Abstract
Infancy is the foundational period for learning from adults, and the dynamics of the social environment have long been considered central to children’s development. Here, we reveal a novel, naturalistic approach for studying live interactions between infants and adults. Using functional near-infrared spectroscopy (fNIRS), we simultaneously and continuously measured the brains of infants (N = 18; 9–15 months of age) and an adult while they communicated and played with each other. We found that time-locked neural coupling within dyads was significantly greater when dyad members interacted with each other than with control individuals. In addition, we characterized the dynamic relationship between neural activation and the moment-to-moment fluctuations of mutual gaze, joint attention to objects, infant emotion, and adult speech prosody. This investigation advances what is currently known about how the brains and behaviors of infants both shape and reflect those of adults during real-life communication.
Keywords: communication, development, infancy, naturalistic, language, functional near-infrared spectroscopy, neural coupling, open data, open materials
The ability to communicate during the first years of life requires the development of common ground with other people. This involves learning to engage with social environments using a range of behaviors, including eye gaze, facial expressions, and speech. By adulthood, we interact with others following a set of norms shared by our community of speakers.
Recent work with adults indicates that shared understanding is associated with shared neural responses across people in a set of higher-order brain regions (Hasson, Ghazanfar, Galantucci, Garrod, & Keysers, 2012). Responses in these areas correlate strongly across people when input is interpreted in a similar way, irrespective of its form (Honey, Thompson, Lerner, & Hasson, 2012). For example, written and spoken versions of the same story evoke comparable neural responses across readers and listeners (Regev et al., 2019). Furthermore, effective storytelling reflects the successful transfer of information between brains: Responses in a listener’s linguistic and higher-order brain areas are coupled (i.e., correlated with a short temporal lag) to the responses in a speaker’s brain, and stronger speaker–listener coupling is associated with better comprehension (Stephens, Silbert, & Hasson, 2010). Importantly, this coupling in higher-order areas is not simply driven by shared exposure to the same perceptual input; areas of the default-mode network (including medial prefrontal cortex) are coupled only when participants have a shared understanding of a story, not simply when identical stimuli are presented (Lerner, Honey, Silbert, & Hasson, 2011; Simony et al., 2016). Speaker–listener neural coupling has been found using both functional MRI (fMRI; Stephens et al., 2010) and functional near-infrared spectroscopy (fNIRS; Jiang et al., 2012; Liu et al., 2017), showing strong consistency between the two methodologies.
How does this shared neural code emerge in our brains as we begin learning during the first years of life? The ability to understand language input does not emerge automatically. On the contrary, children’s knowledge of the conventional ways of using words to communicate ideas, desires, and intentions must emerge over time from interactions with other members of a community of speakers (Brazelton, Koslowski, & Main, 1974; Tomasello, 1992; Vygotsky, 1978). Two-way interactions provide continuous information about these communicative conventions, in the form of feedback from (and to) caregivers, and transactional models of development emphasize the roles of both infants and caregivers in shaping communication and learning (see Sameroff, 2009). For instance, mothers modify the pitch, rhythm, and overall spectral quality of their voices when talking to their infants (Fernald & Kuhl, 1987; Piazza, Iordan, & Lew-Williams, 2017), even changing their prosody in real time on the basis of their children’s emotional feedback (N. A. Smith & Trainor, 2008). On the other side of the interaction, infants preferentially attend to and learn from communicatively relevant information (Ferguson & Lew-Williams, 2016; Vouloumanos & Werker, 2004), and infants’ gaze following and pointing predict later language outcomes (Brooks & Meltzoff, 2008). This generates an interesting prediction: that dynamic coupling between infant and adult brains may support the successful exchange of information during everyday interactions.
Here, we aimed to understand the ways in which the brains of infants and adults are coupled in real time, both to each other and to natural social behaviors. To do so, we developed a new dual-brain fNIRS paradigm for simultaneously measuring the brains of an adult caregiver and 9- to 15-month-old infants while they engaged in continuous, everyday, two-way interactions, including playing, singing, and reading. fNIRS provides a noninvasive measure of changes in blood oxygenation resulting from neural activity while being minimally sensitive to motion artifacts, thus allowing multiple participants to move and interact freely, face to face, while wearing comfortable caps (Boas, Elwell, Ferrari, & Taga, 2014). Our fNIRS hyperscanning (two-brain) paradigm opens new experimental possibilities for studying the reciprocal dynamics of natural communication among multiple people, such as one person’s brain predicting another person’s upcoming behavior. This approach contrasts with that of previous studies, which used a single fMRI scanner to measure neural coupling between adult speakers and listeners during one-way communication. We predicted that infant-to-caregiver brain-to-brain coupling would be higher when the infant and adult interacted with each other, compared with a control condition in which each member of the dyad interacted with another person in the room. Furthermore, we predicted that activation in areas of the brain involved in mutual understanding (in particular, prefrontal and parietal areas; Liu et al., 2017) would be related to natural communicative behaviors, such as mutual gaze, joint attention to objects, infant smiling, and adult speech prosody.
Method
Participants
Eighteen infants (age: M = 11.3 months, range = 9.8–14.9 months; 9 female) with no history of hearing problems and no known developmental delays participated in the experiment. One experimenter with extensive parenting experience performed the adult role in all experimental sessions. This experimenter was generally aware of the basic hypothesis (i.e., stronger coupling when she interacted with the infant than when she ignored the infant), but she was naive with respect to details, such as the behavioral cues or brain areas that might be relevant. One of the included 18 infants was excluded from behavioral analyses because of video malfunction; 3 additional infants were unable to participate in the experiment because they refused to wear the fNIRS cap, and 21 additional infants were excluded from statistical analyses because of excessive signal noise or artifacts (15 of these infants grabbed the cap or squirmed excessively, resulting in significant head motion). The sample size was based on sizes used in previous studies that measured neural coupling in communication paradigms with fMRI (e.g., Stephens et al., 2010) and fNIRS (Jiang et al., 2012; Liu et al., 2017).
Procedure
We used a dual-brain LABNIRS system (Shimadzu Scientific Instruments, Columbia, MD) to simultaneously record brain activity from the adult and infant in each dyad. We recorded from 57 channels (3 cm in the adult, 2.5 cm in the infants) across the cortex of each participant. These channels covered prefrontal cortex (PFC), temporoparietal junction, and parietal cortex (i.e., areas involved in prediction, language processing, and understanding other people’s perspectives; Lerner et al., 2011). The locations of these channels were homologous across the infant and the adult (see Fig. 1a). Caps were positioned on the basis of known anatomical landmarks according to the international 10-20 system (e.g., the center point of the cap was approximately at Cz). Our primary intersubject correlation (ISC) analyses, which aimed to determine the spatial extent of coupling, focused on deoxyhemoglobin because it is less likely than oxyhemoglobin to include systemic effects and therefore measures more spatially precise cortical activation (Boas et al., 2014; Hirsch, Zhang, Noah, & Ono, 2017).
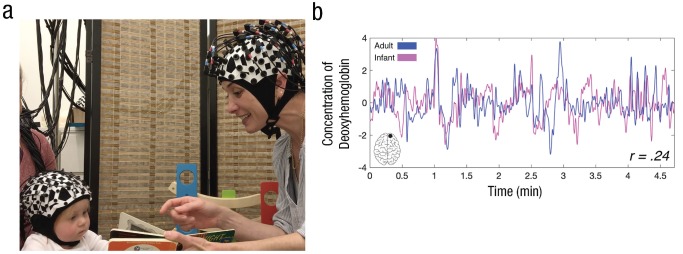
Fig. 1.
Example of an interaction between an adult and infant during the together condition (a) and the corresponding intersubject correlation (ISC) in one channel pair (b). The graph shows the concentration of deoxyhemoglobin across the length of an interaction during the together condition, separately for the adult and infant. The ISC between the adult and infant, computed from a single right prefrontal cortex (PFC) channel, is shown at the bottom right.
All adult–infant dyads participated in two 5-min conditions. In the together condition, the adult experimenter engaged directly with the child by playing with a consistent set of toys, singing nursery rhymes, and reading Goodnight Moon (Fig. 1a). The child sat on his or her parent’s lap, and the parent was told to keep the child comfortable but not to communicate with the child in any way. In the control (apart) condition, the experimenter turned 90° away from the child and told a story to another adult experimenter using adult-directed speech, while the child interacted quietly with his or her parent. The order of the two conditions was counterbalanced across participants. This comparison allowed us to test whether coupling was stronger when the adult and child directly communicated with each other than when they were engaged in a similarly communicative task but not communicating with each other. Importantly, in both conditions, the members of the dyad shared common perceptual input: They could hear the same speech, see the same toys, and look at faces in both conditions. The critical difference was that in the together condition, the members of a dyad interacted directly with each other, and in the apart condition, they each interacted with someone else.
Preprocessing and analysis
We removed motion artifacts using moving standard deviation and spline interpolation (Scholkmann, Spichtig, Muehlemann, & Wolf, 2010); we also low-pass-filtered (0.5 Hz) and high-pass-filtered (0.02 Hz) the signal to remove physiological noise and drift, respectively. We conducted additional control analyses that demonstrated that our ISC results could not be driven by motion artifacts (see Fig. S2 in the Supplemental Material available online). To calculate ISC, we computed a Pearson correlation between one channel in the adult and one channel in the infant (Fig. 1b).
Because of the presence of long-range temporal autocorrelation in fNIRS time series, we estimated the statistical likelihood of each observed correlation using a bootstrapped permutation procedure. This procedure is based on surrogate data generated using phase randomization (see Simony et al., 2016), which preserves the mean and autocorrelation of the original signal but randomizes the phases after applying a fast Fourier transform. For each of 3,249 channel combinations (57 infant × 57 adult), we computed a group-average Pearson correlation coefficient based on phase-scrambled time series in the adult and infant of each dyad. We performed this procedure 20,000 times to yield a null distribution of ISCs for each channel combination and computed a p value by calculating the proportion of null values that exceeded the true ISC value for that channel combination; p values of 0 (in which the entire null distribution fell below the true ISC) were set to 1 divided by the number of bootstrapped samples. To correct for multiple comparisons, we applied the false-discovery-rate procedure (Benjamini & Hochberg, 1995; q < .05).
During the together condition, we continuously recorded video and audio of the dyadic interaction. Later, a research assistant coded every 500 ms whether or not there was mutual gaze (joint eye contact) between the adult and infant, whether or not the infant was smiling, and whether or not the infant and adult were engaged in joint attention to an external object, such as a toy or book. We did not code adult smiling because the adult experimenter smiled more than 95% of the time; the nearly continuous adult smiling left minimal opportunities for the infant to react to individual instances of smiling. Two additional research assistants each coded these behaviors in subsets of the data (two thirds of the data sets each), and the scores were highly reliable across the three raters (Cronbach’s α = .70, averaged across the behavioral measures; Cronbach, 1951). After filtering out background noise from the audio recordings in Adobe Audition, we analyzed continuous fundamental frequency (F0) using Praat software (Version; 6.0.41; Boersma & Weenink, 2009). Only six infants displayed any smiling throughout the videos, and this subset was used in the smiling-related analyses. During the 5-min duration of the together condition, mutual gaze was present 27% of the time (on average across dyads); the corresponding rates of infant smiling, joint attention, and adult speech were 5.5%, 40%, and 49%, respectively.
To perform brain-behavior comparisons, we first downsampled the fNIRS time series in each channel to match the behavioral data (2 Hz for mutual gaze, smiling, and joint attention, 5 Hz for F0 variability) before computing time-lagged correlations. Here, we report oxyhemoglobin for clarity of interpretation, as this signal reflects increases in local cortical activation in relation to the onset of behavioral events and is commonly reported in similar event-related analyses (e.g., Emberson, Boldin, Robertson, Cannon, & Aslin, 2018).
Results
The brain responses of the infants and the adult experimenter were recorded simultaneously using fNIRS (Fig. 1) in two conditions: together and apart. In the together condition, each adult-infant dyad participated in face-to-face sets of playful interactions with each other, and in the apart condition, they interacted with other individuals.
In the first set of analyses (Figs. 2 and 3), we assessed the significance of neural coupling between infants and the adult experimenter in both conditions using a robust bootstrapped phase-scrambling analysis across all available cortical channels and a form of cross-correlation that validated the temporal specificity of coupling in one cortical region of interest—the PFC. In the second and third sets of analyses (Figs. 4 and 5), we measured the relationship between the neural activation of each participant in each dyad (adult, infant) and socially salient, dynamic behaviors measured throughout the together condition (mutual gaze, infant smiling, joint attention, and adult speech prosody).
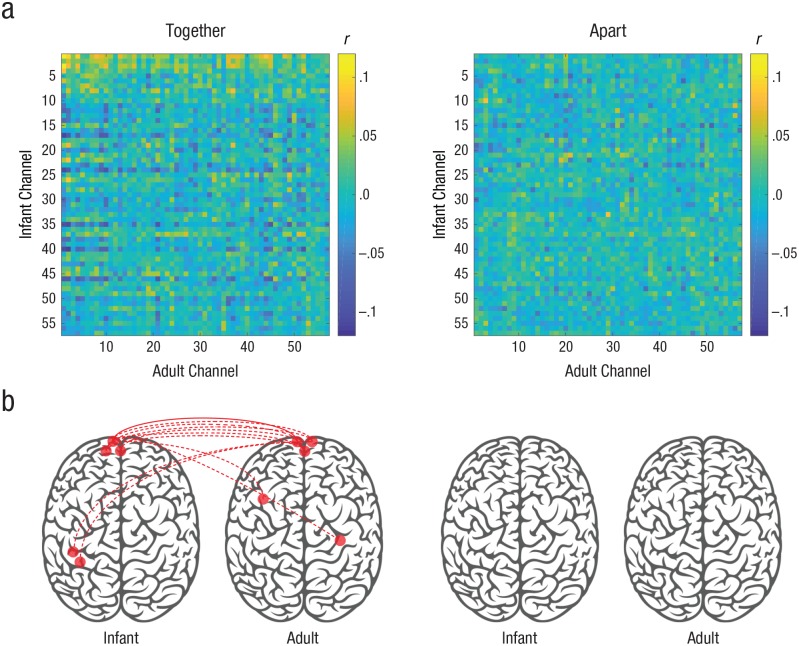
Fig. 2.
Spatial extent of infant–adult neural coupling. The intersubject correlation matrices (a) show the relation between all infant and adult channels, separately for the together and apart conditions. The brain diagrams (b) show significantly coupled channel pairs (in red), determined by phase-scrambling analysis and corrected for multiple comparisons using the false-discovery-rate procedure (q < .05; Benjamini & Hochberg, 1995). The solid line indicates a homologous channel pair; dotted lines indicate nonhomologous channel pairs. N = 18 dyads.
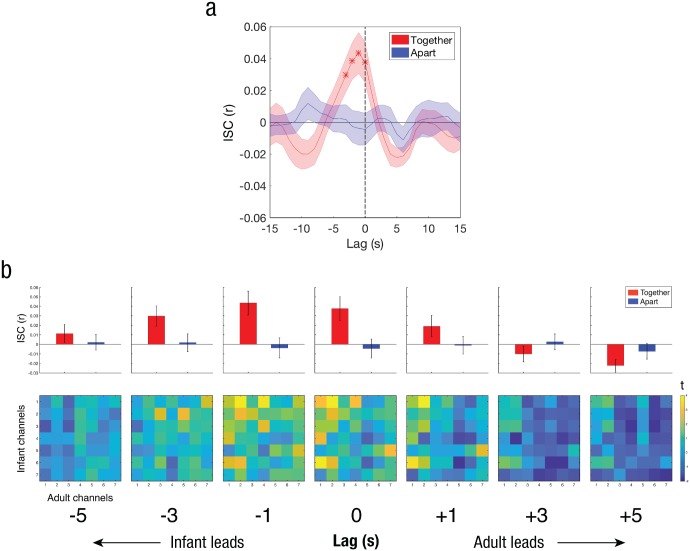
Fig. 3.
Temporal dynamics of infant–adult coupling in the prefrontal cortex (PFC). The graph in (a) shows the intersubject correlation (ISC) between the adult and infant PFC signals across relative shifts (lags) of the two signals, separately for the together and apart conditions. Asterisks indicate significant lags, corrected for multiple comparisons. The top row in (b) shows mean ISC values across all 49 pairwise (infant–adult) combinations of PFC channels, averaged across dyads and shown separately for each condition at lags between −5 s and +5 s. The bottom row in (b) shows t maps comparing infant–adult ISCs (together vs. apart) for all pairwise PFC channel combinations at lags between −5 s and +5 s. Error bands (a) and error bars (b) represent standard errors of the mean. N = 18 dyads.
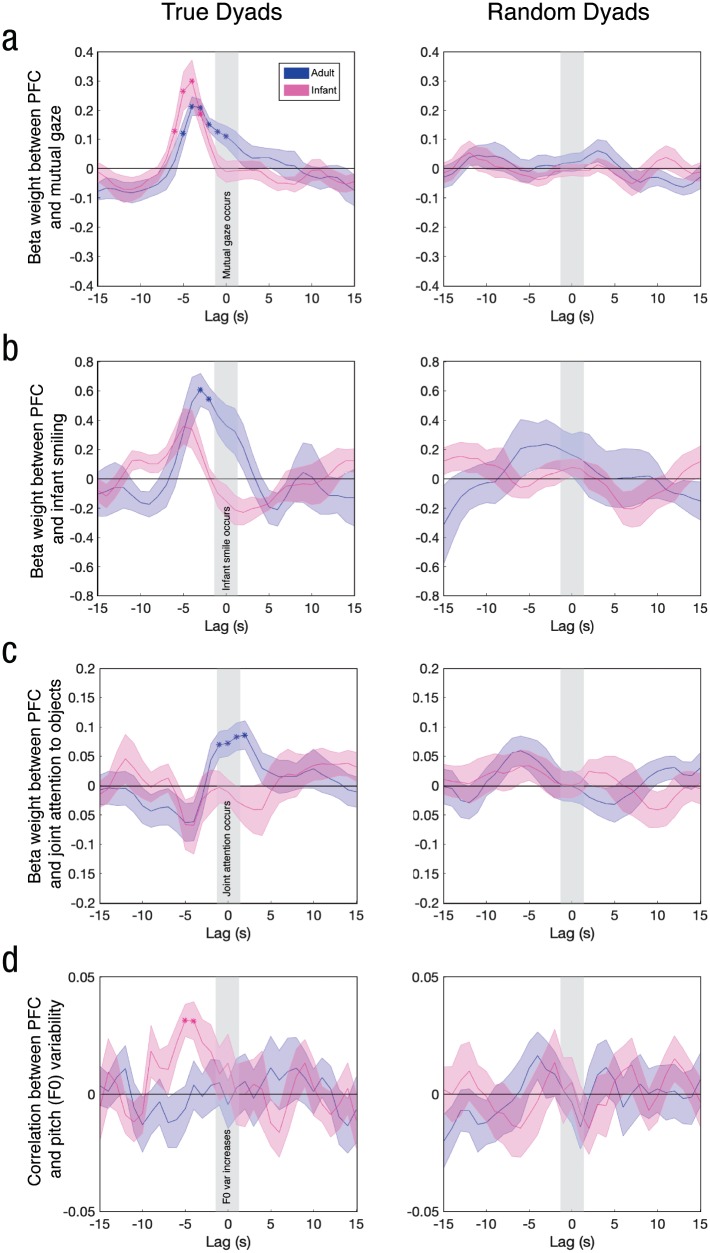
Fig. 4.
Time-lagged relationship between neural responses in the prefrontal cortex (PFC) and continuous measures of (a) mutual gaze, (b) infant smiling, (c) joint attention to objects, and (d) pitch (F0) variability of the adult’s speech. Results are shown separately for the adult and for infants (n = 17 for all analyses except smiling, n = 6). The left column shows results for true dyads, and the right column depicts control results using random brain-behavior assignments. Asterisks indicate time lags at which coefficients significantly exceeded 0 after correction for multiple comparisons across lags. Error bands represent standard errors of the mean.
Fig. 5.
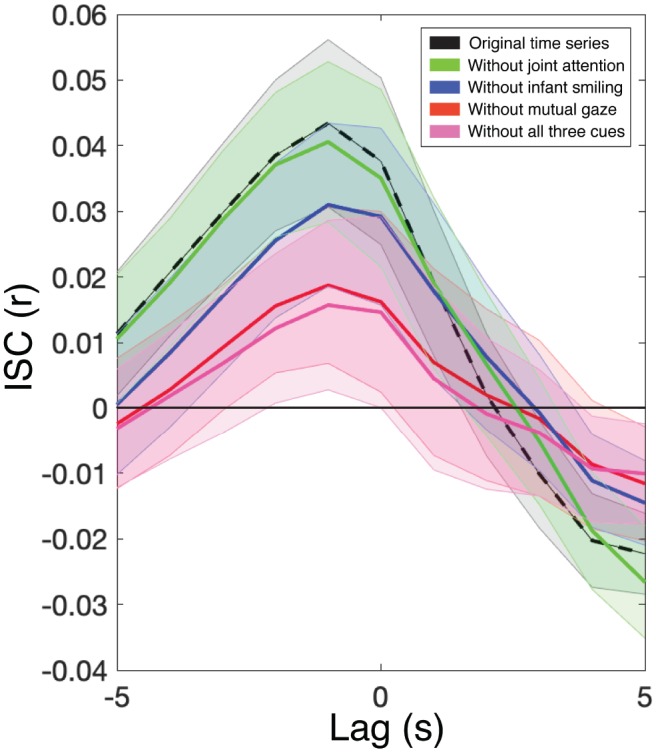
Time-lagged intersubject correlation (ISC). Results are shown for five analyses: one using the original prefrontal cortex (PFC) time series (dashed line, which represents the curve for the together condition shown in Fig. 3a) and the other four after regressing out the time course of joint attention to objects from the PFC time series, the time course of infant smiling, the time course of mutual gaze, and all three cues. Error bands represent standard errors of the mean. N = 17.
Infant–adult neural coupling is present only during joint interaction
In the together (but not the apart) condition, we found significant coupling between many PFC channels and some parietal channels of infants and the adult (see Fig. 2b). ISC between the infant and adult brains was measured during the together and apart conditions, and these actual correlation values were compared with null distributions of correlation values. We included all homologous (i.e., same channel across brains) and nonhomologous (i.e., different channels across brains) channel pairings in this analysis. Statistical significance was determined using a permutation procedure based on phase-randomized surrogate data, and multiple-comparisons correction was performed across channel pairs (see the Method section). These results revealed that communication between an infant and a caregiver was reflected in significant intersubject coupling throughout cortical areas involved in social and narrative processing. Specifically, as shown in Figure 2b, we found eleven significant channel pairs (primarily in the PFC) in the together condition and no significant channel pairs in the apart condition. The spatial selectivity of this pattern of coupling indicated that the effects were not due to widespread, global arousal throughout the brain.
To further assess infant–adult coupling, we compared the strength of ISC between the infant and adult brains during the together and apart phases in the PFC channels, which exhibited the most robust coupling in the whole-brain analysis. Each participant had seven channels covering the PFC. For each dyad, we computed the average ISC across all 49 pairwise combinations of PFC channels (Infant Channel 1 vs. Adult Channel 1, Infant Channel 1 vs. Adult Channel 2, etc.). To assess the temporal dynamics of the infant–adult coupling, we shifted the two signals relative to each other in time and performed the above statistical comparison (together vs. apart) at shifts (lags) of −15 s (infant’s PFC activity leading) to +15 s (adult’s PFC activity leading) in 1-s increments (Fig. 3a).
Significant infant–adult neural coupling was found at a lag of 0 and at negative lags of −1, −2, and −3 s (infant leading), but only for the together condition (Fig. 3a; see also Fig. S1 in the Supplemental Material). Infant–adult neural coupling in the apart condition did not exceed chance at any lag. Similarly, infant–adult neural coupling was significantly greater in the together condition than in the apart condition only at the same set of lags (0, −1, −2, and −3 s; see Fig. S1). The pattern of channel combinations that drove these channel-averaged results can be seen in Figure 3b (bottom row), which shows t statistics (together – apart) for all 49 pairwise channel correlations at each lag. These findings suggest that the infant PFC may have slightly led the adult PFC. For these time-shifted analyses, p values were corrected using false-discovery rate across lags (q < .05; Benjamini & Hochberg, 1995). This lag in neural responses between the child and the experimenter helped to ensure that the neural coupling was not a mere reflection of shared perceptual input. If responses were locked to shared audiovisual input, then neural coupling should have peaked at Lag 0. Additionally, the relative strength of PFC (compared with parietal) coupling reinforces the idea that these results were not driven by coupling in sensorimotor areas as a result of joint motion.
The PFC plays a dynamic role in joint eye contact, infant emotion, and joint attention to objects
Infant–adult coupling emerges from continuous feedback between interlocutors through eye contact, facial expressions, vocal prosody, and other cues. Which behavioral cues are most directly involved in the coupling between the infant and adult brains during the interaction? To start probing this question, we asked an independent rater (whose scores were validated by two additional raters; see the Method section) to view and code the video recording of each infant–adult interaction on a frame-by-frame basis along three behavioral dimensions: (a) the presence of mutual gaze (i.e., whether the adult and infant were looking directly at each other’s faces), (b) the signaling of a smile by the infant, and (c) the presence of joint attention (i.e., whether the adult and infant were looking at the same object, such as a toy or book). We then shifted all fNIRS time series relative to the behavioral time series, taking into account a canonical hemodynamic lag of 4 s to 5 s, before performing regression between the brain and behavioral signals. When we instead convolved the behavioral time series with a canonical hemodynamic response function (Arichi et al., 2012), the results (described in Fig. 4) were highly similar.
Next, we performed linear regression using the behavioral time series to predict the infants’ and the adult’s brain responses. We did so to examine whether responses in PFC increased during moments of mutual gaze, of infant smiling, and of joint attention. For each dyad, we performed this regression in each channel of the adult and infant. We averaged the resulting beta weights within our specific region of interest (PFC) and computed group-level statistics across dyads (see Fig. S1). To assess the temporal alignment between the neural and behavioral time series, we shifted the two signals relative to each other in time and performed the above steps at lags from −15 s (brain leading) to +15 s (behavior leading) in 1-s increments (see the Method section).
In both the infant and adult brains, PFC activation slightly preceded moments of mutual gaze (i.e., joint eye contact between the infant and adult; Fig. 4a, left). Specifically, the relationship between brain and behavior peaked at about 5 s before the initiation of the mutual gaze (see Fig. S1). This suggests that the PFC of both individuals anticipated—or even drove—an increase in joint social behavior. PFC activity also modulated the presence of infants’ smiles. Here, we observed a more distinct pattern of results between infants and the adult (Fig. 4b, left). Specifically, both infant and adult PFC responses increased before the initiation of an infant smile, with the infant PFC responses preceding the adult’s; this confirmed our general finding that the infants’ neural dynamics led the adult’s. Finally, the adult PFC showed a time-locked relationship to the time course of joint attention to objects (Fig. 4c, left). Control analyses randomly reassigning each neural time series to the behavioral data from a different dyad (Figs. 4a–4c, right) showed no significant brain–behavior relationship for any of the three measures at any lag. This result ensured that the effects we observed were due to the unique, dyad-specific dynamics of a given interaction and not simply the fact that a particular task (e.g., singing) might have reliably increased both behavioral and neural activity at a coarse level across all dyads because of similarities in overall task structure.
Finally, we assessed to what extent the direct infant–adult brain-to-brain coupling (reported in Fig. 3) was modulated by mutual gaze, infant smiling, and joint attention to objects. We did this by regressing out these three behavioral time courses from the fNIRS (PFC) signals of each dyad (both infant and adult) before recalculating infant–adult ISC. Specifically, we first regressed out each behavioral time series at all possible lags from −15 s to +15 s relative to each infant and adult fNIRS time series (after accounting for a 4-s hemodynamic lag). This analysis completely removed the influence of each behavior, regardless of the temporal offset at which it was most correlated with brain activation. After regressing out each behavior, we recalculated ISC from lags of −5 s (infant leading) to +5 s (adult leading; see Fig. 5). At the peak of the curve (lag = −1 s), brain-to-brain coupling significantly decreased after the removal of infant smiling, t(16) = 2.09, p < .05, Cohen’s d = 0.52, 95% confidence interval (CI) for d = [–0.16, 1.21]; mutual gaze, t(16) = 3.12, p < .01, Cohen’s d = 0.78, 95% CI for d = [0.08, 1.48]; and all three behaviors together (infant smiling, mutual gaze, and joint attention), t(16) = 3.01, p < .01, Cohen’s d = 0.75, 95% CI for d = [0.06, 1.45]. However, removing joint attention alone did not significantly decrease coupling, t(16) = −0.79, p = .44, Cohen’s d = −0.20, 95% CI for d = [–0.87, 0.48]. This suggests that mutual gaze and infant smiling may have contributed more to neural coupling than did joint attention to objects, but differences in sample size and in the frequency of each behavior suggest the need for further research on this finding.
The infant PFC is dynamically linked to the pitch variability of adult speech
Another factor known to facilitate communication with infants is the intonation of infant-directed speech, also known as “motherese.” We assessed the relationship between infant and adult neural responses and the dynamics of the adult’s infant-directed speech, focusing on F0 variability because of its influence on infant attention (Fernald & Kuhl, 1987) and language processing (Trainor & Desjardins, 2002). To do this, we measured the relationship between F0 variability and the fNIRS signal across time bins in the together condition. We chose a bin size of 200 ms because the syllabic rate of child-directed speech is typically close to 4 Hz to 5 Hz (Ryan, 2000). In each time bin in which there were recorded F0 values, we extracted the standard deviation of F0 of the adult’s voice as well as the average fNIRS activation in that bin. After once again accounting for the hemodynamic lag, we computed the correlation between these two measures, averaged across the two most frontal PFC channels (Bonferroni-corrected for all possible pairs of PFC channels; α = .002).
An increase in infant PFC response amplitude was reliably followed by an increase in pitch variability of the caregiver’s speech. Figure 4d (left) shows that PFC responses in the infant brain were significantly correlated with the F0 variability of the adult’s speech at lags of −4 s to −5 s (see Fig. S1) after false-discovery-rate correction across lags (from −15 s to +15 s, as in the analyses above). The adult PFC was not significantly correlated with this measure at any lag, suggesting that the adult’s own pitch variability was not directly related to the responses in her PFC. This influence of the infants’ neural dynamics on the adult’s speech is consistent with the direction of coupling (infant preceding adult) found in the main ISC analyses and indicates the experimenter’s sensitivity to the state of the infant. For example, she may have increased her vocal excitement in response to a range of possible infant behaviors that could be linked to an infant PFC response. Once again, a control analysis randomly reassigning each neural time series to the behavioral data from a different dyad (Fig. 4d, right) showed no significant brain–behavior relationship at any lag, suggesting that the infant PFC’s relationship to the variability of adult speech was due to dyad-specific dynamics.
In addition, for each dyad, we found time windows containing the highest 10% of F0 variability for that session and compared infant PFC activation (again, in the first two channels) with activation during all other moments (lower 90% in F0 variability). Moments of particularly high pitch variability were preceded by significantly higher infant PFC activation than other moments (see Fig. S3a in the Supplemental Material)—two-tailed t test, t(16) = 4.35, p < .001, Cohen’s d = 1.09, 95% CI for d = [0.37, 1.81].
Finally, we assessed the extent to which direct infant–adult brain-to-brain coupling was modulated by the adult speaker’s pitch variability. We did so by regressing its time course at all lags from −15 s to +15 s relative to each infant and adult fNIRS time series (after accounting for a 4-s hemodynamic lag), as in the partial regression analysis above. After regressing out F0, we recalculated ISC at lags of −5 s to +5 s (see Fig. S3b in the Supplemental Material). The inclusion in this analysis of fNIRS data only at time points containing speech resulted in a slightly noisier baseline ISC curve here (Fig. S3b, black line) than in Figure 3a (red line), but the shape remained highly consistent. At the peak of the curve (Lag 0), we found no significant change in ISC after removing F0 variability, t(16) = −0.84, p = .41, Cohen’s d = −0.21, 95% CI for d = [–0.88, 0.46], suggesting that the infant–adult brain-to-brain coupling observed in the PFC was not significantly mediated by variability in the adult’s speech prosody over time. This finding is consistent with our finding of lack of modulation of the adult PFC responses by the F0 variability of her own voice (Fig. 4d, left).
Discussion
From the beginning of life, infants’ survival depends on successful communication with adult caregivers. Researchers have uncovered important details about how the behaviors of infants and adults are coupled during natural communication (Cohn & Tronick, 1988; Marsh, Richardson, & Schmidt, 2009), but very little is known about how their brains interact during this process. Our research, using an infant-friendly imaging technique, provides the first demonstration of the dynamic role played by both the developing and mature brain during live social interaction. Moreover, we began uncovering the relationships between various communicative behaviors and infant–adult neural alignment.
During coupled interactions, infant and adult brains are dynamically linked to important social cues (gaze, smiling, joint attention, and speech prosody), although to differing extents. Our findings represent a crucial step toward understanding how infants’ brain responses both anticipate and process the most important structure from adults’ input and how adults’ brains represent infants’ emotional feedback as adults strive to engage infants in everyday communication. This opens new possibilities for understanding the independence versus interdependence of two brains, the role of coupling in early learning, individual differences in early processing and learning, and the many dimensions of coupling in infant–caregiver interactions over time.
The findings support transactional development models, which emphasize not only the role of adults’ input but children’s potential role in shaping their own input (Sameroff, 2009; for related ideas, see L. B. Smith, Jayaraman, Clerkin, & Yu, 2018). Our results expand on these frameworks and demonstrate a new application of dynamic-systems theory (Thelen & Smith, 1996) by showing how adults’ and infants’ brains reflect each other during natural, social interaction—mediated, importantly, by sensitivity to each other’s behaviors. Overall, we found that prefrontal activity in infants’ brains slightly preceded similar activity in the adult’s brain, despite the relative lack of overt behavioral influence exhibited by infants (e.g., minimal vocalization relative to the adult). This is consistent with fMRI evidence showing that the activity in the medial and dorsolateral PFC of an adult listener often precedes a speaker’s PFC activity; this anticipatory signal is likely to relate to prediction of upcoming events in a story (Dikker, Silbert, Hasson, & Zevin, 2014; Stephens et al., 2010). Our findings suggest that the adult was sensitive to subtle behavioral cues from the infants (likely via a combination of explicit and implicit processes), which in turn modified the adult’s brain responses and behaviors in real time in order to improve alignment with, and maximize information transfer to, the infants. Recent developmental work has emphasized a feedback loop between developmental changes to the body, the statistics of input, and brain networks (Byrge, Sporns, & Smith, 2014), and our investigation recasts this proposal within a multibrain framework.
Importantly, we measured alignment between brain and behavior from both sides of the dyadic interaction, in support of this dynamic view. In most cases, neural responses preceded behavior, but the two brains were differentially engaged with each cue depending on its social relevance. For instance, both adult and infant PFCs were significantly coupled to the time course of mutual gaze—a social cue common to both individuals—at lags that skewed slightly negatively (Fig. 4a, left), which implies that neural alignment preceded and anticipated joint eye contact. The adult PFC tracked joint attention to objects in a time-locked manner and anticipated infant smiling (Figs. 4b and 4c, left), probably because the adult was motivated to use the toys to engage the infants and elicit positive emotional responses; the infant PFC, on the basis of our conservative corrections, showed a nonsignificant trend toward anticipating the infants’ own smiles (Fig. 4b, left). Finally, the infant PFC anticipated changes in the adult’s infant-directed speech (Fig. 4d, left), likely because the adult used extreme pitch contours in response to a range of infant behaviors, possibly to highlight a certain word. This finding differs from those of previous studies showing that variation in speech prosody drives infants’ attention (Fernald & Kuhl, 1987) and that musical features drive the adult medial PFC (Janata et al., 2002).
Why did PFC activation often precede behavioral cues? It could be that the PFC was predicting behaviors, contributing to the generation of those behaviors, or responding to earlier, mediating social events. Future investigations of the causal link between brain and behavior (via measures of additional brain regions, real-time feedback, or transcranial magnetic stimulation) could help to distinguish these interpretations and further clarify how the PFC and other areas organize the statistics of social and nonsocial input throughout development.
Our investigation of the complementary neural representations of second-to-second social dynamics advances what is currently known about infant–adult interactions, building on previous fNIRS research showing heightened medial PFC activation in infants in response to direct gaze (Urakawa, Takamoto, Ishikawa, Ono, & Nishijo, 2015). It also expands on electroencephalogram research showing enhanced coupling between infants and adults during direct (vs. indirect) gaze (Leong et al., 2017) and showing parents’ neural responsiveness to infants’ attention (Wass et al., 2019). Importantly, our design enabled us not only to simultaneously measure neural responsiveness of both infants and adults to a variety of natural communicative cues but also to compare the relative contributions of these different cues to coupling. For example, mutual gaze appears to play a stronger role in driving ISC than joint attention to objects does (Fig. 5). Future work will benefit from analyses of a broader range of behavioral cues known to be important for successful communication and learning, such as hand actions and other gestures (Brand, Baldwin, & Ashburn, 2002; Yu & Smith, 2013), contingency of adult feedback on infant vocalizations (Goldstein, King, & West, 2003), and facial expressions. Whether in lab manipulations or unconstrained interactions, systematic comparisons of neural coupling and a range of behaviors will unravel how brains synchronize during naturalistic communication.
Because of the constraints of fNIRS research (and developmental neuroscience in general), our current sample was limited to infants who could sit relatively still during an interaction with a stranger. To enhance our understanding of natural variability in how caregivers and children align their brains and behaviors, researchers will need new approaches to study how infants with different temperaments and communicative abilities couple with adults. In particular, our findings prompt comparisons of the reciprocal dynamics of brain-to-brain coupling in typically developing populations and the breakdown of neural responses to social cues in disorders such as autism spectrum disorder (Elsabbagh et al., 2009).
To conclude, our investigation represents an innovative approach to the study of social interaction—namely, by tracking the back-and-forth relationships between brains and behaviors during live communication. Studying this process in 1-year-old infants has the potential to advance models of socially embedded cognition and learning in development. This research highlights the value of examining the perspectives of both the developing and mature members of dyads, an approach that will enable a rich understanding of how infants and adults work together to facilitate playful, shared communication.
Supplemental Material
Supplemental material, Piazza_Open_Practices_Disclosure for Infant and Adult Brains Are Coupled to the Dynamics of Natural Communication by Elise A. Piazza, Liat Hasenfratz, Uri Hasson and Casey Lew-Williams in Psychological Science
Supplemental material, Piazza_Supplemental_Material for Infant and Adult Brains Are Coupled to the Dynamics of Natural Communication by Elise A. Piazza, Liat Hasenfratz, Uri Hasson and Casey Lew-Williams in Psychological Science
Acknowledgments
We thank Ariella Cohen, Alice Wang, Mia Sullivan, Sagi Jaffe-Dax, Eva Fourakis, and Carolyn Mazzei for assistance with data collection, behavioral coding, analysis feedback, and participant recruitment.
Footnotes
Action Editor: Rebecca Treiman served as action editor for this article.
Author Contributions: E. A. Piazza, C. Lew-Williams, and U. Hasson designed the experiment, E. A. Piazza and L. Hasenfratz collected the data, E. A. Piazza analyzed the data with feedback from U. Hasson and C. Lew-Williams. E. A. Piazza drafted the manuscript, and all authors edited the manuscript.
ORCID iD: Elise A. Piazza  https://orcid.org/0000-0001-6729-8559
https://orcid.org/0000-0001-6729-8559
Declaration of Conflicting Interests: The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Funding: This work was supported by the Princeton University C. V. Starr Fellowship (to E. A. Piazza), the Eric and Wendy Schmidt Transformative Technology Award (to E. A. Piazza, U. Hasson, and C. Lew-Williams), National Institutes of Health (NIH) Grant 5DP1HD091948 (to U. Hasson), and NIH Grants R01HD095912 and R03HD079779 (to C. Lew-Williams).
Supplemental Material: Additional supporting information can be found at http://journals.sagepub.com/doi/suppl/10.1177/0956797619878698
Open Practices:


All data, analysis scripts, and materials needed to reproduce this study have been made publicly available via the Open Science Framework and can be accessed at https://osf.io/udxqp/. The design and analysis plans for the experiment were not preregistered. The complete Open Practices Disclosure for this article can be found at http://journals.sagepub.com/doi/suppl/10.1177/0956797619878698. This article has received the badges for Open Data and Open Materials. More information about the Open Practices badges can be found at http://www.psychologicalscience.org/publications/badges.
References
- Arichi T., Fagiolo G., Varela M., Melendez-Calderon A., Allievi A., Merchant N., . . . Edwards A. D. (2012). Development of BOLD signal hemodynamic responses in the human brain. NeuroImage, 63, 663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society B: Methodological, 57, 289–300. [Google Scholar]
- Boas D. A., Elwell C. E., Ferrari M., Taga G. (2014). Twenty years of functional near-infrared spectroscopy: Introduction for the special issue. NeuroImage, 85, 1–5. [DOI] [PubMed] [Google Scholar]
- Boersma P., Weenink D. (2009). Praat: Doing phonetics by computer (Version 6.0.41) [Computer software]. Retrieved from http://www.praat.org/
- Brand R. J., Baldwin D. A., Ashburn L. A. (2002). Evidence for ‘motionese’: Modifications in mothers’ infant-directed action. Developmental Science, 5, 72–83. [Google Scholar]
- Brazelton T. B., Koslowski B., Main M. (1974). The origins of reciprocity: The early mother-infant interaction. In Lewis M., Rosenblum L. A. (Eds.), The effect of the infant on its caregiver (pp. 49–76). Oxford, England: Wiley-Interscience. [Google Scholar]
- Brooks R., Meltzoff A. N. (2008). Infant gaze following and pointing predict accelerated vocabulary growth through two years of age: A longitudinal, growth curve modeling study. Journal of Child Language, 35, 207–220. [DOI] [PubMed] [Google Scholar]
- Byrge L., Sporns O., Smith L. B. (2014). Developmental process emerges from extended brain-body-behavior networks. Trends in Cognitive Sciences, 18, 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn J. F., Tronick E. Z. (1988). Mother-infant face-to-face interaction: Influence is bidirectional and unrelated to periodic cycles in either partner’s behavior. Developmental Psychology, 24, 386–392. [Google Scholar]
- Cronbach L. J. (1951). Coefficient alpha and the internal structure of tests. Psychometrika, 16, 297–334. [Google Scholar]
- Dikker S., Silbert L. J., Hasson U., Zevin J. D. (2014). On the same wavelength: Predictable language enhances speaker-listener brain-to-brain synchrony in posterior superior temporal gyrus. The Journal of Neuroscience, 34, 6267–6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M., Volein A., Csibra G., Holmboe K., Garwood H., Tucker L., . . . Johnson M. H. (2009). Neural correlates of eye gaze processing in the infant broader autism phenotype. Biological Psychiatry, 65, 31–38. [DOI] [PubMed] [Google Scholar]
- Emberson L. L., Boldin A. M., Robertson C. E., Cannon G., Aslin R. N. (2018). Expectation affects neural repetition suppression in infancy. Developmental Cognitive Neuroscience, 37, Article 100597. doi: 10.1016/j.dcn.2018.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson B., Lew-Williams C. (2016). Communicative signals support abstract rule learning by 7-month-old infants. Scientific Reports, 6, Article 25434. doi: 10.1038/srep25434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald A., Kuhl P. (1987). Acoustic determinants of infant preference for motherese speech. Infant Behavior and Development, 10, 279–293. [Google Scholar]
- Goldstein M. H., King A. P., West M. J. (2003). Social interaction shapes babbling: Testing parallels between birdsong and speech. Proceedings of the National Academy of Sciences, USA, 100, 8030–8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U., Ghazanfar A. A., Galantucci B., Garrod S., Keysers C. (2012). Brain-to-brain coupling: A mechanism for creating and sharing a social world. Trends in Cognitive Sciences, 16, 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J., Zhang X., Noah J. A., Ono Y. (2017). Frontal temporal and parietal systems synchronize within and across brains during live eye-to-eye contact. NeuroImage, 157, 314–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey C. J., Thompson C. R., Lerner Y., Hasson U. (2012). Not lost in translation: Neural responses shared across languages. The Journal of Neuroscience, 32, 15277–15283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janata P., Birk J. L., Van Horn J. D., Leman M., Tillmann B., Bharucha J. (2002). The cortical topography of tonal structures underlying Western music. Science, 298, 2167–2170. [DOI] [PubMed] [Google Scholar]
- Jiang J., Dai B., Peng D., Zhu C., Liu L., Lu C. (2012). Neural synchronization during face-to-face communication. The Journal of Neuroscience, 45, 16064–16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong V., Byrne E., Clackson K., Georgieva S., Lam S., Wass S. (2017). Speaker gaze increases information coupling between infant and adult brains. Proceedings of the National Academy of Sciences, USA, 114, 13290–13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner Y., Honey C. J., Silbert L. J., Hasson U. (2011). Topographic mapping of a hierarchy of temporal receptive windows using a narrated story. The Journal of Neuroscience, 31, 2906–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Piazza E. A., Simony E., Shewokis P. A., Onaral B., Hasson U., Ayaz H. (2017). Measuring speaker–listener neural coupling with functional near infrared spectroscopy. Scientific Reports, 7, Article 43293. doi: 10.1038/srep43293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh K. L., Richardson M. J., Schmidt R. C. (2009). Social connection through joint action and interpersonal coordination. Topics in Cognitive Science, 1, 320–339. [DOI] [PubMed] [Google Scholar]
- Piazza E. A., Iordan M. C., Lew-Williams C. (2017). Mothers consistently alter their unique vocal fingerprints when communicating with infants. Current Biology, 27, 3162–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev M., Simony E., Lee K., Tan K. M., Chen J., Hasson U. (2019). Propagation of information along the cortical hierarchy as a function of attention while reading and listening to stories. Cerebral Cortex, 29, 4017–4034. doi: 10.1093/cercor/bhy282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan B. P. (2000). Speaking rate, conversational speech acts, interruption, and linguistic complexity of 20 pre-school stuttering and non-stuttering children and their mothers. Clinical Linguistics & Phonetics, 14, 25–51. [DOI] [PubMed] [Google Scholar]
- Sameroff A. (2009). The transactional model. In Sameroff A. (Ed.), The transactional model of development: How children and contexts shape each other (pp. 3–21). Washington, DC: American Psychological Association. [Google Scholar]
- Scholkmann F., Spichtig S., Muehlemann T., Wolf M. (2010). How to detect and reduce movement artifacts in near-infrared imaging using moving standard deviation and spline interpolation. Physiological Measurement, 31, 649–662. [DOI] [PubMed] [Google Scholar]
- Simony E., Honey C. J., Chen J., Lositsky O., Yeshurun Y., Wiesel A., Hasson U. (2016). Dynamic reconfiguration of the default mode network during narrative comprehension. Nature Communications, 7, Article 12141. doi: 10.1038/ncomms12141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. B., Jayaraman S., Clerkin E., Yu C. (2018). The developing infant creates a curriculum for statistical learning. Trends in Cognitive Sciences, 22, 325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith N. A., Trainor L. J. (2008). Infant-directed speech is modulated by infant feedback. Infancy, 4, 410–420. [Google Scholar]
- Stephens G. J., Silbert L. J., Hasson U. (2010). Speaker–listener neural coupling underlies successful communication. Proceedings of the National Academy of Sciences, USA, 107, 14425–14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen E., Smith L. B. (1996). A dynamic systems approach to the development of cognition and action. Cambridge, MA: MIT Press. [Google Scholar]
- Tomasello M. (1992). The social bases of language acquisition. Social Development, 1, 67–87. [Google Scholar]
- Trainor L. J., Desjardins R. J. (2002). Pitch characteristics of infant-directed speech affect infants’ ability to discriminate vowels. Psychonomic Bulletin & Review, 9, 335–340. [DOI] [PubMed] [Google Scholar]
- Urakawa S., Takamoto K., Ishikawa A., Ono T., Nishijo H. (2015). Selective medial prefrontal cortex responses during live mutual gaze interactions in human infants: An fNIRS study. Brain Topography, 28, 691–701. [DOI] [PubMed] [Google Scholar]
- Vouloumanos A., Werker J. F. (2004). Tuned to the signal: The privileged status of speech for young infants. Developmental Science, 7, 270–276. [DOI] [PubMed] [Google Scholar]
- Vygotsky L. (1978). Interaction between learning and development. In Gauvain M., Cole M. (Eds.), Readings on the development of children (Vol. 23, pp. 34–41). New York, NY: Scientific American Books. [Google Scholar]
- Wass S. V., Noreika V., Georgieva S., Clackson K., Brightman L., Nutbrown R., . . . Leong V. (2019). Parental neural responsivity to infants’ visual attention: How mature brains influence immature brains during social interaction. PLOS Biology, 16(12), Article e2006328. doi: 10.1371/journal.pbio.2006328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Smith L. B. (2013). Joint attention without gaze following: Human infants and their parents coordinate visual attention to objects through eye-hand coordination. PLOS ONE, 8(11), Article e79659. doi: 10.1371/journal.pone.0079659 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Piazza_Open_Practices_Disclosure for Infant and Adult Brains Are Coupled to the Dynamics of Natural Communication by Elise A. Piazza, Liat Hasenfratz, Uri Hasson and Casey Lew-Williams in Psychological Science
Supplemental material, Piazza_Supplemental_Material for Infant and Adult Brains Are Coupled to the Dynamics of Natural Communication by Elise A. Piazza, Liat Hasenfratz, Uri Hasson and Casey Lew-Williams in Psychological Science