Abstract
Background
Analysis of “emerging” pathogens in cystic fibrosis (CF) lung disease has focused on unique pathogens that are rare in other human diseases or are drug resistant. Escherichia coli is recovered in the sputum of up to 25% of patients with CF, yet little is known about the epidemiology or clinical impact of infection.
Methods
We studied patients attending a Canadian adult CF clinic who had positive sputum cultures for E coli from 1978 to 2016. Infection was categorized as transient or persistent (≥3 positive sputum cultures, spanning >6 months). Those with persistent infection were matched 2:1 with age, sex, and time-period controls without history of E coli infection, and mixed-effects models were used to assess pulmonary exacerbation (PEx) frequency, lung function decline, hospitalization, and intravenous antibiotic days.
Results
Forty-five patients (12.3%) had E coli recovered from sputum samples between 1978 and 2016, and 18 patients (40%) developed persistent infection. Nine patients (24%) had PEx at incident infection, and increased bioburden was predictive of exacerbation (P = .03). Risk factors for persistent infection included lower nutritional status (P < .001) and lower lung function (P = .009), but chronic infection with Pseudomonas aeruginosa was protective. There was no difference in annual lung function decline, need for hospitalization or intravenous antibiotics, or risk of PEx in patients with persistent infection.
Conclusions
Persistent E coli infection was frequent and was more common in CF patients with low nutritional status and lung function. However, this does not predict clinical decline. Multicenter studies would allow better characterization of the epidemiology and clinical impact of E coli infection.
Keywords: emerging infection, eradication, infection control, infection transmission, sputum
Progressive airway inflammation from recurrent or chronic bacterial infection is the major source of morbidity and mortality in patients with cystic fibrosis (CF) [1]. A significant effort has been made to understand the prevalence, clinical impact, and natural history of infection with common and/or classic CF pathogens [2]. Indeed, Pseudomonas aeruginosa, Staphylococcus aureus, Haemophilus influenzae, and Burkholderia cepacia complex prevalences are routinely reported in the national registry data of major countries. However, these pathogens represent only a portion of the organisms recovered from CF sputum.
Escherichia coli is a Gram-negative bacterium that is periodically isolated from the respiratory tract of patients with CF. Moreover, E coli is a principle human pathogen that is responsible for a large burden of disease afflicting the genitourinary tract and intra-abdominal compartment and is responsible for secondary bacteremias [3, 4]. Despite more being known about it than any other organism on earth, astonishingly little is known about E coli in CF. When E coli is recovered in sputum, it is often overlooked in favor of unusual organisms due to its commonplace nature in the environment, even though 25% of patients with CF may demonstrate pulmonary infection with the organism [5–7]. Instead, CF research emphasizes infrequent organisms that are deemed more novel or drug resistant (ie, Inquilinus, Ralstonia, Cupriavidus) [8–10]. These reports are then included in reviews of “emerging pathogens” in CF, again overlooking frequently encountered pathogens such as the Enterobacteriaceae [11, 12].
Although it is rarely considered to be a respiratory pathogen, E coli can cause respiratory disease. Escherichia coli community-acquired pneumonia in non-CF patients has a high rate of mortality at 11%–21%, with a disproportionally high rate of bacteremia [13, 14]. Furthermore, E coli along with other members of the Enterobacteriaceae family account for 20% of cases of hospital-associated pneumonia (HAP)—as many as P aeruginosa, which is the quintessential HAP pathogen [15]. Despite this, only 1 study in CF on the epidemiology of E coli has been completed [5]. Pathogenicity in CF patients has not been clearly demonstrated, and it is often viewed as an innocuous colonizer [2]. Therefore, we aimed to determine the epidemiology and clinical consequences of CF pulmonary infection with E coli.
METHODS
Population
We undertook a single-center study utilizing the Calgary Adult CF Clinic, which provides care to all individuals living with CF in Southern Alberta, Canada. Attendees were followed quarterly with serial sputum samples and provided consent to collection and analysis of every sputum-derived pathogen in our biobank. After organisms were identified and enumerated (using colony-forming units [CFU]/mL sputum), they were stored at −80°C. Patients were included in the study if E coli was cultured at least once from sputum samples between January 1978 and December 2016. Patients were classified as “transient” (≥1 sample positive for E coli but not meeting criteria for persistence) and “persistent” (≥3 sputum cultures positive for E coli with carriage ≥6 months). Those with persistent infection were matched with at least 1 (and where possible 2) control CF patients (age [±3 years], time period, and sex matched) without history of E coli through random number generation. Patients who were transferred from other CF centers were censured if data lacked sputum or clinical history making determination of incident infection date or outcome analysis impossible.
Clinical Data
We performed a retrospective chart review collecting clinical data 2 years before and after incident E coli infection. For control patients, we collected data for 2 years after a time point equivalent to the time of first E coli growth for their comparator. Baseline patient data included demographics, comorbidities, medications, and concomitant sputum pathogens. At each visit, we collected percentage predicted value of forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1), pulmonary exacerbations (PEx) necessitating either oral or intravenous (IV) antibiotic therapy, and hospitalizations.
The primary outcome was incidence of PEx at first isolation of E coli in the sputum compared with the visit prior or after. Pulmonary exacerbations were diagnosed by the clinic physician with need for either oral or IV antimicrobial treatment in accordance with Fuch’s criteria [16, 17]. We assessed for risk factors predictive of progression to persistent infection and the impact of persistent E coli infection relative to controls including the following: (1) odds of PEx, (2) differential mean annual decline in pulmonary function (FEV1), (3) hospitalization days for IV antibiotics, (4) days of home IV antibiotics, and (5) progression to transplant or (6) death 5 years postinfection.
Characterization of Escherichia coli Isolates
We determined antimicrobial resistance patterns of “incident” isolates (to avoid recurrence bias from persistent infection) to the following: piperacillin-tazobactam, aztreonam, meropenem, amoxicillin, ceftazidime, tobramycin, trimethoprim-sulfamethoxazole, and ciprofloxacin. Values were recorded in zone size (mm) and compared with Clinical and Laboratory Standards Institute breakpoints to determine susceptibility.
Statistical Analysis
Symmetrical and asymmetrical variables were described as means with standard deviations and medians with interquartile ranges (IQR), respectively. Pairwise comparisons were conducted using a Student’s t test (continuous variables) and the Fisher exact test (proportions). Unadjusted risk ratios were calculated to determine PEx risk at initial acquisition compared with prior encounters. Mixed-effects linear regression models with an exchangeable correlation structure were conducted to assess the rate of lung function decline. Mixed-effects logistic regression models with a Poisson distribution were constructed to assess the risk of PEx and for persistence of infection. The mixed-effects models compared pre- and post-E coli infection within patients, between transient and persistent groups, and between patients with persistent infection and matched controls. All hypotheses were at a 2-sided α of 0.05, and analyses were conducted with STATA, version 14.2 (StataCorp, College Station, TX). The study was approved by the University of Calgary ethics board (nos. REB-15-0854 and REB 15-2744).
RESULTS
Population Characteristics
We found 45 of 366 patients (12.3%) with sputum positive for E coli. Of these, 24 patients had ≥2 positive sputum samples, whereas 18 (40%) met criteria for persistent infection. Seven patients were excluded from outcomes analysis (2 persistent, 5 transient) because they lacked sufficient data before and/or after E coli culture for comparison. Baseline patient data are shown in Table 1. In the 2 years before and after incident E coli infection, the median number of clinic visits for cases was 9 (IQR, 6.25–13) and 10 (IQR, 7–17.25), respectively. In transient infections, the median number of submitted sputum samples in the 2 years after incident infection was 7 (IQR, 5–11).
Table 1.
Baseline Characteristics of Patients With Incident Escherichia coli Infection as a Function of Its Stability Within the CF Airways
| Stability | Comparisons | |||||
|---|---|---|---|---|---|---|
| Variable | Total (n = 38) | Transient (n = 22) | Persistent (n = 16) | Controls (n = 29) | P Value (Transient vs Persistent) | P Value (Persistent vs Control) |
| Age years, mean (SD) | 28 (9.3) | 26 (7.4) | 31 (11.0) | 28 (8.5) | .07 | .82 |
| Male, no. (%) | 18 (47.4) | 11 (50.0) | 7 (43.8) | 13 (44.8) | .75 | 1.00 |
| BMI kg/m2, mean (SD) | 20.0 (2.6) | 20.0 (2.9) | 20.1 (2.1) | 22.7 (3.0) | .45 | <.001 |
| FVC%, mean (SD) | 74.9 (21.7) | 73.9 (23.1) | 76.4 (20.1) | 95.0 (29.3) | .36 | .009 |
| FEV1%, mean (SD) | 54.5 (22.3) | 52.7 (23.3) | 57.2 (21.3) | 74.2 (30.7) | .27 | .02 |
| Incident culture count (CFUa log10), mean (SD) | 4.3 (1.7) | 4.1 (1.9) | 4.4 (1.5) | - | .29 | - |
| Acid suppression, PPI no. (%) | 12 (31.6) | 6 (27.3) | 6 (37.5) | 11 (37.9) | .72 | 1.00 |
| Acid suppression, H2RA, no. (%) | 6 (15.8) | 5 (22.7) | 1 (6.3) | 3 (10.3) | .37 | 1.00 |
| Inhaled tobramycin, no. (%) | 6 (15.8) | 4 (18.2) | 2 (12.5) | 9 (31.0) | 1.00 | .28 |
| Azithromycin, no. (%) | 7 (18.4) | 4 (18.2) | 3 (18.8) | 6 (20.7) | 1.00 | 1.00 |
| Inhaled corticosteroid, no. (%) | 15 (35.5) | 9 (40.9) | 6 (37.5) | 5 (17.2) | 1.00 | .16 |
| b Pseudomonas aeruginosa, no. (%) | 22 (57.9) | 16 (72.7) | 6 (37.5) | 18 (62.1) | .05 | .13 |
| b Staphylococcus aureus, no. (%) | 25 (65.8) | 13 (59.1) | 12 (75.0) | 12 (41.4) | .49 | .06 |
| Comorbidities | - | - | - | - | - | - |
| CFRD, no. (%) | 10 (26.3) | 5 (22.7) | 5 (31.3) | 9 (31.0) | .71 | 1.00 |
| Pancreatic insufficiency, no. (%) | 31 (83.8) | 19 (86.4) | 12 (75.0) | 25 (86.2) | .43 | .43 |
| Home O2, no. (%) | 8 (21.1) | 4 (18.2) | 4 (25.0) | 7 (24.1) | .70 | 1.00 |
Abbreviations: BMI, body mass index; CF, cystic fibrosis; CFRD, CF-related diabetes; CFU, colony-forming units; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; H2RA, histamine-2 receptor antagonist; PPI, proton pump inhibitor; SD, standard deviation.
NOTE: Baseline data: at time of incident E coli culture, except for FVC% and FEV1%, which were collected at visit before incident.
aAzithromycin or tobramycin at time of E coli culture or control study entry.
bChronic coinfection in 2 years before and/or after E coli incident culture.
Pulmonary exacerbations occurred in 9 patients (24%; 95% confidence interval [CI], 12.8%–39.4%) at incident isolation. By evaluating the primary outcome, we found that PEx was not more likely to occur at incident infection relative to the visit before (relative risk [RR] = 0.9; 95% CI, 0.4–2.0; P = 1.00) or after (RR = 1.2; 95% CI, 0.6–2.4; P = .80). The mean age was not different between those who exacerbated and those who did not (difference, −0.3 years; 95% CI, −6.4 to 5.8). There was no difference in baseline FEV1 in those who exacerbated (FEV1, 51.3%) and those who did not (FEV1, 55.5%) (difference, −4.2; 95% CI, −22.6 to 14.2). Patients on chronic antibacterials exacerbated at similar frequency to those who did not (22.2% vs 24.1%; P = 1.00). Increased sputum E coli density at incident infection was associated with increased PEx risk (5.5 log10 CFU/mL in those who exacerbated versus 3.9 log10 CFU/mL in those who did not; difference, 1.7; 95% CI, 0.4–3.0; P = .03). Patients who developed persistent infection were not more likely to experience PEx at incident infection (5 of 16 [31%] vs 4 of 22 [18%]; RR, 1.7; 95% CI, 0.5–5.4; P = .35). No patient with E coli pulmonary infection had a secondary bloodstream infection.
Epidemiology of Persistent Infection
Patients with E coli infection who progressed to persistent infection were not significantly different than those with transient infection, although coinfection with P aeruginosa trended towards being less likely in those with persistent infection. Patients who received a change in antimicrobial therapy at incident infection to agents with activity against E coli had no decreased risk of developing persistent infection (P = .62).
Sixteen of 18 patients with persistent infection were able to be matched to 29 control patients with no history of E coli infection. Relative to controls, patients with persistent infection were more likely to have reduced baseline lung function and nutritional status (Table 1).
Escherichia coli Infections and Long-Term Outcomes in Cystic Fibrosis
All patients with E coli infection, in unadjusted analysis, had decreased mean annual decline in pulmonary function (FEV1) after E coli infection relative to before infection: (−1.38%/year vs −2.08%/year; difference, −0.70%/year; 95% CI, −0.49 to −0.91; P < .001). In addition, the odds of PEx after infection were lower after infection (odds ratio [OR] = 0.65; 95% CI, 0.47–0.89; P = .008). There was no difference in mean annual home IV days (14.5 vs 10.0; 95% CI, −14.0 to 23.2 days) but less mean annual hospital IV days (9.5 vs 12.2; difference, −2.7 days; 95% CI, −4.7 to −0.71 days; P = .008) after infection with E coli.
When comparing persistent versus transient infection, there was a trend to increased mean annual lung function decline (−3.20%/year vs −1.72%/year; 95% CI, −3.34 to 0.34) and odds of PEx (OR = 1.7; 95% CI, 0.9–3.1; P = .09). There was no difference in mean annual home IV days (5.5 vs 22.5; 95% CI, −50.5 to 16.5 days) or mean annual hospital IV days (9.9 vs 10.5; 95% CI, −5.5 to 4.3 days) with persistent infection. There was no difference in progression to lung transplant (P = .63) or death (P = .25) at 5 years.
Comparing persistently infected patients to matched controls, there was no evidence of differential annual lung function decline (difference, −1.06%/year; 95% CI, −2.82 to 0.70; P = .24) or odds of PEx (OR = 1.4; 95% CI, 0.8–2.3; P = .26). There was also no difference in mean annual home IV days (5.2 vs 9.7; 95% CI, −13.3–4.4 days) or mean annual hospital IV days (10.1 vs 9.8; 95% CI, −4.97 to 5.59 days). There was no difference in progression to lung transplant (P = .33) or death (P = .54) at 5 years.
Escherichia coli Antimicrobial Susceptibility
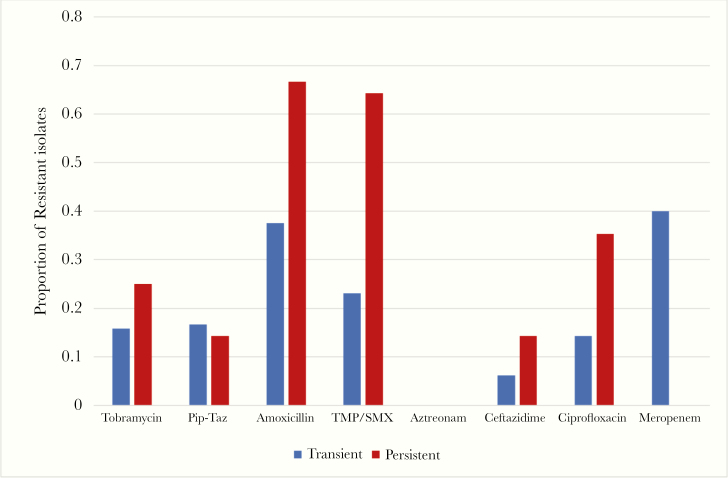
Across antibacterials there were no differences in resistance profiles (Figure 1). There was a trend to higher proportion of resistance to trimethoprim-sulfamethoxazole (TMP/SMX) in persistent infections (64% vs 23%, P = .05). In reviewing the exposure to TMP/SMX in the 2 years before incident infection, there was a trend towards increased incidence of exposure in those who developed persistent infection (63% vs 32%, P = .10). We recovered 3 isolates that were producers of extended-spectrum beta lactamases, and these were not associated with persistent infection (P = .56).
Figure 1.
Proportion of resistant Escherichia coli isolates at incident infection to 8 commonly used antibacterial agents. PIP-TAZ, piperacillin-tazobactam; TMP/SMX, trimethoprim-sulfamethoxazole.
Evolution of Escherichia coli in Cystic Fibrosis Airways
Persistent infections were genotypically confirmed with pulse-field gel electrophoresis (data not shown) [18]. We sought to determine the duration and patterns of persistence with E coli airways infection. Of the 18 patients with persistent infection, 14 eventually cleared the organism after a median of 1279 days (IQR, 348–2176 days). Of these, 3 appeared to do so spontaneously without new antimicrobial treatment (acute or chronic) or new sputum pathogens identified. Eight patients were initiated on new antimicrobials and/or treated for PEx at time of clearance.
Discussion
In this retrospective cohort study, we found that despite frequent isolation and persistence of E coli in the sputum of adult patients with CF, particularly those with poor nutritional status and lung function, this did not portend worse outcomes.
Much of the limited information relating to E coli epidemiology comes from cross-sectional studies reviewing incidence of multiple organisms [6, 7] or case reports [19, 20]. In the ARREST CF study of children with annual bronchoalveolar lavage samples, E coli was the fourth most frequently recovered bacterial species, behind P aeruginosa, S aureus, and H influenzae [7]. In the only prior epidemiologic study, Barillova et al [5] identified E coli in 25% of their CF cohort over 7 years. National registries have not reported E coli in their prevalence analyses [21], but single-center studies have reported prevalence as high as 11%–25% [5, 6]. Our cumulative prevalence of 12.3% still rivals that of other classic pathogens such as Achromobacter species and Stenotrophomonas maltophilia—organisms that have received extensive study [11].
In our study, there was a trend to increased age associating with persistence of infection, but this did not meet significance. However, the predilection of persistent infection in patients with worse nutrition and lung function suggests that E coli may target comparatively compromised CF patients to establish long-term infection. The near association of persistence with TMP/SMX-resistant strains is curious. The rates of E coli resistance to TMP/SMX in our transiently infected population closely mirror the rates of our general population (25%–30%). However, the high rates of resistance in the persistent infection population are discordant (Figure 1). Increased exposure to this commonly used antimicrobial in nonpseudomonal infections may breed resistant strains. This also may support the theory of aspiration of endogenous gastrointestinal microbiota leading to pulmonary infection, rather than environmental acquisition. For all other antimicrobials investigated, our data suggest that antimicrobial resistance was not a factor associated with persistent E coli infection.
Barillova et al [5] found that E coli was coisolated with S aureus and/or P aeruginosa in approximately 50% of infections; however, in those who grew E coli repeatedly, Pseudomonas coinfection was less common. We observed similar findings, with 58% of our cohort demonstrating coinfection with P aeruginosa. More important, those with persistent E coli infection were less likely to have chronic P aeruginosa infection. Furthermore, during persistent infection, only 1 patient acquired new, incident P aeruginosa infection to the subsequent exclusion of E coli that was never observed again and another who spontaneously cleared E coli before acquiring chronic P aeruginosa. As the archetypal CF pathogen, P aeruginosa may be able to outcompete invading organisms such as E coli in some circumstances, preventing the development of persistent infection. However, in only 1 instance, an individual with chronic P aeruginosa infection who acquired incident E coli infection subsequently lost the originally colonizing P aeruginosa. It is interesting to note that spontaneous clearance of E coli in persistent infection was uncommon relative to other investigated emerging pathogens such as Achromobacter spp where up to 90% of isolates ultimately cleared from our cohort [22].
The pathogenicity of E coli in CF is debated because it is known to commonly colonize mucosal surfaces [4]. Others have argued for the pathogenicity of E coli in CF given a persistent, relatively high bacterial load of a virulent strain of the organism [5]. Although we did not note an increased overall risk of PEx at incident E coli culture, we found that those with a high organism bioburden were more likely to experience PEx. Likewise, we found limited data to support worse outcome in individuals with chronic E coli recovery from sputum. This may support the notion that E coli is colonizing damaged lung parenchyma rather than serving a pathogenic role, and this is supported by prior findings of limited local lung inflammatory reaction to the organism’s presence [7]. Coinciding with this observation, coinfection with P aeruginosa was lower in the persistent E coli infection group. Barillova et al [5] also found E coli to be the lone recovered pathogen in persistent infection disproportionally. One hypothesis is that E coli actually served some type of protective role against the deleterious nature of pseudomonal infection by competing for territory in some individuals and thus preserving lung function that may have been lost due to Pseudomonas. A predilection of some clonal E coli strains for lung parenchyma based on genetic virulence factors has been hypothesized [5, 23], which may support this theory.
The limitations of this study include the retrospective nature risking selection and information bias including misclassification of infection and missing information. We also must consider the effect of different treatments for CF over 30 years of patient follow-up. Not all samples were recoverable from our biobank, thus limiting our analysis of strains. Multicenter studies are warranted to further characterize the epidemiology of infection and determine whether clinical decline becomes more apparent with greater patient numbers, as has become apparent with methicillin-resistant S aureus whose pathogenicity was initially debated [24–26].
Conclusions
When followed longitudinally, E coli pulmonary infection in CF patients occurred surprisingly often in our population and frequently resulted in persistent infection. Infection with E coli did not portend clinical worsening in any cohorts studied: any infection, transient infection, or persistent infection. However, persistent infection was more likely to be established in patients with worse baseline nutrition and lung disease, suggesting an opportunistic behavior.
Acknowledgments
Author contributions. B. D. E. conducted the chart reviews and collected clinical data. J. G.-W. and B. W. performed and analyzed bacterial susceptibility testing. B. D. E. wrote the initial draft of the manuscript, and all authors contributed to its revision. B. D. E. and R. S. performed statistical testing. D. G. S., R. S., H. R. R., M. G. S., and M. D. P. collect and maintain the Calgary Adult CF Biobank. The project was envisioned and supervised by M. D. P.
Potential conflicts of interest. This study was funded by a grant to M. D. P. from Cystic Fibrosis (CF) Canada (3185). M. D. P., H. R. R., and M. G. S. have received research support from Gilead Sciences, CF Canada, and Canadian Institutes of Health Research (CIHR). R. S. has received research support from CF Canada and CIHR. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ratjen F. Update in cystic fibrosis 2008. Am J Respir Crit Care Med 2009; 179:445–8. [DOI] [PubMed] [Google Scholar]
- 2. Lipuma JJ. The changing microbial epidemiology in cystic fibrosis. Clin Microbiol Rev 2010; 23:299–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arancibia F, Bauer TT, Ewig S, et al. Community-acquired pneumonia due to Gram-negative bacteria and Pseudomonas aeruginosa: incidence, risk, and prognosis. Arch Intern Med 2002; 162:1849–58. [DOI] [PubMed] [Google Scholar]
- 4. Donnenberg MS. Enterobacteriaeae. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell , Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier Inc; 2015:2503–2518. [Google Scholar]
- 5. Barillova P, Tchesnokova V, Dübbers A, et al. Prevalence and persistence of Escherichia coli in the airways of cystic fibrosis patients - an unrecognized CF pathogen? Int J Med Microbiol 2014; 304:415–21. [DOI] [PubMed] [Google Scholar]
- 6. Seidmon EJ, Mosovich LL, Neter E. Colonization by Enterobacteriaceae of the respiratory tract of children with cystic fibrosis of the pancreas and their antibody response. J Pediatr 1975; 87:528–33. [DOI] [PubMed] [Google Scholar]
- 7. Gangell C, Gard S, Douglas T, et al. ; AREST CF Inflammatory responses to individual microorganisms in the lungs of children with cystic fibrosis. Clin Infect Dis 2011; 53:425–32. [DOI] [PubMed] [Google Scholar]
- 8. Coenye T, Goris J, Spilker T, et al. Characterization of unusual bacteria isolated from respiratory secretions of cystic fibrosis patients and description of Inquilinus limosus gen. nov., sp. nov. J Clin Microbiol 2002; 40:2062–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bittar F, Richet H, Dubus JC, et al. Molecular detection of multiple emerging pathogens in sputa from cystic fibrosis patients. PLoS One 2008; 3:e2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalka-Moll WM, LiPuma JJ, Accurso FJ, et al. Airway infection with a novel Cupriavidus species in persons with cystic fibrosis. J Clin Microbiol 2009; 47:3026–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parkins MD, Floto RA. Emerging bacterial pathogens and changing concepts of bacterial pathogenesis in cystic fibrosis. J Cyst Fibros 2015; 14:293–304. [DOI] [PubMed] [Google Scholar]
- 12. Spicuzza L, Sciuto C, Vitaliti G, et al. Emerging pathogens in cystic fibrosis: ten years of follow-up in a cohort of patients. Eur J Clin Microbiol Infect Dis 2009; 28:191–5. [DOI] [PubMed] [Google Scholar]
- 13. Marrie TJ, Fine MJ, Obrosky DS, et al. Community-acquired pneumonia due to Escherichia coli. Clin Microbiol Infect 1998; 4:717–23. [DOI] [PubMed] [Google Scholar]
- 14. Ruiz LA, Zalacain R, Gómez A, et al. Escherichia coli: an unknown and infrequent cause of community acquired pneumonia. Scand J Infect Dis 2008; 40:424–7. [DOI] [PubMed] [Google Scholar]
- 15. Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis 2010; 51(Suppl 1):S81–7. [DOI] [PubMed] [Google Scholar]
- 16. Fuchs HJ, Borowitz DS, Christiansen DH, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med 1994; 331:637–42. [DOI] [PubMed] [Google Scholar]
- 17. Lam JC, Somayaji R, Surette MG, et al. Reduction in Pseudomonas aeruginosa sputum density during a cystic fibrosis pulmonary exacerbation does not predict clinical response. BMC Infect Dis 2015; 15:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Izydorczyk C, Edwards B, Greysson-Wong J, et al. Genomic epidemiology of Escherichia coli infections in adults with cystic fibrosis. North American Cystic Fibrosis Conference, Nashville, Tennessee, 31 October 2019; 54(S4):308. [Google Scholar]
- 19. Macone AB, Pier GB, Pennington JE, et al. Mucoid Escherichia coli in cystic fibrosis. N Engl J Med 1981; 304:1445–9. [DOI] [PubMed] [Google Scholar]
- 20. Crémet L, Caroff N, Giraudeau C, et al. Detection of clonally related Escherichia coli isolates producing different CMY β-lactamases from a cystic fibrosis patient. J Antimicrob Chemother 2013; 68:1032–5. [DOI] [PubMed] [Google Scholar]
- 21.2017 Patient Registry Annual Data Report. Bethesda, MD: Cystic Fibrosis Foundation, 2017. [Google Scholar]
- 22. Edwards BD, Greysson-Wong J, Somayaji R, et al. Prevalence and outcomes of Achromobacter species infections in adults with cystic fibrosis: a North American Cohort study. J Clin Microbiol 2017; 55:2074–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vali P, Shahcheraghi F, Seyfipour M, et al. Phenotypic and genetic characterization of carbapenemase and ESBLs producing Gram-negative bacteria (GNB) isolated from patients with cystic fibrosis (CF) in Tehran hospitals. J Clin Diagn Res 2014; 8:26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ren CL, Morgan WJ, Konstan MW, et al. ; Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis Presence of methicillin resistant Staphylococcus aureus in respiratory cultures from cystic fibrosis patients is associated with lower lung function. Pediatr Pulmonol 2007; 42:513–8. [DOI] [PubMed] [Google Scholar]
- 25. Dasenbrook EC, Checkley W, Merlo CA, et al. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA 2010; 303:2386–92. [DOI] [PubMed] [Google Scholar]
- 26. Dasenbrook EC, Merlo CA, Diener-West M, et al. Persistent methicillin-resistant Staphylococcus aureus and rate of FEV1 decline in cystic fibrosis. Am J Respir Crit Care Med 2008; 178:814–21. [DOI] [PubMed] [Google Scholar]