (OR: 0.124, 95% CI: 0.022–0.689), chemerin (OR: 5.175, 95% CI: 1.079–24.834) and VEGF (OR: 12.752, 95% CI: 2.146–75.760) were independent risk factors for vascular remodeling in CAD patients (Tables II–IV).
Table II.
Univariate analysis of risk factors for vascular remodeling [n (%), mean ± SD].
| Factors | Patients in the remodeling group (n=27) | Patients in the non-remodeling group (n=23) | t/χ2 | P-value |
|---|---|---|---|---|
| Sex | 0.004 | 0.951 | ||
| Male | 19 (70.37) | 16 (69.57) | ||
| Female | 8 (29.63) | 7 (30.43) | ||
| Age (years) | 0.080 | 0.777 | ||
| ≥60 | 13 (48.15) | 12 (52.17) | ||
| <60 | 14 (51.85) | 11 (47.83) | ||
| BMI (kg/m2) | 0.415 | 0.520 | ||
| ≥24 | 22 (81.48) | 17 (73.91) | ||
| <24 | 5 (18.52) | 6 (26.09) | ||
| Family history of CAD | 0.215 | 0.643 | ||
| Yes | 10 (37.04) | 10 (43.48) | ||
| No | 17 (62.96) | 13 (56.52) | ||
| Smoking history | 0.004 | 0.951 | ||
| Yes | 19 (76.00) | 16 (69.57) | ||
| No | 8 (24.00) | 7 (30.43) | ||
| Alcohol abuse history | 0.438 | 0.508 | ||
| Yes | 2 (7.41) | 3 (13.04) | ||
| No | 25 (92.59) | 20 (86.96) | ||
| Place of residence | 0.483 | 0.487 | ||
| City | 15 (55.56) | 15 (65.22) | ||
| Country | 12 (44.44) | 8 (34.78) | ||
| Adiponectin | 0.563±0.148 | 0.710±0.214 | 2.858 | 0.006 |
| Chemerin | 1.638±0.240 | 1.504±0.207 | 2.094 | 0.042 |
| VEGF | 1.735±0.161 | 1.601±0.215 | 2.516 | 0.015 |
| Epicardial fat volume (cm3) | 198.57±27.11 | 178.25±27.95 | 2.604 | 0.012 |
BMI, body mass index; CAD, coronary artery disease; VEGF, vascular endothelial growth factor.
Table IV.
Multivariate analysis.
| 95% CI of Exp (β) | |||||||
|---|---|---|---|---|---|---|---|
| Factors | β | S.E | Wals | Sig. | Exp (β) | Lower limit | Upper limit |
| Adiponectin | −2.089 | 0.876 | 5.692 | 0.017 | 0.124 | 0.022 | 0.689 |
| Chemerin | 1.644 | 0.800 | 4.221 | 0.040 | 5.175 | 1.079 | 24.834 |
| VEGF | 2.546 | 0.909 | 7.840 | 0.005 | 12.752 | 2.146 | 75.760 |
| Epicardial fat volume | 1.938 | 0.823 | 5.551 | 0.018 | 6.945 | 1.385 | 34.825 |
VEGF, vascular endothelial growth factor.
Multivariate ROC curve analysis
A ROC curve was drawn for indexes with multifactorial significance. It was found that the areas under adiponectin, chemerin, VEGF and epicardial fat volume curves were 0.697, 0.652, 0.696, and 0.689, respectively (Table V and Fig. 4).
Table V.
ROC parameters.
| Factors | Adiponectin | Chemerin | VEGF | Epicardial fat volume |
|---|---|---|---|---|
| AUC | 0.697 | 0.652 | 0.696 | 0.689 |
| 95% CI | 0.548–0.846 | 0.500–0.804 | 0.547–0.844 | 0.542–0.836 |
| Sensitivity | 77.78% | 29.63% | 62.96% | 88.88% |
| Specificity | 60.87% | 95.65% | 73.91% | 43.48% |
| Youden index | 38.65% | 25.28% | 36.88% | 32.37% |
| Cut-off value | <0.661 | >1.837 | >1.694 | >168.91 |
ROC, receiver operating characteristic; VEGF, vascular endothelial growth factor.
Figure 4.
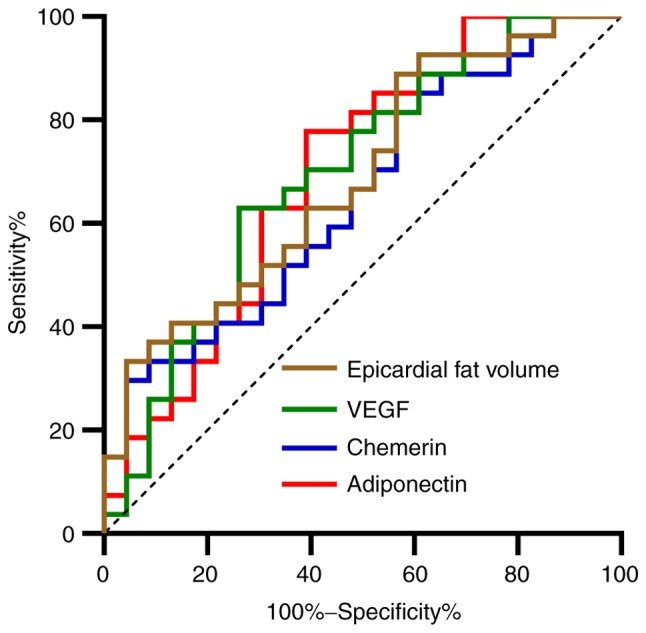
ROC curves. Red line represents adiponectin, blue line represents chemerin, green line represents VEGF, and the brown line represents epicardial fat volume. ROC, receiver operating characteristic; VEGF, vascular endothelial growth factor.
Discussion
Epicardial adipose tissue is a relatively unique visceral adipose tissue between myocardium and pericardium. According to previous studies, there is a close relationship between epicardial adipose tissue and vascular remodeling and atherosclerosis (15), and epicardial adipose tissue can be adopted as an independent risk factor for coronary artery stenosis and plaque vulnerability (16). However, epicardial fat volume is mainly detected through imaging in clinic, which may aggregate the burden of patients due to its long imaging time and high cost. Compared with imaging, serological detection has the advantages of requiring shorter time and have low cost. Therefore, it is critical to find serum indexes to reflect changes in the epicardial fat volume of patients.
The epicardial fat volumes of the observation and control groups were first detected showing that the observation group had obviously larger epicardial fat volume than the control group. Previous studies have shown that epicardial fat accumulates during the development of CAD, which may cause the release of a large number of epicardial adipose cell cytokines and inflammatory factors, promoting calcified plaque in coronary artery, leading eventually to coronary artery occlusion in patients (17,18). Therefore, the question is whether adipocyte factors or vascular factors related to CAD can be detected to determine the epicardial fat volume of patients. To find the answer, adiponectin, chemerin and VEGF in the serum of CAD patients were detected. A study conducted by Eiras and González-Juanatey (19) showed that low adiponectin expression in CAD patients is expected to be a potential biomarker for CAD, while a study conducted by Madonna et al (20) and Nakamura et al (21) showed that both chemerin and VEGF have the effect of promoting blood vessel survival. The present study detected the expression of adiponectin, chemerin and VEGF in the serum of the observation group, finding that the expression of chemerin and VEGF in the serum of the observation group was significantly higher than those of the control group, while the expression of adiponectin was significantly lower than that of the control group, in consistency with previous studies (22,23). The present study further detected and analyzed the correlation of adiponectin, chemerin, and VEGF with epicardial fat volume by performing a Pearson's correlation analysis. The results revealed that adiponectin expression gradually decreased with the increase of epicardial fat volume, showing a negative correlation, while the expression of chemerin and VEGF gradually increased with the increase of epicardial fat volume, showing a possitive correlation. This suggests that adiponectin, chemerin, and VEGF could be taken as potential observation indexes for changes in epicardial fat volume. The relevant mechanisms of indexes and epicardial fat volume may be the following: i) Both adiponectin and chemerin can be produced by epicardial adipose tissue. The accumulation of epicardial fat in patients after CAD will cause the release of a large number of inflammatory factors and adipocyte factors, promoting CAD (24). ii) VEGF is an angiogenic growth factor. When a body is hypoxic-ischemic, VEGF expression will significantly increase, which will alleviate myocardial apoptosis induced by myocardial ischemia by promoting angiogenesis and collateral circulation; while with the increase of epicardial fat volume and gradual aggravation of CAD, VEGF expression in serum increases (25).
Statistics on remodeling of CAD patients was performed. Vascular remodeling is a morphological change in the lumen for the change of structure of the patient's vascular wall. Vascular remodeling can cause endothelial cell proliferation, inflammatory cell aggregation and increase of atherosclerosis area (26). Therefore, it is especially important to explore the risk factors for vascular remodeling. We conducted multivariate analysis, finding that increase of epicardial fat volume, chemerin and VEGF and decrease of adiponectin are independent risk factors for vascular remodeling, and we mapped ROC curves, finding that each index has a certain clinical diagnostic value in vascular remodeling.
The present study can preliminarily explain the correlation between adiponectin, chemerin, VEGF and epicardial fat volume of CAD patients, and the expression of adiponectin, chemerin and VEGF can reflect epicardial fat volume in CAD patients. Epicardial fat volume, adiponectin, chemerin and VEGF can be adopted as potential observation indexes of vascular remodeling. However, this study still has certain limitations. ROC curves of adiponectin, chemerin, and VEGF in CAD patients were not drawn and expression of adiponectin, chemeri and VEGF in adipose tissue of CAD patients were not detected. Therefore, further study is still required.
In summary, epicardial fat volume, adiponectin, chemerin and VEGF are independent risk factors for vascular remodeling, and the expression of adiponectin, chemerin and VEGF can reflect the epicardial fat volume.
Table III.
Valuation.
| Factors | Valuation |
|---|---|
| Epicardial fat volume | ≥189.22 cm3=1, <189.22 cm3=0 |
| Adiponectin | ≥0.631=1, <0.631=0 |
| Chemerin | ≥1.571=1, <1.571=0 |
| VEGF | ≥1.674=1, <1.674=0 |
| Remodeling | Remodeling =1, non-remodeling =0 |
VEGF, vascular endothelial growth factor.
Acknowledgements
Not applicable.
Funding
The study was supported by the Foundation of Guangxi Health Department (nos. Z20170726 and Z20170729) and the Programs for Science and Technology Development of Chongzuo (no. FA20170729).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
QW and YC analyzed and interpreted the patient general data. SC performed PCR. XW and WN were responsible for the analysis of the observation indicators. QW and YC wrote the manuscript. All the authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Chongzuo People's Hospital (Chongzuo, China). Patients who participated in this research had complete clinical data. Signed informed consents were obtained from the patients or their guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wong MCS, Zhang DX, Wang HH. Rapid emergence of atherosclerosis in Asia: A systematic review of coronary atherosclerotic heart disease epidemiology and implications for prevention and control strategies. Curr Opin Lipidol. 2015;26:257–269. doi: 10.1097/MOL.0000000000000191. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, et al. Writing Group Members; American Heart Association Statistics Committee; Stroke Statistics Subcommittee Executive summary: heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 3.Laclaustra M, Casasnovas JA, Fernández-Ortiz A, Fuster V, León-Latr M, Jiménez-Borreguero LJ, Pocovi M, Hurtado-Roca Y, Ordovas JM, Jarauta E, et al. Femoral and carotid subclinical atherosclerosis association with risk factors and coronary calcium: The AWHS study. J Am Coll Cardiol. 2016;67:1263–1274. doi: 10.1016/j.jacc.2015.12.056. [DOI] [PubMed] [Google Scholar]
- 4.Blaha MJ, Cainzos-Achirica M, Greenland P, McEvoy JW, Blankstein R, Budoff MJ, Dardari Z, Sibley CT, Burke GL, Kronmal RA, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2016;133:849–858. doi: 10.1161/CIRCULATIONAHA.115.018524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piché ME, Poirier P, Lemieux I, Després JP. Overview of epidemiology and contribution of obesity and body fat distribution to cardiovascular disease: An update. Prog Cardiovasc Dis. 2018;61:103–113. doi: 10.1016/j.pcad.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Meenakshi K, Rajendran M, Srikumar S, Chidambaram S. Epicardial fat thickness: A surrogate marker of coronary artery disease - Assessment by echocardiography. Indian Heart J. 2016;68:336–341. doi: 10.1016/j.ihj.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKenney-Drake ML, Rodenbeck SD, Bruning RS, Kole A, Yancey KW, Alloosh M, Sacks HS, Sturek M. Epicardial adipose tissue removal potentiates outward remodeling and arrests coronary atherogenesis. Ann Thorac Surg. 2017;103:1622–1630. doi: 10.1016/j.athoracsur.2016.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kianoush S, Al Rifai M, Cainzos-Achirica M, Al-Mallah MH, Tison GH, Yeboah J, Miedema MD, Allison MA, Wong ND, DeFilippis AP, et al. Thoracic extra-coronary calcification for the prediction of stroke: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2017;267:61–67. doi: 10.1016/j.atherosclerosis.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamada T, Sone T, Higashi H, Jo Y, Yamamoto A, Kanki A, Ito K. Prostate cancer detection in patients with total serum prostate-specific antigen levels of 4–10 ng/ml: Diagnostic efficacy of diffusion-weighted imaging, dynamic contrast-enhanced MRI, and T2-weighted imaging. AJR Am J Roentgenol. 2011;197:664–670. doi: 10.2214/AJR.10.5923. [DOI] [PubMed] [Google Scholar]
- 10.Moazzami K, Ostovaneh MR, Ambale Venkatesh B, Habibi M, Yoneyama K, Wu C, Liu K, Pimenta I, Fitzpatrick A, Shea S, et al. Left ventricular hypertrophy and remodeling and risk of cognitive impairment and dementia: MESA (Multi-Ethnic Study of Atherosclerosis) Hypertension. 2018;71:429–436. doi: 10.1161/HYPERTENSIONAHA.117.10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaur J, Mattu HS, Chatha K, Randeva HS. Chemerin in human cardiovascular disease. Vascul Pharmacol. 2018;110:1–6. doi: 10.1016/j.vph.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Dashkevich A, Hagl C, Beyersdorf F, Nykänen AI, Lemström KB. VEGF pathways in the lymphatics of healthy and diseased heart. Microcirculation. 2016;23:5–14. doi: 10.1111/micc.12220. [DOI] [PubMed] [Google Scholar]
- 13.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:2564–2603. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Nagy E, Jermendy AL, Merkely B, Maurovich-Horvat P. Clinical importance of epicardial adipose tissue. Arch Med Sci. 2017;13:864–874. doi: 10.5114/aoms.2016.63259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keresztesi AA, Asofie G, Simion MA, Jung H. Correlation between epicardial adipose tissue thickness and the degree of coronary artery atherosclerosis. Turk J Med Sci. 2018;48:40–45. doi: 10.3906/sag-1604-58. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita K, Yamamoto MH, Igawa W, Ono M, Kido T, Ebara S, Okabe T, Saito S, Amemiya K, Isomura N, et al. Association of epicardial adipose tissue volume and total coronary plaque burden in patients with coronary artery disease. Int Heart J. 2018;59:1219–1226. doi: 10.1536/ihj.17-709. [DOI] [PubMed] [Google Scholar]
- 18.Tekin I, Edem E. Association of epicardial fat tissue with coronary artery disease and left ventricle diastolic function indicators. Med Sci Monit. 2018;24:6367–6374. doi: 10.12659/MSM.910989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eiras S, González-Juanatey JR. Adiponectin as biomarker in coronary artery disease. In: Patel V, Preedy V, editors. Biomarkers in Cardiovascular Disease. Biomarkers in Disease: Methods, Discoveries and Applications. Springer; Dordrecht: 2016. pp. 635–651. [DOI] [Google Scholar]
- 20.Madonna R, Petrov L, Teberino MA, Manzoli L, Karam JP, Renna FV, Ferdinandy P, Montero-Menei CN, Ylä-Herttuala S, De Caterina R. Transplantation of adipose tissue mesenchymal cells conjugated with VEGF-releasing microcarriers promotes repair in murine myocardial infarction. Cardiovasc Res. 2015;108:39–49. doi: 10.1093/cvr/cvv197. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura N, Naruse K, Kobayashi Y, Miyabe M, Saiki T, Enomoto A, Takahashi M, Matsubara T. Chemerin promotes angiogenesis in vivo. Physiol Rep. 2018;6:e13962. doi: 10.14814/phy2.13962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lurins J, Lurina D, Tretjakovs P, Mackevics V, Lejnieks A, Rapisarda V, Baylon V. Increased serum chemerin level to predict early onset of aortic valve stenosis. Biomed Rep. 2018;8:31–36. doi: 10.3892/br.2017.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.İnci S, Aksan G, Doğan P. Chemerin as an independent predictor of cardiovascular event risk. Ther Adv Endocrinol Metab. 2016;7:57–68. doi: 10.1177/2042018816629894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smekal A, Vaclavik J. Adipokines and cardiovascular disease: A comprehensive review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2017;161:31–40. doi: 10.5507/bp.2017.002. [DOI] [PubMed] [Google Scholar]
- 25.Alber HF, Frick M, Dulak J, Dörler J, Zwick RH, Dichtl W, Pachinger O, Weidinger F. Vascular endothelial growth factor (VEGF) plasma concentrations in coronary artery disease. Heart. 2005;91:365–366. doi: 10.1136/hrt.2003.021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kul S, Karadeniz A, Dursun İ, Şahin S, Faruk Çırakoğlu Ö, Raşit Sayın M, Turan T, Hakan Ateş A. Non-alcoholic fatty pancreas disease is associated with increased epicardial adipose tissue and aortic intima-media thickness. Acta Cardiol Sin. 2019;35:118–125. doi: 10.6515/ACS.201903_35(2).20181009A. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.