Abstract
Background
The novel Fujifilm SILVAMP TB-LAM (FujiLAM) assay detects mycobacterial lipoarabinomannan in urine and has demonstrated superior sensitivity to the Alere Determine TB-LAM Ag (AlereLAM) assay for detection of tuberculosis among hospitalized people with human immunodeficiency virus (PWH). This is the first study to evaluate the assay among a broad population referred for antiretroviral therapy including both outpatients (mainly) and inpatients.
Methods
We assessed diagnostic accuracy of FujiLAM and AlereLAM assays in biobanked urine samples from a cohort of adults referred for antiretroviral therapy in Ghana against a microbiological and a composite (including clinical judgement) reference standard, and we assessed the association of FujiLAM test positivity with mortality.
Results
We evaluated urine samples from 532 PWH (462 outpatients, 70 inpatients). Against a microbiological reference standard, the sensitivity of FujiLAM was 74.2% (95% confidence interval [CI], 62.0–84.2) compared to 53.0% (95% CI, 40.3–65.4) for AlereLAM, a difference of 21.2% (CI, 13.1–32.5). Specificity was 89.3% (95% CI, 85.8–92.2) versus 95.6% (95% CI, 93.0–97.4) for FujiLAM and AlereLAM, a difference of −6.3% (95% CI −9.6 to −3.3). Specificity estimates for FujiLAM increased markedly to 98.8% (95% CI, 96.6–99.8) in patients with CD4 >100 cells/µL and when using a composite reference standard. FujiLAM test positivity was associated with increased cumulative risk of mortality at 6 months (hazard ratio, 4.80; 95% CI, 3.01–7.64).
Conclusions
FujiLAM offers significantly increased diagnostic sensitivity in comparison to AlereLAM. Specificity estimates for FujiLAM were lower than for AlereLAM but were affected by the limited ability of the reference standard to correctly diagnose tuberculosis in individuals with low CD4 counts.
Keywords: diagnostic accuracy, HIV, LAM, tuberculosis, urine
Tuberculosis remains the leading cause of morbidity and mortality among people with human immunodeficiency virus (PWH) [1]. It is estimated that only 51% of tuberculosis cases among PWH are diagnosed and notified to health authorities [1]. This gap is partly explained by the limitations of available methods to diagnose tuberculosis in PWH, who are often unable to produce sputum and have frequent extrapulmonary and/or paucibacillary disease [2, 3]. To improve tuberculosis case detection and treatment in PWH, a rapid test that does not rely on sputum is a key priority [1, 4].
In 2015, the World Health Organization (WHO) published recommendations for the urine lateral flow Alere Determine TB-LAM Ag test (AlereLAM; Abbott, Palatine, IL) to assist diagnosis of tuberculosis in PWH with a CD4 cell count ≤100 cells/μL or “who are seriously ill” [5]. The WHO policy was updated in 2019 to include recommendation for use of AlereLAM in a broader group of people, both in inpatient and outpatient settings [6]. The AlereLAM is simple to use and provides a result within 25 minutes. However, implementation of the test has been limited in parts due to its modest sensitivity [7]. A systematic review found a pooled sensitivity of 42% (95% credible interval [CrI], 31–55), increasing to 54% (CrI, 38–69) among people with CD4 ≤100 cells/μL, but reducing to 17% (CrI, 10–27) in people with CD4 >100 cells/μL [8]. Other factors that may have hindered implementation of AlereLAM in programmatic settings are the need of a reference scale card to interpret the AlereLAM test results and the emphasis on CD4 cell count to identify the target group for lipoarabinomannan (LAM) testing [9, 10].
The novel Fujifilm SILVAMP TB-LAM (FujiLAM; Fujifilm, Tokyo, Japan) also detects mycobacterial LAM in urine as a lateral flow test, through a 5-step process, with a result available within 1 hour [11, 12]. The FujiLAM test was developed through careful selection of a pair of high-affinity monoclonal antibodies for distinct Mycobacterium tuberculosis LAM epitopes present in urine samples of patients with tuberculosis [12]. The selection of detection antibodies in the FujiLAM test is further combined with a silver amplification step to increase visibility of the test lines [11]. FujiLAM has demonstrated superior sensitivity to AlereLAM in hospitalized patients with HIV (~70% vs 42%) [11].
This study is the first to assess diagnostic accuracy of FujiLAM for the detection of tuberculosis compared with AlereLAM among patient with HIV referred for antiretroviral therapy (ART), including mainly outpatients (expected to have lower pretest probability and higher CD4 cell count than inpatients), and to assess the predictive value of FujiLAM test positivity for mortality.
METHODS
Design, Setting, and Study Population
The diagnostic accuracy and predictive value of FujiLAM was evaluated in frozen urine samples stored from the DETECT HIV-TB study cohort of adults with human immunodeficiency virus (HIV) referred for ART to the Korle-Bu Teaching Hospital in Accra, Ghana [13, 14]. Participants for the DETECT HIV-TB study were recruited prospectively between January 2013 and March 2014 from the out- and inpatient departments at the Fevers unit. Adults were consecutively enrolled whether or not they reported tuberculosis symptoms if they met the following criteria: HIV-positive, ≥18 years, and referred for ART initiation (ie, WHO clinical stage 3 or 4, CD4 ≤350 cells/µL, or pregnant as per recommendations at the time of the study) [15]. Participants who were receiving treatment for tuberculosis or unable to produce any samples for mycobacterial testing (no sputum and no urine) were excluded.
Demographic, clinical, and routine laboratory data including CD4 cell count were collected, the research team systematically collected urine and spontaneously expectorated sputum samples upon enrollment, and participants were asked to bring in an additional early morning sputum sample. At 6-month follow up, medical records were reviewed for vital status, loss to follow up, ART, and tuberculosis treatment status. If medical records were unavailable, contact with the participants or next of kin was attempted by phone.
Informed consent was obtained from all participants, and the study was approved by the Institutional Review Board of University of Ghana Medical School and the Danish National Committee on Health Research Ethics. We followed the Standards for the Reporting of Diagnostic accuracy studies (STARD) criteria [16].
Procedures
Urine specimens collected as part of the DETECT HIV-TB cohort were stored at −20°C at the Department of Medical Microbiology, University of Ghana and shipped on dry ice to the Research Institute of Tuberculosis/Japan Anti-Tuberculosis Association in Tokyo, Japan for LAM testing between January and March 2019. At the time of testing, frozen urine aliquots were thawed to ambient temperature and mixed manually. FujiLAM and AlereLAM testing were done from the same aliquot.
FujiLAM testing was done in accordance with the manufacturer’s instructions that involve a 5-step procedure as previously described [11] (see Supplementary Figure S1). In brief, approximately 200 µL urine was added to the reagent tube up to the indicator line, mixed, and incubated for 40 minutes at ambient temperature. After mixing again, 2 drops of sample were added to the test strip. Then, 2 buttons were pressed sequentially within 3–10 minutes for the silver amplification. The final result was available within 50–60 minutes.
AlereLAM testing was done according to the test’s package insert [17]. In brief, 60 µL urine was applied to the sample pad on the test strip and the result was read after 25 minutes. We used the updated reference scale card with 4 color band intensities to grade the test as negative or grade 1, 2, 3, or 4 and considered the recommended grade 1 cutoff point as the positivity threshold [17].
The AlereLAM and FujiLAM test results were read independently by 2 readers blinded to their counterpart’s observations, to the test result of the respective other test, to patient status as well as the results of the tuberculosis reference tests. In the event of discordance, the 2 readers reinspected the test to establish a final consensus result. In case of test failure, the test was repeated once.
For reference standard testing, sputum samples were processed as previously described [13, 14]. Testing included smear microscopy for acid-fast bacilli using both Ziehl-Neelsen and Auramine O staining, sputum culture for mycobacteria using both solid Löwenstein-Jensen medium and the BACTEC mycobacteria growth indicator tube (MGIT) liquid culture system (BD Diagnostics, Sparks, MD), and Xpert MTB/RIF (Xpert; Cepheid, Sunnyvale, CA). Positive mycobacterial isolates were sent to the German National Reference Centre for Mycobacteria in Borstel for speciation using the GenoType Mycobacterium CM/AS (Hain Lifescience, Nehren, Germany) assay and analysis of 16S ribosomal ribonucleic acid gene. In addition, urinary Xpert testing was performed using 6 mL biobanked urine thawed and centrifuged. After removal of the supernatant, the pellet was resuspended in 1 mL sterile phosphate-buffered saline and mixed with the Xpert sample reagent.
Diagnostic Classification and Statistical Analysis
We categorized participants according to the tuberculosis diagnostic categories shown in Table 1 while blinded to the LAM test results. Definite tuberculosis included participants that had a culture (sputum) or Xpert (sputum or urine) positive result for M tuberculosis complex in any of the samples obtained at baseline; not tuberculosis included participants that were alive at 2 months follow up with negative microscopy, culture, and Xpert tests at baseline and follow up and no empirical tuberculosis treatment; for “possible” tuberculosis, the participant did not meet the criteria for “definite” tuberculosis but was started and responded to tuberculosis treatment based on clinical grounds or had a positive test result at follow up; “unclassifiable” was determined if the participant did not meet any of the above categories.
Table 1.
Tuberculosis Diagnostic Classification
| Category | Description |
|---|---|
| Definite TB | Any culture or any Xpert (baseline) positive for MTB ≥1 Positive culture (solid, liquid, sputum) and confirmed MTB complex at baseline OR ≥1 Positive Xpert (sputum or urine) at baseline |
| Possible TB | Any patient not meeting definite TB or not TB classification who is started on TB treatment or has positive laboratory findings on follow up Empiric TB treatment started by the healthcare provider OR Positive sputum culture and/or sputum Xpert and/or sputum smear on follow up |
| Not TB | All microscopy, culture, and Xpert tests negative for MTB, not started on TB treatment, recovers, and has negative follow-up tests All cultures negative (sputum, blood, including follow-up where available) AND All Xpert negative (sputum, urine, including follow-up where available) AND All smear microscopy negative (sputum including follow-up where available) AND Treatment not initiated by healthcare providers AND Improvement or full recovery of symptoms at 2 months of follow up in the absence of TB treatment |
| Unclassifiable | All participants that do not fall into groups “definite TB,” “not TB” or “possible TB” ie, No symptom resolution at follow up (same or worse) in patients with a mycobacterial work-up that are negative at baseline or in follow up at 2 months OR Loss to follow up at 2 months (patients with a mycobacterial work-up that is negative at baseline) OR Passed away (patients with a mycobacterial work-up that is negative at baseline) OR Baseline smear microscopy positive but culture and Xpert negative |
Abbreviations: MTB, Mycobacterium tuberculosis complex; TB, tuberculosis.
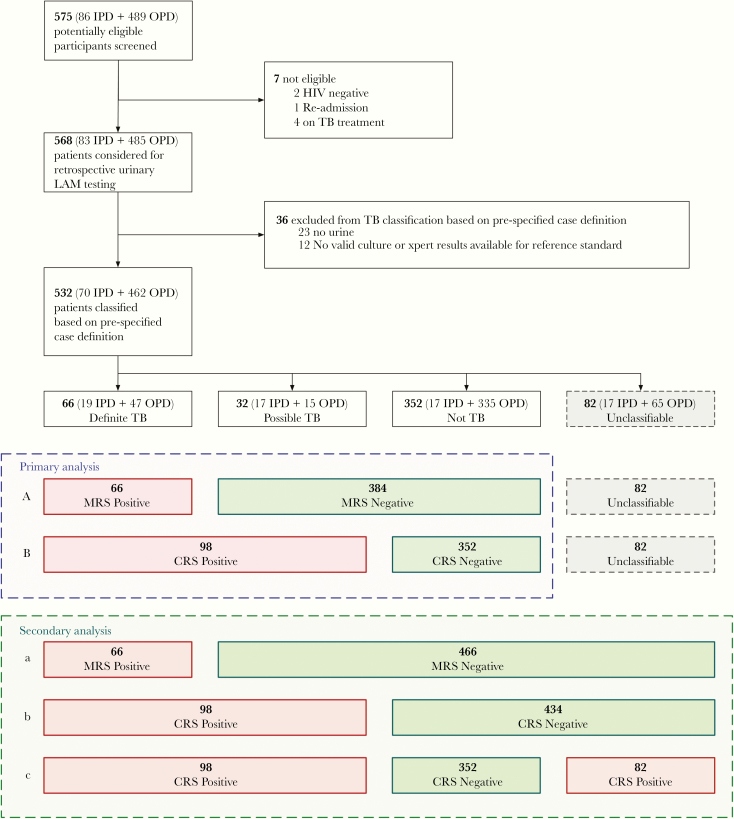
Figure 1 outlines diagnostic classification of participants and analysis. In primary analysis, using a microbiological reference standard (MRS), participants categorized as definite tuberculosis were considered reference standard positive, and participants with not tuberculosis and possible tuberculosis were considered as negative. In a composite reference standard (CRS), participants with possible tuberculosis were reclassified as positive together with definite tuberculosis. Participants that were unclassifiable were excluded from the primary analysis. The effect on performance of including the unclassifiable participants was assessed in a secondary analysis.
Figure 1.
Flow diagram of study population and classification. (Analysis A) Primary diagnostic accuracy analysis including all human immunodeficiency virus (HIV)+ against a Microbiological Reference Standard (MRS). (Analysis B) Primary diagnostic accuracy analysis including all HIV + against a Composite Reference Standard (CRS). (Analysis a) Secondary sensitivity analysis including all HIV+ with unclassifiable reclassified as MRS negative. (Analysis b) Secondary sensitivity analysis including all HIV + with unclassifiable reclassified as CRS negative. (Analysis c) Secondary sensitivity analysis including all HIV+ with unclassifiable reclassified as CRS positive. IPD, inpatients; OPD, outpatients; TB, tuberculosis.
Descriptive analyses were used to characterize the study population. We determined sensitivity and specificity with 95% confidence interval (CI) as well as positive predictive value (PPV) and negative predictive value and likelihood ratios of AlereLAM and FujiLAM against both a microbiological and CRS for the study population overall and for predefined subgroups. The 95% CI around differences between FujiLAM and AlereLAM sensitivities and specificities were calculated according to the Tango [18] method, and the difference was considered significant if the 95% CIs did not include zero. Interreader agreement for each of the FujiLAM test and AlereLAM test results, respectively, was determined by Cohen’s kappa coefficient. To evaluate the association between LAM positivity (by FujiLAM and AlereLAM) and mortality at 2 and 6 months, we used Cox regression analysis and reported the unadjusted hazard ratio (HR) with 95% CI. We constructed Kaplan-Meier curves to examine risk of mortality after 6 months in the population overall and among definite tuberculosis cases, and we compared data stratified by FujiLAM and AlereLAM test results with the log-rank test. All analyses were conducted using STATA version 15.1 software.
RESULTS
Study Population and Tuberculosis Diagnostic Status
Of 575 individuals screened for participation in the DETECT HIV-TB study cohort, 568 were considered eligible for retrospective LAM testing, and 532 had a urine sample and a reference standard result available to enter the study (Figure 1). Of 532 eligible participants, 82 participants (15.4%) were unclassifiable according to tuberculosis diagnostic status and excluded from our primary analysis. The main reason for participants to be unclassifiable was lost to follow up as listed in Supplementary Table S1. Of the 450 participants included in the primary analysis, 66 (14.7%) were classified as definite tuberculosis, 32 (7.1%) were classified as possible tuberculosis, and 352 (78.2%) were classified as not tuberculosis.
Participants had a median CD4 cell count of 152 (interquartile range [IQR], 44–349), and the majority (462 [86.8%]) were outpatients (Table 2). A total of 1937 sputum and urine samples were collected from participants (mean, 3.6 samples/participant). This provided basis for a total of 2448 reference standard test results (mycobacterial solid/liquid culture and Xpert; mean, 4.6 tests/participant) including 2018 tests on sputum samples and 430 tests on urine samples. Lowenstein-Jensen (LJ) contamination rate was 6.0% (9 of 501) for spot sputum samples and 7.0% (31 of 442) for early morning samples. For MGIT, contamination rates were 12.4% (62 of 501) for spot sputum and 17.1% (76 of 442) for early morning samples. For 3 participants, sputum was not available or contaminated in both spot and early morning samples for both LJ and MGIT, and tuberculosis diagnostic categorization relied on the urine Xpert result.
Table 2.
Patient Characteristics
| Key Characteristics of Study Population | |||||
|---|---|---|---|---|---|
| All n = 532 (%) | Definite TB n = 66 (%) | Possible TB n = 32 (%) | Not TB n = 352 (%) | Unclassifiablea n = 82 (%) | |
| Enrollment Site | |||||
| Outpatients | 462 (86.8) | 47 (71.2) | 15 (46.9) | 335 (95.2) | 65 (79.3) |
| Inpatients | 70 (13.2) | 19 (28.8) | 17 (53.1) | 17 (4.8) | 17 (20.7) |
| Median age in years (IQR) | 38 (31–45) | 37 (29–43) | 37 (34–44) | 39 (31–45) | 38 (32–45) |
| Female | 354 (66.5) | 39 (59.4) | 19 (59.4) | 245 (69.6) | 51 (62.2) |
| Median CD4 cell count per µL (IQR)b | 152 (44–349) | 108 (43–190) | 45 (12–179) | 216 (76–410) | 56 (16–220) |
| CD4 ≤100 cells/µL | 192 (37.7) | 32 (50.0) | 19 (63.3) | 97 (28.4) | 44 (59.5) |
| CD4 >100 cells/µL | 318 (62.3) | 32 (50.0) | 11 (36.7) | 245 (71.6) | 30 (40.5) |
| Median BMI (IQR) | 21 (18–23) | 18 (16–20) | 18 (16–22) | 21 (19–25) | 20 (17–22) |
| Positive WHO symptom screenc | 457 (86.2) | 63 (95.5) | 30 (93.8) | 295 (83.8) | 69 (86.3) |
| Previous history of TBd | 35 (6.6) | 5 (7.6) | 3 (9.4) | 22 (6.3) | 5 (6.3) |
| FujiLAM positive | 97 (18.2) | 49 (74.2) | 14 (43.8) | 27 (7.8) | 7 (8.5) |
| AlereLAM positive | 57 (10.7) | 35 (53.3) | 8 (25.0) | 9 (2.6) | 5 (6.1) |
| Vital Status at 6 Months | |||||
| Alive | 372 (69.9) | 32 (48.5) | 15 (46.9) | 321 (91.2) | 4 (4.9) |
| Died | 71 (13.4) | 22 (33.3) | 13 (40.6) | 7 (2.0) | 29 (35.4) |
| LTFU | 89 (16.7) | 12 (18.2) | 4 (12.5) | 24 (6.8) | 49 (59.8) |
Abbreviations: BMI, body mass index; IQR, interquartile range; LTFU, loss to follow up; TB, tuberculosis; WHO, World Health Organization.
aUnclassifiables were excluded from primary analysis.
bCD4 count missing for 22 participants.
cWHO symptoms score missing for 2 participants.
dTB history missing for 4 participants.
Comparison of Accuracy of Fujifilm SILVAMP TB-LAM and Alere Determine TB-LAM Ag
Against an MRS, FujiLAM detected 49 and AlereLAM 35 of 66 of participants with definite tuberculosis, giving an overall sensitivity of 74.2% (49 of 66; 95% CI, 62.0–84.2) for FujiLAM versus 53.0% (35 of 66; 95% CI, 40.3–65.4) for AlereLAM (difference of 21.2%; CI, 13.1–32.5) (Table 3). Among outpatients, FujiLAM sensitivity was 68.1% (32 of 47; 95% CI, 52.9–80.9) compared with AlereLAM sensitivity of 44.7% (21 of 47; 95% CI, 30.2–59.9); difference of 23.4% (95% CI, 13.6–37.2) (Table 4).
Table 3.
Sensitivity and specificity of FujiLAM versus AlereLAM against MRS (A) and CRS (B)
| A | MRS | Test | N | TP | FP | FN | TN | Sensitivity (95% CI) | Specificity (95% CI) | TB% | PPV (95% CI) | NPV (95% CI) | LR+ (95% CI) | LR− (95% CI) |
| All | FujiLAM | 450 | 49 | 41 | 17 | 343 | 74.2% (62.0–84.2) | 89.3% (85.8–92.2) | 14.7% | 54.4% (43.6–65.0) | 95.3% (92.5–97.2) | 7.0 (5.0–9.6) | 0.3 (0.2–0.4) | |
| AlereLAM | 450 | 35 | 17 | 31 | 367 | 53.0% (40.3–65.4) | 95.6% (93.0–97.4) | 14.7% | 67.3% (52.9–79.7) | 92.2% (89.1–94.6) | 12.0 (7.1–20.1) | 0.5 (0.4–0.6) | ||
| △Sn and △Sn | 21.2% (13.1–32.5) | −6.3% (−9.6 to −3.3) | ||||||||||||
| B | CRS | Test | N | TP | FP | FN | TN | Sensitivity (95% CI) | Specificity (95% CI) | TB% | PPV (95% CI) | NPV (95% CI) | LR+ (95% CI) | LR− (95% CI) |
| All | FujiLAM | 450 | 63 | 27 | 35 | 325 | 64.3% (54.0–73.7) | 92.3% (89.0–94.9) | 21.8% | 70.0% (59.4–79.2) | 90.3% (86.7–93.1) | 8.4 (5.7–12.4) | 0.4 (0.3–0.5) | |
| AlereLAM | 450 | 43 | 9 | 55 | 343 | 43.9% (33.9–54.3) | 97.4% (95.2–98.8) | 21.8% | 82.7% (69.7–91.8) | 86.2% (82.4–89.4) | 17.2 (8.7–34.0) | 0.6 (0.5–0.7) | ||
| △Sn and △Sn | 20.4% (11.8–30.0) | −5.1% (−8.4 to −2.3) |
Abbreviations: AlereLAM, Alere Determine TB LAM Ag assay; CI, confidence interval; CRS, composite reference standard; FN, false negative; FP, false positive; FujiLAM, Fujifilm SILVAMP TB LAM assay; HIV, human immunodeficiency virus; LAM, lipoarabinomannan; LR+, likelihood ratio (+); LR−, likelihood ratio (−); MRS, microbiological reference standard; NPV, negative predictive value; PPV, positive predictive value; Sn, sensitivity; Sp, specificity; TB%, tuberculosis prevalence; TN, true negatives; TP, true positives.
a(A) Diagnostic accuracy using a MRS: Definite tuberculosis were considered reference standard positive. Not tuberculosis and Possible tuberculosis were considered reference standard negative. (B) Diagnostic accuracy using a CRS: Definite tuberculosis and Possible tuberculosis were considered reference standard positive. Not tuberculosis were considered reference standard negative.
Table 4.
Sensitivity and specificity of FujiLAM versus AlereLAM against MRS (A) and CRS (B) by patient status
| MRS | Test | N | TP | FP | FN | TN | Sensitivity (95% CI) | Specificity (95% CI) |
| Outpatients | FujiLAM | 397 | 32 | 33 | 15 | 317 | 68.1% (52.9–80.9) | 90.6% (87.0–93.4) |
| AlereLAM | 397 | 21 | 11 | 26 | 339 | 44.7% (30.2–59.9) | 96.9% (94.4–98.4) | |
| △Sn and △Sn | 23.4% (13.6 to 37.2) | −6.3% (−9.7 to −3.5) | ||||||
| Inpatients | FujiLAM | 53 | 17 | 8 | 2 | 26 | 89.5% (66.9–98.7) | 76.5% (58.8–89.3) |
| AlereLAM | 53 | 14 | 6 | 5 | 28 | 73.7% (48.8–90.9) | 82.4% (65.5–93.2) | |
| △Sn and △Sn | 15.8% (−3.7 to 37.6) | −5.9% (−23.7 to 11.8) | ||||||
| CRS | Test | N | TP | FP | FN | TN | Sensitivity (95% CI) | Specificity (95% CI) |
| Outpatients | FujiLAM | 397 | 39 | 26 | 23 | 309 | 62.9% (49.7–75.8) | 92.2% (88.8–94.9) |
| AlereLAM | 397 | 24 | 8 | 38 | 327 | 38.7% (26.6–51.9) | 97.6% (95.3–99.0) | |
| △Sn and △Sn | 24.2% (15.3 to 36.2) | −5.4% (−8.7 to −2.6) | ||||||
| Inpatients | FujiLAM | 53 | 24 | 1 | 12 | 16 | 66.7% (49.0–81.4) | 94.1% (71.3–99.9) |
| AlereLAM | 53 | 19 | 1 | 17 | 16 | 52.8% (35.5–69.6) | 94.1% (71.3%–99.9%) | |
| △Sn and △Sn | 13.9% (−2.9 to 31.0) | 0.0% (−22.7 to 22.7) |
Abbreviations: AlereLAM, Alere Determine TB LAM Ag assay; CI, confidence interval; CRS, composite reference standard; FN, false negatives; FP, false positives; FujiLAM, Fujifilm SILVAMP TB LAM assay; LAM, lipoarabinomannan; MRS, microbiological reference standard; Sn, sensitivity; Sp, specificity; TN, true negatives; TP, true positives.
(A) Diagnostic accuracy using a MRS: Definite tuberculosis were considered reference standard positive. Not tuberculosis and Possible tuberculosis were considered reference standard negative. (B) Diagnostic accuracy using a CRS: Definite tuberculosis and Possible tuberculosis were considered reference standard positive. Not tuberculosis were considered reference standard negative.
The sensitivity of both tests increased with lower CD4 cell counts. The sensitivity in participants with CD4 ≤100 cells/µL was 84.4% (27 of 32; 95% CI, 67.2–94.7) for FujiLAM compared with 65.6% (21 of 32; 95% CI, 46.8–81.4) for Alere LAM (difference of 18.8%; 95% CI, 6.0–35.3) (Table 5). The difference in sensitivity was greatest for participants with CD4 >200 cells/µL in favor of FujiLAM (26.6%; 95% CI, 0.8–52.0), but overall numbers were small (Table 4). Against a CRS, overall sensitivity decreased to 64.3% (63 of 98; 95% CI, 54.0–73.7) for FujiLAM and to 43.9% (43 of 98; 95% CI, 33.9–54.3) for AlereLAM (retaining a difference of 20.4%; 95% CI, 11.8–30.0) (Table 3).
Table 5.
Sensitivity and specificity of FujiLAM versus AlereLAM against MRS (A) and CRS (B) by CD4 strata
| Test | N | TP | FP | FN | TN | Sensitivity (95% CI) | Specificity (95% CI) | |
| 0–100 cells/µL | FujiLAM | 148 | 27 | 35 | 5 | 81 | 84.4% (67.2–94.7) | 69.8% (60.6–78.0) |
| AlereLAM | 148 | 21 | 14 | 11 | 102 | 65.6% (46.8–81.4) | 87.9% (80.6–93.2) | |
| △Sn and △Sn | 18.8% (6.0 to 35.3) | −18.1% (−27.6 to −9.0) | ||||||
| >100 cells/µL | FujiLAM | 288 | 20 | 3 | 12 | 253 | 62.5% (43.7–78.9) | 98.8% (96.6–99.8) |
| AlereLAM | 288 | 12 | 2 | 20 | 254 | 37.5% (21.1–56.3) | 99.2% (97.2–99.9) | |
| △Sn and △Sn | 25.0% (11.6 to 42.1) | −0.4% (−2.5 to 1.5) | ||||||
| 0–200 cells/µL | FujiLAM | 237 | 39 | 35 | 10 | 153 | 79.6% (65.7–89.8) | 81.4% (75.1–86.7) |
| AlereLAM | 237 | 29 | 15 | 20 | 173 | 59.2% (44.2–73.0) | 92.0% (87.2–95.5) | |
| △Sn and △Sn | 20.4% (11.5 to 33.6) | −10.6% (−17.0 to −4.8) | ||||||
| >200 cells/µL | FujiLAM | 199 | 8 | 3 | 7 | 181 | 53.3% (26.6–78.7) | 98.4% (95.3–99.7) |
| AlereLAM | 199 | 4 | 1 | 11 | 183 | 26.7% (7.8–55.1) | 99.5% (97.0–100.0) | |
| △Sn and △Sn | 26.6% (0.8 to 52.0) | −1.1% (−3.9 to 1.0) | ||||||
| 101–200 cells/µL | FujiLAM | 89 | 12 | 0 | 5 | 72 | 70.6% (44.0–89.7) | 100.0% (95.0–100.0) |
| AlereLAM | 89 | 8 | 1 | 9 | 71 | 47.1% (23.0–72.2) | 98.6% (92.5–100.0) | |
| △Sn and △Sn | 23.5% (−0.8 to 47.3) | 1.4% (−3.7 to 7.5) | ||||||
| CRS | Test | N | TP | FP | FN | TN | Sensitivity (95% CI) | Specificity (95% CI) |
| 0–100 cells/µL | FujiLAM | 148 | 38 | 24 | 13 | 73 | 74.5% (60.4–85.7) | 75.3% (65.5–83.5) |
| AlereLAM | 148 | 28 | 7 | 23 | 90 | 54.9% (40.3–68.9) | 92.8% (85.7–97.0) | |
| △Sn and △Sn | 19.6% (6.0 to 33.9) | −17.5% (−27.6 to −8.1) | ||||||
| >100 cells/µL | FujiLAM | 288 | 21 | 2 | 22 | 243 | 48.8% (33.3–64.5) | 99.2% (97.1–99.9) |
| AlereLAM | 288 | 12 | 2 | 31 | 243 | 27.9% (15.3–43.7) | 99.2% (97.1–99.9) | |
| △Sn and △Sn | 20.9% (11.0 to 35.2) | 0.0% (−1.9 to 1.9) | ||||||
| 0–200 cells/µL | FujiLAM | 237 | 50 | 24 | 22 | 141 | 69.4% (57.5–79.8) | 85.5% (79.1–90.5) |
| AlereLAM | 237 | 36 | 8 | 36 | 157 | 50.0% (38.0–62.0) | 95.2% (90.7–97.9) | |
| △Sn and △Sn | 19.4% (9.0 to 31.0) | −9.7% (−16.1 to −3.9) | ||||||
| >200 cells/µL | FujiLAM | 199 | 9 | 2 | 13 | 175 | 40.9% (20.7–63.6) | 98.9% (96.0–99.9) |
| AlereLAM | 199 | 4 | 1 | 18 | 176 | 18.2% (5.2–40.3) | 99.4% (96.9–100.0) | |
| △Sn and △Sn | 22.7% (0.4 to 43.4) | −0.6% (−3.1 to 1.6) | ||||||
| 101–200 cells/µL | FujiLAM | 89 | 12 | 0 | 9 | 68 | 57.1% (34.0–78.2) | 100.0% (94.7–100.0) |
| AlereLAM | 89 | 8 | 1 | 13 | 67 | 38.1% (18.1–61.6) | 98.5% (92.1–100.0) | |
| △Sn and △Sn | 19.0% (0.6 to 40.0) | 1.5% (−4.0 to 7.9) |
Abbreviations: AlereLAM, Alere Determine TB LAM Ag assay; CI, confidence interval; CRS, composite reference standard; FN, false negatives; FP, false positives; FujiLAM, Fujifilm SILVAMP TB LAM assay; LAM, lipoarabinomannan; MRS, microbiological reference standard; Sn, sensitivity; Sp, specificity; TN, true negatives; TP, true positive.
(A) Diagnostic accuracy using a MRS: Definite tuberculosis were considered reference standard positive. Not tuberculosis and Possible tuberculosis were considered reference standard negative. (B) Diagnostic accuracy using a CRS: Definite tuberculosis and Possible tuberculosis were considered reference standard positive. Not tuberculosis were considered reference standard negative.
Against an MRS, the specificity was 89.3% (343 of 384; 95% CI, 85.8–92.2) for FujiLAM versus 95.6% (367 of 384; 95% CI, 93.0–97.4) for AlereLAM (difference of −6.3%; 95% CI, −9.6 to −3.3) (Table 3). FujiLAM specificity was lowest in participants with CD4 ≤100 cells/µL (69.8%; 81 of 116; 95% CI, 60.6–78.0) but increased markedly to 98.8% (253 of 256; 95% CI, 96.6–99.8) in participants with CD4 >100 cells/µL, where it was comparable to AlereLAM specificity 99.2% (254 of 256; 95% CI, 97.2–99.9; difference of −0.4% [95% CI, −2.5 to 1.5]) (Table 5).
Specificity increased when using a CRS to 92.3% (325 of 352; 95% CI, 89.0–94.9) for FujiLAM and to 97.4% (343 of 352; 95% CI, 95.2–98.8) for AlereLAM (Table 3). Of note, 25 of 27 FujiLAM-positive participants classified as tuberculosis negative against the CRS had a CD4 cell count below 100, and 22% (6 of 27) died or were lost to follow up after 2 months (Supplementary Table S2).
Against an MRS restricted to the results of urine Xpert testing (n = 419), we found that FujiLAM was positive in 83.3% (15 of 18) of participants with a positive urine Xpert result (sensitivity), whereas AlereLAM was positive in 72.2% (13 of 18) of participants. Additional analysis of FujiLAM and AlereLAM accuracy by smear status is shown in Supplementary Table S3. Our secondary analysis, reclassifying the unclassifiable, is reported in the Supplementary Table S4.
For FujiLAM testing, 8 of 532 (1.5%) required a second test to produce valid results. For AlereLAM, 2 of 532 (0.4%) required a second test for a valid result. The following 2 types of errors were reported for FujiLAM: (1) insufficient volume of reducing reagent released after pressing button 1 so that the liquid did not reach the end of the test strip, and (2) negative control line. Interreader agreement between the readers was high for both tests (FujiLAM 98.5% [kappa 0.9487; Standard Error (SE) 0.043], AlereLAM 99.3% [kappa 0.961; SE 0.043]) (Supplementary Table S5).
Nontuberculous Mycobacteria and Fujifilm SILVAMP TB-LAM Positivity
Nontuberculous mycobacteria (NTM) were cultured from sputum of 53 of 532 participants (10.0%; 95% CI, 7.7–12.9) (Supplementary Table S6). Of these, NTM were cultured in 2 sputum specimens for 9 of 53 (17.0%) cases and in a single sputum sample for the remaining cases. Among not tuberculosis participants with NTM isolated, FujiLAM was negative in 25 of 27 (92.6%) cases and positive in 2 of 27 (7.4%) with the slow-growing Mycobacterium intracellulare and Mycobacterium avium cultured from sputum. Both of these participants had low CD4 cell count (2 and 65) and survived 6 months of follow up without any treatment for mycobacteria, suggesting against a disseminated systemic infection. Among possible tuberculosis participants with NTM isolated, FujiLAM was positive in 6 of 7 (85.7%) cases, and clinical characteristics suggested a severely sick population with extreme immunosuppression (median CD4 of 3 cells/µL; IQR, 2–5) and high mortality (50%; 3 of 6 died within 6 months). Data for AlereLAM are presented in Supplementary Table S6.
Association of Lipoarabinomannan Test Positivity and Mortality
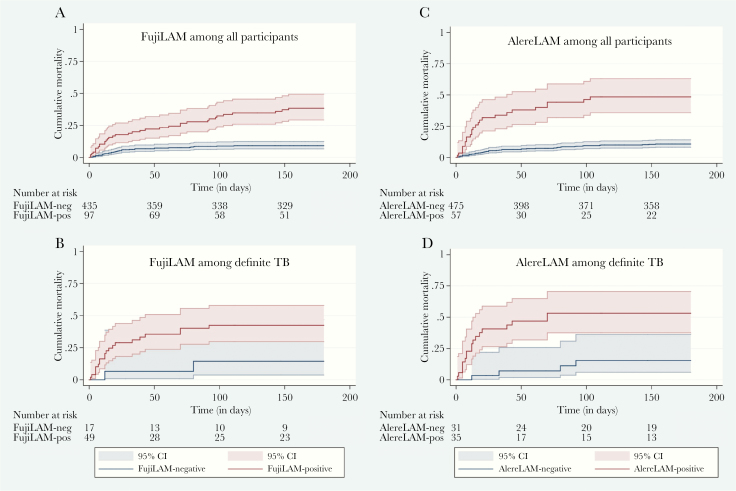
By 6 months, 71 (13%) of all participants had died and an additional 89 (17%) were lost to follow up. The median time to death for FujiLAM-positive participants was 30 days (IQR, 8–91) compared with 13 days (IQR, 7–39) for AlereLAM. FujiLAM was positive in 49.3% (35 of 71) of participants who had died by 6 months versus 35.2% (25 of 71) for AlereLAM (difference 14%; 95% CI, 2.1–26.0). Mortality proportions for all participants and those with definite tuberculosis stratified by FujiLAM and AlereLAM status are presented in Supplementary Table S7. A positive FujiLAM test result was associated with higher risk of death at 2 and 6 months compared with FujiLAM negative (Table 6). AlereLAM-positive participants had a higher HR for death than FujiLAM. The Kaplan-Meier mortality curves are shown in Figure 2.
Table 6.
Cox Regression Analyses for Mortality at 2 and 6 Months According to FujiLAM and AlereLAM Status Among All Participants (n = 532) and Among Definite TB Participants (n = 66)
| Population | Unadjusted Hazard Ratio (95% CI) | P Value |
|---|---|---|
| At 2 Months, All Participants (n = 532) | ||
| FujiLAM negative | Ref. | |
| FujiLAM positive | 3.6 (2.0–6.2) | <.001 |
| AlereLAM negative | Ref. | |
| AlereLAM positive | 6.7 (3.8–11.7) | <.001 |
| At 2 Months, Definite TB (n = 66) | ||
| FujiLAM negative | Ref. | |
| FujiLAM positive | 6.2 (0.8–46.4] | .077 |
| AlereLAM negative | Ref. | |
| AlereLAM positive | 8.7 (2.0–38.0) | .004 |
| At 6 Months, All participants (n = 532) | ||
| FujiLAM negative | Ref. | |
| FujiLAM positive | 4.8 (3.0–7.6) | <.001 |
| AlereLAM negative | Ref. | |
| AlereLAM positive | 6.2 (3.8–10.1) | <.001 |
| At 6 Months, Definite TB (n = 66) | ||
| FujiLAM negative | Ref. | |
| FujiLAM positive | 3.7 (0.9–16.0) | .075 |
| AlereLAM negative | Ref. | |
| AlereLAM positive | 5.2 (1.7–15.3) | .003 |
Abbreviations: CI, confidence interval; Ref., reference; TB, tuberculosis.
Figure 2.
Kaplan-Meier curves for cumulative 6-month mortality stratified by FujiLAM and AlereLAM, respectively. Kaplan-Meier curves shown for (A) FujiLAM among all participants (n = 532; log rank <0.001); (B) FujiLAM among definite tuberculosis (TB) participants (n = 66; log rank = 0.054); (C) AlereLAM among all participants (n = 532; log rank <0.001); (D) AlereLAM among definite TB participants (n = 66; log rank = 0.001).
Discussion
In this first tuberculosis diagnostic accuracy study of the urinary FujiLAM point-of-care test among adult outpatients with HIV, we demonstrate superior sensitivity compared with that of AlereLAM in our study population overall and across CD4 strata (sensitivity gain ranging from 15% to 25% difference). FujiLAM alone could detect 74% of definite tuberculosis cases. Specificity was lower for FujiLAM than for AlereLAM, but the majority of FujiLAM false-positive results were observed among participants with CD4 ≤100 cells/μL, whereas specificity of the 2 tests was comparable in participants with CD4 >100 cells/μL. Specificity increased using a CRS.
Results from our study are in line with the findings of a recent assessment of FujiLAM among hospitalized patients with HIV and mirror the same trend of highest FujiLAM sensitivity in participants with low CD4 cell count [11]. We further demonstrate a significant sensitivity gain of using FujiLAM compared with AlereLAM among outpatients and participants with higher CD4 cell count. Overall, sensitivity was above the minimum WHO suggested target of 65% for rapid nonsputum TB tests and approaching the optimal requirement of 80% [4]. The estimated specificity was low in participants with advanced immunosuppression (CD4 ≤100 cells/μL), but otherwise it met the optimal target of 98% against an MRS [4].
Our MRS was based on cultures from 2 sputum samples and Xpert testing of sputum and urine. Although culture is considered the best available reference standard for tuberculosis, it is not a perfect reference standard due to the paucibacillary nature and the common extrapulmonary and disseminated disease among PWH, particularly among patients with low CD4 count [3, 19, 20]. Moreover, culture, and in particular liquid culture (MGIT), is prone to contamination, as also found in our study. Our reference standard was further limited by sputum Xpert not being available for all participants, no use of sputum induction, and use of a low volume of urine (6 mL) for urinary Xpert testing. This may have impaired the quality and diagnostic yield of the reference standard and would be expected to disproportionally affect a more sensitive test [19, 21]. By recognizing these limitations to an otherwise comprehensive reference standard, we may have misclassified a truly positive FujiLAM result as a false positive. Misclassification may in part explain the low FujiLAM specificity among participants with CD4 ≤100 cells/μL, where the tuberculosis diagnosis is hardest to ascertain. Low specificity among patients with low CD4 cell count was also noted for AlereLAM test in this study as well as in systematic reviews of AlereLAM diagnostic accuracy studies [8, 22]. These findings highlight the challenge of assessing diagnostic specificity for a test that is not site-specific, and that it is important to recognize that specificity estimates may suffer from reference-standard bias.
We could not determine whether heterogeneity in specificity estimates was fully attributable to misclassification bias. In studies of AlereLAM, we previously found some signs of cross-reactivity between AlereLAM and NTM [13, 23], and others also suggested that disseminated NTM disease may cause false-positive AlereLAM results [24, 25]. In analytical studies of the antibodies used in FujiLAM, there was no observed cross-reactivity to rapid-growing NTM [12]. In our study, isolation of NTM in sputum was common and likely represented a mix of NTM contaminants (eg, the vast majority had a single sputum with NTM isolation) and some with true NTM pulmonary/disseminated disease. The majority of not tuberculosis with NTM cultured were FujiLAM negative (25 of 27), whereas 2 participants were FujiLAM positive both with slow-growing NTMs isolated. Given the limitations of our reference standard, it is possible that the participants may have had mixed infection with M tuberculosis that was missed. Participants with possible tuberculosis and sputum NTM isolation (mainly slow-growing) had advanced immunosuppression, high mortality, and negative urine Xpert to suggest possible pulmonary/disseminated NTM disease, and the majority (6 of 7) were FujiLAM positive. Although this raises concerns that clinically important slow-growing NTMs among PWH may affect FujiLAM test result and contribute to low specificity estimate, it needs to be evaluated in studies with a higher quality of reference standard to discriminate between isolated tuberculosis disease, pulmonary/disseminated NTM diseases, and the possibility of a mixed infections with tuberculosis and NTM. The background prevalence of NTM disease relative to the burden of tuberculosis among PWH must also be considered when assessing the PPV of FujiLAM results (and implications of possible cross-reactivity) [26]. Concerns about specificity may need to be assessed with the potential biases in mind and also considering the potential benefits of increased sensitivity for a population in which there may be no other means of making a diagnosis of tuberculosis and mortality of tuberculosis is high.
The turnaround time is longer for the FujiLAM test (50–60 minutes) than the AlereLAM (25 minutes) test and requires additional steps in the workflow. On the other hand, the FujiLAM test interpretation is easier because no reference scale card, such as that used for the AlereLAM test, is required [9]. Implications of these differences on the feasibility and acceptance of the FujiLAM test in clinical practice and for integration in existing algorithms should be addressed in future studies.
A positive FujiLAM test predicted mortality in our study population and identified a higher proportion of participants who died than the AlereLAM test. We do not know whether FujiLAM testing could have prevented some of these deaths, as demonstrated for AlereLAM in clinical trials [27, 28]. We speculate that because the FujiLAM test is developed to detect LAM at a lower concentration than AlereLAM [12], FujiLAM detects LAM from early stages of tuberculosis disease where patients are less sick and survive the follow-up period. Previous quantitative LAM studies have demonstrated that LAM concentration positively correlates with poor prognosis, which supports this explanation [29, 30].
The strengths of this study are a large and representative cohort of well characterized PWH with follow up of 6 months. We have already discussed the limitations of our reference standard. Other limitations include that only ART-naive patients were included in the study and the retrospective testing of stored samples; however, a recent study showed high agreement of FujiLAM test results using fresh versus biobanked samples [31]. We could not classify 82 (15%) participants according to our predefined diagnostic classification mainly due to loss to follo w up, but sensitivity analysis including the unclassifiable did not affect specificity, and the higher sensitivity of FujiLAM compared with AlereLAM was maintained. Prospective studies in clinical setting is the next step needed to evaluate the diagnostic accuracy of FujiLAM. Future studies should consider systematic sampling of multiple specimens from pulmonary and extrapulmonary sites to improve the quality of the reference standard and assess the effect of clinically important disseminated/pulmonary NTM disease.
Conclusions
In conclusion, with significantly higher sensitivity compared with AlereLAM, the FujiLAM test has the potential to improve early detection and treatment of tuberculosis among PWH and translate this into reduced morbidity and mortality. Prospective studies of FujiLAM in clinical settings with a comprehensive mycobacterial reference test are now needed.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the participants in the DETECT HIV-TB study. We also thank Johanna Åhsberg and Kwaku Appiah-Korang Labi for organizing samples before shipment to Japan as well as the staff at the Department of Medical Microbiology, School of Biomedical and Allied Health, University of Ghana, and at the designated laboratory at Research Institute of Tuberculosis/Japan Anti-Tuberculosis Association for organizing transport and testing of samples.
Author contributions. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Disclaimer. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript. The Foundation for Innovative New Diagnostics (FIND) is a not-for-profit foundation, whose mission is to find diagnostic solutions to overcome diseases of poverty in low- and middle-income countries (LMICs). It works closely with the private and public sectors and receives funding from some of its industry partners (no funding received from Cepheid for the development or the evaluation of Ultra). It has organizational firewalls to protect it against any undue influences in its work or the publication of its findings. All industry partnerships are subject to review by an independent Scientific Advisory Committee or another independent review body, based on due diligence, TTPs, and public sector requirements. FIND catalyzes product development, leads evaluations, takes positions, and accelerates access to tools identified as serving its mission. It provides indirect support to industry (eg, access to open specimen banks, a clinical trial platform, technical support, expertise, laboratory capacity strengthening in LMICs, etc) to facilitate the development and use of products in these areas. FIND also supports the evaluation of publicly prioritized tuberculosis (TB) assays and the implementation of World Health Organization-approved (guidance and PQ) assays using donor grants. FIND has product evaluation agreements with several private sector companies for TB and other diseases, which strictly define its independence and neutrality vis-a-vis the companies whose products get evaluated and describes roles and responsibilities.
Financial support. This work was funded by the Global Health Innovative Technology Fund (GHIT Grant Number G2017-207) and the KfW (Grant Number 2020 60 457), whereas the DETECT HIV-TB study was funded by a grant from Odense University Hospital Free Research Fund (Grant Number 11/28764), the Danish AIDS Foundation (Grant Number F12-10), and the Aase and Ejnar Danielsens Fond (Grant Number 10-001013).
Potential conflicts of interests. R. S., A. M., S. G. S., and E. M. are employed by FIND, and T. B. and C. M. D. were employed at FIND during the conduct of the study. T. B. further reports patents in the field of lipoarabinomannan detection. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global Tuberculosis Report. Geneva: World Health Organization; 2018. [Google Scholar]
- 2. Pai M, Behr MA, Dowdy D, et al. Tuberculosis. Nat Rev Dis Primers 2016; 2:16076. [DOI] [PubMed] [Google Scholar]
- 3. Shivakoti R, Sharma D, Mamoon G, Pham K. Association of HIV infection with extrapulmonary tuberculosis: a systematic review. Infection 2017; 45:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. High-Priority Target Product Profiles for New Tuberculosis Diagnostics, Report of a Consenus Meeting. Geneva: World Health Organization; 2014. [Google Scholar]
- 5. World Health Organization. The Use of Lateral Flow Urine Lipoarabinomannan Assay (LF-LAM) for the Diagnosis and Screening of Active Tuberculosis in People Living with HIV. Policy Guidance. Geneva: World Health Organization; 2015. [Google Scholar]
- 6. World Health Organization. Lateral Flow Urine Lipoarabinomannan Assay (LF-LAM) for the Diagnosis of Active Tuberculosis in People Living with HIV. Policy Update (2019). Geneva: World Health Organization; 2019. [Google Scholar]
- 7. Saran K, Masini T, Chikwanha I, et al. Countries are out of step with international recommendations for tuberculosis testing, treatment, and care: findings from a 29-country survey of policy adoption and implementation. bioRxiv 2019. Available at: https://www.biorxiv.org/content/10.1101/533851v1
- 8. Bjerrum S, Schiller I, Dendukuri N, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in people living with HIV. Cochrane Database Syst Rev 2019; 10:CD011420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mathabire Rucker SC, Cossa L, Harrison RE, et al. Feasibility of using Determine TB-LAM to diagnose tuberculosis in HIV-positive patients in programmatic conditions: a multisite study. Glob Health Action 2019; 12:1672366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huerga H, Mathabire Rucker SC, Cossa L, et al. Diagnostic value of the urine lipoarabinomannan assay in HIV-positive, ambulatory patients with CD4 below 200 cells/μl in 2 low-resource settings: a prospective observational study. PLoS Med 2019; 16:e1002792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Broger T, Sossen B, du Toit E, et al. Novel lipoarabinomannan point-of-care tuberculosis test for people with HIV: a diagnostic accuracy study. Lancet Infect Dis 2019; 19:852–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sigal GB, Pinter A, Lowary TL, et al. A novel sensitive immunoassay targeting the 5-methylthio-d-xylofuranose-lipoarabinomannan epitope meets the WHO's performance target for tuberculosis diagnosis. J Clin Microbiol 2018; 56:e01338-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bjerrum S, Kenu E, Lartey M, et al. Diagnostic accuracy of the rapid urine lipoarabinomannan test for pulmonary tuberculosis among HIV-infected adults in Ghana-findings from the DETECT HIV-TB study. BMC Infect Dis 2015; 15:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bjerrum S, Oliver-Commey J, Kenu E, et al. Tuberculosis and non-tuberculous mycobacteria among HIV-infected individuals in Ghana. Trop Med Int Health 2016; 21:1181–90. [DOI] [PubMed] [Google Scholar]
- 15. National HIV/AIDS/STI Control Programme, Guidelines for antiretroviral therapy in Ghana, 3rd ed. Accra: Ministry of Health/Ghana Health Service;. 2010. [Google Scholar]
- 16. Bossuyt PM, Reitsma JB, Bruns DE, et al. ; STARD Group STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015; 351:h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abbott. Alere DetermineTM TB LAM Ag product information. Waltham, MA: Alere Inc; 2007. Available at: alere.com/en/home/product-details/determine-tb-lam.html [Google Scholar]
- 18. Tango T. Equivalence test and confidence interval for the difference in proportions for the paired-sample design. Stat Med 1998; 17:891–908. [DOI] [PubMed] [Google Scholar]
- 19. Kohli M, Schiller I, Dendukuri N, et al. Xpert((R)) MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database Syst Rev 2018; 8:CD012768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Horne DJ, Kohli M, Zifodya JS, et al. Xpert MTB/RIF and Xpert MTB/RIF Ultra for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2019; 6:CD009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peter JG, Theron G, Singh N, et al. Sputum induction to aid diagnosis of smear-negative or sputum-scarce tuberculosis in adults in HIV-endemic settings. Eur Respir J 2014; 43:185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shah M, Hanrahan C, Wang ZY, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in HIV-positive adults. Cochrane Database Syst Rev 2016; CD011420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qvist T, Johansen IS, Pressler T, et al. Urine lipoarabinomannan point-of-care testing in patients affected by pulmonary nontuberculous mycobacteria–experiences from the Danish Cystic Fibrosis cohort study. BMC Infect Dis 2014; 14:655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nel JS, Lippincott CK, Berhanu R, et al. Does disseminated nontuberculous mycobacterial disease cause false-positive determine TB-LAM lateral flow assay results? A retrospective review. Clin Infect Dis 2017; 65:1226–8. [DOI] [PubMed] [Google Scholar]
- 25. Songkhla MN, Tantipong H, Tongsai S, Angkasekwinai N. Lateral flow urine lipoarabinomannan assay for diagnosis of active tuberculosis in adults with human immunodeficiency virus infection: a prospective cohort study. Open Forum Infect Dis 2019; 6:ofz132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gupta-Wright A, Kerkhoff AD, Meintjes G, Corbett EL. Urinary lipoarabinomannan detection and disseminated nontuberculous mycobacterial disease. Clin Infect Dis 2018; 66:158. [DOI] [PubMed] [Google Scholar]
- 27. Peter JG, Zijenah LS, Chanda D, et al. Effect on mortality of point-of-care, urine-based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV-positive hospital inpatients: a pragmatic, parallel-group, multicountry, open-label, randomised controlled trial. Lancet 2016; 387:1187–97. [DOI] [PubMed] [Google Scholar]
- 28. Gupta-Wright A, Corbett EL, van Oosterhout JJ, et al. Rapid urine-based screening for tuberculosis in HIV-positive patients admitted to hospital in Africa (STAMP): a pragmatic, multicentre, parallel-group, double-blind, randomised controlled trial. Lancet 2018; 392:292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shah M, Martinson NA, Chaisson RE, et al. Quantitative analysis of a urine-based assay for detection of lipoarabinomannan in patients with tuberculosis. J Clin Microbiol 2010; 48:2972–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kerkhoff AD, Wood R, Vogt M, Lawn SD. Prognostic value of a quantitative analysis of lipoarabinomannsan in urine from patients with HIV-associated tuberculosis. PLoS One 2014; 9:e103285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Broger T, Muyoyeta M, Kerkhoff AD, et al. High agreement of tuberculosis test results using fresh versus biobanked urine samples with Fujifilm SILVAMP TB LAM. Lancet Infect Dis 2019. doi:10.1016/S1473-3099(19)30001-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.