Abstract
Background:
Prostate cancer is one of the most common health issues among men, especially older men. In recent years, incidences of prostate cancer is increasing.
Objective:
The aim of this study was to provide a comprehensive estimate of the survival of prostate cancer in Asian countries.
Methods:
We searched five international databases including Medline/PubMed, Scopus, Embase, Web of Knowledge and ProQuest until June 1, 2018. The Newcastle-Ottawa Quality Assessment was used to evaluate the quality of selected papers. The review protocol was registered in PROSPERO (CRD42019117044).
Results:
A total of 714 titles were retrieved. Thirty-seven studies met the inclusion criteria. Based on the random-effect model one-year, five-year and ten-year survival rate of prostate cancer were 81% (95% CI 77.8–84.2), 61.9% (95% CI 59.5–64.3) and 36.2% (95% CI 9.2–63.2) respectively. Survival rates based on HDI level for five-year were 30.07, 43.43 and 70.84 percent for medium, high and very high levels, respectively.
Conclusion:
According to the results of our study, the prostate cancer survival rate in Asian countries is relatively lower than in Europe and North America.
Introduction
Prostate cancer, the sixth most common cancer of all cancers, is the second most frequent malignancy in men around the world as well as the most common cancer in men in Europe, North America and parts of Africa [1,2]. The number of new cases in 2000 amounted to 513,000 people, in 2008 nearly 900,000 people (33 per 100,000 population) and in 2012, about 1.1 million cases were estimated; it is expected that in 2030, the number of new cases of the disease will reach about 1.7 million people and the number of deaths is estimated to extend to 499,000 people globally [3,4,5]. Recent international studies on the epidemiology of prostate cancer indicate a high incidence of this disease in Western countries [2,6]. The highest incidences of this cancer are observed in Australia/New Zealand, North America, and northern and western parts of Europe [7,8,9]. On the other hand, over the past few decades, the incidence of prostate cancer in Asian countries has been much lower [7,10,11]. However, significant economic growth as well as social and cultural adjustments in some Asian countries have led to an increase in life expectancy, which has ultimately expanded the incidence and mortality of these cancers in these countries [12,13]. The average mortality rate from this cancer in Asian countries was 3.8 per 100,000 [14]. The low incidence of prostate cancer in Asian countries can be due to various reasons, such as the lack of access to screening tests for diagnosis, other features including nutritional status, genetics, lifestyle, environmental factors, physical activity, smoking, race and registration cancer system [10,15,16]. Survival rates are one of the most important indicators for assessing the quality of cancer control and treatment programs. According to studies, the survival rate of patients with cancer has increased in recent years [17]. Several studies which are conducted on the survival of this cancer in Asia have reported various results, as an illustration, see Chen et al. In the period of 1992 to 2000 in China, a five-year relative survival rate of prostate cancer estimated about 32.5% [18]. However, Jung et al.’s study in South Korea for the two periods of 1996 and 2010–2014 revealed 67.2% and 93.3% survival rate respectively [18,19,20,21]. In a study in Iran, the overall five-year survival rate was 36.1% [22]. In another study conducted among different ethnic groups in China, the survival rate was reported to be 26.6% to 78% over the years, which showed a noticeable fluctuation trend and there was a significant difference between various ethnic groups [23]. In another study by Xu et al. in China, there was a significant difference between the five-year survival of prostate cancer patients with hypertension (28.5%) and the control population (48.3%) [24].
Accurate information on the survival rate of prostate cancer is essential, as it is used in various health and diagnostic planning [25,26]. There is no comprehensive estimate of the survival rate of prostate cancer in Asian countries. Considering the importance of knowledge of the survival rate of this cancer in hospital planning and the different results between published articles, the purpose of this study was to provide a comprehensive estimate of the survival of prostate cancer patients in Asian countries through systematic review and meta-analysis.
Material and methods
The present study is a systematic review and meta-analysis study of prostate cancer survival in Asian countries. This study was designed and conducted in 2018. The methodology of the present study is based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement [27]. The review protocol was registered in PROSPERO (CRD42019117044).
Search strategy
The researchers searched five international databases including Medline/PubMed, Scopus, Embase, Web of Knowledge, and ProQuest until June 1, 2018. We also searched the Google Scholar for detecting grey literature. Selected keywords for international databases included: (“Neoplasm”, “Cancer”, “Carcinoma”, “Malignancy”, “prostate Cancer”, “prostate Neoplasms”, “prostate carcinoma”, “prostate Tumor”, “Cancer of prostate”, “Neoplasms of prostate”, “Survival”, “Survival Analysis”, “Survival Rate”, “Afghanistan”, “Armenia”, “Azerbaijan”, “Bahrain”, “Bangladesh”, “Bhutan”, “Brunei”, “Myanmar”, “Cambodia”, “China”, “Georgia”, “Hong Kong”, “India”, “Indonesia”, “Iran”, “Iraq”, “Israel”, “Japan”, “Jordan”, “Kazakhstan”, “North Korea”, “South Korea”, “Kuwait”, “Kyrgyzstan”, “Laos”, “Lebanon”, “Macau”, “Malaysia”, “Maldives”, “Mongolia”, “Nepal”, “Oman”, “Pakistan”, “Philippines”, “Qatar”, “Saudi Arabia”, “Singapore”, “Sri Lanka”, “Syria”, “Taiwan”, “Tajikistan”, “Thailand”, “Timor-Leste”, “Turkmenistan”, “United Arab Emirates”, “Uzbekistan”, “Vietnam”, and “Yemen”).
The initial search was conducted by two researchers (SR and MF). The searched record entered the EndNote X7 software, and duplicate articles were deleted.
Inclusion and exclusion criteria
All observational studies (cross-sectional, case-control, and cohort) stated the survival rate of localized prostate cancer in Asian countries were included in the study. Articles of other cancers reported survival in people who reported regional, metastatic, as well as review and meta-analysis studies were excluded. It should be noted that studies that did not report the sample size or confidence interval of survival rates were not included in the meta-analysis.
Quality assessment
The Newcastle-Ottawa Quality Assessment Form was used to evaluate the quality of selected papers. This tool has three different parts including selection (4 questions), comparability (1 question) and outcome (3 questions) and based on the final scores divided into three categories: good (3 or 4 stars in selection domain and 1 or 2 stars in comparability domain and 2 or 3 stars in outcome/exposure domain), fair (2 stars in selection domain and 1 or 2 stars in comparability domain and 2 or 3 stars in outcome/exposure domain) and poor (0 or 1 star in selection domain or 0 stars in comparability domain or 0 or 1 stars in outcome/exposure domain) [28]. Results of quality assessment are presented in Appendix 1.
Screening of studies
Screening of studies, extraction of results, and evaluation of quality control of articles were performed separately independently by two authors (HD and EA). If there was no agreement between the two, the supervisor (SH) would announce the final comment on that article.
Data extraction form
All final articles entered into the study process were provided by a checklist that was previously prepared, and were arranged to extract the data. This checklist includes the name of the author, the year of publication, the period of the study, the country of origin, the survival rate by year for each survival period.
Statistical analysis
The heterogeneity of the studies was assessed by Cochran test (with significance less than 0.1) and its composition using I2 statistics. In the case of heterogeneity, the random-effects model was utilized with the inverse-variance method, and in the absence of heterogeneity, the fixed-effects model was applied. In the case of a heterogeneity in the studies, methods such as subgroup analysis were used and factors like the geographical area and the HDI considered in the analysis of subgroups. All analyzes were performed by the STATA (version 13) software.
Additional analysis
Due to the heterogeneity of the studies, the subgroups analysis was used. The indicator applied for this purpose is Human Development Index (HDI). The HDI is a relative measure of life expectancy, education, quality and education level, and in general, it is the living standard in human societies. This index is estimated using the measure of welfare, especially among children and people of low age. These statistics can be used to measure the development of countries, the impact of economic policies on living standards, and the survival of prostate cancer in each of the countries was reported to provide a clear indication of the prostate cancer survival status in each country [29].
Results
Study selection
After searching all the international databases, 714 articles were selected and after removing duplicate articles, the total was 556. After reviewing the titles and abstract of articles, 144 articles entered the next stage, at which point the full text was examined and 37 articles entered the final analysis. It should be noted that the referenced articles were also reviewed to add related studies. In the screening stages, some articles were excluded for a variety of reasons, which included unrelated topic (N = 289), unrelated population (N = 182), inadequate information (N = 44) and repeated results (N = 4). The study selection process is outlined in Figure 1.
Figure 1.
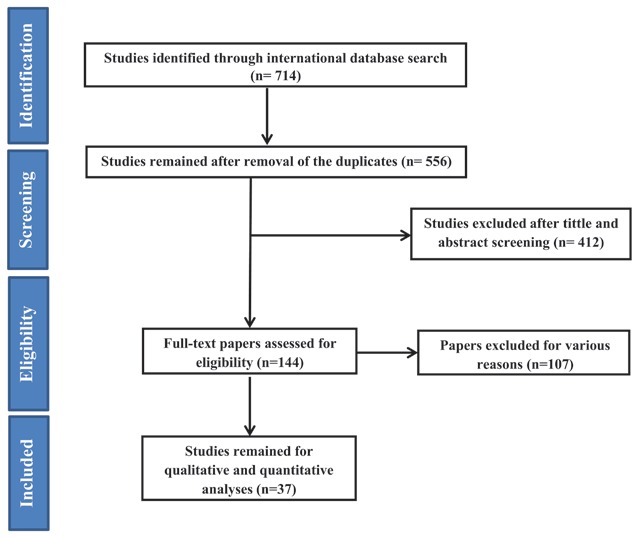
Flowchart of the included eligible studies in systematic review.
Study characteristics
The included studies were published from 1998 to 2018. Based on geographical locations, nine studies were conducted in Korea [19,20,21,30,31,32,33,34,35], five in China [18,36,37,38,39], five in Japan [40,41,42,43,44], four in India [45,46,47,48], four in Thailand [49,50,51,52], two in Singapore [53,54], two in Taiwan [55,56], one in Hong Kong [57], one in the Philippines [58], one involved Chinese residents in Singapore [59], one involved countries (China, India and Singapore) [60], one involved the Asian population in England [61]. Characteristics of the included studies are presented in Table 1.
Table 1.
Basic information of included studies.
| Order | Author (year) | Location | Time period | Sample size | Survival Rate | ||
|---|---|---|---|---|---|---|---|
| 1 | 5 | 10 | |||||
| 1 | Esteban (1998) | Philippines | 1987 | 58 | 66.00 | 15.60 | NR |
| 2 | Fan Jin (1998) | China | 1988–1991 | 373 | 62.50 | 29.60 | NR |
| 3 | Martin (1998) | Thailand | 1983–1992 | 157 | 65.20 | 28.70 | NR |
| 4 | Sato (2002) | Japan | 1962–1966 | 27 | NR | 36.30 | NR |
| 1967–1971 | 32 | NR | 19.4 | NR | |||
| 1972–1976 | 46 | NR | 37.6 | NR | |||
| 1977–1981 | 51 | NR | 31.3 | NR | |||
| 1982–1986 | 58 | NR | 24.2 | NR | |||
| 1987–1991 | 88 | NR | 73.4 | NR | |||
| 1992–1994 | 114 | NR | 84.5 | NR | |||
| 5 | Tsukuma (2006) | Japan | 1993–1996 | 4348 | 88.6 | 50.2 | NR |
| 6 | Jung (2007) | Korea | 1993–1997 | NR | NR | 59.1 | NR |
| 1998–2002 | NR | 70.6 | NR | ||||
| 7 | Lim (2009) | Singapore | 1978–1982 | NR | NR | NR | 16.5 |
| 1983–1987 | NR | NR | 30.1 | ||||
| 1988–1992 | NR | NR | 31.6 | ||||
| 1993–1997 | NR | NR | 41.6 | ||||
| 1998–2002 | NR | NR | 45.2 | ||||
| 8 | Chia (2010) | Chinese Residents in Singapore | 1973–1977 | NR | NR | 51.3 | NR |
| 1978–1982 | NR | 47.7 | |||||
| 1983–1987 | NR | 55.7 | |||||
| 1988–1992 | NR | 76.5 | |||||
| 1993–1997 | NR | 76.3 | |||||
| 1998–2002 | NR | 76.1 | |||||
| 9 | Chen (2011) | China | 1992–2000 | 55 | 37.7 | 15.2 | NR |
| 1982–1991 | NR | 20.7 | NR | ||||
| 10 | Chia (2011) | Singapore | 1993–1997 | 752 | 82.4 | 44.6 | NR |
| 11 | Jayalekshmi (2011) | India | 1991–1997 | 32 | 93.5 | 22.1 | NR |
| 12 | Jung (2011) | Korea | 1993–1995 | NR | NR | 55.9 | NR |
| 1996–2000 | NR | NR | 67.2 | NR | |||
| 2001–2005 | NR | NR | 79.5 | NR | |||
| 2004–2008 | NR | NR | 86.2 | NR | |||
| 13 | Law (2011) | Hong Kong | 1996–2001 | 3206 | 87.8 | 55.6 | NR |
| 14 | Martin (2011) | Thailand | 1990–2000 | 171 | 80.7 | 53.1 | NR |
| 15 | Matsuda (2011) | Japan | 1993–1996 | 4220 | NR | 66.8 | NR |
| 1997–1999 | 4508 | NR | 75.5 | NR | |||
| 1993–1996 | NR | NR | 96.5 | NR | |||
| 1997–1999 | NR | NR | 97.6 | NR | |||
| 16 | Sankaranarayanan (2011) | China | 1996–2001 | NR | NR | 78.2 | NR |
| China | 1992–2000 | NR | NR | 36.3 | NR | ||
| China | 1992–1995 | NR | NR | 54.2 | NR | ||
| China | 1991–1999 | NR | NR | 68.4 | NR | ||
| India | 1991–1997 | NR | NR | 32.6 | NR | ||
| India | 1992–1999 | NR | NR | 42.3 | NR | ||
| Singapore | 1993–1997 | NR | NR | 64.3 | NR | ||
| 17 | Sriplung (2011) | Thailand | 1990–1999 | 144 | 80.8 | 32.1 | NR |
| 18 | Sumitsawan (2011) | Thailand | 1993–1997 | 107 | 78.8 | 30.4 | NR |
| 19 | Xiang (2011) | China | 1992–1995 | 639 | 68.8 | 36.9 | NR |
| 20 | Xishan (2011) | China | 1991–1999 | 423 | 74.5 | 52.5 | NR |
| 1981–1990 | NR | NR | 41.0 | NR | |||
| 21 | Yeole (2011) | India | 1992–1999 | 1463 | 64.3 | 24.0 | NR |
| 22 | Chang (2012) | Taiwan | 2002 | 5933 | NR | 77.1 | NR |
| 2002 | 1392 | NR | 66.7 | NR | |||
| 2002 | 1591 | NR | 69.3 | NR | |||
| 2002 | 2761 | NR | 58.9 | NR | |||
| 23 | Jung (2012) | Korea | 1993–1995 | NR | NR | 55.9 | NR |
| 1996–2000 | NR | NR | 67.2 | NR | |||
| 2001–2005 | NR | NR | 79.9 | NR | |||
| 2005–2009 | NR | NR | 87.6 | NR | |||
| 24 | Balasubramaniam (2013) | India | 1999–2002 | 371 | 92.0 | 62.0 | NR |
| 25 | Ito (2013) | Japan | 2000–2004 | NR | 81.0 | 87.0 | NR |
| 26 | Jung (2013) | Korea | 1993–1995 | NR | NR | 55.9 | NR |
| 1996–2000 | NR | NR | 67.2 | NR | |||
| 2001–2005 | NR | NR | 80.1 | NR | |||
| 2006–2010 | NR | NR | 90.2 | NR | |||
| 27 | Ito (2014) | Japan | 2002–2006 | 19519 | NR | 89.2 | 78.0 |
| 2001–2004 | NR | 94.2 | 79.6 | NR | |||
| 28 | Jung (2014) | Korea | 2007–2011 | NR | NR | 92.0 | NR |
| 29 | Takiar (2014) | India | 2011 | 1495 | 64.9 | 23.9 | NR |
| 30 | Jung (2015) | Korea | 2008–2012 | 92.3 | NR | ||
| 31 | Maringe (2015) | England (South Asians) | 1986–1995 | 310 | 92.3 | 54.4 | NR |
| England (Non-South Asians) | 1986–1995 | 35550 | 84.4 | 51.0 | NR | ||
| England (South Asians) | 1996–2004 | 1052 | 95.2 | 78.4 | NR | ||
| England (Non-South Asians) | 1996–2004 | 72724 | 95.0 | 81.1 | NR | ||
| 32 | Chang-Mo Oh (2016) | Korea | 2008–2013 | NR | NR | 92.5 | NR |
| 33 | Jung (2017) | Korea | 2010–2014 | NR | NR | 93.3 | NR |
| 34 | Chen (2018) | China | 2002–2014 | NR | 69.05 | 18.80 | 9.40 |
| 2002–2014 | NR | NR | 13.80 | NR | |||
| 2002–2014 | NR | NR | 24.00 | NR | |||
| 35 | Chien (2018) | Taiwan | 2000–2010 | NR | NR | 80.06 | NR |
| 2000–2010 | NR | NR | 71.62 | NR | |||
| 2000–2010 | NR | NR | 76.6 | NR | |||
| 36 | Jung (2018) | Korea | 2011–2015 | NR | NR | 94.1 | NR |
| 37 | Zeng (2018) | China | 2003–2005 | 11690 | NR | 53·8 | NR |
| 2006–2008 | NR | 60·8 | NR | ||||
| 2009–2011 | NR | 59·2 | NR | ||||
| 2012–2015 | NR | 66·4 | NR | ||||
NR: Not Reported.
Quality appraisal
The results of the quality assessment of the articles have been shown in Appendix 2. Based on our results, 35 studies have good and 2 studies have a fair quality.
Results of the meta-analysis
First, the articles were sorted according to the year of publication and then analyzed by the one-, five- and ten-year survival rate. It should be noted that the number of papers discussing two- and four-year survival rate was very low, and the results of the three-year survival rate were not very reasonable due to that most of the less-developed countries and lower HDI were reported.
One-year survival rate
Of the most recent papers, 23 number reported one-year survival rate. Based on the random-effect model, the results of the study demonstrated that one-year survival rate in Asian countries was 81% with a 95% confidence interval of 77.8 to 84.2. One-year survival rate of prostate cancer based on HDI has been shown in Figure 2. According to the results, the highest one-year survival rate in countries with a high HDI level was 91% (95% CI, 95–87) and the lowest was for countries with high HDI levels 71.3% (95% CI, 77.3–65.3).
Figure 2.
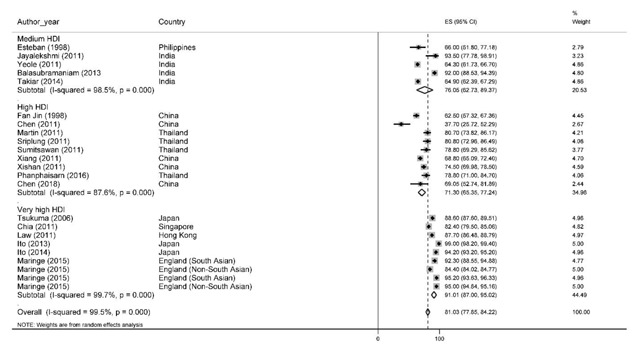
Forest plot of one-year survival rate of prostate cancer in Asian countries.
Five-year survival rate
The five-year survival rate was 61.9% with a 95% confidence interval of 59.5 to 64.3. The results of the five-year survival rate by the HDI has been illustrated in figure Figure 3. Based on the findings of our study, the highest five-year survival rate for countries with a high HDI level was 70.8% (95% CI, 68.5–73.1) and the lowest among countries with a medium HDI level of 30% (95% CI, 17.5–42.5).
Figure 3.
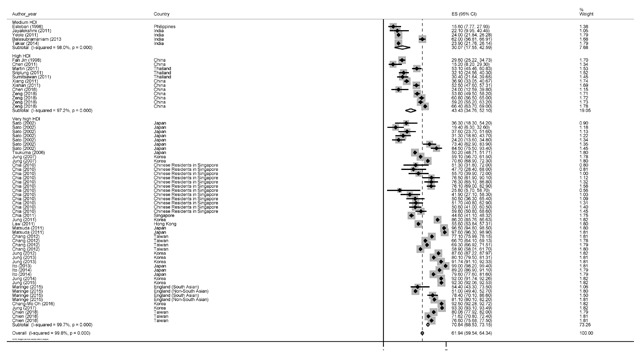
Forest plot of five-year survival rate of prostate cancer in Asian countries.
Ten-year survival rate
A total of 11 studies reported 10-year survival rate. Based on the results, the 10-year survival rate was 36.2% with 95% confidence interval of 9.2 to 63.2. Ten-year survival rate of prostate cancer by HDI has been shown in Figure 4. Based on the results, the highest survival rates for countries with a high HDI level were 40.8% (95% CI, 14.5–67) and the lowest for countries with high HDI levels 9.4 (95% CI, 3–23.5). It should be noted that countries with medium HDI levels did not report the 10-year survival rate.
Figure 4.
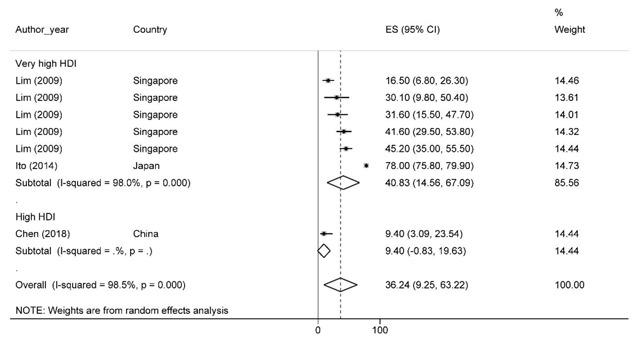
Forest plot of ten-year survival rate of prostate cancer in Asian countries.
Survival rate of prostate cancer in each country
Overall, the results of the survival of prostate cancer in nine countries and three other areas has been showed in the Table 2. The highest survival rates of one, five, and ten-years were reported in Japan (93), Korea (84.9), and Japan (78), respectively, and the lowest survival rates for these years were observed in China (64.1), Philippines (15.6), and China (9.4), respectively.
Table 2.
Result of meta-analysis and heterogeneity of survival rate of prostate cancer in Asia based on each country and years of survival.
| Country | Year of Survival | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 10 | ||||||||||
| # of Study | Effect estimate | I2 | P-value | # of Study | Effect estimate | I2 | P-value | # of Study | Effectestimate | I2 | P-value | |
| China | 5 | 64.1 (56.2–72) | 88.1 | <0.001 | 9 | 45 (35–54.9) | 97.7 | <0.001 | 1 | 9.4 (3–23.5) | – | – |
| Hong Kong | 1 | 87.7 (86.5–88.8) | – | – | 1 | 55.6 (53.8–57.3) | – | – | – | NR | NR | NR |
| India | 4 | 78.2 (63.6–93.1) | 98.9 | <0.001 | 4 | 33.4 (19.3–47.5) | 98.5 | <0.001 | – | NR | NR | NR |
| Japan | 3 | 93.9 (87.6–99.8) | 99.4 | <0.001 | 13 | 64.2 (53.2–75.3) | 99.7 | <0.001 | 1 | 78 (75.9–80) | – | – |
| Philippines | 1 | 66 (51.8–77.1) | – | – | 1 | 15.6 (5.5–25.6) | – | – | – | NR | NR | NR |
| Singapore | 1 | 82.4 (79.6–85.1) | – | – | 1 | 44.6 (40.9–48.2) | – | – | 5 | 33 (20.7–45.4) | 78.3 | <0.001 |
| Korea | – | NR* | NR | NR | 10 | 84.9 (82.8–97) | 99.7 | <0.001 | – | NR | NR | NR |
| Taiwan | – | NR | NR | NR | 7 | 71.5 (67.2–75.7) | 98.6 | <0.001 | – | NR | NR | NR |
| Thailand | 4 | 79.9 (76.4–83.3) | 0 | 0.960 | 3 | 38.6 (23.9–53.3) | 89.7 | <0.001 | – | NR | NR | NR |
| Chinese Residents in Singapore | – | NR | NR | NR | 12 | 57.1 (48.9–65.3) | 80 | <0.001 | – | NR | NR | NR |
| England (South Asian) | 2 | 94.1 (91.3–96.8) | 63.4 | 0.099 | 2 | 67.2 (43.8–90.7) | 86.6 | 0.006 | – | NR | NR | NR |
| England (Non-South Asian) | 2 | 89.7 (79.3–99.6) | 99.9 | <0.001 | 2 | 66 (36.5–95.5) | 99.9 | <0.001 | – | NR | NR | NR |
| Overall | 24 | 81 (77.8–84.2) | 99.5 | <0.001 | 65 | 61.9 (59.5–64.3) | 99.8 | <0.001 | 7 | 36.2 (9.2–63.2) | 98.5 | <0.001 |
* NR: Not reported.
Meta-regression
Results of meta-regression showed a significant association between publication year and five-year survival rate. Thus, year of study is a cause of variability in results of five-year survival rate (Reg Coef = 0.022, p < 0.001). This association for one-year survival rate not statistically significant (Reg Coef = –0.010, p = 0.084). According to results, an increasing survival rate across the study period was observed.
Another factor for inconsistency in results is HDI. HDI is a cause of variability in results of one (Reg Coef = 0.022, p < 0.001) and five-year survival rate (Reg Coef = 2.73, p < 0.001). According to results, an increasing survival rate was observed with higher HDI in countries.
Also, we examined the sample size as other explanatory factor to variability in our study and results showed sample size was another reason for this inconsistency (Reg Coef = –2.94 e–6, p = 0.194 for one-year, Coef = –0.00001, p = 0.001 for five-year survival rate). Studies with larger sample size had a higher survival rate, but this finding not statistically significant for one-year survival rate. Results of meta-regression is shown in Figure 5.
Figure 5.
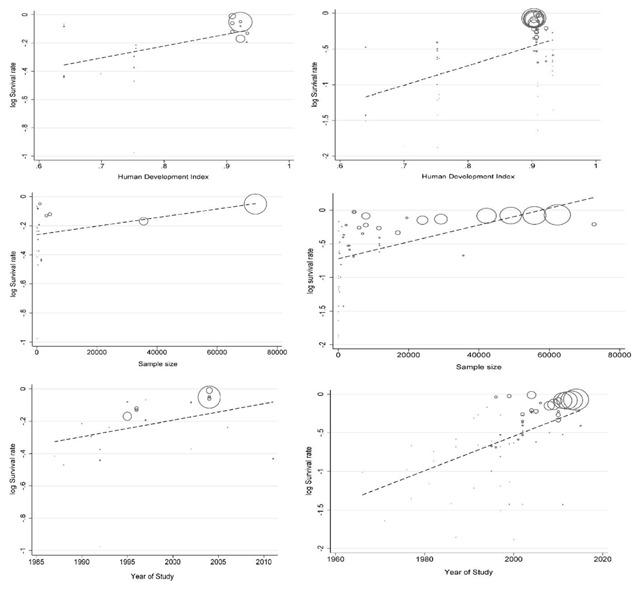
Result of meta-regression for one (left) and five-years (right) survival rate based on HDI, sample size and year of study.
Discussion
Prostate cancer is one of the most principal and arising health issues among elderly men, with a recent increase in its incidence[2]. Given that the importance of this cancer among men, especially in developing countries, as well as the many differences in the incidence and survival of this cancer in the world, awareness of survival is an important issue [62].
In this study, the survival rate of prostate cancer has been reported in Asia for one-, five- and ten-year survival rate. These values for the one-, five- and ten-year survival rate are 81.0 (77.8–84.2), 61.9 (59.5–64.3) and 36.2 (9.2–63.2) respectively.
One-year survival rates for countries with high HDI were lower than those with a medium HDI index. The reason for this difference is the low number of studies and those are the medium HDI often reported in India, while articles in the category of high HDI are mostly from China.
According to the results of this study, the highest survival rate has been reported in the Asians residence in England, followed by Japan in the second place. On the other hand, China has the lowest one-year survival rate. It seems that the higher HDI is directly related to the higher survival rate, as countries such as Japan and Singapore, which have a much higher HDI, have higher survival rates. But this is not true in India, because it has a higher survival rate, although it has a lower HDI than China. With regard to the three levels of the HDI, countries at the second level of this index have the lowest survival rates. However, given the low levels of countries at each level, especially in countries with a medium HDI, there can certainly not be any reason for this difference, and it seems to be due to variations in demographic characteristics [7]. Another potential reason can be observed in the short run of one-year, since studies have reveal that in longer periods of time, the survival rate is closer to real value [63].
This difference has not beeen seen in five-year survival rate and the aggregate survival rate for the three levels of HDI has a certain trend, so that countries with a medium HDI have the lowest survival rates and countries with a very high HDI have the highest aggregate survival rates. This higher survival rate could be better for reasons such as improving lifestyle, nutritional status, physical activity, and the use of health care [64].
According to the results of the Coleman et al. study, the five-year survival rate in 31 countries manifested that variations in the standardized survival of the age range are very wide between countries. This difference, even after adjusting for dissimilarities in mortality due to other causes, remains the highest in the United States, which was more inflated in Caucasians compared to African-Americans (92.4% versus 85.8%). These variations are due to reasons such as differences in treatment care and the stages of the diagnosed disease [65,66].
In another study by Steele et al., conducted in 2017, the results showed that the survival rates of one and five-years were significantly higher than the findings of the present study in the years 2002–2003 and 2004–2009. In the former study, the one-year survival rate for both periods was 98.6 and 98.8, while in the present study, this rate was 81%. The five-year survival rate was 96.7% and 96.9%, respectively, which was much higher than the results of the present study, which was 61.9% [67].
Critz et al. reported a 10-year survival rate of prostate cancer in their study in 2013 approximately 75% [68]. The results of this study, conducted in the United States, have revealed that survival rate is nearly twice as high as the results of the present study (75% versus 36.2%).
In general, and according to the results of this study, the survival rate of 1, 5 and 10 years for prostate cancer among Asian countries is lower than normal worldwide. Also, countries with higher HDI have higher survival values. These values are higher for Asian people outside of Asia.
In this study, we performed meta-regression for three variables including publication year, HDI level and sample size. The reason for the meta-regression for these three variables was access to data through the included studies. The meta-regression result shows a significant relationship between published year of the studies with five-year survival rate, HDI with one- and five-year survival rate, and sample size with five-year survival rate. The reason for the increase in survival rate over time can be due to introducing new treatments and interventions. Higher HDI can also lead to better access to new treatments and interventions, and this can increase survival rate of patient with prostate cancer.
Study Limitations
There are certain limitations in systematic review studies, most notably in the absence of access to some of the information that attempts were made to contact the authors of the study to resolve the problem, which in several cases did not receive an adequate response. One of the main limitations of our study was the failure to report sample size and the inability to calculate the confidence interval for survival, which did not allow the study to include the meta-analysis stage. Other limitations included a survival report of less than one-year (6 and 9 months), which, given the low level of these, had no significant effect on our results. Ultimately, due to the lack of studies reporting 10-year survival, the correct estimate of survival requires more robust studies.
Recommendations for future research
According to the results of this study, estimating the survival rate of prostate cancer requires more extensive studies at the level of other Asian countries, especially in the West and Central Asia, as most studies in this study were conducted in South and Southeast of Asia, and estimates are somewhat incorrect. Another suggestion could be a study of the survival of prostate cancer in patients who metastasized, which was not our study goal, and is an important issue in clinical decision making and the continuation of treatment.
Conclusion
According to the results of our study, the prostate cancer survival rate in Asian countries is relatively lower than in Europe and North America, which may be due to less access to diagnostic facilities and higher age at recognition of disease than to advanced countries. Another result of our study was the higher survival rate of prostate cancer in countries with very high HDI (such as South Korea and Japan), with similar survival rates as those of advanced countries such as Europe and North America.
Additional File
The additional file for this article can be found as follows:
Quality assessment of included studies.
Acknowledgements
This study was supported financially by Alborz University of Medical Sciences, Karaj, Iran.
Funding Statement
This study was supported financially by Alborz University of Medical Sciences, Karaj, Iran.
Competing Interests
The authors have no competing interests to declare.
References
- 1.Siegel RL, Miller KD and Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018; 68(1): 7–30. DOI: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2.Hassanipour-Azgomi S, Mohammadian-Hafshejani A, Ghoncheh M, Towhidi F, Jamehshorani S and Salehiniya H. Incidence and mortality of prostate cancer and their relationship with the Human Development Index worldwide. Prostate Int. 2016; 4(3): 118–124. DOI: 10.1016/j.prnil.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bashir MN. Epidemiology of Prostate Cancer. Asian Pac J Cancer Prev. 2015; 16(13): 5137–5141. DOI: 10.7314/APJCP.2015.16.13.5137 [DOI] [PubMed] [Google Scholar]
- 4.Brawley OW. Prostate cancer epidemiology in the United States. World J Urol. 2012; 30(2): 195–200. DOI: 10.1007/s00345-012-0824-2 [DOI] [PubMed] [Google Scholar]
- 5.McDavid K, Lee J, Fulton JP, Tonita J and Thompson TD. Prostate cancer incidence and mortality rates and trends in the United States and Canada. Public Health Rep. 2004; 119(2): 174–186. DOI: 10.1177/003335490411900211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly SP, Rosenberg PS, Anderson WF, et al. Trends in the Incidence of Fatal Prostate Cancer in the United States by Race. Eur Urol. 2017; 71(2): 195–201. DOI: 10.1016/j.eururo.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang K, Bangma CH and Roobol MJ. Prostate cancer screening in Europe and Asia. Asian J Urol. 2017; 4(2): 86–95. DOI: 10.1016/j.ajur.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou CK, Check DP, Lortet-Tieulent J, et al. Prostate cancer incidence in 43 populations worldwide: An analysis of time trends overall and by age group. Int J Cancer. 2016; 138(6): 1388–1400. DOI: 10.1002/ijc.29894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carducci MA, Cetin K, Markus R and Fryzek JP. Time trends in the epidemiology of newly diagnosed stage IV prostate cancer in the United States: An analysis of data from the Surveillance, Epidemiology, and End Results (SEER) Program (1988–2003). Journal of Clinical Oncology. 2008; 26(15_suppl): 5059 DOI: 10.1200/jco.2008.26.15_suppl.5059 [DOI] [Google Scholar]
- 10.Salehiniya H, Pakzad R, Hassanipour S and Mohammadian M. The incidence and mortality of thyroid cancer and its relationship with HDI in the world. World Cancer Research Journal. 2018; 5(2): 1–6. [Google Scholar]
- 11.Chornokur G, Dalton K, Borysova ME and Kumar NB. Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. Prostate. 2011; 71(9): 985–997. DOI: 10.1002/pros.21314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajjar RR, Atli T, Al-Mandhari Z, Oudrhiri M, Balducci L and Silbermann M. Prevalence of aging population in the Middle East and its implications on cancer incidence and care. Ann Oncol. 2013; 24(Suppl 7): vii11–24. DOI: 10.1093/annonc/mdt268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu X, Zheng R, Xia C, et al. Interactions between life expectancy and the incidence and mortality rates of cancer in China: A population-based cluster analysis. Cancer Commun (Lond). 2018; 38(1): 44 DOI: 10.1186/s40880-018-0308-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pakzad R, Mohammadian-Hafshejani A, Ghoncheh M, Pakzad I and Salehiniya H. The incidence and mortality of prostate cancer and its relationship with development in Asia. Prostate Int. 2015; 3(4): 135–140. DOI: 10.1016/j.prnil.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baade PD, Youlden DR, Cramb SM, Dunn J and Gardiner RA. Epidemiology of prostate cancer in the Asia-Pacific region. Prostate International. 2013; 1(2): 47–58. DOI: 10.12954/PI.12014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cullen J, Elsamanoudi S, Brassell SA, et al. The burden of prostate cancer in Asian nations. J Carcinog. 2012; 11: 7 DOI: 10.4103/1477-3163.94025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen SL, Wang SC, Ho CJ, et al. Prostate cancer mortality-to-incidence ratios are associated with cancer care disparities in 35 countries. Sci Rep. 2017; 7: 40003 DOI: 10.1038/srep40003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen JG, Zhu J, Zhang YH and Lu JH. Cancer survival in Qidong, China, 1992–2000. IARC Sci Publ. 2011; 162: 43–53. [PubMed] [Google Scholar]
- 19.Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH and Lee JS. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2011. Cancer Res Treat. 2014; 46(2): 109–123. DOI: 10.4143/crt.2014.46.2.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung KW, Park S, Kong HJ, et al. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat. 2011; 43(1): 1–11. DOI: 10.4143/crt.2011.43.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung KW, Won YJ, Oh CM, et al. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2014. Cancer Res Treat. 2017; 49(2): 292–305. DOI: 10.4143/crt.2017.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zahir ST, Nazemian MR, Zand S and Zare S. Survival of patients with prostate cancer in Yazd, Iran. Asian Pac J Cancer Prev. 2014; 15(2): 883–886. DOI: 10.7314/APJCP.2014.15.2.883 [DOI] [PubMed] [Google Scholar]
- 23.Wang F, Feng J, Chen P, et al. Probiotics in Helicobacter pylori eradication therapy: Systematic review and network meta-analysis. Clinics and Research in Hepatology and Gastroenterology. 2017; 41(4): 466–475. DOI: 10.1016/j.clinre.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 24.Xu H, Zhang LM, Liu J, Ding GX, Ding Q and Jiang HW. The association between overall survival of prostate cancer patients and hypertension, hyperglycemia, and overweight in Southern China: A prospective cohort study. J Cancer Res Clin Oncol. 2013; 139(6): 943–951. DOI: 10.1007/s00432-013-1407-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelloff GJ, Choyke P and Coffey DS. Prostate Cancer Imaging Working G. Challenges in clinical prostate cancer: Role of imaging. AJR Am J Roentgenol. 2009; 192(6): 1455–1470. DOI: 10.2214/AJR.09.2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myint Z, Huang B, Chen Q and Wang P. Epidemiology and survival disparities of prostate cancer in Appalachian Kentucky. Journal of Clinical Oncology. 2018; 36(6_suppl): 292–292. DOI: 10.1200/JCO.2018.36.6_suppl.292 [DOI] [Google Scholar]
- 27.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015; 4(1): 1 DOI: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penson DF, Krishnaswami S, Jules A, Seroogy JC and McPheeters ML. Evaluation and treatment of cryptorchidism. 2012. [PubMed] [Google Scholar]
- 29.Human Development Reports. 2018. http://hdr.undp.org/en/2018-update.
- 30.Jung KW, Yim SH, Kong HJ, et al. Cancer survival in Korea 1993–2002: A population-based study. J Korean Med Sci. 2007; 22(Suppl): S5–S10. DOI: 10.3346/jkms.2007.22.S.S5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung KW, Park S, Kong HJ, et al. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat. 2012; 44(1): 11–24. DOI: 10.4143/crt.2012.44.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG and Lee JS. Cancer statistics in Korea: Incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013; 45(1): 1–14. DOI: 10.4143/crt.2013.45.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung KW, Won YJ, Kong HJ, et al. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015; 47(2): 127–141. DOI: 10.4143/crt.2015.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh CM, Won YJ, Jung KW, et al. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat. 2016; 48(2): 436–450. DOI: 10.4143/crt.2016.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung KW, Won YJ, Kong HJ, Lee ES and Community of Population-Based Regional Cancer R. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2015. Cancer Res Treat. 2018; 50(2): 303–316. DOI: 10.4143/crt.2018.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin F, Xiang YB and Gao YT. Cancer survival in Shanghai, People’s Republic of China. IARC Sci Publ. 1998; 145: 37–50. [PubMed] [Google Scholar]
- 37.Xishan H, Chen K, Min H, Shufen D and Jifang W. Cancer survival in Tianjin, China, 1991–1999. IARC Sci Publ. 2011; 162: 69–84. [PubMed] [Google Scholar]
- 38.Chen JG, Chen HZ, Zhu J, et al. Cancer survival in patients from a hospital-based cancer registry, China. J Cancer. 2018; 9(5): 851–860. DOI: 10.7150/jca.23039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng H, Chen W, Zheng R, et al. Changing cancer survival in China during 2003–15: A pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018; 6(5): e555–e567. DOI: 10.1016/S2214-109X(18)30127-X [DOI] [PubMed] [Google Scholar]
- 40.Sato Y. Survival Trends of Patients Treated at the National Cancer Center Hospital, Japan, from 1962 to 1994. Japanese Journal of Clinical Oncology. 2002; 32(5): 181–186. DOI: 10.1093/jjco/hyf037 [DOI] [PubMed] [Google Scholar]
- 41.Tsukuma H, Ajiki W, Ioka A, Oshima A and Research Group of Population-Based Cancer Registries of J. Survival of cancer patients diagnosed between 1993 and 1996: A collaborative study of population-based cancer registries in Japan. Jpn J Clin Oncol. 2006; 36(9): 602–607. DOI: 10.1093/jjco/hyl068 [DOI] [PubMed] [Google Scholar]
- 42.Matsuda T, Ajiki W, Marugame T, et al. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: A chronological and international comparative study. Jpn J Clin Oncol. 2011; 41(1): 40–51. DOI: 10.1093/jjco/hyq167 [DOI] [PubMed] [Google Scholar]
- 43.Ito Y, Nakayama T, Miyashiro I, Ioka A and Tsukuma H. Conditional survival for longer-term survivors from 2000–2004 using population-based cancer registry data in Osaka, Japan. BMC Cancer. 2013; 13: 304 DOI: 10.1186/1471-2407-13-304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ito Y, Miyashiro I, Ito H, et al. Long-term survival and conditional survival of cancer patients in Japan using population-based cancer registry data. Cancer Sci. 2014; 105(11): 1480–1486. DOI: 10.1111/cas.12525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jayalekshmi P, Gangadharan P and Sebastian P. Cancer survival in Karunagappally, India, 1991–1997. IARC Sci Publ. 2011; 162: 125–132. [PubMed] [Google Scholar]
- 46.Yeole BB, Kurkure AP and Sunny L. Cancer survival in Mumbai (Bombay), India, 1992–1999. IARC Sci Publ. 2011; 162: 133–142. [PubMed] [Google Scholar]
- 47.Balasubramaniam G, Talole S, Mahantshetty U, Saoba S and Shrivastava S. Prostate cancer: a hospital-based survival study from Mumbai, India. Asian Pac J Cancer Prev. 2013; 14(4): 2595–2598. DOI: 10.7314/APJCP.2013.14.4.2595 [DOI] [PubMed] [Google Scholar]
- 48.Takiar R, Krishnan SK and Shah VP. A model approach to calculate cancer prevalence from 5 years survival data for selected cancer sites in India—Part II. Asian Pac J Cancer Prev. 2014; 15(14): 5681–5684. DOI: 10.7314/APJCP.2014.15.14.5681 [DOI] [PubMed] [Google Scholar]
- 49.Martin N, Srisukho S, Kunpradist O and Suttajit M. Cancer survival in Chiang Mai, Thailand. IARC Sci Publ. 1998; 145: 109–121. [PubMed] [Google Scholar]
- 50.Martin N, Pongnikorn S, Patel N and Daoprasert K. Cancer survival in Lampang, Thailand, 1990–2000. IARC Sci Publ. 2011; 162: 217–226. [PubMed] [Google Scholar]
- 51.Sriplung H and Prechavittayakul P. Cancer survival in Songkhla, Thailand, 1990–1999. IARC Sci Publ. 2011; 162: 227–235. [PubMed] [Google Scholar]
- 52.Sumitsawan Y, Srisukho S, Sastraruji A, Chaisaengkhum U, Maneesai P and Waisri N. Cancer survival in Chiang Mai, Thailand, 1993–1997. IARC Sci Publ. 2011; 162: 199–209. [PubMed] [Google Scholar]
- 53.Lim GH, Wong CS, Chow KY, Bhalla V and Chia KS. Trends in long-term cancer survival in Singapore: 1968–2002. Ann Acad Med Singapore. 2009; 38(2): 99–105. [PubMed] [Google Scholar]
- 54.Chia KS. Cancer survival in Singapore, 1993–1997. IARC Sci Publ. 2011; 162: 183–198. [PubMed] [Google Scholar]
- 55.Chang CM, Huang KY, Hsu TW, et al. Multivariate analyses to assess the effects of surgeon and hospital volume on cancer survival rates: A nationwide population-based study in Taiwan. PLoS One. 2012; 7(7): e40590 DOI: 10.1371/journal.pone.0040590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chien LH, Tseng TJ, Tsai FY, et al. Patterns of age-specific socioeconomic inequalities in net survival for common cancers in Taiwan, a country with universal health coverage. Cancer Epidemiol. 2018; 53: 42–48. DOI: 10.1016/j.canep.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 57.Law SC and Mang OW. Cancer survival in Hong Kong SAR, China, 1996–2001. IARC Sci Publ. 2011; 162: 33–41. [PubMed] [Google Scholar]
- 58.Esteban D, Ngelangel C, Lacaya L, Robles E and Monson M. Cancer survival in Rizal, Philippines. IARC Sci Publ. 1998; 145: 101–108. [PubMed] [Google Scholar]
- 59.Chia SE, Tan CS, Lim GH, Sim X, Lau W and Chia KS. Incidence, mortality and five-year relative survival ratio of prostate cancer among Chinese residents in Singapore from 1968 to 2002 by metastatic staging. Annals Academy of Medicine Singapore. 2010; 39(6): 466–471. [PubMed] [Google Scholar]
- 60.Sankaranarayanan R, Swaminathan R, Jayant K and Brenner H. An overview of cancer survival in Africa, Asia, the Caribbean and Central America: The case for investment in cancer health services. IARC Sci Publ. 2011; 162: 257–291. [PubMed] [Google Scholar]
- 61.Maringe C, Li R, Mangtani P, Coleman MP and Rachet B. Cancer survival differences between South Asians and non-South Asians of England in 1986–2004, accounting for age at diagnosis and deprivation. Br J Cancer. 2015; 113(1): 173–181. DOI: 10.1038/bjc.2015.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taitt HE. Global trends and prostate cancer: A review of incidence, detection, and mortality as influenced by race, ethnicity, and geographic location. Am J Mens Health. 2018; 12(6): 1807–1823. DOI: 10.1177/1557988318798279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kimura T. East meets West: Ethnic differences in prostate cancer epidemiology between East Asians and Caucasians. Chin J Cancer. 2012; 31(9): 421–429. DOI: 10.5732/cjc.011.10324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cuzick J, Thorat MA, Andriole G, et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014; 15(11): e484–492. DOI: 10.1016/S1470-2045(14)70211-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coleman MP, Quaresma M, Berrino F, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol. 2008; 9(8): 730–756. DOI: 10.1016/S1470-2045(08)70179-7 [DOI] [PubMed] [Google Scholar]
- 66.Bekelman JE, Rumble RB, Chen RC, et al. Clinically Localized Prostate Cancer: ASCO Clinical Practice Guideline Endorsement of an American Urological Association/American Society for Radiation Oncology/Society of Urologic Oncology Guideline. J Clin Oncol. 2018; 36(32): JCO1800606 DOI: 10.1200/JCO.18.00606 [DOI] [PubMed] [Google Scholar]
- 67.Steele CB, Li J, Huang B and Weir HK. Prostate cancer survival in the United States by race and stage (2001–2009): Findings from the CONCORD-2 study. Cancer. 2017; 123(Suppl 24): 5160–5177. DOI: 10.1002/cncr.31026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Critz FA, Benton JB, Shrake P and Merlin ML. 25-Year disease-free survival rate after irradiation for prostate cancer calculated with the prostate specific antigen definition of recurrence used for radical prostatectomy. J Urol. 2013; 189(3): 878–883. DOI: 10.1016/j.juro.2012.10.061 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quality assessment of included studies.


