Abstract
A worldwide increase in the Mycobacterium abscessus (M. abscessus) complex has been observed. Therefore, the aim of the present study was to investigate the diversity of the rrl and erm(41) genes, both of which are associated with macrolide sensitivity in the M. abscessus complex. The current study also examined the efficacy of mass spectrometry as an alternative to molecular testing to classify subspecies of the M. abscessus complex. A total of 14 strains of the M. abscessus complex were obtained, and based on conventional analyses using housekeeping genes, 57% were determined to be M. abscessus subsp. abscessus, 43% were M. abscessus subsp. massiliense, and none were identified as M. abscessus subsp. bolletii. However, depending on the strain, it was not always possible to distinguish between the subspecies by mass spectrometry. Consequently, PCR products for the rrl and erm(41) genes were directly sequenced. Overall, 7.1% of the strains were identified to have a rrl mutation, and 92.9% carried a T at position 28 of erm(41). Results presented here suggest that the principal cause of treatment failure for M. abscessus complex infections is inducible macrolide resistance encoded by the erm(41) gene. From a strictly pragmatic standpoint, the phenotypic function of a putative erm(41) gene is the most important piece of information required by clinicians in order to prescribe an effective treatment. Although PCR amplification of erm(41) is not sufficient to differentiate between the M. abscessus complex subspecies, PCR can be easily and efficiently used to predict the sensitivity of members of the M. abscessus complex to clarithromycin.
Keywords: Mycobacterium abscessus complex, rrl, erm(41), matrix-assisted laser desorption ionization-time of flight mass spectrometry, macrolide resistance
Introduction
Recent epidemiology research has revealed a worldwide increase in non-tuberculous mycobacteria (NTM) infections. In Japan in particular, the Mycobacterium abscessus (M. abscessus) complex is the third most common pathogen in pulmonary diseases caused by NTM, after the Mycobacterium avium complex and Mycobacterium kansasii (1). The M. abscessus complex is categorized as rapidly growing mycobacteria, defined by visible growth within seven days, and is one of the most difficult pathogens to treat. Over the past decade, the M. abscessus complex has been subclassified into three new subspecies: M. abscessus subsp. abscessus, M. abscessus subsp. massiliense and M. abscessus subsp. bolletii (2). Macrolides are the key drugs used for the treatment of M. abscessus complex infection; however, macrolides are not always effective or in some cases they lose effectiveness during the course of treatment. Acquired macrolide resistance is associated with point mutations in the rrl gene, which encodes 23S rRNA (3). An erythromycin ribosomal methylase, encoded by erm(41) in the M. abscessus complex, confers inducible resistance to macrolides (4). The functionality of the erm(41) gene differs depending on the subspecies. Most notably, M. abscessus subsp. massiliense has been proposed to have an incomplete erm(41) gene, which is associated with macrolide sensitivity. In addition, some M. abscessus subsp. abscessus strains have substitutions in the erm(41) gene that also lead to macrolide susceptibility. Thus, it is important to distinguish the three kinds of subspecies and to analyze the sequences of the rrl and erm(41) genes. Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) has been used for microbial identification in recent years, and several researchers have attempted to apply this tool to differentiate the subspecies of the M. abscessus complex. However, different diagnostic criteria have been used at different institutions and the results of the method are inconsistent (5–10).
Few studies have investigated the ratio of subspecies of the M. abscessus complex in Japan, or examined their macrolide resistance genes (11). It is likely that regional differences in the ratios of the subspecies and the clinical features of such isolates may exist. In the present study, we aimed to examine the sequence of the erm(41) gene in M. abscessus complex subspecies. We also compared the efficacy of using molecular testing and mass spectrometry to classify subspecies of the M. abscessus complex.
Materials and methods
Samples and data collection
Fourteen strains of the M. abscessus complex were obtained from each patient between July 2016 and April 2018 at Showa University Hospital (Tokyo) or at Showa University Fujigaoka Hospital (Yokohama). For reference, one strain of Mycobacterium fortuitum (M. fortuitum) was collected during the period. All strains were of sputum origin except for one M. abscessus complex isolate from a bronchoscopy. Clinical isolates were cultured in mycobacteria growth indicator tubes (MGIT) and in 2% Ogawa solid medium. M. abscessus complex and M. fortuitum were distinguished by DNA-DNA hybridization. All clinical data were collected from medical records. Official approval for the study was obtained in advance from the Ethics Committee for Research at Showa University (approved numbers 371 and 2016127). Informed consent was waived because of the retrospective nature of the study.
Molecular testing
DNA was extracted from mycobacterial clinical isolates using InstaGene matrix (Bio-Rad Laboratories) and stored at −20°C. The amount of DNA extracted ranged from 104 to 452 ng/µl. Primers for nucleic acid amplification were designed as indicated in Table I. PCRs were performed to amplify mutation hot spot regions in the housekeeping genes hsp65, rpoB and ITS to classify the strains into the three subspecies using a Mycycler ver.10.65 thermal cycler (Bio-Rad Laboratories). The rrl and erm(41) genes were also amplified in a similar manner. All PCR assays were carried out in 25-µl volumes containing 200 ng of template DNA, 0.1 units of Taq DNA polymerase (Roche Diagnostics), 25 pmol of each primer, and 10 nmol of dNTPs. Cycling parameters were 30 sec at 95°C, 30 sec at 60°C, and 60 sec at 72°C for 30 cycles. PCR products were separated on a 5% polyacrylamide gel or 1% agarose gel. The gels were stained with ethidium bromide and photographed under UV illumination. The PCR products were purified and were directly sequenced using a BigDye terminator kit and ABI Prism 3130 xl (Applied Biosystems). When sequences could not be obtained by direct sequencing, the PCR products were ligated into a pGEM T easy vector (Promega), which was then used to transform JM109 cells, as reported previously (12). Multiple clones were selected and plasmid DNA was purified from each and sequenced. The reference sequences for each gene were obtained from GenBank (accession numbers CU458896.1: M. abscessus subsp. abscessus, AP_014547.1: M. abscessus subsp. massiliense.).
Table I.
Primer design.
| Target | Primers | Sequence | bp |
|---|---|---|---|
| hsp65 | hsp65F | 5′-ACCAACGATGGTGTGTCCAT-3′ | 441 |
| hsp65R | 5′-CTTGTCGAACCGCATACCCT-3′ | ||
| rpoB | rpoBF | 5′-GAGGGTCAGACCACGATGAC-3′ | 408 |
| rpoBR | 5′-AGCCGATCAGACCGATGTT-3′ | ||
| ITS | ITSF | 5′-TTGTACACACCGCCCGTC-3′ | 490 |
| ITS336R | 5′-CTTCTAGTGCCAAGGCATTCACC-3′ | ||
| rrl | rrl2145F | 5′-GCGAAATTCCTTGTCGGGTAAGT-3′ | 283 |
| rrl2427R | 5′-GGATATACGGTCCGAGGTTAG-3′ | ||
| erm(41) | erm-86F | 5′-GACCGGGGCCTTCTTCGTGAT-3′ | 673 |
| erm64R | 5′-GACTTCCCCGCACCGATTCC-3′ |
bp, base pair.
Antibiotic susceptibility test
Minimum inhibitory concentrations (MICs) of amikacin and clarithromycin were determined by the broth microdilution method and were interpreted according to the Clinical and Laboratory Standards Institute document M24-A2 (13). Briefly, an appropriate volume of the culture was transferred into 3 ml of sterilized saline until the turbidity matched that of a 0.5 McFarland standard. A 10 µl aliquot of the suspension was used to inoculate 11 ml of cation-adjusted Mueller-Hinton medium and 100 µl was distributed into each of the 96 well panels. The panels were incubated for 72 h at 30°C, and growth was determined. To test for inducible resistance to clarithromycin, the MICs for clarithromycin were also determined after 7 and 14 days of incubation.
Mass spectrometry
Colonies were transferred into microcentrifuge tubes containing 300 µl of sterile deionized water, and the tubes were incubated for 30 min at 95°C. Then samples were mixed with 900 µl of 70% ethanol by vortexing for 1 min. The suspensions were centrifuged at 13,000 rpm for 2 min, and the pellets were dried for 5 min at room temperature and resuspended in 20 µl of 100% acetonitrile with zirconia beads. The mixtures were vortexed for 1 min. The samples were then suspended with 20 µl of 70% formic acid and centrifuged at 13,000 rpm for 2 min. Subsequently, 1 µl of the supernatant from each extract was spotted on a target plate. After drying, 1 µl of matrix solution (saturated α-cyano-4-hydroxycinnamic acid in 47.5% acetonitrile and 2.5% trifluoroacetic acid) was added onto each spot. Mass spectra were obtained on a MALDI Biotyper ver 4.0 configured with Micro flex LT/SH with Mycobacteria Library ver.5.0 (Bruker Daltonik). Spectra were analyzed by Flex Analysis software 3.4 and MBT compass explore ver 4.1 (Bruker Daltonik).
Statistical analysis
The significance in each group was evaluated with Fisher's exact test or Pearson's Chi-square test, unpaired student's t-test, and the nonparametric Mann-Whitney test on ranks. P<.05 was considered significant. All analyses were performed using JMP 13.0 software (SAS Institute).
Results
Determination of M. abscessus complex subspecies by sequencing housekeeping genes
The results of sequence analyses of housekeeping genes are shown in Table II. To distinguish the three subspecies, hsp65, rpoB and ITS sequences were determined by direct sequencing and compared to reference sequences. The sequences of the hsp65 genes from eight strains were consistent with the reference sequence from M. abscessus subsp. abscessus, while those from six strains were consistent with the hsp65 reference sequence from M. abscessus subsp. massiliense, with the exception of one strain (no. 9626), which had a change at position 280T>A. High heterogeneity of rpoB in the M. abscessus complex has been reported (14). The rpoB genes from eight strains were identical to the reference gene from M. abscessus subsp. abscessus, while a 37C>T change was present in two strains (no. 71740 and no. 9614), and two changes (52C>T and 391C>T) were found in another strain (no. 8548). Six strains had rpoB sequences identical to the reference sequence from M. abscessus subsp. massiliense, with the exception of one substitution, 316T>C that was detected in four strains (nos. 74369, 77944, 9626, and 9388). No amino acid changes resulted from these nucleotide sequence differences. Together, eight strains were identified as M. abscessus subsp. abscessus, and six strains as M. abscessus subsp. massiliense. The results of sequence analyses of the ITS region were consistent with these findings. However, a novel insertion sequence (180_181GTTGT) was found in one strain of M. abscessus subsp. abscessus (no. 71740).
Table II.
Sequence differences in clinical isolates of the M. abscessus complex.
| hsp65a | rpoBb | ITSc | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain number | 115 | 118 | 127 | 190 | 280 | 340 | 10 | 31 | 37 | 52 | 88 | 124 | 127 | 136 | 202 | 277 | 316 | 343 | 376 | 379 | 391 | 25 | 60 | 98 | 180 | 276 | Insertion |
| CU458896d | T | T | C | C | T | C | T | T | C | C | T | G | C | T | C | C | T | C | C | C | C | T | A | – | C | G | |
| 9016 | T | T | C | C | T | C | T | T | C | C | T | G | C | T | C | C | T | C | C | C | C | T | A | – | C | G | |
| 8377 | T | T | C | C | T | C | T | T | C | C | T | G | C | T | C | C | T | C | C | C | C | T | A | – | C | G | |
| 9944 | T | T | C | C | T | C | T | T | C | C | T | G | C | T | C | C | T | C | C | C | C | T | A | – | C | G | |
| 71740 | T | T | C | C | T | C | T | T | T | C | T | G | C | T | C | C | T | C | C | C | C | T | A | – | T | G | c.180_181insGTTGT |
| 9614 | T | T | C | C | T | C | T | T | T | C | T | G | C | T | C | C | T | C | C | C | C | T | A | – | C | G | |
| 9854 | T | T | C | C | T | C | T | T | C | C | T | G | C | T | C | C | T | C | C | C | C | T | A | – | C | G | |
| 8548 | T | T | C | C | T | C | T | T | C | T | T | G | C | T | C | C | T | C | C | C | T | T | A | – | C | G | |
| 9419 | T | T | C | C | T | C | T | T | C | C | T | G | C | T | C | C | T | C | C | C | C | T | A | – | C | G | |
| 74369 | G | C | T | T | T | T | C | C | C | C | C | A | T | C | G | T | C | T | T | T | C | C | G | C | T | A | |
| 9835 | G | C | T | T | T | T | C | C | C | C | C | A | T | C | G | T | T | T | T | T | C | T | G | C | C | A | |
| 77944 | G | C | T | T | T | T | C | C | C | C | C | A | T | C | G | T | C | T | T | T | C | C | G | C | T | A | |
| 9626 | G | C | T | T | A | T | C | C | C | C | C | A | T | C | G | T | C | T | T | T | C | T | G | C | C | A | |
| 9388 | G | C | T | T | T | T | C | C | C | C | C | A | T | C | G | T | C | T | T | T | C | C | G | C | T | A | |
| 8006 | G | C | T | T | T | T | C | C | C | C | C | A | T | C | G | T | T | T | T | T | C | T | G | C | C | A | |
| AP014547e | G | C | T | T | T | T | C | C | C | C | C | A | T | C | G | T | T | T | T | T | C | T | G | C | C | A | |
Bold letters demonstrate base-pair changes compared to sequences in CU458896. ins, insertion.
Nucleotide positions are based on the M. abscessus subsp. massiliense sequence (accession no. AB548601).
Nucleotide positions are based on the M. abscessus subsp. massiliense sequence (accession no. AB548600).
Nucleotide positions are based on the M. abscessus subsp. massiliense sequence (accession no. AB548603).
CU458896 (ATCC19977) is a reference sequence for M. abscessus subsp. Abscessus.
AP014547 (JCM15300) is a reference sequence for M. abscessus subsp. massiliense.
Patients and characteristics
As mentioned above, results were obtained for all patients, 57% (8 of 14) of whom were infected with M. abscessus subsp. abscessus, and 43% (6 of 14) of whom were infected with M. abscessus subsp. massiliense. None were infected with M. abscessus subsp. bolletii. Table III shows the patient characteristics. There were seven males and seven females whose ages at diagnosis ranged from 30 to 83 years: Thirteen were Japanese and one was Indian. According to the guidelines published by the American Thoracic Society/Infectious Diseases Society of America (15), all patients were newly diagnosed with M. abscessus complex pulmonary disease, based on at least two positive culture results derived from pulmonary samples. As shown in Table III, there was no significant association of the subspecies with age, body-mass index, sex, smoking history, radiological findings, hemoptysis, sputum smear, or C-reactive protein.
Table III.
Characteristics of patients.
| Characteristics | M. abscessus subsp. abscessus (n=8) | M. abscessus subsp. massiliense (n=6) | P-value |
|---|---|---|---|
| Age (years) | 69.1±18.1 | 62.5±9.7 | 0.435 |
| BMI, kg/m2 | 19.6±3.4 | 18.6±1.8 | 0.552 |
| Sex | |||
| Male | 3 (21.4) | 4 (28.5) | 0.592 |
| Female | 5 (35.7) | 2 (14.2) | |
| Smoking | |||
| Never | 6 (42.8) | 2 (14.2) | 0.277 |
| Ever | 2 (14.2) | 4 (28.5) | |
| Radiological findings | |||
| Cavity | 1 (7.1) | 2 (14.2) | 0.538 |
| Symptom of hemoptysis | |||
| Yes | 2 (14.2) | 3 (21.4) | 0.58 |
| No | 6 (42.8) | 3 (21.4) | |
| Positive smear | |||
| Yes | 6 (42.8) | 3 (21.4) | 0.58 |
| No | 2 (14.2) | 3 (21.4) | |
| Laboratory findings | |||
| CRP, mg/dl | 0.96±1.22 | 1.68±2.17 | 0.492 |
Date are expressed as numbers (%), values are means ± standard deviation. BMI, body mass index; CRP, C-reactive protein.
Gene status of rrl and erm(41)
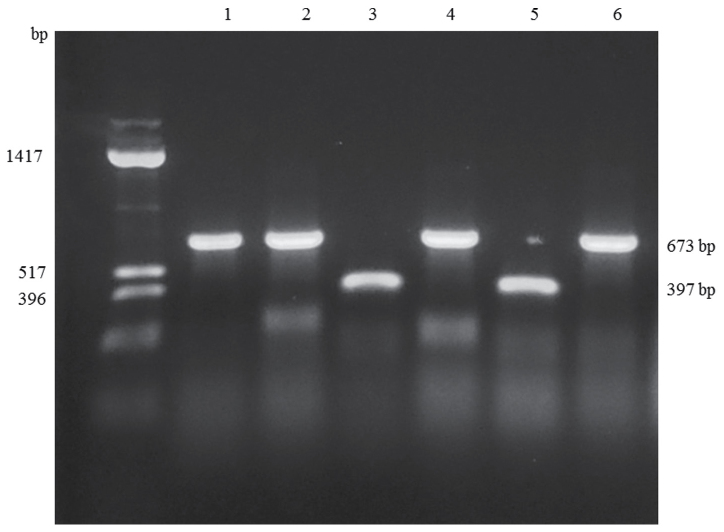
Sequence differences identified in the rrl and erm(41) genes are summarized in Table IV. In the rrl gene, a A>G change was detected at position 2059 in one strain (no. 8006), but no other alterations were found. As for the erm(41) gene, nucleotides at positions 64_65 and 159_432 were deleted in strains of M. abscessus subsp. massiliense, compared to the M. abscessus subsp. abscessus strains. Eight substitutions were found in the M. abscessus subsp. abscessus isolates, whereas no substitutions were found in the strains of M. abscessus subsp. massiliense. In those isolates of M. abscessus subsp. abscessus, 28T>C, 238A>G and 419C>T substitutions were responsible for the amino-acid changes W10R, I80V and P140L, respectively. As shown in Fig. 1, the sizes of the PCR products amplified from the erm(41) genes were consistent with sequencing results (673 base pairs for M. abscessus subsp. abscessus, and 397 base pairs for M. abscessus subsp. massiliense), and identifications based on the size of the erm(41) gene were consistent with those based on hsp65, rpoB and ITS sequences.
Table IV.
Sequence differences in the rrl and erm(41) genes from clinical isolates of the M. abscessus complex.
| rrla | erm(41)b | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain number | 2058 | 2059 | −28 | −4 | 28 | 41 | 46 | 64 | 65 | 85 | 90 | 109 | 120 | 123 | 159 | 238 | 255 | 279 | 330 | 336 | 419 | 432 | 438 | 466 | Amino acid change | ||
| CU458896c | A | A | A | C | T | C | A | C | G | G | C | G | A | A | T | A | G | G | A | T | C | G | A | G | |||
| 9016 | A | A | A | C | T | C | A | C | G | G | C | G | A | A | C | Gf | G | G | C | T | C | G | A | G | I80Vf | ||
| 8377 | A | A | A | C | T | C | A | C | G | G | C | G | A | A | T | A | G | G | A | T | C | G | A | G | |||
| 9944 | A | A | A | C | Ce | C | A | C | G | G | C | G | A | A | C | Gf | G | G | C | T | C | G | A | G | W10Re | I80Vf | |
| 71740 | A | A | A | C | T | C | A | C | G | G | C | G | A | A | C | Gf | A | T | C | C | C | G | A | G | I80Vf | ||
| 9614 | A | A | A | C | T | C | A | C | G | G | C | G | G | A | C | Gf | A | T | C | C | C | G | A | G | I80Vf | ||
| 9854 | A | A | A | C | T | C | A | C | G | G | C | G | A | A | T | A | G | G | A | T | C | G | A | G | |||
| 8548 | A | A | A | C | T | C | A | C | G | G | C | G | A | A | C | Gf | A | T | C | C | Tg | G | A | G | I80Vf | P140Lg | |
| 9419 | A | A | A | C | T | C | A | G | G | G | C | G | A | A | C | Gf | A | T | C | C | C | G | A | G | I80Vf | ||
| 74369 | A | A | G | T | T | A | G | – | – | T | T | A | A | G | – | – | – | – | – | – | – | – | C | A | |||
| 9835 | A | A | G | T | T | A | G | – | – | T | T | A | A | G | – | – | – | – | – | – | – | – | C | A | |||
| 77944 | A | A | G | T | T | A | G | – | – | T | T | A | A | G | – | – | – | – | – | – | – | – | C | A | |||
| 9626 | A | A | G | T | T | A | G | – | – | T | T | A | A | G | – | – | – | – | – | – | – | – | C | A | |||
| 9388 | A | A | G | T | T | A | G | – | – | T | T | A | A | G | – | – | – | – | – | – | – | – | C | A | |||
| 8006 | A | G | G | T | T | A | G | – | – | T | T | A | A | G | – | – | – | – | – | – | – | – | C | A | |||
| AP014547d | A | A | G | T | T | A | G | – | – | T | T | A | A | G | – | – | – | – | – | – | – | – | C | A | |||
Numbering system for the rrl gene from Escherichia coli;
Numbering system for the erm(41) gene, with the GTG start codon as 1
CU458896 (ATCC19977) is a reference sequence for M. abscessus subsp. Abscessus
AP014547 (JCM15300) is a reference sequence for M. abscessus subsp. Massiliense
T to C transition at position 28 (28T>C) leading to a Trp>Arg amino acid change at codon 10
A to G transition at position 238 (238A>G) leading to a Ile>Val amino acid change at codon 80
C to T transition at position 419 (419C>T) leading to a Pro>Leu amino acid change at codon 140.
Figure 1.
Representative PCR products for the erm(41) gene. The amplified products from M. abscessus subsp. abscessus strains (no. 9016, no. 8377, no. 9944 and no. 9854) were 673 bp in length, whereas those from M. abscessus subsp. massiliense strains (no. 9835 and no. 9626) were 397 bp in length. Far left lane, DNA size standard; Lane 1, no. 8377; Lane 2, 9016; Lane 3, 9626; Lane 4, 9944; Lane 5, 9835; Lane 6, 9854.
Antimicrobial sensitivity
Table V shows the antibiotic susceptibility of the M. abscessus strains to amikacin and clarithromycin. The MICs of amikacin ranged from 2 to 16 µg/ml, which indicated that all strains were sensitive. There was no difference in the MICs between the two subspecies. M. abscessus subsp. abscessus isolates were sensitive to clarithromycin on day 3, but the MICs were significantly higher on day 14 with one exception (no. 9944). In contrast, the strains of M. abscessus subsp. massiliense were susceptible to clarithromycin on days 3 through 14, except for one strain (no. 8006), which showed resistance from the start. Strain no. 9626 was sensitive early in the testing period, but the MIC was about 4-fold higher on day 14.
Table V.
Antibiotic susceptibilities of M. abscessus complex isolates based on MIC (µg/ml) values.
| Amikacin | Clarithromycin | ||||
|---|---|---|---|---|---|
| Isolate | Strains no. | Day 3 | Day 3 | Day 7 | Day 14 |
| M. abscessus subsp. abscessus | 9016 | 16 | 0.125 | 64 | 64 |
| 8377 | 16 | 0.5 | 64 | >128 | |
| 9944 | 8 | ≤0.06 | ≤0.06 | ≤0.06 | |
| 71740 | 8 | 0.25 | 32 | 32 | |
| 9614 | 8 | 0.25 | 64 | 64 | |
| 9854 | 8 | 0.125 | >128 | >128 | |
| 8548 | 8 | ≤0.06 | 64 | >128 | |
| 9419 | 8 | 0.5 | 32 | 32 | |
| M. abscessus subsp. massiliense | 74369 | 16 | 0.25 | 0.25 | 0.25 |
| 9835 | 16 | ≤0.06 | ≤0.06 | ≤0.06 | |
| 77944 | 4 | ≤0.06 | ≤0.06 | ≤0.06 | |
| 9626 | 16 | 0.125 | 0.25 | 0.5 | |
| 9388 | 8 | ≤0.06 | ≤0.06 | ≤0.06 | |
| 8006 | 2 | >128 | >128 | >128 | |
MIC, minimum inhibitory concentration.
MALDI-TOF MS analysis
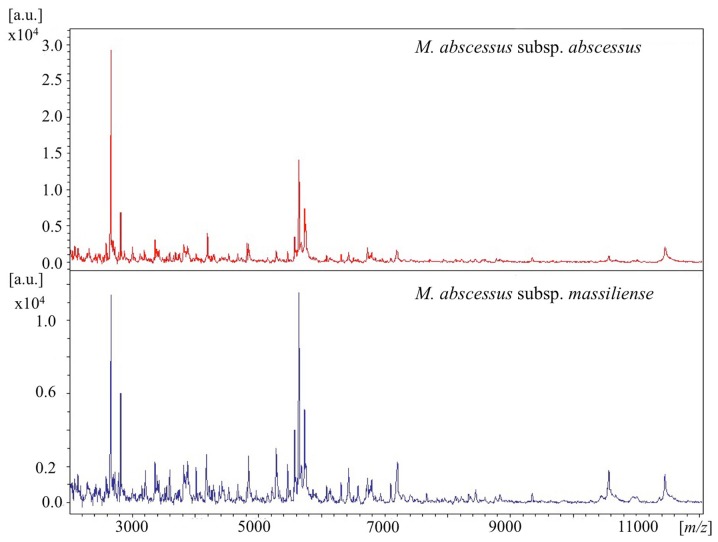
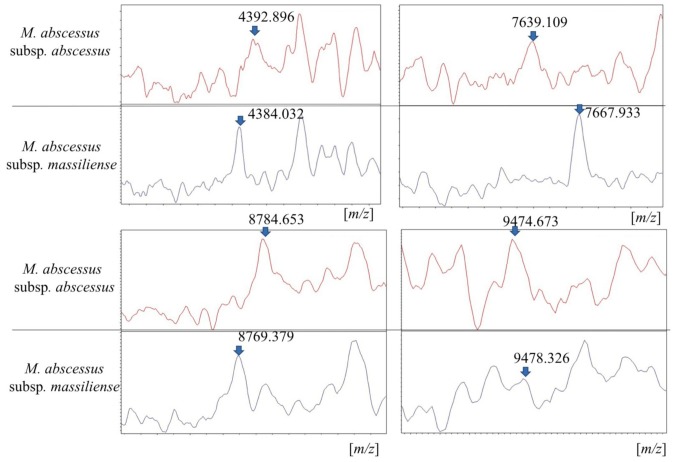
The details of mass spectra are shown in Figs. 2 and 3. Out of the 14 isolates, 11 were analyzed using MALDI-TOF MS. For reference, one clinical isolate of M. fortuitum was simultaneously analyzed in a similar manner. The 11 strains were identified as M. abscessus complex (score range, 1.66 to 2.14) and were correctly differentiated from the strain of M. fortuitum (Fig. S1). As shown in Fig. 2, the representative spectra of M. abscessus complex subspecies were similar at a laser frequency of 50 Hz across 2,000 to 12,000 m/z. When magnifying the spectrum (Fig. 3), distinctive peaks reported previously (8,10) were detected in some cases, but not in all samples. More specifically, peaks around 4390, 7639, 8781 and 9473 m/z for M. abscessus subsp. abscessus and peaks around 4385, 7669, and 8767 m/z for M. abscessus subsp. massiliense were found. However, each baseline was unstable and the peaks were wide and low for these strains. Moreover, discriminating peaks were mostly of low intensity or were overlapping. Thus, detection of clearly identifiable differences between strains of different subspecies was extremely difficult. Overall, it was possible to discriminate between M. abscessus subsp. abscessus and M. abscessus subsp. massiliense by using mass spectra only in some cases.
Figure 2.
Representative MALDI-TOF spectra of M. abscessus subsp. abscessus and M. abscessus subsp. massiliense (2,000–12,000 m/z). MALDI-TOF, Matrix-assisted laser desorption ionization-time of flight mass spectrometry.
Figure 3.
Eight peaks differentiate the two M. abscessus complex subspecies. The diagram shows the relative intensity (%) against the mass-to-charge ratio values of the discriminating peak regions of MALDI-TOF averaged mass spectral profiles for each subspecies. MALDI-TOF, Matrix-assisted laser desorption ionization-time of flight mass spectrometry.
Discussion
In the current study, M. abscessus complex isolates obtained from patients treated at our institutes located in the Tokyo-Yokohama area were analyzed. Overall, 57% (8 of 14) of the isolates were identified as M. abscessus subsp. abscessus, 43% (6 of 14) as M. abscessus subsp. massiliense, and none as M. abscessus subsp. bolletii. It has been reported that M. abscessus subsp. abscessus is the most predominant subspecies of the complex followed by M. abscessus subsp. massiliense, and that M. abscessus subsp. bolletii is quite rare, ranging from 0–3% in Japan (11,16–19). There were no significant differences in clinical features between M. abscessus subsp. abscessus and M. abscessus subsp. massiliense. Clinical characteristics of the M. abscessus complex did not help us to distinguish those subspecies, which is consistent with findings reported in the literature (11,20).
The proportion of the M. abscessus complex subspecies varies depending on the region from which they are isolated. Compared to Western Europe, the prevalence of M. abscessus subsp. bolletii is lower in East Asian countries (21–24). Although, M. abscessus subsp. abscessus is the most predominant subspecies of the complex in most parts of the world, some studies have reported that M. abscessus subsp. massiliense is more abundant than M. abscessus subsp. abscessus in some parts of East Asia such as Taiwan and Korea (5,21,22,25). Actually whole-genome sequencing analyses revealed genetic distinctions between M. abscessus subsp. abscessus isolates in Asia and Western Europe (26), suggesting that the phylogenetic diversity correlates with the regional ratio of the subspecies.
The current commercial system for NTM differentiation in Japan consists of the DNA-DNA hybridization method, which is unable to differentiate subspecies of the M. abscessus complex. The three subspecies also cannot be distinguished by 16S rRNA gene sequencing, which is commonly used for bacterial taxonomy in academic research, because the 16S rRNA genes in the three subspecies are 100% identical (27). The differentiation requires sequencing of several housekeeping genes, which is not easy to accomplish in most mycobacteriology laboratories. Hence, these three subspecies have not been distinguished in hospital laboratories. Sequencing of a single target gene may lead to inaccurate identification of closely related subspecies; however, multilocus sequence analyses of the M. abscessus complex have been described using hsp65, rpoB, ITS, gyrB, dnaA, recA and secA (28). Although some other methods based on technology developed by multilocus sequence analyses have been designed, such as variable-number tandem repeat analysis (18,19,29,30) and multiplex PCR (17,31), those methods are complicated. In the present study, subspecies of the M. abscessus complex were differentiated based on partial sequences of the hsp65 and rpoB genes, and results of ITS sequencing were also consistent in differentiating the two subspecies. In a subset of M. abscessus complex isolates, a hybrid genetic pattern for the hsp65 and rpoB genes has been reported (32,33), presumably the result of horizontal gene transfer between the subspecies. In such cases ITS gene analysis was essential to identify the subspecies. Sequencing of at least three housekeeping genes should therefore be carried out for subspecies identification.
Numerous institutions are seeking an alternative way to distinguish the M. abscessus subspecies in clinical practice. MALDI-TOF MS has been evaluated for the identification of microorganisms including mycobacteria. However in this study, it was not possible to distinguish between the M. abscessus subspecies of all isolates by MALDI-TOF MS. Although several institutes have reported the efficacy of MALDI-TOF MS in differentiating the three subspecies of the M. abscessus complex (5–10), methods for sample preparation and analysis, and diagnostic criteria have not been standardized. When defining the range from 2,000 to 20,000 m/z, ribosomal protein accounts for 50 to 70% of the peptide detected by MALDI-TOF MS. Thus, it is easy to distinguish between species that contain diverse ribosomal proteins. In fact, the current study revealed obvious differences between M. fortuitum and the M. abscessus complex by mass spectra (Fig. S1). However, the mass spectrometry peaks may be affected by variations in culture media formulations, duration of growth, and other conditions. The utility of MGIT liquid medium (10), 5% sheep blood agar (6), Middlebrook 7H11 (7), and Lowenstein-Jensen agar (9) have been reported for MALDI-TOF MS. In the present study, samples were prepared from colonies incubated on 2% Ogawa solid medium. Thus, the diagnostic criteria for MALDI-TOF analysis differ depending on the laboratory carrying out the analyses. Since there are few differences in the ribosomal proteins between the M. abscessus complex subspecies, and the sample preparation for mass spectra is even affected by climate, the procedure is often poorly reproducible. Considerable effort would be necessary to optimize and standardize a protocol to obtain reproducible mass spectrometry results for differentiating the M. abscessus complex subspecies. A proteomics study revealed specific immunogenic proteins in the M. abscessus complex; therefore, the subspecies may be differentiated using immunoassays (34).
Mutations in rrl gene confer acquired resistance and nucleotide T28 of erm(41) is associated with inducible resistance. Since most of the alterations identified in this study were in the erm(41) gene (92.9%; 13 of 14 isolates) rather than the rrl gene (7.1%;1 of 14), we concluded that the main cause of treatment failure for M. abscessus complex infections was inducible resistance encoded by the erm(41) gene. One strain of M. abscessus subsp. massiliense (no. 8006) harboring a point mutation 2059A>G in the rrl gene showed resistance to clarithromycin from day 3 through day 14 (MIC > 128 µg/ml). The presence of rrl mutations was reported in up to 30% of newly isolated strains in East Asia (16,35), whereas no rrl mutations were identified at the time of diagnosis in Spain (36). It has been reported that mutations in the rrl gene rapidly accumulate following clarithromycin use in monotherapy (3). Conversely, several studies have reported on acquired resistance due to rrl mutations in the absence of any macrolide exposure (16,37). However, an in vitro study reported that rrl mutations at position 2058 or 2059 were observed during incubation with clarithromycin (38). In East Asia, low dose macrolides are often administered for prolonged periods to treat chronic respiratory disorders, such as diffuse panbronchiolitis, to stimulate an immunomodulatory effect (39). Hence, careful attention is needed when prescribing macrolides to patients initially diagnosed with pulmonary disease caused by M. abscessus complex. One strain of M. abscessus subsp. abscessus (no. 9944) carried a substitution at position 28T>C in the erm(41) gene, which resulted in an amino acid change (W10R), and showed a low MIC value for clarithromycin. Other strains that harbored amino acid substitutions at I80V (238A>G) or P140L (419C>T) showed inducible resistance. These data are in agreement with previous reports (4,37) suggesting that the 5′ end of the erm(41) gene is a key region because this region is also predicted to carry a second open reading frame encoding a leader peptide that regulates expression of the erm(41) gene itself (4). In M. abscessus subsp. massiliense, two base deletions (61_62del) have been commonly detected, in addition to longer deletions (159_432del), which are consistent with the findings of the present study. Strains of M. abscessus subsp. abscessus harboring a C>T substitution at position 19, resulting in a stop codon (R7stop), have been previously identified in the findings of a case report (40). The data also highlight the importance of the 5′ end of the erm(41) gene. With rare exceptions, such as the presence of a full-length erm(41) gene (41,42), M. abscessus subsp. massiliense is generally not associated with inducible macrolide resistance because most strains harbor a truncated erm(41) gene (4,21,43). Interestingly, in the present study, one strain (no. 9626) showed a low initial MIC but the MIC increased 4-fold by 14 days. This observation suggests that mechanisms not involving erm(41) can cause inducible resistance. No strains that simultaneously harbored both a rrl mutation and nucleotide T28 in erm(41) were detected in this study; however, numerous studies have revealed that both resistance mechanisms can occur concurrently (36,38), and that a functional erm(41) gene does not exclude selection for rrl mutations (44). Transcriptome analysis of M. abscessus complex revealed the presence of several novel open reading frames, which could be activated by stressful conditions, such as hypoxia, to persist (45). Therefore, in addition to antimicrobial resistance genes, pathogenetic gene alterations should also be investigated in the future.
As described above, the two subspecies of the M. abscessus complex were found, which is consistent with the results of other studies showing the predominance of M. abscessus subsp. abscessus and M. abscessus subsp. massiliense in East Asia (11,16,21,24). Data from the current study indicate that the principal difference between the two subspecies was the size of the erm(41) gene. Several studies have revealed the presence of a complete erm(41) gene in M. abscessus subsp. massiliense strains (41,42) and a truncated erm(41) gene in M. abscessus subsp. bolletii (36), suggesting that M. abscessus subsp. massiliense acquired a full-length erm(41) gene by horizontal transfer from M. abscessus subsp. abscessus or M. abscessus subsp. bolletii, and that a truncated erm(41) gene was transferred from M. abscessus subsp. massiliense to M. abscessus subsp. bolletii. Additionally, a change at position 28T>C has been reported in M. abscessus subsp. bolletii (21). Therefore, although horizontal gene transfer between subspecies is probably quite rare, erm(41) PCR is not proposed as the best way to differentiate M. abscessus complex subspecies. However, erm(41) PCR can be easily and efficiently used for the prediction of sensitivity to clarithromycin in the M. abscessus complex. The phenotypic function of a putative erm(41) gene is important for the clinician from a strictly pragmatic standpoint. Likewise, M. abscessus complex subspecies should be categorized based on the presence of a functional erm(41) gene and macrolide sensitivity especially if horizontal gene transfer increases in the future.
In conclusion, the present study demonstrates the features of M. abscessus subsp. abscessus and M. abscessus subsp. massiliense, isolated in the Tokyo-Yokohama area. No strains of M. abscessus subsp. bolletii were detected. It was not possible to differentiate the two subspecies by clinical features in pulmonary infection, and results of mass spectrometry analysis of both these subspecies were highly similar; however, it is possible to predict clarithromycin susceptibility in strains of the two species by PCR amplification of the erm(41) gene. This is a simple and useful method that can be carried out routinely in hospital laboratories, and is recommended to predict inducible resistance to macrolides before determining the MICs, which requires 14 days of incubation for M. abscessus complex subspecies.
Supplementary Material
Acknowledgements
The authors would like to thank Mr. Sadahiro Ichimura and Mr. Hidetoshi Yamamoto (BML Inc., Saitama, Japan) for their microbiological techniques and Ms. Azumi Fujinaga (Bruker Japan K.K., Yokohama, Japan) for help with the mass spectra analysis.
Funding
This work was supported by Grant-in-Aid for Scientific Research(C) (grant no. 17K09021).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
AM, FY, TF, YY and YS examined and cared for the patients. AM, FY, TF, YY, YS and KF developed the concept, designed the experiments and analyzed the data. AM wrote the manuscript with contributions from all authors, who commented on it at all stages.
Ethics approval and consent to participate
Official approval for the study was obtained in advance from the Ethics Committee for Research at Showa University (approval nos. 371 and 2016127). Informed consent was waived due to the retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Namkoong H, Kurashima A, Morimoto K, Hoshino Y, Hasegawa N, Ato M, Mitarai S. Epidemiology of pulmonary nontuberculous Mycobacterial disease, Japan. Emerg Infect Dis. 2016;22:1116–1117. doi: 10.3201/eid2206.151086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee MR, Sheng WH, Hung CC, Yu CJ, Lee LN, Hsueh PR. Mycobacterium abscessus complex infections in humans. Emerg Infect Dis. 2015;21:1638–1646. doi: 10.3201/2109.141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallace RJ, Meier A, Brown BA, Zhang Y, Sander P, Onyi GO, Böttger EC. Genetic basis for clarithromycin resistance among isolates of Mycobacterium chelonae and Mycobacterium abscessus. Antimicrob Agents Chemother. 1996;40:1676–1681. doi: 10.1128/AAC.40.7.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nash KA, Brown-Elliott AB, Wallace RJ., Jr A Novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from mycobacterium chelonae. Antimicrob Agents Chemother. 2009;53:1367–1376. doi: 10.1128/AAC.01275-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teng SH, Chen CM, Lee MR, Lee TF, Chien KY, Teng LJ, Hsueh PR. Matrix-assisted laser desorption ionization-time of flight mass spectrometry can accurately differentiate between Mycobacterium masilliense (M. abscessus subspecies bolletti) and M. abscessus (Sensu Stricto) J Clin Microbiol. 2013;51:3113–3116. doi: 10.1128/JCM.01239-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fangous MS, Mougari F, Gouriou S, Calvez E, Raskine L, Cambau E, Payan C, Hery-Arnaud G. Classification algorithm for subspecies identification within the Mycobacterium abscessus species, based on matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2014;52:3362–3369. doi: 10.1128/JCM.00788-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panagea T, Pincus DH, Grogono D, Jones M, Bryant J, Parkhill J, Floto RA, Gilligan P. Mycobacterium abscessus complex identification with matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2015;53:2355–2358. doi: 10.1128/JCM.00494-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki H, Yoshida S, Yoshida A, Okuzumi K, Fukusima A, Hishinuma A. A novel cluster of Mycobacterium abscessus complex revealed by matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF MS) Diagn Microbiol Infect Dis. 2015;83:365–370. doi: 10.1016/j.diagmicrobio.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Buckwalter SP, Olson SL, Connelly BJ, Lucas BC, Rodning AA, Walchak RC, Deml SM, Wohifiel SL, Wengenack NL. Evaluation of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of Mycobacterium species, Nocardia species, and other aerobic actinomycetes. J Clin Microbiol. 2016;54:376–384. doi: 10.1128/JCM.02128-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kehrmann J, Wessel S, Murali R, Hampel A, Bange FC, Buer J, Mosel F. Principal component analysis of MALDI TOF MS mass spectra separates M. abscessus (sensu stricto) from M. massiliense isolates. BMC Microbiol. 2016;16:24. doi: 10.1186/s12866-016-0636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harada T, Akiyama Y, Kurashima A, Nagai H, Tsuyuguchi K, Fujii T, Yano S, Shigeto R, Kuraoka T, Kajiki A, et al. Clinical and microbiological differences between Mycobacterium abscessus and Mycobacterium massiliense lung diseases. J Clin Microbiol. 2012;50:3556–3561. doi: 10.1128/JCM.01175-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukuchi K, Hagiwara T, Nakamura K, Ichimura S, Tatsumi K, Gomi K. Identification of the regulatory region required for ubiquitination of the cyclin kinase inhibitor, p21. Biochem Biophys Res Commun. 2002;293:120–125. doi: 10.1016/S0006-291X(02)00198-5. [DOI] [PubMed] [Google Scholar]
- 13.Woods GL, Brown-Elliott BA, Conville PS, Desmond EP, Hall GS, Lin G. Susceptibility testing of Mycobacteria, Nocardia and other aerobic actinomycetes; Approved Standard-Second edition. Clin Lab Stand Inst. 2011;26:1–61. [PubMed] [Google Scholar]
- 14.Macheras E, Roux AL, Bastian S, Leao SC, Palaci M, Tardy VS, Gutierrez C, Richter E, Gerdes SR, Pfyffer G, et al. Multilocus sequence analysis and rpoB sequencing of Mycobacterium abscessus (sensu lato) strains. J Clin Microbiol. 2011;49:491–499. doi: 10.1128/JCM.01274-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida S, Tsuyuguchi K, Suzuki K, Tomita M, Okada M, Hayashi S, Iwamoto T, Saito H. Further isolation of Mycobacterium abscessus subsp. abscessus and subsp. bolletii in different regions of Japan and susceptibility of these isolates to antimicrobial agents. Int J Antimicrob Agents. 2013;42:226–231. doi: 10.1016/j.ijantimicag.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 17.Nakanaga K, Sekizuka T, Fukano H, Sakakibara Y, Takeuchi F, Wada S, Ishii N, Makino M, Kuroda M, Hoshino Y. Discrimination of Mycobacterium abscessus subsp. massiliense from Mycobacterium abscessus subsp. abscessus in clinical isolates by multiplex PCR. J Clin Microbiol. 2014;52:251–259. doi: 10.1128/JCM.01327-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida S, Arikawa K, Tsuyuguchi K, Kurashima A, Harada T, Nagai H, Suzuki K, Iwamoto T, Hayashi S. Investigation of the population structure of Mycobacterium abscessus complex strains using 17-locus variable number tandem repeat typing and the further distinction of mycobacterium massiliense hsp65 genotypes. J Med Microbiol. 2015;64:254–261. doi: 10.1099/jmm.0.000016. [DOI] [PubMed] [Google Scholar]
- 19.Kusuki M, Osawa K, Arikawa K, Tamura M, Shigemura K, Shirakawa T, Nakamura T, Nakamachi Y, Fujisawa M, Saegusa J, Tokimatsu I. Determination of the antimicrobial susceptibility and molecular profile of clarithromycin resistance in the Mycobacterium abscessus complex in Japan by variable number tandem repeat analysis. Diagn Microbiol Infect Dis. 2018;91:256–259. doi: 10.1016/j.diagmicrobio.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, Park YK, Kim CK, Shin SJ, Huitt GA, et al. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med. 2011;183:405–410. doi: 10.1164/rccm.201003-0395OC. [DOI] [PubMed] [Google Scholar]
- 21.Kim HY, Kim BJ, Kook Y, Yun YJ, Shin JH, Kim BJ, Kook YH. Mycobacterium massiliense is differentiated from Mycobacterium abscessus and mycobacterium bolletii by erythromycin ribosome methyltransferase gene (erm) and clarithromycin susceptibility patterns. Microbiol Immunol. 2010;54:347–353. doi: 10.1111/j.1348-0421.2010.00221.x. [DOI] [PubMed] [Google Scholar]
- 22.Kim BJ, Yi SY, Shim TS, Do SY, Yu SK, Park YG, Kook YH, Kim BJ. Discovery of a novel hsp65 genotype within Mycobacterium massiliense associated with the rough colony morphology. PLoS One. 2012;7:e38420. doi: 10.1371/journal.pone.0038420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SY, Kang YA, Bae IK, Yim JJ, Park MS, Kim YS, Kim SK, Chang J, Jeong SH. Standardization of multilocus sequence typing scheme for Mycobacterium abscessus and Mycobacterium massiliense. Diagn Microbiol Infect Dis. 2013;77:143–149. doi: 10.1016/j.diagmicrobio.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 24.Luo L, Li B, Chu H, Huang D, Zhang Z, Zhang J, Gui T, Xu L, Zhao L, Sun X, Xiao H. Characterization of Mycobacterium abscessus subtypes in Shanghai of China: Drug sensitivity and bacterial epidemicity as well as clinical manifestations. Medicine (Baltimore) 2016;95:e2338. doi: 10.1097/MD.0000000000002338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koh WJ, Jeong BH, Kim SY, Jeon K, Park KU, Jhun BW, Lee H, Park HY, Kim DH, Huh HJ, et al. Mycobacterial characteristics and treatment outcomes in Mycobacterium abscessus lung disease. Clin Infect Dis. 2017;64:309–316. doi: 10.1093/cid/ciw724. [DOI] [PubMed] [Google Scholar]
- 26.Davidson RM, Hasan NA, Reynolds PR, Totten S, Garcia B, Levin A, Ramamoorthy P, Heifets L, Daley CL, Strong M. Genome sequencing of Mycobacterium abscessus isolates from patients in the United States and comparisons to globally diverse clinical strains. J Clin Microbiol. 2014;52:3573–3582. doi: 10.1128/JCM.01144-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leao SC, Tortoli E, Viana-Niero C, Ueki SY, Lima KV, Lopes ML, Yubero J, Menendez MC, Garcia MJ. Characterization of mycobacteria from a major Brazilian outbreak suggests that revision of the taxonomic status of members of the Mycobacterium chelonae-M. abscessus group is needed. J Clin Microbiol. 2009;47:2691–2698. doi: 10.1128/JCM.00808-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan JL, Khang TF, Ngeow YF, Choo SW. A phylogenomic approach to bacterial subspecies classification: Proof of concept in Mycobacterium abscessus. BMC Genomics. 2013;14:879. doi: 10.1186/1471-2164-14-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kikuchi T, Watanabe A, Gomi K, Sakakibara T, Nishimori K, Daito H, Fujimura S, Tazawa R, Inoue A, Ebina M, et al. Association between mycobacterial genotypes and disease progression in Mycobacterium avium pulmonary infection. Thorax. 2009;64:901–907. doi: 10.1136/thx.2009.114603. [DOI] [PubMed] [Google Scholar]
- 30.Wong YL, Ong CS, Ngeow YF. Molecular typing of Mycobacterium abscessus based on tandem-repeat polymorphism. J Clin Microbiol. 2012;50:3084–3088. doi: 10.1128/JCM.00753-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mougari F, Raskine L, Ferroni A, Marcon E, Sermet-Gaudelus I, Veziris N, Heym B, Gaillard JL, Nassif X, Cambau E. Clonal relationship and differentiation among Mycobacterium abscessus isolates as determined using the semiautomated repetitive extragenic palindromic sequence PCR-based diversilab system. J Clin Microbiol. 2014;52:1969–1977. doi: 10.1128/JCM.03600-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim HY, Kook Y, Yun YJ, Park CG, Lee NY, Shim TS, Kim BJ, Kook YH. Proportions of Mycobacterium massiliense and Mycobacterium bolletii strains among Korean Mycobacterium chelonae-Mycobacterium abscessus group isolates. J Clin Microbiol. 2008;46:3384–3390. doi: 10.1128/JCM.00319-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim BJ, Kim GN, Kim BR, Shim TS, Kook YH, Kim BJ. Phylogenetic analysis of Mycobacterium massiliense strains having recombinant rpoB gene laterally transferred from Mycobacterium abscessus. PLoS One. 2017;12:e0179237. doi: 10.1371/journal.pone.0179237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steindor M, Nkwouano V, Stefanski A, Stuehler K, Loerger TR, Bogumil D, Jacobsen M, Mackenzie CR, Kalscheuer R. A proteomics approach for the identification of species-specific immunogenic proteins in the Mycobacterium abscessus complex. Microbes Infect. 2018 Nov 13; doi: 10.1016/j.micinf.2018.10.006. (Epub ahead of print). doi: 10.1016/j.micinf.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Lee SH, Yoo HK, Kim SH, Koh WJ, Kim CK, Park YK, Kim HJ. Detection and assessment of clarithromycin inducible resistant strains among Korean Mycobacterium abscessus clinical strains: PCR methods. J Clin Lab Anal. 2014;28:409–414. doi: 10.1002/jcla.21702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubio M, March F, Garrigó M, Moreno C, Español M, Coll P. Inducible and acquired clarithromycin resistance in the Mycobacterium abscessus complex. PLoS One. 2015;10:e0140166. doi: 10.1371/journal.pone.0140166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bastian S, Veziris N, Roux AL, Brossier F, Gaillard JL, Jarlier V, Cambau E. Assessment of clarithromycin susceptibility in strains belonging to the Mycobacterium abscessus group by erm(41) and rrl sequencing. Antimicrob Agents Chemother. 2011;55:775–781. doi: 10.1128/AAC.00861-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mougari F, Bouziane F, Crockett F, Nessar R, Chau F, Veziris N, Sapriel G, Raskine L, Cambau E. Selection of resistance to clarithromycin in Mycobacterium abscessus subspecies. Antimicrob Agents Chemother. 2016;61(pii):e00943–16. doi: 10.1128/AAC.00943-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kudoh S, Keicho N. Diffuse panbronchiolitis. Clin Chest Med. 2012;33:297–305. doi: 10.1016/j.ccm.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Kim SY, Shin SJ, Jeong BH, Koh WJ. Successful antibiotic treatment of pulmonary disease caused by Mycobacterium abscessus subsp. abscessus with C-to-T mutation at position 19 in erm(41) gene: Case report. BMC Infect Dis. 2016;16:207. doi: 10.1186/s12879-016-1554-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shallom SJ, Gardina PJ, Myers TG, Sebastian Y, Conville P, Calhoun LB, Tettelin H, Olivier KN, Uzal G, Sampaio EP, et al. New rapid scheme for distinguishing the subspecies of the Mycobacterium abscessus group and identifying Mycobacterium massiliense isolates with inducible clarithromycin resistance. J Clin Microbiol. 2013;51:2943–2949. doi: 10.1128/JCM.01132-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown-Elliott BA, Vasireddy S, Vasireddy R, Iakhiaeva E, Howard ST, Nash K, Parodi N, Strong A, Gee M, Smith T, Wallace RJ., Jr Utility of sequencing the erm(41) gene in isolates of Mycobacterium abscessus subsp. abscessus with low and intermediate clarithromycin MICs. J Clin Microbiol. 2015;53:1211–1215. doi: 10.1128/JCM.02950-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshida S, Tsuyuguchi K, Suzuki K, Tomita M, Okada M, Shimada R, Hayashi S. Rapid identification of strains belonging to the Mycobacterium abscessus group through erm(41) gene pyrosequencing. Diagn Microbiol Infect Dis. 2014;79:331–336. doi: 10.1016/j.diagmicrobio.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Maurer FP, Rüegger V, Ritter C, Bloemberg GV, Böttger EC. Acquisition of clarithromycin resistance mutations in the 23S rRNA gene of Mycobacterium abscessus in the presence of inducible erm(41) J Antimicrob Chemother. 2012;67:2606–2611. doi: 10.1093/jac/dks279. [DOI] [PubMed] [Google Scholar]
- 45.Miranda-CasoLuengo AA, Staunton PM, Dinan AM, Lohan AJ, Loftus BJ. Functional characterization of the Mycobacterium abscessus genome coupled with condition specific transcriptomics reveals conserved molecular strategies for host adaptation and persistence. BMC Genomics. 2016;17:553. doi: 10.1186/s12864-016-2868-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.