Abstract
Purpose
Gastrointestinal symptoms are often related to antibiotic treatment. Their incidence, risk and protective conditions in children are not well defined and represent the aims of this study.
Methods
We prospectively enrolled inpatient children submitted to antibiotic treatment. Indication, type, dose and duration of treatment, probiotic supplementation and gastrointestinal symptoms were recorded at recruitment, after two and four weeks. Antibiotic-associated diarrhea (AAD) was defined as the presence of at least 3 loose/liquid stools within 14 days from antibiotic onset.
Results
AAD occurred in 59/289 (20.4%) of patients, with increased risk in children younger than 3 years (relative risk [RR]=4.25), in lower respiratory (RR=2.11) and urinary infections (RR=3.67), intravenous administration (RR=1.81) and previous AAD episodes (RR=1.87). Abdominal pain occurred in 27/289 (9.3%), particularly in children >6 years (RR=4.15), with previous abdominal pain (RR=7.2) or constipation (RR=4.06). Constipation was recorded in 23/289 (8.0%), with increased risk in children having surgery (RR=2.56) or previous constipation (RR=7.38). Probiotic supplementation significantly reduced AAD (RR=0.30) and abdominal pain (RR=0.36). Lactobacillus rhamnosus GG (LGG) and L. reuteri significantly reduced AAD (RR=0.37 and 0.35) and abdominal pain (RR=0.37 and 0.24).
Conclusion
AAD occurred in 20.4% of children, with increased risk at younger age, lower respiratory and urinary tract infections, intravenous treatment and previous AAD. LGG and L. reuteri reduced both AAD and associated abdominal pain.
Keywords: Abdominal pain, Anti-bacterial agents, Antibiotic-associated diarrhea, Children, Constipation, Diarrhea, Lactobacillus reuteri, Lactobacillus rhamnosus GG, Probiotics
INTRODUCTION
Antibiotics are the most frequently prescribed drugs in children [1]. Gastrointestinal symptoms are often associated with antibiotic treatment because of related alterations in motility, permeability, nociceptors, and microbiota [2,3,4]. Antibiotic-associated diarrhea (AAD), the most commonly reported gastrointestinal manifestation, is defined as the presence of at least 3 loose or liquid bowel movements per day during or after antibiotic treatment excluding other etiologies (infections, food poisoning, use of laxatives, and chronic gastrointestinal diseases) [5,6]. AAD is a mild and self-limiting (309 days) adverse effect [7], but it can eventually cause severe electrolyte/fluid imbalance, hospital admission, and pseudomembranous colitis [2] associated with Clostridium difficile infection in 10–25% of cases [8]. Moreover, AAD may result in lower compliance rates to antibiotic treatment [9], family concerns, and increased health care costs [10]. The European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) reported AAD in up to 33% children taking antibiotics [5]. However, pediatric data are heterogeneous in terms of incidence (4.3–80.0%), patient selection (different age, outpatients or inpatients), and definition of AAD and associated factors [7,11].
A few studies showed an increased rate of AAD in young children [10,12,13], in particular with the use of specific antibiotics such as amoxicillin-clavulanate and ampicillin-sulbactam [12,13,14]. There is minor evidence of other possible risk factors, such as intestinal dysbiosis, hospitalization, immunodeficiency, and prolonged and combined antibiotic therapy [5,8,10]. To prevent and reduce AAD in children, the ESPGHAN recommends the use of specific strains of probiotics [5], which may interfere with pathogenic colonization of the gut by remodeling the gut flora and immune response [2]. Lactobacillus rhamnosus GG (LGG) and Saccharomyces boulardii have demonstrated a 52% reduction in the incidence of AAD in children supplemented with probiotics compared to placebo or non-intervention groups [5]. A specific cost-effective analysis of probiotics for AAD in children is lacking, although they are considered cost-saving in cases of C. difficile-associated diarrhea [15].
This study aimed to assess the incidence of AAD and other gastrointestinal symptoms during and after antibiotic treatment and identify risk and protective factors in inpatient children of different ages.
MATERIALS AND METHODS
Setting and participants
This observational prospective study was performed in a single center at a pediatric hospital from April 2017 to May 2018. Children referred to the Emergency Department and/or who were admitted to the Pediatric Ward and received antibiotic treatment were consecutively enrolled. Exclusion criteria were the presence of diarrhea or vomit at recruitment, known immunodeficiency, food allergy, chronic gastrointestinal, mental or neurological diseases, treatment with acid inhibitors, or previous gastrointestinal surgery. At recruitment, we collected each patient's demographic data; site of infection; dose, duration, and kind of antibiotic; and previous AAD or other gastrointestinal symptoms reported in the last 12 months. Two weeks after the onset of antibiotic treatment, we interviewed the parents regarding the occurrence of fever or new infections and of gastrointestinal symptoms such as abdominal pain, constipation or diarrhea (considering day of onset and duration, number of stools, stool investigations, and results), and the eventual use of probiotics (specifying strain, period of intake, and indication from health care practitioners). If gastrointestinal symptoms persisted, we repeated the recall every 2 weeks until they resolved.
Study aim
The primary objective of the study was to measure the incidence of AAD, abdominal pain, and constipation in inpatient children. The secondary aims were to assess the duration and possible risk factors related to antibiotic treatment and the efficacy of specific probiotic strains.
Definitions
AAD was defined as the presence of 3 or more loose or liquid bowel movements per day during antibiotic treatment (early AAD [7]) or within 14 days from the antibiotics course excluding other etiologies [5,6]. We used the term ‘protected penicillins’ to indicate the combination of penicillins plus clavulanate or sulbactam or tazobactam molecules.
We considered antibiotic-associated abdominal pain (AAAP) as the presence of abdominal pain not reduced by defecation and not related to other recognizable conditions [16]. We also recorded fussiness and persisting crying without any other obvious causes in infants and younger children [17].
We considered antibiotic-associated constipation (AAC) the presence during antibiotic treatment and the period of recall of at least 2 of the following: difficult or painful evacuation, hard or voluminous stools, and need to use a laxative or enema [18].
The choice of different antibiotic treatment and probiotic products was not established a priori but was determined by clinicians based on the clinical presentation, experience, and attitude.
Informed consent was obtained by all parents of the enrolled children and the study was approved by our local institutional review board. This was an independent and spontaneous study in all stages of its design and conduction; data collection, management, analysis, and interpretation; and preparation, review, and approval of the paper. No pharmacological or probiotic manufacturer was ever involved in any way or at any stage.
Statistical analysis
All data were collected on an Microsoft Excel 2007 (Microsoft, Redmond, WA, USA) spreadsheet and the statistical analysis was performed by an external statistician blinded to the study design and outcomes using Medcalc v.18.6 (MedCalc Software bvba, Ostend, Belgium) and R v.3.5.0 software. The kinds of probiotics and antibiotics and their indications were divided into categories and analyzed. For probiotics, we considered ‘assumed’ if the product was taken at least 24 hours prior to the gastrointestinal manifestations [19]. We compared each strain of probiotics in terms of assumption or no assumption. To determine significant differences between two dichotomous variables, such as a risk evaluation of site of infection, antibiotic type, probiotic type, sex, and previous gastrointestinal medical history, we determined the relative risk (RR) with 95% confidence intervals (CI) and, when indicated, the number needed to treat (NNT) [20]. We analyzed mean±standard deviations and medians of age, day of onset, and duration of gastrointestinal symptoms and used a t-test to define significant variations in duration [21]. Significance was considered at values of p<0.05.
Sample size
Based on the observational design of our study, a proper power size calculation was not performed. However, based on previous pediatric studies of AAD and probiotics, we arbitrarily decided to reach a similar sample size of inpatient children [7,11].
RESULTS
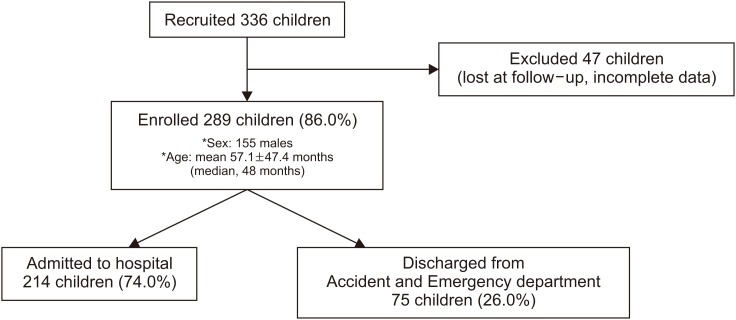
We enrolled 336 children who received antibiotic treatment; of them, 289 (86.0%) completed the follow-up. Our population included 155 male and 134 female patients aged 1 month to 17 years (mean±standard deviation: 57.1±47.4 months; median, 48 months). Of the 289 children, 214 (74.0%) were admitted to the hospital and 75 (26.0%) were discharged from the Accident and Emergency Department (A&E). A flow diagram of the study is shown in Fig. 1. The median hospital stay was 4 days (mean, 5.7±4 days).
Fig. 1. Flow chart of participant enrollment process.
Antibiotic-associated diarrhea
At the first recall, 62/289 (21.4%) children reported diarrhea; 3 were excluded because of positive rotavirus or norovirus results on stool tests during the follow-up period. Hence, AAD was found in 59/289 (20.4%) children, of whom 47 (79.7%) were admitted to the hospital and 12 (20.3%) were discharged from the A&E. The median AAD duration was 5 days (mean, 4.3±3.8 days): In 22/59 (37.3%) cases, it lasted less than 3 days; in 32/59 (54.2%) cases, it lasted 3–7 days; and in 5/59 (8.5%) cases, it lasted more than 1 week, with the longest duration being 20 days. The onset of AAD occurred after a median of 5 days (mean, 6.2±4.2) since starting antibiotic treatment; in 48/59 (81.4%) cases, the antibiotic treatment was still ongoing when the diarrhea occurred (early AAD) (Table 1). We reported a mean 5.2±1.6 bowel movements per day in children with AAD.
Table 1. Characteristics of gastrointestinal symptoms associated with antibiotic treatment in our population.
| Gastrointestinal manifestation | n° | Duration (n° d) | Onset (d) | ||
|---|---|---|---|---|---|
| Median | Mean±SD | Median | Mean±SD | ||
| AAD | 59/289 (20.4) | 5 | 4.3±3.8 | 5 | 6.2±4.2 |
| AAAP | 27/289 (9.3) | 4 | 5.4±3.9 | 5 | 6.4±5.4 |
| AAC | 23/289 (8.0) | 3 | 4.6±3.1 | 3 | 5.0±4.3 |
Values are presented as number (%).
SD: standard deviation, AAD: antibiotic-associated diarrhea, AAAP: antibiotic-associated abdominal pain, AAC: antibiotic-associated constipation.
Other gastrointestinal symptoms
AAAP was reported in 27/289 (9.3%) children; 21 episodes (77.8%) occurred in children admitted to the hospital and 6 (22.2%) in children discharged from the A&E. The median AAAP duration was 4 days (mean, 5.4±3.9 days); the onset of AAAP occurred after a median 5 days (mean, 6.4±5.4 days) since starting antibiotic treatment. AAC was reported in 23/289 (8.0%) children; the median duration of AAC was 3 days (mean, 4.6±3.1 days) and the onset occurred after a median of 3 days (mean, 5.0±4.3 days) from antibiotic treatment.
None of the patients required hospitalization for the antibiotic-associated gastrointestinal symptoms.
Different antibiotics
The incidence and characteristics of the gastrointestinal manifestations by the different antibiotics are shown in Table 2.
Table 2. Gastrointestinal manifestations associated with antibiotic treatment in our population stratified by antibiotic type.
| Antibiotic class | n° | AAD | AAAP | AAC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n° | Duration (n° d) | Onset (d) | n° | Duration (n° d) | Onset (d) | n° | Duration (n° d) | Onset (d) | |||||
| Protected penicillins | 194 | 38/194 (19.6) | 4.1 | 6.1 | 23/194 (11.9) | 5.5 | 6.5 | 18/194 (9.3) | 4.7 | 4.9 | |||
| Oral only | 117 | 15/117 (12.8) | 3.4 | 5.3 | 13/117 (11.1) | 4.8 | 5.2 | 13/117 (7.3) | 4.5 | 3.8 | |||
| IV | 77 | 23/77 (29.9) | 4.6 | 6.7 | 10/77 (13.0) | 6.5 | 8.2 | 5/77 (6.5) | 5.4 | 8 | |||
| Amoxicillin | 7 | 0/7 (0) | 0 | 0 | 1/7 (14.3) | 7 | 2 | 0/7 (0.0) | 0 | 0 | |||
| Cephalosporins | 70 | 15/70 (21.4) | 4.7 | 6.9 | 2/70 (2.9) | 5 | 8 | 4/70 (5.7) | 4 | 5.8 | |||
| Oral only | 15 | 2/15 (13.3) | 3 | 14 | 0/15 (0.0) | 0 | 0 | 1/15 (6.7) | 5 | 3 | |||
| IV | 55 | 13/55 (23.7) | 5 | 5.8 | 2/55 (3.6) | 5 | 8 | 3/55 (5.5) | 3.7 | 6.7 | |||
| Macrolides | 64 | 16/64 (25.0) | 3.2 | 5.9 | 4/64 (6.2) | 8.3 | 8 | 1/64 (1.6) | 5 | 3 | |||
| Others | 10 | 0/10 (0.0) | 0 | 0 | 2/10 (20.0) | 3 | 4 | 1/10 (10.0) | 3 | 3 | |||
| Oral only | 5 | 0/5 (0.0) | 0 | 0 | 0/5 (0.0) | 0 | 0 | 0/5 (0.0) | 0 | 0 | |||
| IV | 5 | 0/5 (0) | 0 | 0 | 2/5 (40.0) | 3 | 4 | 1/5 (20.0) | 3 | 3 | |||
| Total | 289 | 59/289 (20.4) | 4.3 | 6.2 | 27/289 (9.3) | 5.4 | 6.4 | 23/289 (8.0) | 4.6 | 5 | |||
| Overall oral only | 155 | 23/155 (14.8) | 3.7 | 5.8 | 13/155 (8.4) | 5.1 | 5.2 | 14/155 (9.0) | 4.6 | 3.7 | |||
| Overall IV | 134 | 36/134 (26.9) | 4.8 | 6.4 | 14/134 (10.4) | 5.8 | 7.6 | 9/134 (6.7) | 4.6 | 7 | |||
Values are presented as number only or number (%).
AAD: antibiotic-associated diarrhea, AAAP: antibiotic-associated abdominal pain, AAC: antibiotic-associated constipation, IV: intravenous administration.
The most frequently prescribed antibiotics were protected penicillins (in 194/289 children [67.1%]), cephalosporins (in 70/289 children [24.0%]), and macrolides (in 64/289 children [22.1%]). Fifty-four patients (18.7%) assumed 2 different antibiotic classes, and 2 children took 3 different antibiotics. The mean duration of antibiotic treatment was 10.7±6.5 days (median, 10 days). In 46/289 children (15.9%), a previous antibiotic treatment was reported within 2 weeks prior to recruitment: among these children, 23 assumed penicillins, 5 amoxicillin, 11 macrolides, and 7 cephalosporins (of which 1 was associated with penicillins, 1 with macrolides, and 1 with sulfamethoxazole-trimethoprim). In this group of patients, the antibiotic was stopped by the time of referral in 20/46 (43.5%) of cases at a mean 6.4±3.3 days before recruitment.
Of the 194 children assuming protected penicillins, 38 reported AAD (19.6%), 23 AAAP (11.9%) and 18 AAC (9.3%). Of the 70 assuming cephalosporins, 15 reported AAD (21.4%), 2 AAAP (2.9%), and 4 AAC (5.7%). Of the 64 assuming macrolides, 16 reported AAD (25.0%), 4 AAAP (6.2%), and 1 AAC (1.6%). None of these three classes of antibiotics reached a statistically significant increase in gastrointestinal symptoms or duration compared to the other classes of antibiotics.
Overall, 134/289 (46.4%) children were given intravenous antibiotics (3 were administered 2 different classes). In this group, AAD was significantly more frequent compared to oral antibiotic treatment (36/134 vs. 23/155, 26.9% vs. 14.8%; RR, 1.81; 95% CI, 1.13–2.89). In particular, 23/77 (29.9%) children administered protected penicillins intravenously reported AAD compared to 15/117 (12.8%) of those administered the equivalent oral drug (RR, 2.33; 95% CI, 1.30–4.18). None of the other classes of antibiotics reached a statistically significant difference in gastrointestinal symptom incidence or duration.
Additional risk factors
The indications for antibiotic treatment were acute infections (231 children [79.9%]) or surgical intervention (58 cases [20.1%]). Risk factors for gastrointestinal symptoms according to patient characteristics are shown in Table 3. We recognized the following as additional risk factors for AAD:
Table 3. Additional risk factors for antibiotic-associated gastrointestinal symptoms in our population.
| Category | AAD | AAAP | AAC | ||||
|---|---|---|---|---|---|---|---|
| n° | RR (CI) | n° | RR (CI) | n° | RR (CI) | ||
| Sex | |||||||
| Male | 34/155 (21.9) | 1.17 (0.74–1.87) | 15/155 (9.7) | 1.08 (0.52–2.23) | 12/155 (7.7) | 0.94 (0.43–2.07) | |
| Female | 25/134 (18.7) | 0.85 (0.54–1.35) | 12/134 (9.0) | 0.93 (0.45–1.90) | 11/134 (8.2) | 1.06 (0.48–2.32) | |
| Age (yr) | |||||||
| 0–3 | 44/118 (37.2) | 4.25 (2.49–7.27) | 3/118 (2.5) | 0.18 (0.06–0.58) | 7/118 (5.9) | 0.63 (0.27–1.49) | |
| <1 | 22/47 (46.1) | 3.06 (2.00–4.68) | 1/47 (2.1) | 0.20 (0.03–1.42) | 2/47 (4.2) | 0.49 (0.12–2.02) | |
| >3 | 15/171 (8.8) | 0.24 (0.14–0.40) | 24/171 (14.0) | 5.52 (1.70–17.91) | 16/171 (9.3) | 1.57 (0.67–3.71) | |
| >6 | 5/84 (6.0) | 0.23 (0.09–0.54) | 17/84 (20.2) | 4.15 (1.98–8.68) | 9/84 (10.7) | 1.57 (0.71–3.48) | |
| Site of infection | |||||||
| Ear | 4/25 (16.0) | 0.77 (0.30–1.94) | 3/25 (12.0) | 1.32 (0.43–4.08) | 2/25 (8.0) | 1.01 (0.25–4.04) | |
| Upper resp. airways | 18/88 (20.5) | 1.00 (0.61–1.64) | 9/88 (10.2) | 1.14 (0.53–2.44) | 5/88 (5.7) | 0.63 (0.24–1.65) | |
| Lower resp. airways | 24/71 (33.8) | 2.11 (1.35–3.29) | 6/71 (8.5) | 0.88 (0.37–2.09) | 2/71 (2.8) | 0.29 (0.07–1.22) | |
| Urinary tract | 7/21 (33.3) | 3.67 (1.85–7.28) | 1/21 (4.8) | 0.49 (0.07–3.44) | 1/21 (4.8) | 0.58 (0.08–4.10) | |
| Surgery | 4/58 (6.9) | 0.29 (0.11–0.77) | 7/58 (12.1) | 1.39 (0.62–3.14) | 9/58 (15.5) | 2.56 (1.17–5.62) | |
| Lymph nodes | 1/10 (10.0) | 0.48 (0.07–3.13) | 1/10 (10.0) | 1.07 (0.16–7.14) | 1/10 (10.0) | 1.26 (0.19–8.50) | |
| Soft tissues | 2/14 (14.3) | 0.69 (0.19–2.54) | 1/14 (7.1) | 0.76 (0.11–5.17) | 1/14 (7.1) | 0.89 (0.13–6.16) | |
| Other | 3/17 (17.6) | 0.86 (0.30–2.46) | 0/17 (0.0) | 0.28 (0.02–4.34) | 3/17 (17.6) | 2.40 (0.79–7.28) | |
| Previous gastrointestinal symptoms | |||||||
| AAD | 13/38 (34.2) | 1.87 (1.12–3.12) | 5/38 (13.2) | 1.50 (0.60–3.73) | 3/38 (7.9) | 0.99 (0.31–3.17) | |
| Recurrent abdominal pain | 3/33 (9.1) | 0.42 (0.14–1.25) | 13/33 (39.4) | 7.20 (3.71–13.97) | 4/33 (4.1) | 1.63 (0.59–4.51) | |
| Constipation | 2/19 (10.5) | 0.50 (0.13–1.89) | 6/19 (31.6) | 4.06 (1.86–8.85) | 8/19 (42.1) | 7.58 (3.69–15.59) | |
| No AAD or FGIDs | 42/214 (19.6) | 0.87 (0.53–1.43) | 10/214 (4.7) | 0.21 (0.10–0.43) | 11/214 (5.1) | 0.39 (0.17–0.87) | |
| Positive family history | |||||||
| AAD | 3/23 (13.0) | 0.62 (0.21–1.83) | 1/23 (4.3) | 0.45 (0.06–3.13) | 0/23 (0.0) | 0.42 (0.03–6.66) | |
| FGIDs | 7/22 (31.8) | 1.63 (0.85–3.16) | 4/22 (18.2) | 2.11 (0.80–5.56) | 3/22 (13.6) | 1.82 (0.59–5.65) | |
| AAD or FGIDs | 9/43 (20.9) | 1.03 (0.55–1.94) | 5/43 (11.6) | 1.30 (0.52–3.25) | 3/43 (7.0) | 0.86 (0.27–2.76) | |
| None | 50/246 (20.3) | 0.97 (0.52–1.83) | 22/246 (8.9) | 0.77 (0.31–1.92) | 20/246 (8.1) | 1.16 (0.36–3.75) | |
Values are presented as number (%).
AAD: antibiotic-associated diarrhea, AAAP: antibiotic-associated abdominal pain, AAC: antibiotic-associated constipation, RR: relative risk, CI: confidence interval, resp: respiratory, FGIDs: functional gastrointestinal disorders.
- Age: AAD was significantly more common among children younger than 3 years (44/118 vs. 15/171 [37.2% vs. 8.8%]; RR, 4.25; 95% CI, 2.49–7.27) and in infants less than 12 months of age (22/47 vs. 37/242 [46.1% vs. 15.3%] in older children; RR, 3.06; 95% CI, 2.00–4.68);
- Infection site: We noticed an increased incidence of AAD in cases of lower respiratory infections (24/71 vs. 35/218 [33.8% vs. 16%]; RR, 2.11; 95% CI, 1.35–3.29) or urinary infections (7/21 vs. 52/268 [33.3% vs. 19.4%]; RR, 3.67; 95% CI, 1.85–7.29); and
- Previous AAD: Children reporting previous episodes of AAD were at increased risk of AAD concomitant with a new course of antibiotics (13/38 vs. 46/251 [34.2% vs. 18.3%]; RR, 1.87; 95% CI, 1.12–3.12).
AAAP was significantly associated with:
- Age: AAAP incidence was significantly increased in children older than 6 years (17/84 vs. 10/205 [20.2% vs. 4.9%]; RR, 4.15; 95% CI, 1.98–8.68);
- Previous recurrent abdominal pain: These children were at higher risk of developing AAAP concurrently with antibiotic use (13/33 vs. 14/256 [39.4% vs. 5.5%]; RR, 7.20; 95% CI, 3.71–13.97); and
- Previous constipation: These children were at higher risk of developing abdominal pain concurrently with antibiotic use (6/19 vs. 21/270 [31.6% vs. 7.8%]; RR, 4.06; 95% CI, 1.86–8.85).
The following were additional risk factors for AAC:
- Surgery: Children admitted for surgical intervention and treated with antibiotics reported an increased incidence of AAC than those treated for infections (9/58 vs. 14/231 [15.5% vs. 6.6%]; RR, 2.56; 95% CI, 1.17–5.62); and
- Previous constipation: These children were more likely to report constipation concurrently with antibiotic use (8/19 vs. 15/270 [42.1% vs. 5.6%]; RR, 7.58; 95% CI, 3.69–15.59).
Neither sex nor family history of AAD or functional gastrointestinal disorders (FGIDs) was associated with a significant increase in gastrointestinal symptoms related to antibiotic therapy.
Efficacy of probiotics
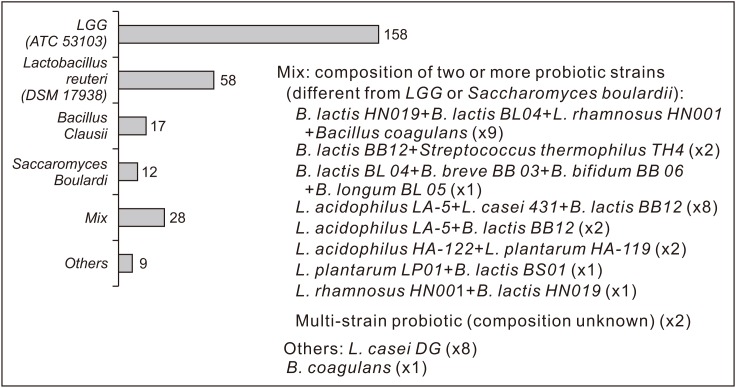
In our population, 234/289 (81.0%) children assumed a probiotic product to prevent or treat a gastrointestinal symptom, with a mean supplementation of 13.1±6.9 days. Probiotic intake started after a median of 2 days (mean, 3.9±3.4) since beginning the antibiotic treatment. The total numbers of children assuming the different probiotic strains (pre, during, or post-occurrence of gastrointestinal symptoms) are shown in Fig. 2. Among them, 42/234 (17.9%) assumed 2 different probiotic products with different strains. In general, 212/234 (90.6%) children reported a daily intake of the probiotic product according to the dose and method of administration shown on the package leaflet. No increased incidence of gastrointestinal symptoms was found in the 22 children who reported decreased compliance with the probiotic instructions. Among children who received probiotics, 8/234 (3.4%) were on probiotic supplementation at recruitment because of a previous antibiotic therapy; in the other 226/234 (96.6%), the probiotic product was assumed after enrollment, at the beginning of treatment, or during or even after the antibiotic treatment for concomitant gastrointestinal symptoms. Physicians recommended probiotics in 177/289 (61.2%) cases; the most indicated strains were LGG ATCC 53103 (in 110/177 children) and L. reuteri DSM 17938 (in 37/177). On the other hand, 57/289 children (19.7%) assumed probiotics without a medical prescription, and the most independently chosen probiotics were LGG (in 23 children), Bacillus clausii (in 11), Saccharomyces boulardii (in 4), and other strains (in 19).
Fig. 2. Total probiotic intake in our population by different strains. LGG, Lactobacillus rhamnosus GG; L., Lactobacillus; B., Bifidobacterium.
Gastrointestinal manifestations according to the reported assumed probiotic strain are shown in Table 4. Prophylaxis with a probiotic significantly reduced AAD and AAAP but not AAC. Compared to the group of patients who did not assume probiotics, AAD decreased from 33/79 (41.8%) to 26/210 (12.4%) (RR, 0.30; 95% CI, 0.19-0.46; and NNT, 3.4; 95% CI, 2.55–5.12), with a delayed onset (from mean 4.6 to 8.1 days) (p=0.03), without any significant reduction in duration (from mean 4.9 to 3.6 days). In our population, the most effective strains for the prevention of AAD were: LGG ATCC 53103, with occurrence of AAD in 18/117 (15.4%) children (RR, 0.37; 95% CI, 0.22–0.61; and NNT, 3.8; 95% CI, 2.61–6.93); L. reuteri DSM 17938, with AAD in 6/41 (14.6%) (RR, 0.35; 95% CI, 0.16-0.77; and NNT, 3.7; 95% CI, 2.27–9.85) and different multi-strain formulations, with AAD in 1/25 (4%) children (RR, 0.10; 95% CI, 0.01–0.67; and NNT, 2.6; 95% CI, 1.74–5.57).
Table 4. Gastrointestinal manifestations associated with antibiotic treatment in our population stratified by probiotic used.
| Probiotic strains | AAD | AAAP | AAC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AAD n° | AAD (N/O) n° | RR (CI) (vs. N/O) | RR (CI) (vs. N) | NNT (CI) (vs. N) | AAAP n° | AAAP (N/O) n° | RR (CI) (vs. N/O) | RR (CI) (vs. N) | NNT (CI) (vs. N) | AAC n° | AAC (N/O) n° | RR (CI) (vs. N/O) | RR (CI) (vs. N) | NNT (CI) (vs. N) | |
| LGG (ATCC 53103) | 18/117 (15.4) | 41/172 (23.8) | 0.65 (0.39–1.07) | 0.37 (0.22–0.61) | 3.8 (2.61–6.93) | 9/129 (7.0) | 18/160 (11.3) | 0.62 (0.29–1.33) | 0.37 (0.17–0.84) | 8.5 (4.78–38.60) | 10/132 (7.6) | 13/157 (8.3) | 0.91 (0.41–2.02) | 0.63 (0.25–1.57) | 22.3 (−23.27–7.53) |
| Lactobacillus reuteri (DSM 17938) | 6/41 (14.6) | 53/248 (21.4) | 0.68 (0.31–1.49) | 0.35 (0.16–0.77) | 3.7 (2.27–9.85) | 2/45 (4.4) | 25/244 (10.2) | 0.43 (0.11–1.77) | 0.24 (0.06–1.01) | 7.0 (3.73–54.53) | 1/46 (2.2) | 22/243 (9.1) | 0.24 (0.03–1.74) | 0.18 (0.02–1.41) | 10.1 (−417.2–4.99) |
| Bacillus clausii | 1/14 (7.1) | 58/275 (21.1) | 0.34 (0.05–2.27) | 0.17 (0.03–1.15) | 2.9 (1.64–12.23) | 1/14 (7.1) | 26/275 (9.5) | 0.76 (0.11–5.17) | 0.38 (0.06–2.69) | 8.6 (−10.21–3.03) | 2/15 (13.3) | 21/274 (7.7) | 1.74 (0.45–6.74) | 1.10 (0.26–4.78) | 79.1 (−5.02–5.75) |
| Saccharomyces boulardii | 0/11 (0.0) | 59/278 (21.2) | 0.20 (0.01–2.97) | 0.09 (0.01–1.39) | 2.7 (1.52–10.58) | 0/10 (0.0) | 27/279 (9.7) | 0.46 (0.03–7.11) | 0.24 (0.02–3.71) | 6.8 (−10.93–2.60) | 1/11 (9.1) | 22/278 (7.9) | 1.15 (0.17–7.77) | 0.75 (0.10–5.53) | 33.6 (−5.67–4.24) |
| Mix | 1/25 (4.0) | 58/264 (22.0) | 0.18 (0.03–1.26) | 0.10 (0.01–0.67) | 2.6 (1.74–5.57) | 2/24 (8.3) | 25/265 (9.4) | 0.88 (0.22–3.51) | 0.44 (0.11–1.84) | 9.6 (−15.14–3.65) | 2/23 (8.7) | 21/264 (8.0) | 1.01 (0.25–4.04) | 0.66 (0.15–2.97) | 24.6 (−9.54–5.37) |
| Other | 0/9 (0.0) | 59/280 (21.1) | 0.29 (0.02–4.03) | 0.12 (0.01–1.80) | 2.7 (1.47–16.88) | 1/9 (11.1) | 26/280 (9.3) | 1.20 (0.18–7.87) | 0.54 (0.08–3.67) | 11.4 (−6.05–2.94) | 1/9 (11.1) | 22/280 (7.9) | 1.41 (0.21–9.37) | 0.92 (0.13–6.63) | 104.4 (−4.59–4.22) |
| Any probiotic | 26/210 (12.4) | 33/79 (41.8) | 0.30 (0.19–0.46) | 0.30 (0.19–0.46) | 3.4 (2.55–5.12) | 15/225 (6.7) | 12/64 (18.8) | 0.36 (0.18–0.72) | 0.36 (0.18–0.72) | 8.3 (5.00–24.25) | 16/231 (6.9) | 7/58 (12.1) | 0.57 (0.25–1.33) | 0.57 (0.25–1.33) | 19.4 (−38.08–7.75) |
Values are presented as number (%).
AAD: antibiotic-associated diarrhea, AAAP: antibiotic-associated abdominal pain, AAC: antibiotic-associated constipation, N/O: group without any probiotic plus probiotic products with different strains, RR: relative risk, CI: confidence interval, N: group without any probiotic, NNT: number needed to treat, LGG: Lactobacillus rhamnosus GG.
Compared to the group who did not assume probiotics as prophylaxis, AAAP decreased from 12/64 (18.8%) to 15/255 (6.7%) (RR, 0.36; 95% CI, 0.18–0.72; and NNT, 8.3; 95% CI, 5.00–24.25) with no significant reduction in duration (from 4.7 to 5.5 days) but with a significant delay in symptom onset (from 2.7 to 8.3 days) (p=0.04). The most efficient probiotics for the prevention of AAAP were LGG ATCC 53103, with AAAP in 9/129 children (7%) (RR, 0.37; 95% CI, 0.17–0.84; and NNT, 8.5; 95% CI, 4.78–38.60) and L. reuteri DSM 17938, with 2/45 (4.4%) AAAP (RR, 0.24; 95% CI, 0.06–1.01; and NNT, 7; 95% CI, 3.73–54.53).
Probiotic use did not significantly reduce AAC incidence (16/231 vs. 7/58 [6.9% vs. 12.1%]; RR, 0.57; 95% CI, 0.25–1.33) or duration (from 2.7 to 5.4 days) (p=0.06).
DISCUSSION
Several studies reported AAD and related factors in adults, but data among inpatient children are limited [7]. In our study, AAD occurred in 20.4% of children, a similar incidence to that reported by a recent pediatric meta-analysis (including 22 randomized control trials and 4,155 children) [11]. The incidence of AAD in children remains uncertain because of small sample sizes and absent or heterogenous definitions and follow-up [11,22]. In a previous large pediatric study, Turck et al. [12] reported a lower incidence of AAD (11%) in 650 outpatient children followed for only 1 week. However, compared to that population, 75% of our patients were hospitalized, with likely more severe infections requiring more aggressive treatment. We also considered AAD over a longer time frame (up to 14 days after stopping antibiotic treatment) and used the same definitions of diarrhea and AAD suggested by the World Health Organization [6], ESPGHAN [5] and most related systematic reviews [22] to reduce other possible confounding factors in these patients, who are more prone to infections. Compared to the results reported by Turck et al. [12], our results were similar in terms of AAD duration (4.3 days) and onset (80% early-onset AAD). We also confirmed the inverse association with age: children 0–3 years old were significantly more affected (37%) than children 3–6 years old (11%) or >6 years old (6%). This could be partially explained by GI tract immaturity or more frequent alterations in the microbiota after antibiotics, but some authors simply allocate a more frequent and less consistent fecal pattern in this age [23]. In contrast with Turck et al. [12], we did not recognize significant differences in AAD risk with amoxicillin-clavulanate or other antibiotic types: protected penicillins or cephalosporins or macrolides had 20–25% AAD rates, while the other classes were not associated with diarrhea, likely due to an insufficient sample size.
We reported an increased incidence of AAD with intravenous antibiotics, ampicillin-sulbactam in particular, of 30%. This aspect was never fully investigated in previous works on AAD in children; rather, these studies focused more on outpatients or oral antibiotic products, whereas intravenous antibiotic therapy was often considered an exclusion criterion. Only one study reported an increased AAD risk of ampicillin-sulbactam compared to oral azithromycin (37/234 vs. 19/232 [15.5% vs. 8.1%]) [13]. Despite being previously considered a ‘safer for gut’ method of administration, many intravenous drugs may affect the gut microbiota regardless [24]. A recent trial of 521 patients including 85% children and nearly all hospitalized participants strengthens this hypothesis of an increased rate of diarrhea after ampicillin-sulbactam, ceftazidime, and piperacillin-tazobactam and 4-fold more complications (in particular neutropenia and rash) after intravenous antibiotics [14]. Moreover, a study recruiting adult patients in A&E departments found a double incidence of AAD with intravenous antibiotics (25%) compared to oral administration (12%) [25].
Our report is the first to recognize lower respiratory and urinary tract infections as risk factors for AAD in children [10], possibly related to the more severe clinical pattern and prolonged antibiotic treatments compared to other sites of infection.
As expected, no significant sex-based differences were found [7,10]. We evaluated individual factors such as previous episodes or family history of gastrointestinal symptoms and found statistical significance only for previously reported AAD.
Probiotics and AAD
We reported a 70% lower incidence of AAD in the probiotic group similar to the 52% decrease reported in the most recent ESPGHAN recommendations (based on 21 randomized controlled trials, n=3,255) [5]. The same paper also made a strong recommendation for LGG and Saccharomyces boulardii. We can partly confirm this statement: We obtained a 63% decrease with LGG ATCC 53103 (as the most commonly used probiotic class, with 57% of total assumptions and 70% of the probiotics indicated by the physician), but we have not accumulated a sufficient sample size of Saccharomyces boulardii, which is less commonly used in our country. However, we report a similar 65% decrease in AAD for L. reuteri DSM 17938, a strain with a minor level of evidence [26,27]. The best preventive result for AAD in our study (1/25, with a 90% decrease) was obtained by different products containing a mixture of at least two different probiotic strains. However, the heterogeneity of this group and the small sample size prevented a precise analysis and general conclusions about their efficacy [5]. Specific probiotic strains have previously shown a decrease in duration of diarrhea of approximately 24 hours [26]; we obtained a comparable decrease (from 4.9 to 3.6 days), but the difference was not significant. We also found a few days' delay in the onset of AAD in the group that used probiotics, especially L. reuteri DSM 17938.
Incidence and risk factors for AAAP and AAC
We found an incidence of 9.3% for abdominal pain and 8% for constipation reported during or soon after antibiotic treatment. However, data assessing the correlation between antibiotic treatment and abdominal pain or constipation in children are very limited in the literature. A Swedish cohort study of 2700 adolescents found that broad-spectrum antibiotics, or at least 2 antibiotic cycles in the first 2 years of life, were associated with a significant increase in abdominal pain in female teenagers, reaching an incidence similar to our AAAP rate [28]. Another study suggested a consistent increase in FGIDs (including irritable bowel syndrome and functional abdominal pain) after both viral and bacterial gastrointestinal infections [29]. Previous studies showed a significant increase in FGIDs 6 months after bacterial infections (36% vs. 11% in controls) [30] but not after rotavirus infections [31]. Like infections, antibiotic treatments perturb the microbiota, leading to dysregulation of neuroimmune functions and triggering of inflammation or alterations in gastrointestinal motility and behavior [32]. An individual predisposition to an antibiotic-related effect could explain our finding of a 7-fold higher risk of post-antibiotic abdominal pain in our group of children who reported the same symptoms in the previous year. We found no significant sex-based differences in the incidence of AAAP or AAC despite a longer AAAP duration among girls (7.1 vs. 4.1 days). Different antibiotic class, administration route, and infection site did not significantly affect AAAP and AAC. Our children admitted for surgical intervention reported an increased rate of constipation as previously reported in literature [33]; this could be explained by different factors including bedding, anesthetic agents, and stress as previously suggested by other authors [18,34]. We also found that children reporting constipation in the past year were more prone to experience recurrence under antibiotic treatment and have significantly more AAAP.
Probiotics, AAAP, and AAC
Probiotics as prophylaxis overall reduced the AAAP incidence of 64%, particularly L. reuteri DSM 17938 and, even more significantly, LGG ATCC 53103. Several meta-analyses and systematic reviews indicated a significant decrease in abdominal pain–related FGIDs with these probiotic strains: L. reuteri for functional abdominal pain and infantile colic and LGG for irritable bowel syndrome [26,32,34,35,36]. Conversely, probiotics were not effective for constipation as already reported [18,32,34].
The major strength of our study lies in its prospective design, relatively large group of inpatient children, standardized recall, and analysis of different possible risk and protective factors for AAD and other gastrointestinal symptoms. Moreover, this was a spontaneous real-life study with a comparative analysis of different antibiotic treatments and probiotic strains. We are aware of some limitations of our results. First, it was an open observational study with symptoms mostly based on parental reports. Furthermore, we had a heterogeneous subgroup of children and treatments with a limited sample size to gather evidences on some classes of antibiotics and probiotics.
In conclusion, AAD occurred in 1/5 of our inpatient children, with a significant increase seen in children younger than 3 years, with lower respiratory or urinary tract infections, in whom intravenous antibiotics were administered, and who reported previous AAD episodes. The incidence of abdominal pain was 9%, a value that was significantly increased in children >6 years old and in those with recurrent abdominal pain or constipation in the previous year. In 8% of children, constipation was reported with an increased risk in children having surgery or previous constipation. LGG and L. reuteri DSM 17938 proved to be an effective method of preventing AAD and abdominal pain and should be considered, particularly for at-risk children.
ACKNOWLEDGEMENTS
We greatly thank the participants' parents and the physicians and nurses of the A&E Department and Pediatric Ward.
Footnotes
Funding: This study received no external funding.
Conflict of Interest: The authors have no financial conflicts of interest.
References
- 1.Chai G, Governale L, McMahon AW, Trinidad JP, Staffa J, Murphy D. Trends of outpatient prescription drug utilization in US children, 2002-2010. Pediatrics. 2012;130:23–31. doi: 10.1542/peds.2011-2879. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg JZ, Lytvyn L, Steurich J, Parkin P, Mahant S, Johnston BC. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2015;(12):CD004827. doi: 10.1002/14651858.CD004827.pub4. [DOI] [PubMed] [Google Scholar]
- 3.Videlock EJ, Cremonini F. Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2012;35:1355–1369. doi: 10.1111/j.1365-2036.2012.05104.x. [DOI] [PubMed] [Google Scholar]
- 4.Reed DE, Vanner SJ. Emerging studies of human visceral nociceptors. Am J Physiol Gastrointest Liver Physiol. 2017;312:G201–7. doi: 10.1152/ajpgi.00391.2016. [DOI] [PubMed] [Google Scholar]
- 5.Szajewska H, Canani RB, Guarino A, Hojsak I, Indrio F, Kolacek S, et al. ESPGHAN Working Group for ProbioticsPrebiotics. Probiotics for the Prevention of Antibiotic-Associated Diarrhea in Children. J Pediatr Gastroenterol Nutr. 2016;62:495–506. doi: 10.1097/MPG.0000000000001081. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Diarrhoeal Disease Fact Sheet [Internet] Geneva: World Health Organization; 2017. [cited 2019 Aug date]. Available from: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease. [Google Scholar]
- 7.McFarland LV, Ozen M, Dinleyici EC, Goh S. Comparison of pediatric and adult antibiotic-associated diarrhea and Clostridium difficile infections. World J Gastroenterol. 2016;22:3078–3104. doi: 10.3748/wjg.v22.i11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai J, Zhao C, Du Y, Zhang Y, Zhao M, Zhao Q. Comparative efficacy and tolerability of probiotics for antibiotic-associated diarrhea: systematic review with network meta-analysis. United European Gastroenterol J. 2018;6:169–180. doi: 10.1177/2050640617736987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reyes H, Guiscafré H, Muñoz O, Pérez-Cuevas R, Martinez H, Gutiérrez G. Antibiotic noncompliance and waste in upper respiratory infections and acute diarrhea. J Clin Epidemiol. 1997;50:1297–1304. doi: 10.1016/s0895-4356(97)00197-2. [DOI] [PubMed] [Google Scholar]
- 10.McFarland LV. [Risk factor for antibiotic-associated diarrhea. A review of the literature] Ann Med Interne (Paris) 1998;149:261–266. French. [PubMed] [Google Scholar]
- 11.McFarland LV, Goh S. Preventing pediatric antibiotic-associated diarrhea and Clostridium difficile infections with probiotics: a meta-analysis. World J Meta-Anal. 2013;1:102–120. [Google Scholar]
- 12.Turck D, Bernet JP, Marx J, Kempf H, Giard P, Walbaum O, et al. Incidence and risk factors of oral antibiotic-associated diarrhea in an outpatient pediatric population. J Pediatr Gastroenterol Nutr. 2003;37:22–26. doi: 10.1097/00005176-200307000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Erdeve O, Tiras U, Dallar Y. The probiotic effect of Saccharomyces boulardii in a pediatric age group. J Trop Pediatr. 2004;50:234–236. doi: 10.1093/tropej/50.4.234. [DOI] [PubMed] [Google Scholar]
- 14.Murphy JL, Fenn N, Pyle L, Heizer H, Hughes S, Nomura Y, et al. Adverse events in pediatric patients receiving long-term oral and intravenous antibiotics. Hosp Pediatr. 2016;6:330–338. doi: 10.1542/hpeds.2015-0069. [DOI] [PubMed] [Google Scholar]
- 15.Li N, Zheng B, Cai HF, Chen YH, Qiu MQ, Liu MB. Cost-effectiveness analysis of oral probiotics for the prevention of Clostridium difficile-associated diarrhoea in children and adolescents. J Hosp Infect. 2018;99:469–474. doi: 10.1016/j.jhin.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Hyams JS, Di Lorenzo C, Saps M, Shulman RJ, Staiano A, van Tilburg M. Functional disorders: children and adolescents. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Benninga MA, Faure C, Hyman PE, St James Roberts I, Schechter NL, Nurko S. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.016. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Tabbers MM, DiLorenzo C, Berger MY, Faure C, Langendam MW, Nurko S, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition; North American Society for Pediatric Gastroenterology. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr. 2014;58:258–274. doi: 10.1097/MPG.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 19.Patro-Golab B, Shamir R, Szajewska H. Yogurt for treating antibiotic-associated diarrhea: systematic review and meta-analysis. Nutrition. 2015;31:796–800. doi: 10.1016/j.nut.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Akobeng AK. Communicating the benefits and harms of treatments. Arch Dis Child. 2008;93:710–713. doi: 10.1136/adc.2008.137083. [DOI] [PubMed] [Google Scholar]
- 21.Guo B, Yuan Y. A comparative review of methods for comparing means using partially paired data. Stat Methods Med Res. 2017;26:1323–1340. doi: 10.1177/0962280215577111. [DOI] [PubMed] [Google Scholar]
- 22.Johnston BC, Shamseer L, da Costa BR, Tsuyuki RT, Vohra S. Measurement issues in trials of pediatric acute diarrheal diseases: a systematic review. Pediatrics. 2010;126:e222–31. doi: 10.1542/peds.2009-3667. [DOI] [PubMed] [Google Scholar]
- 23.Castelluzzo MA, Tarsitano F, Pensabene F. Approccio al bambino con disturbi funzionali gastrointestinali. Prosp in Ped. 2016;46:276–289. [Google Scholar]
- 24.Robinson CJ, Young VB. Antibiotic administration alters the community structure of the gastrointestinal micobiota. Gut Microbes. 2010;1:279–284. doi: 10.4161/gmic.1.4.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haran JP, Hayward G, Skinner S, Merritt C, Hoaglin DC, Hibberd PL, et al. Factors influencing the development of antibiotic associated diarrhea in ED patients discharged home: risk of administering IV antibiotics. Am J Emerg Med. 2014;32:1195–1199. doi: 10.1016/j.ajem.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szajewska H. What are the indications for using probiotics in children? Arch Dis Child. 2016;101:398–403. doi: 10.1136/archdischild-2015-308656. [DOI] [PubMed] [Google Scholar]
- 27.Urbańska M, Gieruszczak-Białek D, Szajewska H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for diarrhoeal diseases in children. Aliment Pharmacol Ther. 2016;43:1025–1034. doi: 10.1111/apt.13590. [DOI] [PubMed] [Google Scholar]
- 28.Uusijärvi A, Bergström A, Simrén M, Ludvigsson JF, Kull I, Wickman M, et al. Use of antibiotics in infancy and childhood and risk of recurrent abdominal pain--a Swedish birth cohort study. Neurogastroenterol Motil. 2014;26:841–850. doi: 10.1111/nmo.12340. [DOI] [PubMed] [Google Scholar]
- 29.Pensabene L, Talarico V, Concolino D, Ciliberto D, Campanozzi A, Gentile T, et al. Post-Infectious Functional Gastrointestinal Disorders Study Group of Italian Society for Pediatric Gastroenterology, Hepatology and Nutrition. Postinfectious functional gastrointestinal disorders in children: a multicenter prospective study. J Pediatr. 2015;166:903–907.e1. doi: 10.1016/j.jpeds.2014.12.050. [DOI] [PubMed] [Google Scholar]
- 30.Saps M, Pensabene L, Di Martino L, Staiano A, Wechsler J, Zheng X, et al. Post-infectious functional gastrointestinal disorders in children. J Pediatr. 2008;152:812–816.e1. doi: 10.1016/j.jpeds.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 31.Saps M, Pensabene L, Turco R, Staiano A, Cupuro D, Di Lorenzo C. Rotavirus gastroenteritis: precursor of functional gastrointestinal disorders? J Pediatr Gastroenterol Nutr. 2009;49:580–583. doi: 10.1097/MPG.0b013e31819bcbd2. [DOI] [PubMed] [Google Scholar]
- 32.Salvatore S, Pensabene L, Borrelli O, Saps M, Thapar N, Concolino D, et al. Mind the gut: probiotics in paediatric neurogastroenterology. Benef Microbes. 2018;9:883–898. doi: 10.3920/BM2018.0013. [DOI] [PubMed] [Google Scholar]
- 33.Smith JT, Smith MS. Does a preoperative bowel preparation reduce bowel morbidity and length of stay after scoliosis surgery? A randomized prospective study. J Pediatr Orthop. 2013;33:e69–71. doi: 10.1097/BPO.0b013e318296e032. [DOI] [PubMed] [Google Scholar]
- 34.Korterink JJ, Ockeloen L, Benninga MA, Tabbers MM, Hilbink M, Deckers-Kocken JM. Probiotics for childhood functional gastrointestinal disorders: a systematic review and meta-analysis. Acta Paediatr. 2014;103:365–372. doi: 10.1111/apa.12513. [DOI] [PubMed] [Google Scholar]
- 35.Hojsak I. Probiotics in functional gastrointestinal disorders. Adv Exp Med Biol. 2019;1125:121–137. doi: 10.1007/5584_2018_321. [DOI] [PubMed] [Google Scholar]
- 36.Newlove-Delgado T, Abbott RA, Martin AE. Probiotics for children with recurrent abdominal pain. JAMA Pediatr. 2019;173:183–184. doi: 10.1001/jamapediatrics.2018.4575. [DOI] [PubMed] [Google Scholar]