Abstract
Attention-deficit/hyperactivity disorder (ADHD) is a common neurodevelopmental disorder characterized by hyperactivity/impulsivity and inattentiveness. Efforts toward the development of a biologically based diagnostic test have identified differences in the EEG power spectrum; most consistently reported is an increased ratio of theta to beta power during resting state in those with the disorder, compared with controls. Current approaches calculate theta/beta ratio using fixed frequency bands, but the observed differences may be confounded by other relevant features of the power spectrum, including shifts in peak oscillation frequency and altered slope or offset of the aperiodic 1/f-like component of the power spectrum. In the present study, we quantify the spectral slope and offset, peak alpha frequency, and band-limited and band-ratio oscillatory power in the resting-state EEG of 3- to 7-yr-old children with and without ADHD. We found that medication-naive children with ADHD had higher alpha power, greater offsets, and steeper slopes compared with typically developing children. Children with ADHD who were treated with stimulants had comparable slopes and offsets to the typically developing group despite a 24-h medication-washout period. We further show that spectral slope correlates with traditional measures of theta/beta ratio, suggesting the utility of slope as a neural marker over and above traditional approaches. Taken with past research demonstrating that spectral slope is associated with executive functioning and excitatory/inhibitory balance, these results suggest that altered slope of the power spectrum may reflect pathology in ADHD.
NEW & NOTEWORTHY This article highlights the clinical utility of comprehensively quantifying features of the EEG power spectrum. Using this approach, we identify, for the first time, differences in the aperiodic components of the EEG power spectrum in children with attention-deficit/hyperactivity disorder (ADHD) and provide evidence that spectral slope is a robust indictor of an increase in low- relative to high-frequency power in ADHD.
Keywords: attention-deficit/hyperactivity disorder, electroencephalography, power spectrum, spectral slope, theta/beta ratio
INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is a common neurodevelopmental disorder characterized by hyperactivity/impulsivity and inattentiveness. Children with ADHD are more likely to exhibit poor educational outcomes (Loe and Feldman 2007), social-emotional problems (Wehmeier et al. 2010), and substance use disorders (Wilens et al. 2011) that persist into adulthood. Recent estimates place the worldwide prevalence of ADHD between 5.3% and 7.2% (Polanczyk et al. 2007, 2014; Thomas et al. 2015), although the rate of diagnosis in the United States is higher, estimated at 7.7% for 4- to 11-yr-olds and 13.5% for 12- to 17-yr-olds (Xu et al. 2018). In addition to varying by age, diagnostic rates vary by sex, race, and ethnicity. Specifically, females and Hispanic and African American children are diagnosed at lower rates than Caucasian males (Polanczyk et al. 2014; Visser et al. 2014; Xu et al. 2018). These inconsistencies appear to reflect disproportionate diagnosis rather than true differences in prevalence between these populations (Bruchmüller et al. 2012; Merten et al. 2017).
One potential solution to the misdiagnosis of ADHD is a sensitive and specific biologically based diagnostic test. Toward this, a large body of research has sought to identify biomarkers of ADHD diagnosis and symptomology. Many of these efforts have focused on resting state electroencephalography (EEG), due in part to the clinical accessibility and cost-effectiveness of EEG. One of the more consistent findings differentiating ADHD from controls comes from analysis of the EEG power spectrum. Children with ADHD tend to have relatively greater power in the low-frequency theta range along with relatively reduced power in the high-frequency beta range compared with typically developing children; this is referred to as the theta/beta ratio and has commonly been proposed as a potential biomarker of ADHD (Barry et al. 2003; Loo and Makeig 2012; Monastra et al. 1999, 2001; Snyder and Hall 2006). In addition to elevated theta/beta ratio in ADHD, a recent study found reductions in theta/beta ratio following treatment with methylphenidate, a common stimulant used to treat ADHD, which persisted after a 24-h medication washout (Isiten et al. 2017). This finding is consistent with reports that treatment with stimulant medications ameliorates EEG and cortical structure abnormalities in ADHD patients (Clarke et al. 2003, 2017; Nakao et al. 2011; Shaw et al. 2009; reviewed in Spencer et al. 2013).
Despite the fact that reduced theta/beta ratio is one of the more consistently observed differences between ADHD and control subjects, its diagnostic utility is low due to failed replications and diminishing effect sizes over time (Arns et al. 2013; Loo and Makeig 2012; Saad et al. 2018). One potential explanation for this variability is that current approaches calculate theta/beta ratio using fixed frequency bands, defining theta as EEG power between 4 and 8 Hz, and beta as EEG power between 13 and 21 Hz (Monastra et al. 1999). Importantly, observed group differences in theta/beta ratio could be explained not just by differences in narrowband oscillatory power, but by other dynamic and physiologically relevant features of the power spectrum, including a shift in peak oscillation frequencies and altered slope or offset of the aperiodic, 1/f-like component of the power spectrum (Gao 2016; Haller et al. 2018).
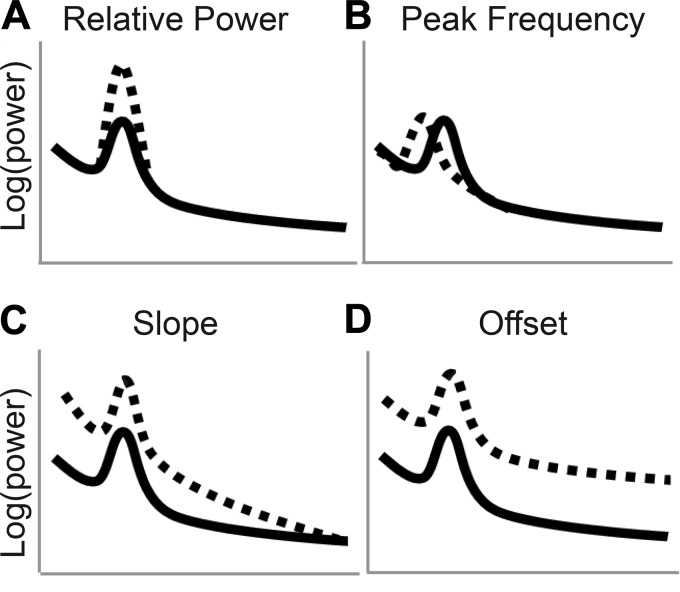
Differences in oscillatory power across conditions are the most extensively studied feature of the EEG power spectrum (Fig. 1A). Differences in oscillatory power have been linked to disease states as well as to a wide variety of cognitive processes (Başar et al. 1999, 2001; Klimesch 1999; Makeig et al. 2002). For example, studies have linked task-related increases in theta oscillations with enhanced cognitive performance, including working memory (Hsieh and Ranganath 2014) and attention (Makeig et al. 2002). Conversely, chronic elevations in theta power have been associated with cognitive impairment observed in old age (reviewed in Klimesch 1999) and in disease states, including ADHD (Barry et al. 2003) and Alzheimer’s disease (Fernández et al. 2002). In addition to differences in oscillatory power, the peak frequency within these frequency bands can also vary (Fig. 1B). For example, the location of the peak frequency within the alpha band increases with age during childhood (Epstein 1980; Marshall et al. 2002), peaks in early adulthood, and then decreases during older adulthood (Aurlien et al. 2004), at which point a lower peak frequency is associated with diminished executive function (Grandy et al. 2013). Furthermore, oscillatory peaks within defined frequency bands exist atop an aperiodic signal reflecting diminished power with increasing frequency, which varies in terms of slope and offset (He 2014). The slope of the aperiodic signal, or rate of decline in power with increasing frequency (Fig. 1C), fluctuates with cognitive state (Podvalny et al. 2015) and is associated with aging, executive function (Voytek et al. 2015), and synaptic excitatory/inhibitory balance (Gao et al. 2017). In contrast, the offset, or broadband power of the signal (Fig. 1D), may reflect the firing rate of neuronal populations (Manning et al. 2009). Thus typical EEG approaches that do not fully characterize the power spectrum may conflate differences in the ratio of low-frequency to high-frequency oscillations with shifts in peak frequencies, power spectral slope, and/or offset. For example, increased power in a low-frequency band (theta) relative to a higher frequency band (beta) may be better assessed by measuring the slope of the aperiodic signal, because this would implicitly measure the relative power in high and low frequencies without relying on arbitrarily defined frequency bands.
Fig. 1.
Schematic of the 4 components of the electrophysiological power spectrum. A: low (solid line) and high (dashed line) power in the alpha range. B: low (dashed line) and high (solid line) peak alpha frequency. C: flat (solid line) and steep (dashed line) slopes. D: low (solid line) and high (dashed line) offsets.
In the present study, we took such a comprehensive approach and compared the slope, offset, peak alpha frequency, and band-limited and band-ratio relative power of the resting-state EEG signal in a sample of 3- to 7-yr-old medication-naive children with ADHD (n = 50) and age- and sex-matched typically developing controls (TD; n = 50). In addition, we compared these aspects of the EEG power spectra in 3- to 7-yr-old children with ADHD and a history of stimulant treatment (n = 26) with those in age- and sex-matched medication-naive children with ADHD (n = 26) and typically developing controls (n = 26). Given previous literature documenting theta/beta ratio differences associated with childhood ADHD and suggesting normalization of the EEG power spectra with stimulant treatment, we hypothesized that medication-naive children with ADHD would have steeper slopes compared with typically developing controls and that treatment with stimulants would flatten the EEG power spectral slope. We further hypothesized that slope estimates would correlate with traditional estimates of theta/beta ratio, reflecting the utility of measuring EEG power spectral slope as a robust indicator of relative low- to high-frequency power in children with ADHD.
MATERIALS AND METHODS
Participants
A total of 127 children (26.8% female) between the ages of 3 yr 0 mo and 7 yr 4 mo (M = 5 yr 9 mo, SD = 1 yr 2 mo) participated in the present study from a sample of children (N = 197) in a longitudinal study evaluating stability of ADHD diagnosis. Participants were recruited from schools, community events, and databases consisting of children seen for ADHD at Boston Children’s Hospital or whose families expressed interest in participating in research within the Laboratories of Cognitive Neuroscience at Boston Children’s Hospital. From the larger sample, we excluded participants due to parent report of genetic abnormalities (n = 1), prenatal substance exposure (n = 2), parent report of autism spectrum disorder confirmed during study assessments (n = 1), parental language barriers (n = 1), refusal to participate after time of consent (n = 1), active use of a nonstimulant psychotropic medication (n = 19), or insufficient artifact-free EEG data as determined by a trained experimenter (n = 18; 11 ADHD, 7 control). Of the remaining participants, 76 met criteria for ADHD and 78 were classified as typically developing controls. Of those who met criteria for ADHD, 50 were medication naive (ADHD−) and 26 were actively treated with stimulant medications but underwent a 24-h medication washout before study procedures (ADHD+). The 24-h washout period was determined based on parent report and is the standard washout period used for stimulants given their short half-life (Cole et al. 2008; Isiten et al. 2017; Valera et al. 2010; Wigal et al. 2007). A group of 50 typically developing (TD) participants was selected to match the ADHD− group regarding both age and sex, and a subset of participants from the TD and ADHD− groups were selected to age and sex match the group of 26 ADHD+ participants. See Table 1 for demographics. All study procedures complied with the Helsinki Declaration and were approved by the Institutional Review Board at Boston Children’s Hospital. All child participants provided verbal assent, and their primary caregivers provided written informed consent.
Table 1.
Group demographics for the full ADHD− and TD samples, as well as subgroups selected for age and sex matching with the ADHD+ group
|
Samples |
Subgroups |
||||
|---|---|---|---|---|---|
| ADHD− | TD | ADHD− | ADHD+ | TD | |
| n | 50 | 50 | 26 | 26 | 26 |
| Female | 28 (14) | 28 (14) | 23.1 (6) | 23.1 (6) | 23.1 (6) |
| Handedness (R) | 86 (43) | 90 (45) | 84.6 (22) | 76.9 (20) | 96.2 (25) |
| Race | |||||
| White | 66 (33) | 62 (31) | 69.2 (18) | 88.5 (23) | 73.1 (19) |
| Black/African American | 12 (6) | 12 (6) | 11.5 (3) | 3.8 (1) | 11.5 (3) |
| Asian | 0 (0) | 6 (3) | 0 (0) | 0 (0) | 3.8 (1) |
| Other/multiracial | 18 (9) | 20 (10) | 15.5 (4) | 7.7 (2) | 11.5 (3) |
| Hispanic/Latino | 18 (9) | 6 (3) | 23.1 (6) | 15.4 (4) | 7.7 (2) |
| Age, mo | 67.70 ± 14.66 | 67.76 ± 14.76 | 74.50 ± 10.38 | 74.88 ± 9.71 | 74.81 ± 10.12 |
Values are percentages of total group, with the raw number in parentheses; age is expressed as mean ± SD. ADHD−, mediation-naive attention-deficit/hyperactivity disorder (ADHD) group; TD, typically developing control group; ADHD+, stimulant-treated ADHD group after 24-h medication washout.
ADHD Diagnosis
ADHD diagnosis was determined during the study visit using the Diagnostic Structured Interview Schedule–young child version (DISC-IV; Shaffer et al. 2000). In some cases, additional information was obtained from the Achenbach child behavior checklist (CBCL 1.5–5 or 6–18 depending on age; Achenbach 1994) and the Swanson Nolan and Pelham checklist (SNAP-IV; Swanson 2011). Children included in the ADHD group either met diagnostic criteria on the DISC-IV (n = 64) or received a subthreshold score on the DISC-IV (n = 8) but met clinical thresholds on either the CBCL (ADHD subscale t score ≥ 70, n = 3), the SNAP-IV (caregiver endorsed 6/9 inattention or hyperactivity symptoms, n = 4), or both (n = 1). In addition, two participants met neither clinical nor subclinical threshold on the DISC-IV but met clinical threshold on the SNAP-IV (n = 1) or both the SNAP-IV and the CBCL (n = 1). Furthermore, due to technical difficulties, two participants did not have DISC-IV scores but met criteria on both the CBCL and the SNAP-IV (n = 2).
Teacher report of ADHD symptoms was assessed using either the Teacher Report Form of the CBCL (TRF; Achenbach 1994) or the Conners-3 Teacher Rating Scale (Conners 2001) in 48% of participants (n = 61) due to complications in data collection. There was no difference in ADHD symptoms between participants with and without teacher report on the DISC, CBCL, or SNAP-IV (P values >0.40). ADHD symptoms by group membership for each of the measures is shown in Table 2 for the full ADHD− and TD samples and in Table 3 for the ADHD+ sample and the age- and sex-matched TD and ADHD− subsamples.
Table 2.
Average ADHD symptoms for the complete ADHD− and TD samples
| ADHD− Versus TD |
||||
|---|---|---|---|---|
| Group Differences |
||||
| ADHD− | TD | t | P | |
| n | 50 | 50 | ||
| DISC symptoms (0–23) | 16.31 ± 4.10 (48) |
3.78 ± 3.84 (45) |
−15.2 | <0.001* |
| CBCL Attention Problems t score | 66.88 ± 6.97 (50) |
52.00 ± 3.47 (49) |
−13.41 | <0.001* |
| SNAP-IV (0–9) | ||||
| Inattentiveness | 5.47 ± 2.53 (47) |
0.69 ± 1.13 (48) |
−11.94 | <0.001* |
| Hyperactivity | 6.21 ± 2.56 (48) |
1.25 ± 1.71 (48) |
−11.17 | <0.001* |
| Teachers Conners | ||||
| Inattention t score | 61.25 ± 11.77 (16) |
46.69 ± 8.53 (13) |
−3.73 | 0.001* |
| Hyperactive t score | 74.69 ± 12.97 (16) |
53.64 ± 16.93 (11) |
−3.66 | 0.001* |
| TRF ADHD t score | 58.83 ± 11.91 (6) |
53.25 ± 4.30 (8) |
−1.237 | 0.24 |
Values are means ± SD, with the number of participants with scores for each measure in parentheses. As expected, the medication-naive attention-deficit/hyperactivity disorder (ADHD−) group had significantly more ADHD symptoms compared with the typically developing (TD) group on all measures with the exception of the Teacher Report Form (TRF), which was completed in a small number of total cases. DISC, Diagnostic Structured Interview Schedule–young child version; CBCL, Child Behavior Checklist; SNAP-IV, Swanson Nolan and Pelham checklist.
P < 0.05, significant between-group difference.
Table 3.
Average ADHD symptoms for the ADHD+ group and the TD and ADHD− subgroups
| ADHD− Versus ADHD+ Versus TD |
||||||
|---|---|---|---|---|---|---|
| Group Differences |
||||||
| ADHD− | ADHD+ | TD | ADHD− versus ADHD+ | ADHD− versus TD | ADHD+ versus TD | |
| n | 26 | 26 | 26 | |||
| DISC symptoms (0–23) | 17.27 ± 3.08 (26) |
18.31 ± 3.67 (26) |
3.88 ± 3.70 (25) |
0.862 | <0.001* | <0.001* |
| CBCL Attention Problems t score | 67.31 ± 6.45 (26) |
68.78 ± 5.74 (23) |
52.04 ± 3.87 (26) |
>0.99 | <0.001* | <0.001* |
| SNAP-IV (0–9) | ||||||
| Inattentiveness | 5.67 ± 2.24 (24) |
7.46 ± 2.11 (24) |
0.46 ± 1.14 (24) |
0.005* | <0.001* | <0.001* |
| Hyperactivity | 6.0 ± 2.71 (25) |
7.54 ± 2.23 (24) |
0.75 ± 1.29 (24) |
0.045* | <0.001* | <0.001* |
| Teachers Conners | ||||||
| Inattention t score | 59.27 ± 11.73 (11) |
53.92 ± 8.39 (12) |
44.82 ± 5.33 (11) |
0.473 | 0.002* | 0.059 |
| Hyperactive t score | 74.47 ± 13.02 (11) |
62.08 ± 16.04 (12) |
51.00 ± 12.85 (9) |
0.137 | 0.003* | 0.261 |
| TRF ADHD t score | 50 (1) |
60.2 ± 7.92 (5) |
54.0 ± 4.95 (5) |
0.176 | ||
Values are means ± SD, with the number of participants with scores for each measure in parentheses. The stimulant-treated attention-deficit/hyperactivity disorder after medication washout (ADHD+) and medication-naive (ADHD−) groups have significantly more symptoms on all parent report measures compared with the typically developing (TD) group. However, ADHD−, but not ADHD+, had significantly more symptoms than TD on teacher report measures, likely due to effects of medication during school hours. CBCL, Child Behavior Checklist; DISC, Diagnostic Structured Interview Schedule–young child version; SNAP-IV, Swanson Nolan and Pelham checklist; TRF, Teacher Report Form.
P < 0.05, significant between-group difference.
EEG Acquisition
EEG data were obtained during eyes-open and eyes-closed resting-state conditions for a total of 7 min. During the recording period, the participants cycled through 30 s of eyes-open data collection in which the child directed attention toward a cartoon image of open eyes, a 15-s break in which a research assistant encouraged the child’s continued compliance, and 30 s of eyes-closed data collection in which the child was instructed to sit calmly with eyes closed. This process was repeated seven times. Although this is a nonstandard procedure for collecting resting-state EEG data, it was designed to maximize the amount of artifact-free data given the young age of the children participating in the study, and similar procedures have been used elsewhere with children in this age range (Vuga et al. 2008). Even within this specially designed procedure, young children were unable to follow the direction to sit calmly with their eyes closed. Specifically, during the eyes-closed segments, children tended to squeeze their eyes shut, squint, or open and close their eyes repeatedly to observe the room. This resulted in an excessive amount of muscle and movement artifact for the eyes-closed segments, and thus these were excluded from further analysis and only eyes-open segments were used.
EEG data were recorded with a 128-channel HydroCel geodesic sensor net system (Electrical Geodesics Inc., Eugene, OR) with a NetAmps 200 amplifier and NetStation software at an effective sampling rate of 250 Hz. Electrodes were maintained such that at least 90% of the 128 electrodes had impedances below 50 kΩ before the resting-state recording was initiated.
EEG Preprocessing
Data were preprocessed using NetStation. Recordings were high-pass filtered to 0.1 Hz and low-pass filtered to 100 Hz. Data were then segmented into the eyes-open and eyes-closed conditions. The best two to four eyes-open segments were selected, and these were concatenated to form a 1- to 2-min block of eyes-open resting-state data. Whereas data length did not differ between the ADHD+ group and the age- and sex-matched TD and ADHD− subgroups [F(2,75) = 0.833, P = 0.439], there was a trend-level group difference in length of data between the full ADHD group (M = 111.97 s, SD = 18.28) and the TD group [M = 117.99 s, SD = 11.91; t(84.26) = 6.02, P = 0.054]. As a result, we controlled for data length in all analyses.
After the data were segmented and concatenated, any electrodes with artifacts outside of a ±80-mV range were removed and replaced with data interpolated from the remaining electrodes. Eye and other radial electrodes were removed from all analyses. Finally, all channels were re-referenced to the average reference (Liu et al. 2015) and exported to MATLAB (The MathWorks Inc., Natick, MA) for further processing.
We identified and removed eye blinks and muscle movements using independent components analysis (ICA) in EEGLAB (Delorme and Makeig 2004). Before ICA was performed, recordings were high-passed filtered to 1 Hz due to evidence that this improves artifact detection (Winkler et al. 2015). Electrode locations from the 128-channel montage were mapped and reduced to the 10–10 International System (Luu and Ferree 2005) to account for highly correlated signal from nearby electrodes (Onton and Makeig 2006). The ICA decomposition was then calculated in EEGLAB, and we used the MARA EEGLAB plug-in (Winkler et al. 2011, 2014). MARA is a supervised machine-learning algorithm that has been pretrained to identify and label independent components of the EEG signal as artifact or neural activity based on six features described in Winkler et al. (2014). Of the 71 components derived from ICA, only the first 12 accounted for more than 1% of the variance each. As such, a trained experimenter (SF) visually inspected these first 12 components to verify MARA’s artifact classification. In the rare instances when it differed from MARA’s classification, the experimenter’s classification by visual inspection was used. The remaining 59 components were classified solely based on MARA’s calculated probabilities, with those assigned a probability >0.50 marked as artifact and their time series subtracted from the overall signal, creating a cleaned signal that is used for further analysis.
Data Analysis
We first estimated power spectral density (PSD) using Welch’s method with a Hamming window length of 1 s and 50% overlap (Gao et al. 2017). To independently examine the four components of the electrophysiological power spectrum (Fig. 1, A–D), we used the Fitting Oscillations and One-Over-f (FOOOF) toolbox to calculate slope and offset (Haller et al. 2018) and visually detected each individual’s peak alpha frequency (PAF), which was then used to estimate individualized narrow-band power (Doppelmayr et al. 1998). We assessed each of these parameters at 12 midline electrodes across the frontal, central, parietal, and occipital regions (FCz, Fz, F3, F4, C3, C4, Cz, P3, P4, Pz, O1, O2).
Individualized peak alpha frequency.
We determined PAF through visual inspection of the plot of the power spectrum. PAF detection was performed within the predefined alpha band of 5.5–13 Hz (Klimesch 1999; Marshall et al. 2002) and defined as the average point of highest amplitude within that range for the 12 channels tested. Two researchers (MR and MK) independently identified the peak within the alpha range to the nearest 0.25 Hz with 83% concordance. In those instances where the researchers differed in their classifications, the PAF was reevaluated to ensure accurate selection. Cases of discordance were due to either split peaks or minimal deviation from the aperiodic background scaling. If, on reevaluation, the researchers could not agree on a dominant peak, split peaks were averaged together to estimate PAF, whereas those with minimal deviation from background scaling were regarded as having no PAF and were excluded from PAF analysis. Of 100 participants, 91 had a clear alpha peak. Of the nine individuals without an alpha peak, four were in the TD group and five were in the ADHD− group. Those with and without alpha peaks did not differ in regard to group [t(98) = 0.346, P = 0.730], age [t(98) = 0.534, P = 0.595], or data length [t(98) = 1.090, P = 0.278], but there was a trending difference in sex [t(98) = 1.947, P = 0.054], with females being more likely to not have an alpha peak.
Frequency band analysis.
To account for observations that frequency bandwidths vary based on PAF, individualized frequency bands were calculated as a percentage of the PAF as follows: theta (PAF × 0.4 − PAF × 0.6) and alpha (PAF × 0.6 − PAF × 1.2) (Doppelmayr et al. 1998). Previous work has shown that this approach better accounts for variations in bandwidth that occur as a function of PAF (Doppelmayr et al. 1998), which in turn varies with age (Aurlien et al. 2004; Epstein 1980; Marshall et al. 2002). For the nine participants with no clear alpha peak, we instead calculated individualized frequency bands using the average PAF for the ADHD− and TD groups, which were 8.43 and 8.84, respectively. To account for differences in the amplitude of the EEG signal due to noise including skull thickness and electrode impedance, we calculated relative power by dividing the power within each band by the total power (Gasser et al. 1982; Kappenman and Luck 2010). To allow for direct comparison with existing literature, theta/beta ratio was calculated using standard methods described in Monastra et al. (1999), which divides theta-band power between 4 and 8 Hz by beta-band power between 13 and 21 Hz.
Slope and offset.
We used the FOOOF toolbox (Haller et al. 2018) to calculate the slope (Fig. 1C) and offset (Fig. 1D) of the PSD between 4 and 50 Hz. Briefly, we first modeled the aperiodic slope and then found the oscillatory peaks and fit them with Gaussians. We then subtracted the Gaussians iteratively until all peaks were removed. We then refit the aperiodic slope of the power spectrum with the peaks removed using an exponential function in semi-log power space. This procedure provides an estimate for each EEG channel of two key aperiodic features of the power spectrum: slope and offset.
Statistics.
Data were analyzed using IBM SPSS Statistics version 25 and SAS version 9.4. To examine electrophysiological differences related to ADHD diagnoses, we conducted a single-factor analysis of covariance (ANCOVA). To evaluate the relationship between slope and theta/beta ratio, we conducted a partial correlation. All analyses controlled for data length and were corrected for multiple comparisons. Between-group main effects were Bonferroni-corrected to P < 0.05. To account for account for collinearity among EEG electrodes and reduce the risk of type II errors, between-group comparisons of the individual EEG electrodes were instead false discovery rate (FDR)-corrected to P < 0.05.
RESULTS
Electroencephalographic Results
Slope of the power spectrum.
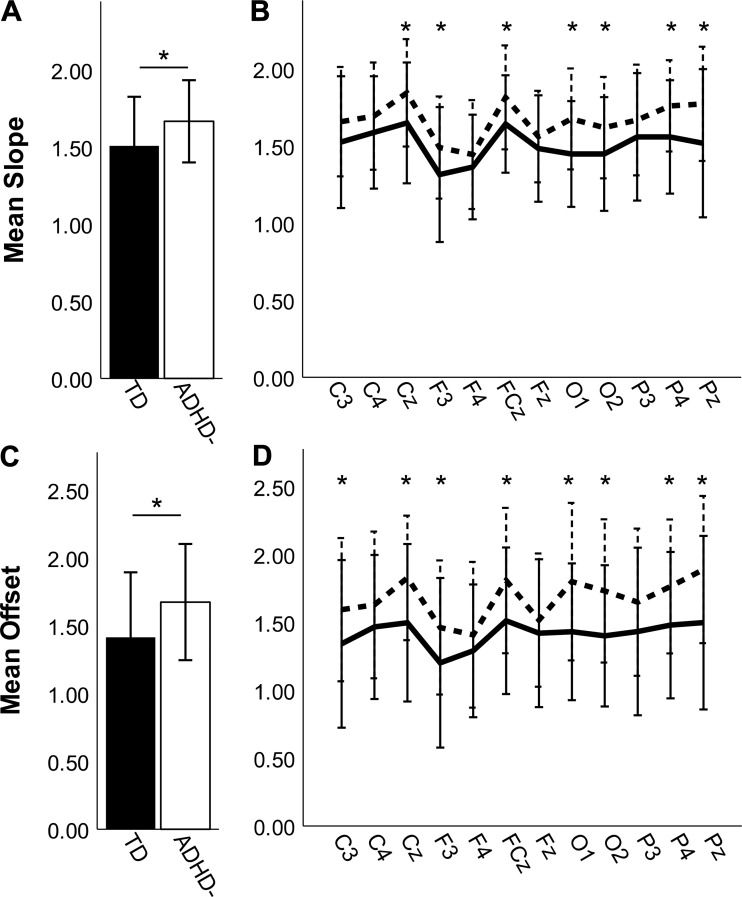
We tested whether the aperiodic spectral slope, averaged across electrodes, differed between the ADHD− and TD groups using ANCOVA, controlling for data segment length. Average slopes were significantly steeper in the ADHD− group (M = 1.67, SD = 0.27) compared with the TD group [M = 1.51, SD = 0.32; F(1,97) = 9.58, P = 0.003, η2 = 0.088; Fig. 2A]. This pattern was consistent across all tested electrode pairs, with statistically significant group differences in electrode pairs Cz (P = 0.008), F3 (P = 0.03), FCz (P = 0.008), O1 (P = 0.003), O2 (P = 0.008), P4 (P = 0.005), and Pz (P = 0.008) after FDR correction (Fig. 2B).
Fig. 2.
Comparisons of slope (A and B) and offset (C and D) in the full sample of typically developing (TD; closed bars, solid lines) and medication-naive attention-deficit/hyperactivity disorder (ADHD−; open bars, dashed lines) participants. Error bars are ±SD. A: ADHD− has steeper slopes compared with TD when averaged across participants and electrodes. B: slopes were steeper in ADHD− for all electrodes tested. C: ADHD− has greater offset compared with TD when averaged across participants and electrodes. D: this pattern holds when electrodes are considered individually. *P < 0.05, statistical significance after false discovery rate correction.
Power spectrum offset.
Next, we evaluated between-group differences in offset of the power spectrum. A single-factor ANCOVA found that the average offsets were greater for ADHD− (M = 1.67, SD = 0.43) than for TD [M = 1.41, SD = 0.48; F(1, 97) = 8.708, P = 0.004, η2 = 0.082; Fig. 2C]. This pattern was consistent across all electrodes tested with C3 (P = 0.042), Cz (P = 0.005), F3 (P = 0.042), FCz (P = 0.012), O1 (P = 0.005), O2 (P = 0.005), P4 (P = 0.01), and Pz (P = 0.005) surviving FDR correction (Fig. 2D).
Individual peak alpha frequency.
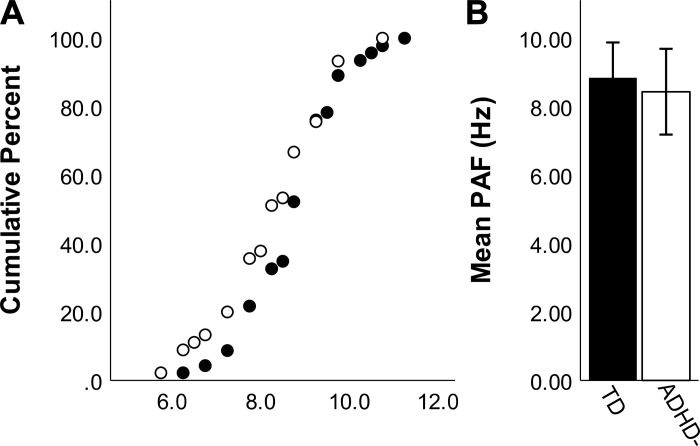
Individual peak alpha frequencies ranged from 5.75 to 11.25 Hz (Fig. 3A). We tested for a difference in the peak alpha frequency between the full TD and ADHD− groups with an ANCOVA and found no significant difference in average peak alpha between the ADHD− (M = 8.43, SD = 1.25) and TD (M = 8.84, SD = 1.03) groups [F(1, 88) = 2.80, P = 0.098; η2 = 0.031; Fig. 3B].
Fig. 3.
Individual alpha frequency as determined by visual inspection of the power spectrums for the sample of typically developing (TD; closed circles, closed bars) and medication-naive attention-deficit/hyperactivity disorder (ADHD−; open circles, open bars) participants. Error bars are ±SD. A: cumulative frequency plot showing the proportion of peaks that fall at various points across the alpha range. B: peak alpha frequency group averages show no significant differences between TD and ADHD−.
Narrow-band alpha and theta.
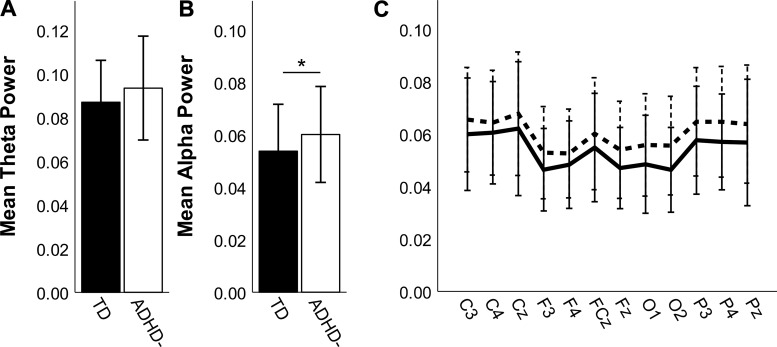
We estimated the individualized alpha and theta power bands based on the location of each person’s peak alpha frequency. Using ANCOVA, we found no significant between-group differences in individualized theta power [Fig. 4A; F(1,97) = 2.15, P = 0.15]. We did find a significant group difference in individualized alpha power [Fig. 4B; F(1,97) = 4.38, P = 0.039, η2 = 0.030], with greater alpha power in the full ADHD− group (M = 0.06, SD = 0.018) compared with the TD group (M = 0.05, SD = 0.018). This pattern was evident across all electrode pairs; group differences at F3 (P = 0.027), Fz (P = 0.015), O1 (P = 0.032), O2 (P = 0.006), and P4 (P = 0.031) were statistically significant, although none survived FDR correction (Fig. 4C).
Fig. 4.
Theta (A) and alpha (B and C) power for the full sample of typically developing (TD; closed bars, solid lines) and medication-naive attention-deficit/hyperactivity disorder (ADHD−; open bars, dashed lines) participants, calculated using individualized frequency bands based on peak alpha. Error bars are ±SD. A: there was no significant group difference in theta power. B: ADHD− has elevated alpha power compared with TD. *P < 0.05. C: although ADHD− had higher alpha power than TD in all tested electrodes, this group difference was not significant for any individual electrode pairs after false discovery rate correction.
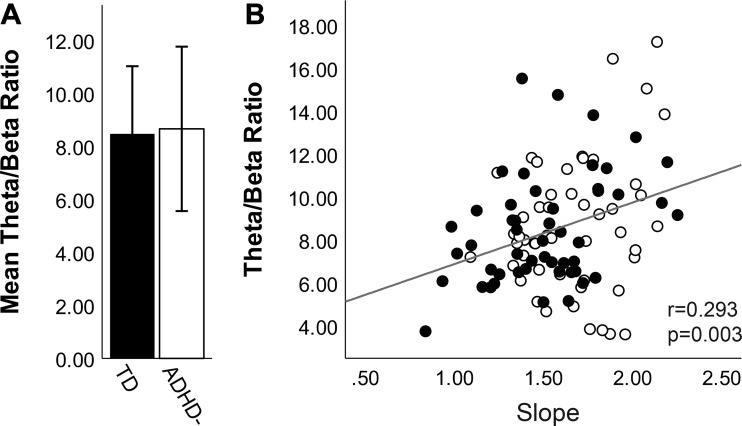
Theta/beta ratio.
Theta/beta ratios have been widely used to compare children with ADHD to TD children. Thus we evaluated theta/beta ratio in this sample to allow direct comparison to data in the literature and to evaluate the relationship between this established metric and the novel EEG measures reported here. We found no overall difference in theta/beta ratio between the full ADHD− (M = 8.66, SD = 3.10) and TD (M = 8.47, SD = 2.55) groups [F(1, 97) = 0.371, P = 0.544, η2 = 0.004; Fig. 5A]. We did observe a significant correlation between theta/beta ratio and aperiodic slope (Fig. 5B; r = 0.293, P = 0.003).
Fig. 5.
Theta/beta ratio for the full sample of typically developing (TD; closed bars, closed circles) and medication-naive attention-deficit/hyperactivity disorder (ADHD−; open bars, open circles) participants. A: there was no significant group difference in theta/beta ratio between TD and ADHD−. Error bars reflect ± SD. B: theta/beta ratio was significantly correlated with slope.
Treatment with Stimulant Medications
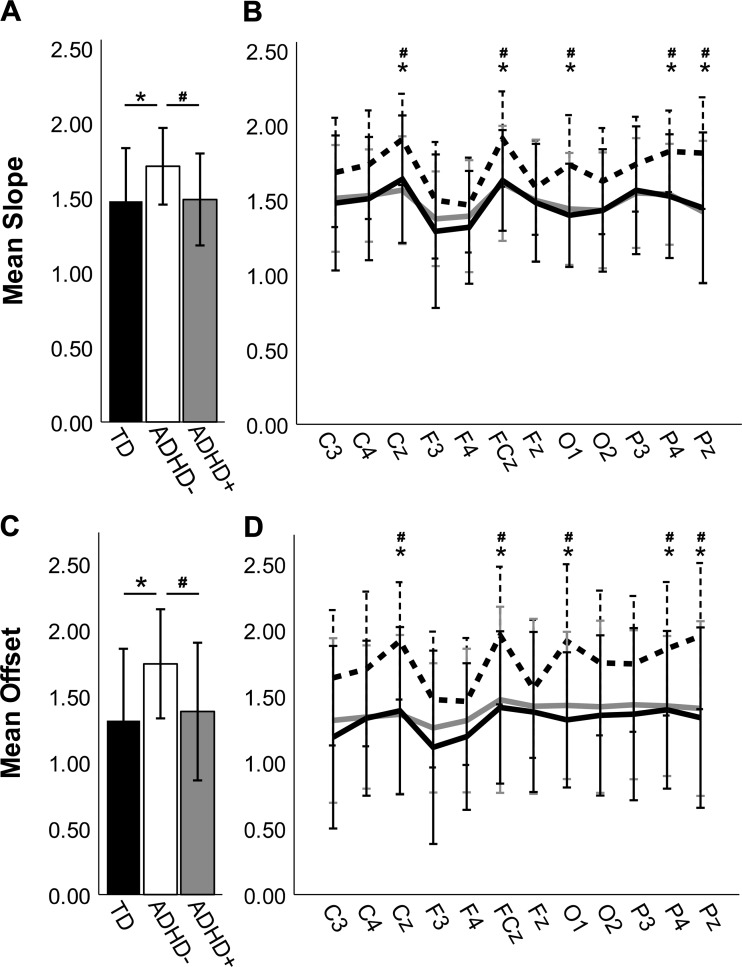
Because this is the first report of power spectrum slope and offset differences between medication-naive children with or without ADHD, we sought to test whether these differences were modified by exposure to stimulant medication. Specifically, we evaluated power spectrum slope and offset in a subsample of the TD and medication-naive (ADHD−) groups that were age and sex matched to a sample of 26 children with ADHD currently treated with stimulants, who underwent a 24-h medication washout before completing the study (ADHD+). An ANCOVA found a main effect of group on mean slope [F(2,74) = 4.76, P = 0.011, η2 = 0.112; Fig. 6A]. As in the larger sample, the ADHD− group (M = 1.71 SD = 0.26) had significantly steeper slopes than the TD group (M = 1.48, SD = 0.36; P = 0.019, Bonferroni corrected) and also had steeper slopes than the ADHD+ group (M = 1.49, SD = 0.31; P = 0.044, Bonferroni corrected). This pattern held across all electrodes (Fig. 6B), with the ADHD− group having significantly steeper slopes than the TD group at Cz (P = 0.024), FCz (P = 0.019), O1 (P = 0.019), P4 (P = 0.019), and Pz (P = 0.019) and significantly steeper slopes than the ADHD+ group at Cz (P = 0.019), FCz (P = 0.019), O1 (P = 0.019), P4 (P = 0.019), and Pz (P = 0.019) after FDR correction. In contrast, the slopes did not differ between the TD and ADHD+ groups at any electrodes (P values >0.642).
Fig. 6.
Slope (A and B) and offset (C and D) for the stimulant-treated attention-deficit/hyperactivity disorder (ADHD) group after medication washout (ADHD+; shaded bars, shaded lines) and the age- and sex-matched typically developing (TD; closed bars, solid lines) and medication-naive ADHD (ADHD−; open bars, dashed lines) subgroups. Error bars are ±SD. A: ADHD− has steeper slopes compared with both TD and ADHD+ when averaged across participants and electrodes. B: slopes were steeper in ADHD− for all electrodes tested. C: ADHD− has greater offset compared with TD and ADHD+ when averaged across participants and electrodes. D: this pattern holds when electrodes are considered individually. *P < 0.05, significant difference between TD and ADHD− after false discovery rate (FDR) correction. #P < 0.05, significant differences between ADHD− and ADHD+ after FDR correction.
We also found a main effect of group on offset [F(2,74) = 5.65, P = 0.005, η2 = 0.132; Fig. 6C], with higher average offset in the ADHD− group (M = 1.74 SD = 0.41) relative to both the TD group (M = 1.31, SD = 0.54; P = 0.007, Bonferroni corrected) and the ADHD+ group (M = 1.38, SD = 0.52; P = 0.038, Bonferroni corrected). In contrast, there were no significant differences in offset between the ADHD+ and TD groups (P values >0.9). Among individual electrode pairs (Fig. 6D), the TD group had significantly lower offset than the ADHD− group for C3 (P = 0.028), with Cz (P = 0.008), FCz (P = 0.011), O1 (P = 0.008), P4 (P = 0.015), and Pz (P = 0.008) withstanding FDR correction. The ADHD+ group had significantly lower offset than the ADHD− group, with Cz (P = 0.008), FCz (P = 0.023), O1 (P = 0.015), P4 (P = 0.023), and Pz (P = 0.015) withstanding FDR correction. Again, there were no significant differences in offset between the TD and ADHD+ groups for any of the electrode pairs (P values >0.50).
DISCUSSION
By quantifying four distinct features of the EEG power spectrum, including aperiodic slope and offset, peak alpha frequency, and power within individualized alpha and theta bands, we identified a novel neural correlate of ADHD. Moreover, our findings may explain discrepancies in the ADHD literature regarding theta/beta ratios. To summarize, we found that medication-naive children with ADHD had steeper spectral slopes and elevated offsets compared with typically developing children. Though this is the first report evaluating spectral slope in children with ADHD, it is consistent with reports of elevated low-frequency:high-frequency power captured by the commonly used theta/beta ratio. Although we did not find a significant group difference in theta/beta ratio in this sample, spectral slope positively correlated with theta/beta ratio, suggesting that band-limited theta/beta ratio calculations may inconsistently capture the shift in low- relative to high-frequency EEG power in ADHD. In contrast, spectral slope considers the full EEG spectrum and may be a better metric because it is not confounded by shifts in aperiodic offset, peak frequencies, or narrow-band power. Together, our findings support the use of spectral slope as a measure of a shift in low- relative to high-frequency power in ADHD. These results are consistent with another recent study that also found relative band power or power ratios predict ADHD diagnosis with only moderate success, whereas entropy measures, which capture non-frequency-specific global activity, are more successful at predicting ADHD diagnosis (Chen et al. 2019).
Stimulant Treatment and Normalization of Aberrant Brain Activity
Because our initial group comparison included only ADHD patients that were medication naive, we next tested whether our observed electrophysiological group differences were modified by treatment with stimulant medications, which improve behavioral symptoms in children with ADHD and are the most common medicinal treatment for the disorder (Storebø et al. 2015). We found aperiodic slopes and offsets in stimulant-treated children with ADHD were similar to those in typically developing controls, but were significantly different from those in the medication-naive ADHD group. These findings are consistent with a growing body of literature showing that stimulant treatment can normalize structural and functional brain abnormalities associated with ADHD (Clarke et al. 2003, 2017; Nakao et al. 2011; Shaw et al. 2009; Spencer et al. 2013). Perhaps most pertinent is a recent study showing a significant reduction in theta/beta ratio in children with ADHD after 1.5 yr of stimulant treatment (Isiten et al. 2017); consistent with our results, this normalization persisted even after a 24-h medication-washout period. This finding taken in conjunction with our work supports the idea that flatter slopes in the stimulant-treated and typically developing groups compared with the medication-naive ADHD group could reflect a posttreatment reduction in low- relative to high-frequency power and a normalization of brain physiology.
Relative Power Across the EEG Power Spectrum
What underlies an abnormal ratio of low- relative to high-frequency power in the brain EEG spectrum? Understanding the relative power across frequencies in brain dynamics is an active area of research, and recent studies evaluating the physiological underpinnings of spectral slope suggest that it reflects neural signal-to-noise ratio (Voytek et al. 2015) and that the spectral slope is an index of the excitatory/inhibitory (E/I) balance of the recorded brain circuits (Gao et al. 2017). Thus our results may reflect abnormal E/I balance in the cortical circuitry of children with unmedicated ADHD. This interpretation is consistent with observations of altered E/I balance in clinical and preclinical models of ADHD, which have shown reductions in GABA signaling (Edden et al. 2012) and/or increases in glutamate signaling (Courvoisie et al. 2004; Hammerness et al. 2012; Zimmermann et al. 2015). Although steeper slope has generally been regarded as reflecting enhanced signal-to-noise ratio and thus increased GABA or reduced glutamate signaling (Gao et al. 2017; Voytek et al. 2015), perhaps there is a range of cognitively optimal spectral slopes at different developmental stages, with slopes that are either too flat or too steep yielding cognitive impairments. Moreover, similar findings have been noted in a clinical study evaluating 1/f slope in patients with schizophrenia. Despite the association of schizophrenia with reduced GABAergic inhibition in the cortex (Lewis et al. 2005), elevated 1/f slopes during an attention task were found in schizophrenia patients compared with controls, which was proposed to reflect a compensatory increase in GABAergic activity (Peterson et al. 2018). Thus it is possible that the steeper 1/f slopes in medication-naive children with ADHD reflect a compensatory mechanism of some sort. For example, our EEG was collected in a quiet resting state, which may have required substantially more cognitive control in the children with ADHD. However, the fact that the previously medicated ADHD group did not show evidence of such compensation argues against this idea. Still, studies assessing E/I balance with the use of transcranial magnetic stimulation (TMS) have shown that stimulants such as methylphenidate, which inhibits reuptake of dopamine and norepinephrine, may rectify E/I balance in ADHD (Buchmann et al. 2006; Moll et al. 2000), consistent with the idea that normalization of slope could reflect normalization of E/I balance. Further work is needed to confirm that the effects we observed reflect a stimulant-induced change in E/I balance.
Study Limitations
Our results indicate a difference in power spectral slope in young children with ADHD compared with typically developing controls, which could represent a transdiagnostic risk factor or an intermediate phenotype, rather than an ADHD-specific feature. Previous work has reported variations in spectral slope associated with age (Voytek et al. 2015) and with other clinical diagnoses, including schizophrenia (Peterson et al. 2018). Additionally, evidence that spectral slope may reflect differences in E/I balance (Gao et al. 2017) suggests that spectral slope differences may be present in other disorders with underlying E/I imbalance, such as autism, epilepsy, and alcohol use disorders (reviewed in Fritschy 2008; Gao and Penzes 2015; Rubenstein and Merzenich 2003; Selten et al. 2018; Wackernah et al. 2014). Though the specificity of this difference in spectral slope remains to be tested, our results do suggest that spectral slope more appropriately captures a shift in low- relative to high-frequency power in ADHD compared with the theta/beta ratio, which has been frequently reported as an EEG biomarker in children with ADHD (Barry et al. 2003; Loo and Makeig 2012; Monastra et al. 1999, 2001; Snyder and Hall 2006).
We acknowledge certain limitations of this study. First, diagnosis in this study was based on parent report of symptoms, which could be subject to inconsistencies. Although we did collect teacher report of symptoms in a subset of participants to confirm diagnostic status, we were unable to do so for all participants. Second, we used a nontraditional EEG data acquisition paradigm; however, this paradigm was chosen because of its superior robustness to the excess movement that occurs in very young study participants (Vuga et al. 2008). Third, in evaluating the chronic impact of stimulant treatment on aperiodic slope and offset, we used a relatively short washout period of 24 h. Previous studies have used a similar washout period (Cole et al. 2008; Isiten et al. 2017; Valera et al. 2010), and given the short half-life of stimulants, even in young children (Wigal et al. 2007), it is unlikely that normalized aperiodic slope and offset in stimulant-treated children are driven entirely by acute drug effects. Still, it is important to note that we did not measure drug levels or compliance with the 24-h medication washout, which was determined by parent report. Thus we cannot rule out the possibility that acute drug action or stimulant withdrawal could at least partly explain our results.
Conclusion
In summary, this study highlights the potential clinical utility of comprehensively quantifying features of the EEG power spectrum. Using this approach, we found that medication-naive children with ADHD had steeper EEG power spectrum slopes and greater EEG power spectrum offsets than typically developing children. Moreover, we show that spectral slope correlates with traditional measures of theta/beta ratio, although theta/beta ratio itself did not differ between groups. This is consistent with spectral slope and offset as a robust and complete measure of relative contributions of low and high frequencies to the overall power spectrum. Interestingly, this difference was not apparent in stimulant-treated children with ADHD, despite a 24-h medication washout. Thus spectral slope may reflect pathology in the brains of children with ADHD that is normalized by stimulant medication. Future studies should evaluate whether these group differences in spectral slope and offset can be replicated in older children and adults with ADHD, determine whether there are interaction effects of age and sex, and assess normalization of slope and offset after stimulant treatment using random assignment.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants T32DA007244 (to M. M. Robertson) and R03-DA037405 (to M. A. Sheridan), Alfred P. Sloan Foundation Grant FG-2015-66057 (to B. Voytek), Whitehall Foundation Grant 2017-12-73 (to B. Voytek), National Science Foundation Grant BCS-1736028 (to B. Voytek), and NIH Grants P60AA011605 (to C. A. Boettiger), R01NR017221 (to C. A. Boettiger), and K01-MH092555 (to M. A. Sheridan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.M.R., C.A.B., and M.A.S. conceived and designed research; M.A.S. performed experiments; M.M.R. and S.F. analyzed data; M.M.R., B.V., T.D., C.A.B., and M.A.S. interpreted results of experiments; M.M.R. prepared figures; M.M.R. drafted manuscript; M.M.R., S.F., B.V., T.D., C.A.B., and M.A.S. edited and revised manuscript; M.M.R., S.F., B.V., T.D., C.A.B., and M.A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Julio Dominguez and Michelle Kim for their insights and technical assistance.
REFERENCES
- Achenbach TM. Child Behavior Checklist and related instruments. In: The Use of Psychological Testing for Treatment Planning and Outcome Assessment, edited by Maruish ME. Hillsdale, NJ: Erlbaum, 1994, p. 517–549. [Google Scholar]
- Arns M, Conners CK, Kraemer HC. A decade of EEG theta/beta ratio research in ADHD: a meta-analysis. J Atten Disord 17: 374–383, 2013. doi: 10.1177/1087054712460087. [DOI] [PubMed] [Google Scholar]
- Aurlien H, Gjerde IO, Aarseth JH, Eldøen G, Karlsen B, Skeidsvoll H, Gilhus NE. EEG background activity described by a large computerized database. Clin Neurophysiol 115: 665–673, 2004. doi: 10.1016/j.clinph.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clin Neurophysiol 114: 171–183, 2003. doi: 10.1016/S1388-2457(02)00362-0. [DOI] [PubMed] [Google Scholar]
- Başar E, Başar-Eroğlu C, Karakaş S, Schürmann M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neurosci Lett 259: 165–168, 1999. doi: 10.1016/S0304-3940(98)00934-3. [DOI] [PubMed] [Google Scholar]
- Başar E, Başar-Eroglu C, Karakaş S, Schürmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol 39: 241–248, 2001. doi: 10.1016/S0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Bruchmüller K, Margraf J, Schneider S. Is ADHD diagnosed in accord with diagnostic criteria? Overdiagnosis and influence of client gender on diagnosis. J Consult Clin Psychol 80: 128–138, 2012. doi: 10.1037/a0026582. [DOI] [PubMed] [Google Scholar]
- Buchmann J, Gierow W, Weber S, Hoeppner J, Klauer T, Wittstock M, Benecke R, Haessler F, Wolters A. Modulation of transcallosally mediated motor inhibition in children with attention deficit hyperactivity disorder (ADHD) by medication with methylphenidate (MPH). Neurosci Lett 405: 14–18, 2006. doi: 10.1016/j.neulet.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Chen H, Chen W, Song Y, Sun L, Li X. EEG characteristics of children with attention-deficit/hyperactivity disorder. Neuroscience 406: 444–456, 2019. doi: 10.1016/j.neuroscience.2019.03.048. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, Baker IE, McCarthy R, Selikowitz M. An investigation of stimulant effects on the EEG of children with attention-deficit/hyperactivity disorder. Clin EEG Neurosci 48: 235–242, 2017. doi: 10.1177/1550059416664657. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, Selikowitz M, Brown CR, Croft RJ. Effects of stimulant medications on the EEG of children with attention-deficit/hyperactivity disorder predominantly inattentive type. Int J Psychophysiol 47: 129–137, 2003. doi: 10.1016/S0167-8760(02)00119-8. [DOI] [PubMed] [Google Scholar]
- Cole WR, Mostofsky SH, Larson JC, Denckla MB, Mahone EM. Age-related changes in motor subtle signs among girls and boys with ADHD. Neurology 71: 1514–1520, 2008. doi: 10.1212/01.wnl.0000334275.57734.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners C. Conners Rating Scales-Revised. North Tonawanda, NY: Multihealth Systems, 2001. [Google Scholar]
- Courvoisie H, Hooper SR, Fine C, Kwock L, Castillo M. Neurometabolic functioning and neuropsychological correlates in children with ADHD-H: preliminary findings. J Neuropsychiatry Clin Neurosci 16: 63–69, 2004. doi: 10.1176/jnp.16.1.63. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134: 9–21, 2004. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Doppelmayr M, Klimesch W, Pachinger T, Ripper B. Individual differences in brain dynamics: important implications for the calculation of event-related band power. Biol Cybern 79: 49–57, 1998. doi: 10.1007/s004220050457. [DOI] [PubMed] [Google Scholar]
- Edden RA, Crocetti D, Zhu H, Gilbert DL, Mostofsky SH. Reduced GABA concentration in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 69: 750–753, 2012. doi: 10.1001/archgenpsychiatry.2011.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein HT. EEG developmental stages. Dev Psychobiol 13: 629–631, 1980. doi: 10.1002/dev.420130608. [DOI] [PubMed] [Google Scholar]
- Fernández A, Maestú F, Amo C, Gil P, Fehr T, Wienbruch C, Rockstroh B, Elbert T, Ortiz T. Focal temporoparietal slow activity in Alzheimer’s disease revealed by magnetoencephalography. Biol Psychiatry 52: 764–770, 2002. doi: 10.1016/S0006-3223(02)01366-5. [DOI] [PubMed] [Google Scholar]
- Fritschy J-M. Epilepsy, E/I balance and GABAA receptor plasticity. Front Mol Neurosci 1: 5, 2008. doi: 10.3389/neuro.02.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R. Interpreting the electrophysiological power spectrum. J Neurophysiol 115: 628–630, 2016. doi: 10.1152/jn.00722.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Penzes P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr Mol Med 15: 146–167, 2015. doi: 10.2174/1566524015666150303003028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Peterson EJ, Voytek B. Inferring synaptic excitation/inhibition balance from field potentials. Neuroimage 158: 70–78, 2017. doi: 10.1016/j.neuroimage.2017.06.078. [DOI] [PubMed] [Google Scholar]
- Gasser T, Bächer P, Möcks J. Transformations towards the normal distribution of broad band spectral parameters of the EEG. Electroencephalogr Clin Neurophysiol 53: 119–124, 1982. doi: 10.1016/0013-4694(82)90112-2. [DOI] [PubMed] [Google Scholar]
- Grandy TH, Werkle-Bergner M, Chicherio C, Schmiedek F, Lövdén M, Lindenberger U. Peak individual alpha frequency qualifies as a stable neurophysiological trait marker in healthy younger and older adults. Psychophysiology 50: 570–582, 2013. doi: 10.1111/psyp.12043. [DOI] [PubMed] [Google Scholar]
- Haller M, Donoghue T, Peterson E, Varma P, Sebastian P, Gao R, Noto T, Knight RT, Shestyuk A, Voytek B. Parameterizing neural power spectra (Preprint). bioRxiv 299859, 2018. doi: 10.1101/299859. [DOI] [PMC free article] [PubMed]
- Hammerness P, Biederman J, Petty C, Henin A, Moore CM. Brain biochemical effects of methylphenidate treatment using proton magnetic spectroscopy in youth with attention-deficit hyperactivity disorder: a controlled pilot study. CNS Neurosci Ther 18: 34–40, 2012. doi: 10.1111/j.1755-5949.2010.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ. Scale-free brain activity: past, present, and future. Trends Cogn Sci 18: 480–487, 2014. doi: 10.1016/j.tics.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh L-T, Ranganath C. Frontal midline theta oscillations during working memory maintenance and episodic encoding and retrieval. Neuroimage 85: 721–729, 2014. doi: 10.1016/j.neuroimage.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isiten HN, Cebi M, Sutcubasi Kaya B, Metin B, Tarhan N. Medication effects on EEG biomarkers in attention-deficit/hyperactivity disorder. Clin EEG Neurosci 48: 246–250, 2017. doi: 10.1177/1550059416675232. [DOI] [PubMed] [Google Scholar]
- Kappenman ES, Luck SJ. The effects of electrode impedance on data quality and statistical significance in ERP recordings. Psychophysiology 47: 888–904, 2010. doi: 10.1111/j.1469-8986.2010.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev 29: 169–195, 1999. doi: 10.1016/S0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 6: 312–324, 2005. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Liu Q, Balsters JH, Baechinger M, van der Groen O, Wenderoth N, Mantini D. Estimating a neutral reference for electroencephalographic recordings: the importance of using a high-density montage and a realistic head model. J Neural Eng 12: 056012, 2015. doi: 10.1088/1741-2560/12/5/056012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loe IM, Feldman HM. Academic and educational outcomes of children with ADHD. Ambul Pediatr 7, Suppl: 82–90, 2007. doi: 10.1016/j.ambp.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Loo SK, Makeig S. Clinical utility of EEG in attention-deficit/hyperactivity disorder: a research update. Neurotherapeutics 9: 569–587, 2012. doi: 10.1007/s13311-012-0131-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu P, Ferree T. Determination of the HydroCel Geodesic Sensor Nets’ Average Electrode Positions and Their 10–10 International Equivalents (Technical Note). Eugene, OR: Electrical Geodesics, 2005. [Google Scholar]
- Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, Sejnowski TJ. Dynamic brain sources of visual evoked responses. Science 295: 690–694, 2002. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- Manning JR, Jacobs J, Fried I, Kahana MJ. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. J Neurosci 29: 13613–13620, 2009. doi: 10.1523/JNEUROSCI.2041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clin Neurophysiol 113: 1199–1208, 2002. doi: 10.1016/S1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Merten EC, Cwik JC, Margraf J, Schneider S. Overdiagnosis of mental disorders in children and adolescents (in developed countries). Child Adolesc Psychiatry Ment Health 11: 5, 2017. doi: 10.1186/s13034-016-0140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll GH, Heinrich H, Trott G, Wirth S, Rothenberger A. Deficient intracortical inhibition in drug-naive children with attention-deficit hyperactivity disorder is enhanced by methylphenidate. Neurosci Lett 284: 121–125, 2000. doi: 10.1016/S0304-3940(00)00980-0. [DOI] [PubMed] [Google Scholar]
- Monastra VJ, Lubar JF, Linden M. The development of a quantitative electroencephalographic scanning process for attention deficit-hyperactivity disorder: reliability and validity studies. Neuropsychology 15: 136–144, 2001. doi: 10.1037/0894-4105.15.1.136. [DOI] [PubMed] [Google Scholar]
- Monastra VJ, Lubar JF, Linden M, VanDeusen P, Green G, Wing W, Phillips A, Fenger TN. Assessing attention deficit hyperactivity disorder via quantitative electroencephalography: an initial validation study. Neuropsychology 13: 424–433, 1999. doi: 10.1037/0894-4105.13.3.424. [DOI] [PubMed] [Google Scholar]
- Nakao T, Radua J, Rubia K, Mataix-Cols D. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am J Psychiatry 168: 1154–1163, 2011. doi: 10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
- Onton J, Makeig S. Information-based modeling of event-related brain dynamics. Prog Brain Res 159: 99–120, 2006. doi: 10.1016/S0079-6123(06)59007-7. [DOI] [PubMed] [Google Scholar]
- Peterson EJ, Rosen BQ, Campbell AM, Belger A, Voytek B. 1/f neural noise is a better predictor of schizophrenia than neural oscillations (Preprint). bioRxiv 113449, 2018. doi: 10.1101/113449. [DOI] [PubMed]
- Podvalny E, Noy N, Harel M, Bickel S, Chechik G, Schroeder CE, Mehta AD, Tsodyks M, Malach R. A unifying principle underlying the extracellular field potential spectral responses in the human cortex. J Neurophysiol 114: 505–519, 2015. doi: 10.1152/jn.00943.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 164: 942–948, 2007. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Polanczyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA. ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol 43: 434–442, 2014. doi: 10.1093/ije/dyt261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav 2: 255–267, 2003. doi: 10.1034/j.1601-183X.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad JF, Kohn MR, Clarke S, Lagopoulos J, Hermens DF. Is the theta/beta EEG Marker for ADHD inherently flawed? J Atten Disord 22: 815–826, 2018. doi: 10.1177/1087054715578270. [DOI] [PubMed] [Google Scholar]
- Selten M, van Bokhoven H, Nadif Kasri N. Inhibitory control of the excitatory/inhibitory balance in psychiatric disorders. F1000 Res 7: 23, 2018. doi: 10.12688/f1000research.12155.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry 39: 28–38, 2000. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shaw P, Sharp WS, Morrison M, Eckstrand K, Greenstein DK, Clasen LS, Evans AC, Rapoport JL. Psychostimulant treatment and the developing cortex in attention deficit hyperactivity disorder. Am J Psychiatry 166: 58–63, 2009. doi: 10.1176/appi.ajp.2008.08050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder SM, Hall JR. A meta-analysis of quantitative EEG power associated with attention-deficit hyperactivity disorder. J Clin Neurophysiol 23: 441–455, 2006. doi: 10.1097/01.wnp.0000221363.12503.78. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Brown A, Seidman LJ, Valera EM, Makris N, Lomedico A, Faraone SV, Biederman J. Effect of psychostimulants on brain structure and function in ADHD: a qualitative literature review of magnetic resonance imaging-based neuroimaging studies. J Clin Psychiatry 74: 902–917, 2013. doi: 10.4088/JCP.12r08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storebø OJ, Krogh HB, Ramstad E, Moreira-Maia CR, Holmskov M, Skoog M, Nilausen TD, Magnusson FL, Zwi M, Gillies D, Rosendal S, Groth C, Rasmussen KB, Gauci D, Kirubakaran R, Forsbøl B, Simonsen E, Gluud C. Methylphenidate for attention-deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta-analyses and trial sequential analyses of randomised clinical trials. BMJ 351: h5203, 2015. doi: 10.1136/bmj.h5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM. The SNAP-IV Teacher and Parent Rating Scale (PhD thesis). Irvine, CA: University of California, 2011. [Google Scholar]
- Thomas R, Sanders S, Doust J, Beller E, Glasziou P. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics 135: e994–e1001, 2015. doi: 10.1542/peds.2014-3482. [DOI] [PubMed] [Google Scholar]
- Valera EM, Spencer RM, Zeffiro TA, Makris N, Spencer TJ, Faraone SV, Biederman J, Seidman LJ. Neural substrates of impaired sensorimotor timing in adult attention-deficit/hyperactivity disorder. Biol Psychiatry 68: 359–367, 2010. doi: 10.1016/j.biopsych.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, Perou R, Blumberg SJ. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003–2011. J Am Acad Child Adolesc Psychiatry 53: 34–46.e2, 2014. doi: 10.1016/j.jaac.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B, Kramer MA, Case J, Lepage KQ, Tempesta ZR, Knight RT, Gazzaley A. Age-Related Changes in 1/f Neural Electrophysiological Noise. J Neurosci 35: 13257–13265, 2015. doi: 10.1523/JNEUROSCI.2332-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuga M, Fox NA, Cohn JF, Kovacs M, George CJ. Long-term stability of electroencephalographic asymmetry and power in 3 to 9 year-old children. Int J Psychophysiol 67: 70–77, 2008. doi: 10.1016/j.ijpsycho.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackernah RC, Minnick MJ, Clapp P. Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst Abuse Rehabil 5: 1–12, 2014. doi: 10.2147/SAR.S37907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehmeier PM, Schacht A, Barkley RA. Social and emotional impairment in children and adolescents with ADHD and the impact on quality of life. J Adolesc Health 46: 209–217, 2010. doi: 10.1016/j.jadohealth.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Wigal SB, Gupta S, Greenhill L, Posner K, Lerner M, Steinhoff K, Wigal T, Kapelinski A, Martinez J, Modi NB, Stehli A, Swanson J. Pharmacokinetics of methylphenidate in preschoolers with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 17: 153–164, 2007. doi: 10.1089/cap.2007.0043. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Martelon M, Joshi G, Bateman C, Fried R, Petty C, Biederman J. Does ADHD predict substance-use disorders? A 10-year follow-up study of young adults with ADHD. J Am Acad Child Adolesc Psychiatry 50: 543–553, 2011. doi: 10.1016/j.jaac.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler I, Brandl S, Horn F, Waldburger E, Allefeld C, Tangermann M. Robust artifactual independent component classification for BCI practitioners. J Neural Eng 11: 035013, 2014. doi: 10.1088/1741-2560/11/3/035013. [DOI] [PubMed] [Google Scholar]
- Winkler I, Debener S, Muller KR, Tangermann M. On the influence of high-pass filtering on ICA-based artifact reduction in EEG-ERP. Conf Proc IEEE Eng Med Biol Soc 2015: 4101–4105, 2015. doi: 10.1109/EMBC.2015.7319296. [DOI] [PubMed] [Google Scholar]
- Winkler I, Haufe S, Tangermann M. Automatic classification of artifactual ICA-components for artifact removal in EEG signals. Behav Brain Funct 7: 30, 2011. doi: 10.1186/1744-9081-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Strathearn L, Liu B, Yang B, Bao W. Twenty-year trends in diagnosed attention-deficit/hyperactivity disorder among US children and adolescents, 1997–2016. JAMA Netw Open 1: e181471, 2018. doi: 10.1001/jamanetworkopen.2018.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann AM, Jene T, Wolf M, Görlich A, Gurniak CB, Sassoè-Pognetto M, Witke W, Friauf E, Rust MB. Attention-deficit/hyperactivity disorder-like phenotype in a mouse model with impaired actin dynamics. Biol Psychiatry 78: 95–106, 2015. doi: 10.1016/j.biopsych.2014.03.011. [DOI] [PubMed] [Google Scholar]