Abstract
The prognosis of patients with advanced melanoma has improved dramatically. However, the clinical outcomes of patients with highly elevated serum lactate dehydrogenase (LDH) remain very poor. The aim of this study was to explore whether patients with normalized LDH after targeted therapy could benefit from subsequent treatment with immune checkpoint inhibitors (ICI). Data from all patients with BRAF-mutant metastatic melanoma with a highly elevated serum LDH at baseline (≥2× upper limit of normal) receiving first-line targeted therapy between 2012 and 2019 in the Netherlands were collected. Patients were stratified according to response status to targeted therapy and change in LDH at start of subsequent treatment with ICI. Differences in overall survival (OS) between the subgroups were compared using log-rank tests. After a median follow-up of 35.1 months, median OS of the total study population (n = 360) was 4.9 months (95% CI 4.4–5.4). Of all patients receiving subsequent treatment with ICI (n = 113), survival from start of subsequent treatment was significantly longer in patients who had normalized LDH and were still responding to targeted therapy compared to those with LDH that remained elevated (median OS 24.7 vs. 1.1 months). Our study suggests that introducing ICI upon response to targeted therapy with normalization of LDH could be an effective strategy in obtaining long-term survival in advanced melanoma patients with initial highly elevated serum LDH.
Keywords: melanoma, metastasis, targeted therapy, immune checkpoint inhibitors, lactate dehydrogenase, real-life data, prognostic factors
1. Introduction
Multiple effective systemic treatment options have emerged for patients with advanced BRAF-mutant melanoma over the last decade. Since the approval of the BRAF inhibitor vemurafenib [1] and the CTLA-4 antibody ipilimumab [2], combination therapy with a BRAF and MEK inhibitor [3] and treatment with anti-PD-1 antibodies as monotherapy [4,5] or combined with a CTLA-4 antibody [6] have broadened the therapeutic arsenal for these patients. Combination therapy with a BRAF and MEK inhibitor has resulted in a median overall survival of over two years [7], while treatment with anti-PD-1 also concurrently showed significant improvements, with 2-year survival rates of 55–58% [8]. Nevertheless, although long-term survival may be achieved in a subgroup of patients, there is still an unmet medical need for patients with unfavorable prognostic factors [7,9]. Elevated serum lactate dehydrogenase (LDH) level is a well-known marker for poor outcome, and a strong negative predictor for response to immune checkpoint inhibitors (ICI) and targeted therapy [7,8]. In previous reports, substantially less activity was demonstrated in patients with elevated serum LDH of ≥2× upper limit of normal (ULN), with a median OS of 2.9 months after ipilimumab therapy [9] and 2.3 months after anti-PD1 therapy [10]; compared to 14.7 months and 16.1 months for patients with normal LDH, respectively. Similarly, LDH has been shown to be one of the key predictors of survival for patients receiving targeted therapy [11]. Although the majority of BRAF mutant patients with elevated serum LDH respond to targeted therapy, responses are usually short-lived, with median progression-free survival shorter than six months for patients with LDH ≥2× ULN, compared to 17 months for the patients with normal LDH [7].
Targeted therapies are capable of inducing rapid anti-tumor responses associated with a decrease in LDH [7], which might enable ICI to work more efficiently in patients with initial elevated serum LDH. Furthermore, BRAF and MEK-inhibition could facilitate immune responses in multiple ways. Preclinical data showed an increase in CD8+ T-cell recognition of tumor cells by inducing rapid upregulation of MHC class I surface expression in BRAF-mutant melanoma cells [12,13]. These data support the potential of BRAF-inhibition to increase response rates to ICI. Although this concept seems promising, clinical data supporting the approach of BRAF inhibitor induction treatment preceding treatment with ICI in patients with aggressive disease are lacking, and little is known about which patients could benefit from induction treatment.
This prospective population-based study focuses on the clinical outcomes of BRAF-mutant metastatic melanoma patients with baseline serum LDH of ≥2× ULN treated with first-line targeted therapy. The main objective of the study was to investigate whether the level of LDH and response status at the switch to ICI was associated with survival.
2. Results
2.1. Overall Study Population
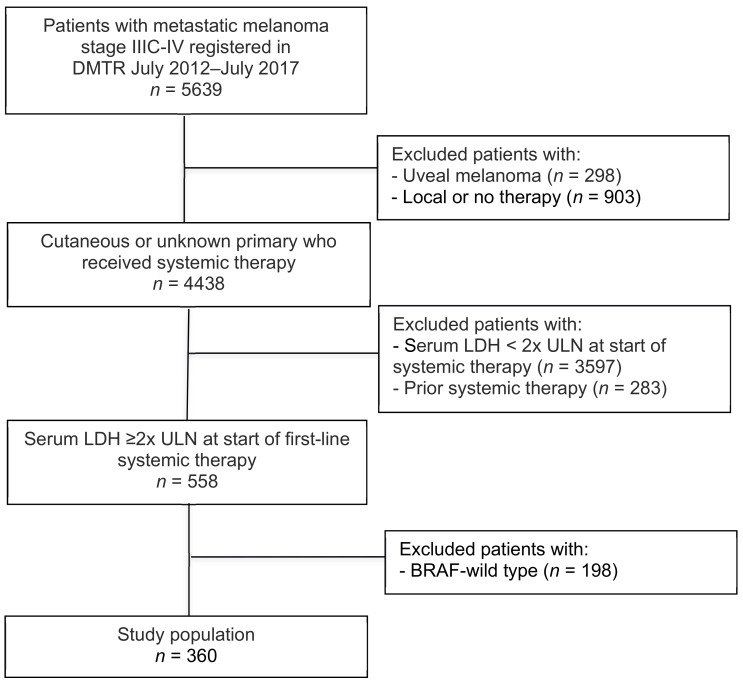
A total of 5639 unresectable stage IIIC or IV melanoma patients were registered in the Dutch Melanoma Treatment Registry (DMTR) between 1 July, 2012 and 1 June 1, 2019 (Figure 1). Of these, 360 BRAF-mutant advanced melanoma patients with a baseline serum LDH of ≥2× ULN received first-line targeted therapy and were included for analyses. Baseline characteristics are shown in Table 1. The median age was 60 years and the majority of patients were male (60%). Median serum LDH was 823 U/L (IQR 625–1419). Thirty eight percent of patients had an Eastern Cooperative Oncology Group performance status (ECOG PS) of ≥2 and most patients had ≥3 organ sites involved (72%). The majority of patients received BRAF monotherapy (76%). BRAF monotherapy was mainly prescribed up to August 2016. Combination therapy with a BRAF- and MEK inhibitor was increasingly used since October 2015. Median follow-up was 35.1 months (95% CI 18.2–52.1) and 308 patients (85%) died during follow-up. At time of analysis, 91% of patients discontinued treatment with targeted therapy, due to disease progression (60%), toxicity (10%), death (9%), planned in advance (11%), patient’s choice (2%), other (4%) and unknown (4%).
Figure 1.
Flowchart of study population.
Table 1.
Patient and treatment characteristics of overall study population.
| Variable | n = 360 |
|---|---|
| n (%) | |
| Median age, years (IQR) | 60 (50–68) |
| Age in categories | |
| <50 | 90 (25) |
| 50–59 | 85 (23) |
| 60–69 | 110 (31) |
| ≥70 | 75 (21) |
| Gender | |
| Male | 214 (60) |
| Female | 146 (40) |
| ECOG PS | |
| 0 | 75 (21) |
| 1 | 110 (31) |
| ≥2 | 136 (38) |
| Unknown | 39 (10) |
| Median baseline LDH (IQR) | 823 (625–1419) |
| No. of organ sites involved | |
| <3 | 60 (17) |
| ≥3 | 261 (72) |
| Unknown | 39 (11) |
| M stage | |
| M1a | 6 (2) |
| M1b | 3 (1) |
| M1c | 349 (96) |
| Unknown | 2 (1) |
| Brain metastases | |
| No | 231 (64) |
| Asymptomatic | 42 (12) |
| Symptomatic | 63 (17) |
| Unknown | 24 (7) |
| Type of targeted therapy | |
| BRAFi monotherapy | 206 (76) |
| BRAFi + MEKi | 154 (24) |
IQR = interquartile range; ECOG PS = Eastern Cooperative Oncology Group performance status; BRAFi = BRAF inhibitor; MEKi = MEK inhibitor.
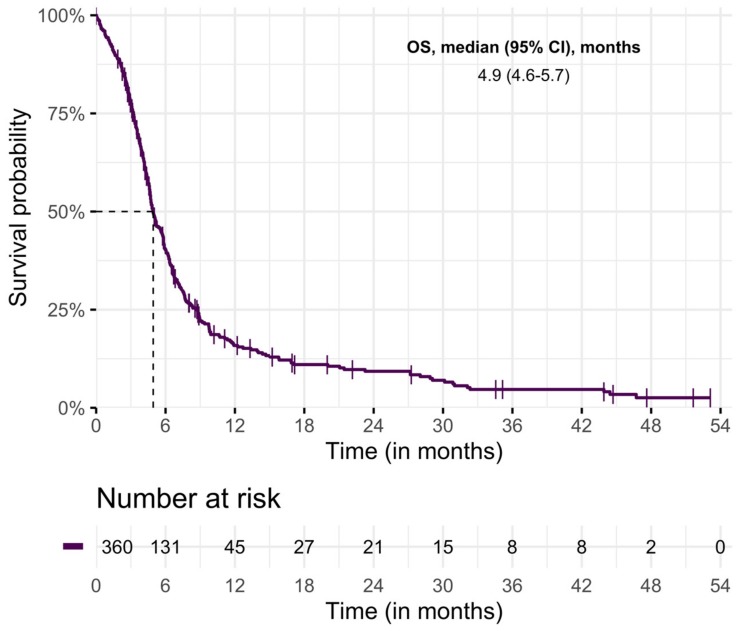
Median OS was 4.9 months (95% CI 4.4–5.4) (Figure 2). Survival rates at six months and one year were 40% (95% CI 35–45) and 16% (95% CI 12–20), respectively.
Figure 2.
Overall survival of study population.
2.2. Patients with Subsequent Treatment with ICI
A total of 113 patients (31%) received subsequent treatment with ICI. Combination therapy of ipilimumab and nivolumab was most often administered (n = 55), followed by anti-PD1 (pembrolizumab (n = 20), nivolumab (n = 16)), and ipilimumab (n = 22). Baseline characteristics at start of subsequent treatment with ICI are shown in Table 2. Median follow up from start of subsequent treatment with ICI was 30.0 months (95% CI 10.6–51.2).
Table 2.
Patient and treatment characteristics at start of subsequent treatment with ICI.
| Variable | n = 113 |
|---|---|
| n (%) | |
| Median age, years (min–max) | 56 (47–67) |
| Age in categories | |
| <50 | 35 (31) |
| 50–59 | 28 (25) |
| 60–69 | 30 (26) |
| ≥70 | 20 (18) |
| Gender | |
| Male | 69 (61) |
| Female | 44 (39) |
| Serum LDH | |
| <ULN | 38 (34) |
| ≥1 to <2× ULN | 42 (37) |
| ≥2× ULN | 33 (29) |
| ECOG PS | |
| 0 | 24 (21) |
| 1 | 61 (54) |
| ≥2 | 15 (13) |
| Unknown | 13 (12) |
| No. of organ sites involved | |
| <3 | 17 (15) |
| ≥3 | 84 (74) |
| Unknown | 12 (11) |
| M stage | |
| M1a | 0 (0) |
| M1b | 0 (0) |
| M1c | 113 (100) |
| Unknown | 0 (0) |
| Brain metastases | |
| No | 68 (60) |
| Asymptomatic | 18 (16) |
| Symptomatic | 19 (17) |
| Unknown | 8 (7) |
| Type of prior targeted therapy | |
| BRAFi monotherapy | 41 (36) |
| BRAFi + MEKi | 72 (64) |
| Response on targeted therapy | |
| Partial response | 27 (24) |
| Stable disease | 7 (6) |
| Progressive disease | 79 (70) |
| Type of subsequent ICI | |
| Ipilimumab | 22 (19) |
| Nivolumab | 16 (14) |
| Pembrolizumab | 20 (18) |
| Ipilimumab and nivolumab | 55 (49) |
ECOG PS = Eastern Cooperative Oncology Group performance status; BRAFi = BRAF-inhibitor; MEKi = MEK inhibitor. LDH = lactate dehydrogenase, ULN = upper limit of normal; ICI = immune checkpoint inhibitors.
The main objective of the study was to investigate whether the response to targeted therapy and level of serum LDH at start of subsequent treatment with ICI affects survival. Outcomes were stratified according to LDH at start of subsequent treatment with ICI and tumor response after targeted therapy. Table 3 shows the median OS and 6-months survival rates, calculated from start of subsequent treatment with ICI.
Table 3.
Kaplan–Meier estimates of median overall survival, and 6-months and 1-year survival rates at start of subsequent treatment with ICI, according to serum LDH at start of subsequent treatment with ICI and tumor response after targeted therapy.
| Serum LDH at Start ICI | Response on Targeted Therapy | Deaths/No. of Patients | Median OS | 6 m Survival Rate | 1 y Survival Rate |
|---|---|---|---|---|---|
| (95% CI), m | (95% CI), % | (95% CI), % | |||
| <ULN | |||||
| PR | 7/16 | 24.7 (16.1–33.4) | 85 (66–100) | 73 (46–100) | |
| SD | 5/6 | 7.0 (0–14.9) | 63 (21–100) | 21 (0–57) | |
| PD | 14/16 | 4.4 (1.3–7.4) | 36 (11–61) | 22 (0–44) | |
| ≥1 to <2× ULN a | |||||
| PR | 5/9 | 10.4 (0–22.5) | 60 (24–96) | 40 (0–80) | |
| PD | 25/32 | 2.7 (1.9–3.5) | 24 (8–40) | 20 (5–35) | |
| ≥2× ULN b | |||||
| PD | 29/31 | 1.1 (0.7–1.6) | 17 (3–30) | 8 (0–19) | |
LDH = lactate dehydrogenase, ULN = upper limit of normal, ICI = immune checkpoint inhibitors, OS = overall survival, m = months, NR = not reached, PR = partial response, SD = stable disease, PD = progressive disease. a Due to low numbers of patients with stable disease (n = 1) in this subgroup, these patients were excluded from analyses. b Due to low numbers of patients with partial response (n = 2) in this subgroup, these patients were excluded from analyses.
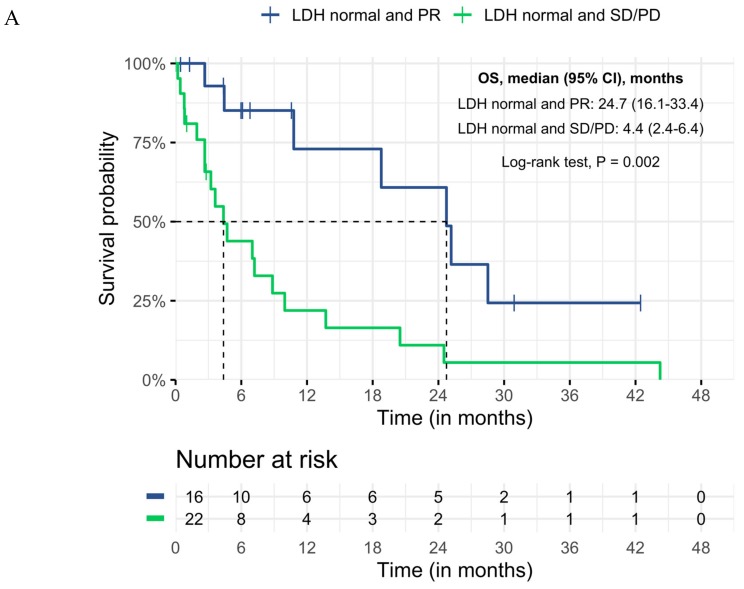
Patients with a normalized LDH who had a partial response to prior targeted therapy (n = 16; combination therapy of BRAF and MEK inhibitor (n = 11), BRAF monotherapy (n = 5)) had the best survival from start of treatment with ICI (median OS 24.7 (95% CI 16.1–33.4) and 6-months and 1-year survival rate of 85% (95% CI 66–100) and 73% (95% CI 46–100), respectively). In this subgroup, most patients received combination therapy of ipilimumab and nivolumab (n = 9), followed by anti-PD1 (n = 6) and ipilimumab (n = 1). Median duration of targeted therapy before switching to ICI in LDH-normalized patients was 3.6 months (range 1.8–30.9). The main reason for treatment switch to ICI was a planned switch (n = 9). Other reasons were toxicity (n = 3) and unknown (n = 4). Baseline characteristics at start of targeted therapy were compared between the subgroup with normalized LDH and partial response, and the other subgroups. No significant differences were found (Table S1).
Most patients who had an elevated LDH at start of treatment with ICI had progressed on targeted therapy (n = 63). Median duration of targeted therapy before switching to ICI was 5.9 months (95% CI 5.3–6.6). Patients who started second-line ICI with LDH ≥ 2× ULN had the worst outcomes, with a median OS of 1.1 months (95% CI 0.7–1.6), and 6-months and 1-year survival rate of 17% (95% CI 3–30) and 8% (95% CI 0–19), respectively. The survival curves demonstrate significant survival differences between the normalized LDH group with partial response, compared to the other subgroups (Figure 3a,b).
Figure 3.
Differences in Kaplan–Meier curves of overall survival at start of subsequent treatment with ICI, in the subgroup with normalized LDH and PR compared to (A) normalized LDH and SD or PD, (B) all other subgroups, LDH = lactate dehydrogenase, OS = overall survival, CI = confidence interval, PR = partial response, SD = stable disease, PD = progressive disease.
The 6-months and 1-year survival rates of the subgroup with normalized LDH and partial response are significantly better when compared to the whole subgroup that received ICI (6 months: 85% (95% CI 66–100) vs. 31% (95% CI 21–41); and 1-year: 73% (95% CI 46–100) vs. 18 (95% CI 10–27)).
3. Discussion
These real-world data support previous reports of the poor prognosis of advanced melanoma patients with highly elevated serum LDH. At the same time, these data provide a potential strategy to improve clinical outcomes. In our cohort of metastatic melanoma patients with baseline serum LDH of ≥2x ULN treated with first-line BRAF(/MEK) inhibitors, median OS was significantly longer in patients with normalized LDH and still responding to initial targeted therapy who started second-line treatment with ICI, compared to those with elevated LDH at start of treatment with ICI. Our data suggest that introducing ICI upon response to targeted therapy with normalization of LDH could be an effective strategy in obtaining long-term survival in patients with initial elevated serum LDH.
The median OS of 4.9 months of the overall study population confirms previous data, as clinical outcomes remain poor in this subgroup of patients [9,10,14]. Patients who received subsequent treatment with ICI with LDH levels that remained ≥2× ULN are unlikely to benefit from ICI with a median OS of 1.1 months and a 6-months and 1-year survival rate of 17% and 8%, respectively.
The exact role of LDH is not completely elucidated. It could simply be a marker of more aggressive disease that requires rapid anti-tumor responses [9]. The delayed tumor responses generally observed with ICI might therefore take too long for these patients to benefit. Moreover, tumor metabolism is characterized by the conversion of pyruvate into lactate, even in the presence of sufficient oxygen. Preclinical data demonstrated that tumor cells producing high levels of lactic acid disturb the function of cytotoxic T lymphocytes, thereby negatively influencing the potency of an immune response [15,16].
Interestingly, our data show that patients who switch to ICI with normalized LDH while still responding to targeted therapy have a real chance of long-term survival. After a median follow-up of 30 months, median OS was 24.7 months, and 6-months and 1-year survival rate was 85% and 73%, respectively. Survival was significantly longer compared to the other subgroups. No differences were found in prognostic factors at start of targeted therapy among the subgroups, indicating that this subgroup is not simply a selection of a best prognosis group.
It should be noted that only a small proportion of patients received this treatment strategy (n = 16; 4% of total study population). However, the majority of the total study population (n = 360) received BRAF monotherapy as first-line targeted therapy. The emergence of combination therapy with a BRAF and MEK inhibitor for this subgroup of patients might lead to a greater proportion of patients with response to targeted therapy and normalization of LDH. A 3-year follow-up pooled analysis of phase III trials with BRAF and MEK inhibitor combination therapy showed promising results, with 50% partial response in patients with initial LDH ≥ 2× ULN [17].
The value of sequencing targeted therapy prior to treatment with ICI in patients with initial elevated LDH has not been investigated thus far. Previous retrospective reports revealed that normalization of LDH while on targeted therapy was associated with ipilimumab cycle completion [18,19]. In another study on 101 advanced melanoma patients with decreased serum LDH after BRAF inhibitor treatment who completed all courses of ipilimumab, showed a significantly longer OS compared to those who did not (median OS 12.7 months vs. 1.2 months) [20].
The real benefit of induction treatment with combined BRAF- and MEK-inhibition in patients with elevated LDH is currently under investigation in multiple prospective randomized trials. The EORTC EBIN study (NCT03235245), compares ipilimumab and nivolumab upfront versus the same treatment preceded by induction therapy with encorafenib and binimetinib in advanced melanoma patients, irrespective of LDH level. One of the arms of the three-arm phase II SECOMBIT study (NCT02631447) will assess whether an induction treatment with encorafenib plus binimetinib of 8 weeks before combination therapy with ICI might help potentiate an immunotherapeutic response. Guidelines are not conclusive on this issue. Our results may therefore be of added value to medical oncologists while awaiting these trial results.
It would be interesting to investigate survival differences between patients who started second-line treatment with ICI with normalized LDH and response to initial targeted therapy vs. responders who stayed on targeted therapy. Unfortunately, this could not be assessed with our data, as we have no information on LDH level during follow-up with patients who stayed on targeted therapy.
Given the observational design of this analysis, we cannot rule out confounding by indication or selection bias. However, its multicentered design attenuates this potential selection bias. Furthermore, observational studies are more susceptible to registration bias. To ensure high-quality data, data managers were extensively trained and supervised by oncologists [21]. Another limitation is the small number of patients in the subgroup analyses. The conclusions drawn need validation in prospective randomized trials. Lastly, other clinical parameters such as lymphocyte counts and CRP level that have also been associated with patient outcome after treatment with ICI were not registered in our database, and could therefore not be included in this study [16].
4. Materials and Methods
4.1. Data: The Dutch Melanoma Treatment Registry (DMTR)
Data were retrieved from the Dutch Melanoma Treatment Registry (DMTR), a prospective population-based registry that was set-up to monitor the safety and effectiveness of the new drugs in real-world clinical practice and to assess the quality of melanoma care in the Netherlands. The DMTR contains information on baseline patient and tumor characteristics, local and systemic treatment modalities, treatment-related adverse events (grade 3 or 4 according to common terminology criteria for adverse events (CTCAE) version 4), and clinical outcomes of all patients with unresectable stage IIIC or IV melanoma. A detailed description of the DMTR was published previously [21].
In compliance with Dutch regulations, the DMTR was approved by the medical ethical committee and was not subject to the Medical Research Involving Human Subjects Act. Patients were offered an opt-out option.
4.2. Patients
All patients with BRAF-mutant unresectable or metastatic (stage IIIC or stage IV) cutaneous melanoma or with a BRAF-mutant melanoma of unknown primary with a baseline serum LDH of ≥2x the upper limit of normal (ULN), who received targeted therapy (either monotherapy with a BRAF inhibitor or combination therapy with BRAF and MEK inhibitors) between 1 July, 2012 and 1 June, 2019 were included. The ULN was defined at 250 U/L. Patients with prior systemic treatment for metastasized disease were excluded to avoid bias of ongoing activity of previous systemic agents.
4.3. Statistical Analysis
Time to next treatment (TTNT) and overall survival (OS) with corresponding two-sided 95% confidence intervals (CI) for medians were analyzed using the Kaplan–Meier method. For the overall study population, TTNT was determined from the start of targeted therapy to the start of subsequent systemic therapy, or death from any cause. Patients who were still on treatment were censored at time of analysis. OS was defined as the time from start of targeted therapy to the date of death from any cause. Patients alive at time of analysis were censored. Follow-up time was calculated from start date of targeted therapy using the inverse Kaplan–Meier method [22].
The main objective of the study was to investigate whether the response to targeted therapy and level of serum LDH at start of subsequent treatment with ICI affects survival. For this analysis, OS was defined from start of subsequent treatment with ICI to the date of death from any cause. Patients were stratified according to LDH at start of subsequent treatment with ICI (<ULN, >1 to <2× ULN, ≥2× ULN) and tumor response after treatment of targeted therapy according to Response Evaluation Criteria in Solid Tumors (RECIST). OS was compared between the subgroups using log-rank tests. Statistical significance was defined as a two-sided p value < 0.05. All statistical analyses were performed in PASW Statistics version 20 (SPSS Inc. Chicago, IL, USA).
5. Conclusions
This population-based study confirms the very poor prognosis of advanced melanoma patients with LDH ≥ 2× ULN. Moreover, our data suggest that switching to ICI upon response to targeted therapy with normalization of LDH may be a strategy to obtain long-term survival for these patients. Nevertheless, randomized trials are needed to assess the real benefit of sequential treatment of targeted therapy and ICI in patients with highly elevated serum LDH.
Acknowledgments
The authors thank all physicians and data managers who registered the patient data in the Dutch Melanoma Treatment Registry.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/11/12/1940/s1, Table S1: Patient and treatment characteristics at start of targeted therapy, according to subgroup of normalized LDH and PR (n = 16) and all other groups (n = 97) after targeted therapy.
Author Contributions
Conceptualization: M.G.S., K.P.M.S., M.W.J.M.W., and R.H.T.K.; methodology: M.G.S. and K.P.M.S.; formal analysis: M.G.S.; resources: M.G.S.; writing—original draft preparation: M.G.S., K.P.M.S., and M.W.J.M.W.; writing—review and editing: M.G.S., K.P.M.S., R.H.T.K., A.J., M.C.T.V.Z., A.J.M.v.d.E., J.B.A.G.H., M.J.B.A., A.C.J.v.A., F.W.P.J.v.d.B., J.W.B.d.G., G.A.P.H., E.K., W.H.K., D.P., R.S.V.D.R., A.J.T.T., G.V., J.J.M.v.d.H., and M.W.J.M.W.; visualization: M.G.S.; supervision: M.W.J.M.W.; project administration: J.B.A., G.A.P.H. and M.W.J.M.W.; funding acquisition: J.H. and M.W.J.M.W.
Funding
This research was funded by The Netherlands Organization for Health Research and Development (ZonMW), grant number 836002002. This subsidy is part of the program of effectiveness research of high-cost medicine. The first four years (2012–2016) of the Dutch Melanoma Treatment Registry (DMTR) were sponsored by Roche Nederland B.V, Bristol-Myers Squibb (BMS), GlaxoSmithKline (GSK)/Novartis and, since 2015, also by Merck Sharp & Dohme (MSD).
Conflicts of Interest
K.P.M.S. has consulting/advisory relationships with BMS and MSD. She received honoraria from Novartis, Pierre Fabre, and Roche. A.C.J.V.A. has consulting/advisory relationships with Amgen, Bristol-Myers Squibb, Novartis, MSD, and Merck-Pfizer. He received research funding from Amgen and Novartis. He received travel, accommodations, and expenses from Amgen and Novartis. J.W.B.D.G. has advisory relationships with BMS, MSD, and Roche. G.A.P.H. has consulting/advisory relationships with Roche, MSD, BMS, and Novartis. Her institution received research funding from BMS. J.H. provided consultation, attended advisory boards, and/or provided lectures for MSD, BMS, Roche, and Novartis, for which NKI received honoraria. His institution received grant support from BMS and Novartis. A.J.M.V.D.E. has consulting/advisory relationships with BMS, Roche, MSD, and Novartis. He received a study grant from Roche. R.K. has received speaker fees from BMS, MSD, and Roche. He has advisory relationships with BMS, MSD, Novartis, and Roche. He received research grants from BMS and Roche. E.K. has advisory relationships with BMS, Novartis, Roche, Merck, Amgen, Pierre-Fabre, EISAI, Bayer, and Genzyme-Sanofi (all paid to institution). She received research grants from BMS. The other authors report no conflict of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
References
- 1.Chapman P.B., Hauschild A., Robert C., Haanen J.B., Ascierto P., Larkin J., Dummer R., Garbe C., Testori A., Maio M., et al. Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation for the BRIM-3 Study Group. N. Engl. J. Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodi F.S., O’Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., Gonzalez R., Robert C., Schadendorf D., Hassel J.C., et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C., Karaszewska B., Schachter J., Rutkowski P., Mackiewicz A., Stroiakovski D., Lichinitser M., Dummer R., Grange F., Mortier L., et al. Improved Overall Survival in Melanoma with Combined Dabrafenib and Trametinib. N. Engl. J. Med. 2014;372:30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 4.Robert C., Long G.V., Brady B., Dutriaux C., Maio M., Mortier L., Hassel J.C., Rutkowski P., McNeil C., Kalinka-Warzocha E., et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 2015 doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 5.Robert C., Schachter J., Long G.V., Arance A., Grob J.J., Mortier L., Daud A., Carlino M.S., McNeil C., Lotem M., et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 6.Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J.J., Cowey C.L., Lao C.D., Schadendorf D., Dummer R., Smylie M., Rutkowski P., et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2017 doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robert C., Ribas A., Hamid O., Daud A., Wolchok J.D., Joshua A.M., Hwu W.J., Weber J.S., Gangadhar T.C., Joseph R.W., et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: A pooled analysis of individual patient data from randomised trials. Lancet Oncol. 2016:1743–1754. doi: 10.1016/S1470-2045(16)30578-2. [DOI] [PubMed] [Google Scholar]
- 8.Robert C., Ribas A., Hamid O., Daud A., Wolchok J.D., Joshua A.M., Hwu W.J., Weber J.S., Gangadhar T.C., Joseph R.W., et al. Durable Complete Response After Discontinuation of Pembrolizumab in Patients With Metastatic Melanoma. J. Clin. Oncol. 2018 doi: 10.1200/JCO.2017.75.6270. [DOI] [PubMed] [Google Scholar]
- 9.Kelderman S., Heemskerk B., van Tinteren H., van den Brom R.R., Hospers G.A., van den Eertwegh A.J., Kapiteijn E.W., de Groot J.W., Soetekouw P., Jansen R.L., et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol. Immunother. 2014:449–458. doi: 10.1007/s00262-014-1528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weide B., Martens A., Hassel J.C., Berking C., Postow M.A., Bisschop K., Simeone E., Mangana J., Schilling B., Di Giacomo A.M., et al. Baseline Biomarkers for Outcome of Melanoma Patients Treated with Pembrolizumab. Clin. Cancer Res. 2016:5487–5496. doi: 10.1158/1078-0432.CCR-16-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long G.V., Eroglu Z., Infante J., Patel S., Daud A., Johnson D.B., Gonzalez R., Kefford R., Hamid O., Schuchter L., et al. Long-Term Outcomes in Patients With BRAF V600—Mutant Metastatic Melanoma Who Received Dabrafenib Combined with Trametinib. J. Clin. Oncol. 2018 doi: 10.1200/JCO.2017.74.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim S.Y., Menzies A.M., Rizos H. Mechanisms and Strategies to Overcome Resistance to Molecularly Targeted Therapy for Melanoma. Cancer. 2017;123:2118–2129. doi: 10.1002/cncr.30435. [DOI] [PubMed] [Google Scholar]
- 13.Bradley S.D., Chen Z., Melendez B., Talukder A., Khalili J.S., Rodriguez-Cruz T., Liu S., Whittington M., Deng W., Li F., et al. BRAF V600E Co-opts a Conserved MHC Class I Internalization Pathway to Diminish Antigen Presentation and CD8 þ T-cell Recognition of Melanoma. Cancer Immunol. Res. 2015;3:602–610. doi: 10.1158/2326-6066.CIR-15-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diem S., Kasenda B.R., Gore M.J. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br. J. Cancer. 2016;114:256–261. doi: 10.1038/bjc.2015.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schadendorf D., Long G.V., Stroiakovski D., Karaszewska B., Hauschild A., Levchenko E., Chiarion-Sileni V., Schachter J., Garbe C., Dutriaux C., et al. Inhibitory effect of tumor cell—Derived lactic acid on human T cells. Blood. 2018;109:3812–3820. doi: 10.1182/blood-2006-07-035972.The. [DOI] [PubMed] [Google Scholar]
- 16.Blank C.U., Angeles L., Khersonsky O., Fleishman S.J. The cancer immunogram. Science. 2016;352:658–660. doi: 10.1126/science.aaf2834. [DOI] [PubMed] [Google Scholar]
- 17.Schadendorf D., Long G.V., Stroiakovski D., Karaszewska B., Hauschild A., Levchenko E., Chiarion-Sileni V., Schachter J., Garbe C., Dutriaux C., et al. Three-year pooled analysis of factors associated with clinical outcomes across dabrafenib and trametinib combination therapy phase 3 randomised trials. Eur. J. Cancer. 2017;82:45–55. doi: 10.1016/j.ejca.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 18.Seifert H., Fisher R., Martin-Liberal J., Edmonds K., Hughes P., Khabra K., Gore M., Larkin J. Prognostic markers and tumour growth kinetics in melanoma patients progressing on vemurafenib. Melanoma Res. 2016;26:138–144. doi: 10.1097/CMR.0000000000000218. [DOI] [PubMed] [Google Scholar]
- 19.Ascierto P.A., Simeone E., Giannarelli D., Grimaldi A.M., Romano A., Mozzillo N. Sequencing of BRAF inhibitors and ipilimumab in patients with metastatic melanoma: A possible algorithm for clinical use. J. Transl. Med. 2012;10:107. doi: 10.1186/1479-5876-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ascierto P.A., Simeone E., Sileni V.C., Del Vecchio M., Marchetti P., Cappellini G.C., Ridolfi R., de Rosa F., Cognetti F., Ferraresi V., et al. Sequential Treatment with Ipilimumab and BRAF Inhibitors in Patients With Metastatic Melanoma: Data From the Italian Cohort of the Ipilimumab Expanded Access Program. Cancer Investig. 2017;32:144–149. doi: 10.3109/07357907.2014.885984. [DOI] [PubMed] [Google Scholar]
- 21.Jochems A., Schouwenburg M.G., Leeneman B., Franken M.G., van den Eertwegh A.J., Haanen J.B., Gelderblom H., Uyl-de Groot C.A., Aarts M.J., van den Berkmortel F.W., et al. ScienceDirect Dutch Melanoma Treatment Registry: Quality assurance in the care of patients with metastatic melanoma in the Netherlands. Eur. J. Cancer. 2017;72:156–165. doi: 10.1016/j.ejca.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Schemper M., Smith T.L. A note on quantifying follow-up in studies of failure time. Control. Clin. Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.