Abstract
Background
The effect of risk-reducing salpingo-oophorectomy (RRSO) on breast cancer risk for BRCA1 and BRCA2 mutation carriers is uncertain. Retrospective analyses have suggested a protective effect but may be substantially biased. Prospective studies have had limited power, particularly for BRCA2 mutation carriers. Further, previous studies have not considered the effect of RRSO in the context of natural menopause.
Methods
A multi-centre prospective cohort of 2272 BRCA1 and 1605 BRCA2 mutation carriers was followed for a mean of 5.4 and 4.9 years, respectively; 426 women developed incident breast cancer. RRSO was modelled as a time-dependent covariate in Cox regression, and its effect assessed in premenopausal and postmenopausal women.
Results
There was no association between RRSO and breast cancer for BRCA1 (HR = 1.23; 95% CI 0.94–1.61) or BRCA2 (HR = 0.88; 95% CI 0.62–1.24) mutation carriers. For BRCA2 mutation carriers, HRs were 0.68 (95% CI 0.40–1.15) and 1.07 (95% CI 0.69–1.64) for RRSO carried out before or after age 45 years, respectively. The HR for BRCA2 mutation carriers decreased with increasing time since RRSO (HR = 0.51; 95% CI 0.26–0.99 for 5 years or longer after RRSO). Estimates for premenopausal women were similar.
Conclusion
We found no evidence that RRSO reduces breast cancer risk for BRCA1 mutation carriers. A potentially beneficial effect for BRCA2 mutation carriers was observed, particularly after 5 years following RRSO. These results may inform counselling and management of carriers with respect to RRSO.
Keywords: Breast cancer, BRCA1, BRCA2, Mutation, Risk-reducing salpingo-oophorectomy
Background
Women carrying germline mutations in BRCA1 or BRCA2 are at high risk of developing breast cancer and ovarian cancer [1, 2]. Mutation carriers undergo enhanced cancer surveillance and may be offered interventions including risk-reducing mastectomy (RRM) or risk-reducing salpingo-oophorectomy (RRSO). While RRSO substantially reduces the risk of developing ovarian cancer, its effect on breast cancer risk is uncertain. Some studies have reported substantial breast cancer risk reduction of up to 50% following RRSO [3–6]. However, these studies may have been subject to bias and confounding [7, 8]. Biases include ‘cancer-induced testing bias’, which can occur if mutation testing is conducted as a result of a breast cancer diagnosis and follow-up before DNA testing is included in the analysis, and ‘immortal person-time bias’, caused by excluding follow-up prior to RRSO uptake. Heemskerk-Gerritsen et al. found no evidence for an association between RRSO and breast cancer after eliminating several sources of bias [9, 10]. Prospective cohort studies can avoid such biases, but large studies with long follow-up are required to provide sufficient power.
Here, we report results from a large international collaborative, multi-centre, prospective cohort of 2272 BRCA1 and 1605 BRCA2 mutation carriers. We examined the association between RRSO and breast cancer risk according to the timing of RRSO relative to menopause and time since RRSO.
Methods
Study design and study population
We combined information from three consortia: The International BRCA1/2 Carrier Cohort Study (IBCCS), Kathleen Cuningham Foundation Consortium for Research Into Familial Breast Cancer (kConFab) Follow-Up Study, and Breast Cancer Family Registry (BCFR) (Tables 1 and 2, Additional file 1: Table S1) [11–15]. In total, 9856 BRCA1/2 mutation carriers were included. Eighty-nine percent of participants were invited into the studies after receiving their clinical genetic test results, while 3% were recruited as an untested member of a mutation-carrying family and opted for a clinical test only after enrolment. Seven percent were tested in a research setting, and it was unknown whether or when they opted for a clinical test. Sixty-six percent of participants were enrolled through one of five ongoing nationwide studies in the UK and Ireland (Epidemiological Study of Familial Breast Cancer [EMBRACE]), France (Gene Etude Prospective Sein Ovaire [GENEPSO]), Netherlands (Hereditary Breast and Ovarian cancer study Netherlands [HEBON]), Australia and New Zealand (kConFab), and Austria (Medical University of Vienna [MUV]). Other studies were centre-based.
Table 1.
Prospective cohort of BRCA1 and BRCA2 mutation carriers
| IBCCS studies | BRCA1 mutation carriers | BRCA2 mutation carriers | ||||||
|---|---|---|---|---|---|---|---|---|
| Number of women | FUP time mean, years (sd) | BC, N | Mean age BC diagnosis, years | Number of women | FUP time mean, years (sd) | BC, N | Mean age BC diagnosis, years | |
| EMBRACE | 471 | 4.4 (3.0) | 41 | 45.4 | 478 | 3.9 (2.5) | 42 | 48.2 |
| GENEPSO | 486 | 3.6 (2.4) | 46 | 45.8 | 325 | 3.2 (1.9) | 18 | 48.8 |
| HEBON | 242 | 7.2 (3.6) | 40 | 47.6 | 75 | 5.9 (2.8) | 4 | 47.3 |
| kConFab | 325 | 6.7 (3.8) | 55 | 42.2 | 288 | 6.4 (3.7) | 38 | 50.5 |
| BCFR | 327 | 7.7 (4.4) | 50 | 47.1 | 255 | 7.5 (4.3) | 33 | 49.8 |
| Other studiesa | 421 | 4.9 (3.2) | 37 | 41.4 | 184 | 4.3 (2.9) | 22 | 47.0 |
| Total | 2272 | 5.4 (3.7) | 269 | 44.9 | 1605 | 4.9 (3.4) | 157 | 49.0 |
BC breast cancer, FUP follow-up, sd standard deviation
aOther studies: MUV-Austria, INHERIT, OUH, GC-HBOC, NIO-Hungary, CNIO, HCSC, LUND-BRCA, STOCKHOLM-BRCA, IHCC, and MODSQUAD (see Additional file 1: Table S1 for details)
Table 2.
Characteristics of the cohort of BRCA1 and BRCA2 mutation carriers
| BRCA1 mutation carriers | BRCA2 mutation carriers | |||
|---|---|---|---|---|
| Unaffected women (N = 2003) | Women with breast cancer (N = 269) | Unaffected women (N = 1448) | Women with breast cancer (N = 157) | |
| Total person-years of follow-up | 11,207 | 1134 | 7286 | 600 |
| Person-years of follow-up (mean (sd)) | 5.60 (3.67) | 4.21 (3.27) | 5.03 (3.44) | 3.82 (3.08) |
| Age at start of follow-up (mean (sd)) | 37.51 (11.80) | 40.68 (10.25) | 40.00 (12.53) | 45.14 (10.11) |
| Age at diagnosis/censoring (mean (sd)) | 43.10 (12.28) | 44.90 (10.33) | 45.00 (13.00) | 48.97 (10.30) |
| Reason for censoring | ||||
| Breast cancer | 0 | 269 | 0 | 157 |
| Ovarian cancer | 49 | 3a | 9 | 1a |
| Other cancer | 45 | 5a | 28 | 2a |
| RRM | 299 | – | 181 | – |
| Death | 5 | – | 8 | – |
| Unaffected at last follow-up time | 1605 | – | 1222 | – |
| Year of birth | ||||
| ≤ 1960 | 604 (83.54) | 119 (16.46) | 500 (84.75) | 90 (15.25) |
| > 1960 | 1399 (90.32) | 150 (9.68) | 948 (93.40) | 67 (6.60) |
| Menopausal status | ||||
| Premenopausal at censoringb | ||||
| Last informationc after censoring | 512 | 69 | 344 | 35 |
| Last information before censoringd | 585 | 58 | 473 | 35 |
| Postmenopausal | ||||
| Natural menopause age known | 194 | 27 | 182 | 31 |
| Natural menopause age unknown | 5 | 0 | 7 | 1 |
| Post-hysterectomy | 70 | 12 | 64 | 11 |
| Unknown menopausal status | 62 | 13 | 33 | 8 |
| RRSO status at censoring | ||||
| No RRSO | ||||
| Last information after censoring | 664 | 110 | 467 | 79 |
| Last information before censoring | 618 | 44 | 535 | 27 |
| RRSO | 721 | 115 | 446 | 51 |
| As reason for menopausee | 574 | 90 | 345 | 36 |
| After natural menopause | 101 | 18 | 76 | 10 |
| After hysterectomy | 46 | 7 | 25 | 5 |
RRSO risk-reducing salpingo-oophorectomy, RRM risk-reducing mastectomy
aDiagnosed at the same time as breast cancer
bFifteen women did not report age at menopause but were older than 60 years at the end of follow-up
cInformation from questionnaire and record linkage
dAge last known to be premenopausal mean 32.3 years, median 31 years for BRCA1 mutation carriers: mean 33.9, median 34 years for BRCA2 mutation carriers. Time between this age and end of censoring: mean 6.3, median 5 years for BRCA1 and mean 6 years, median 5 years for BRCA2 mutation carriers
eSeven women reported RRSO after age 60 years without first reporting natural menopause
Study participants
Women were eligible if they were 18–80 years of age at recruitment and tested positive for a pathogenic BRCA1 or BRCA2 mutation, had no cancer history, and had retained both breasts at the date of genetic testing or study enrolment, whichever was last (N = 3886). One woman was excluded as she had been diagnosed with Turner syndrome and eight excluded as it was unclear whether they had had a hysterectomy or RRSO before recruitment.
Data collection
Study participants were invited to complete a baseline questionnaire and a series of follow-up questionnaires. The questionnaires requested detailed information on known or suspected risk factors for breast and ovarian cancer, including family history, reproductive history, and surgical interventions including RRM or RRSO. The questionnaires also asked for information on age at last menstruation, whether the woman had had any period in the past year, the number of years/months since last menstruation, and reason(s) for the stopping of periods. Age at menopause for those who indicated no period in the past year was determined by adding 1 year to ‘age at last menstruation’. Women were considered premenopausal if they indicated that they had had a period in the past year, or if the ‘reason for periods stopping’ was medication, oral contraceptive use, pregnancy, or breast-feeding. Women reporting RRSO as the reason for menopause were considered premenopausal until RRSO. After hysterectomy, menopausal status was considered unknown.
In addition to questionnaires, some studies obtained RRSO information from medical records or linkage to a pathological registry. For the primary analysis, risk factor information was updated from all available sources, including post-diagnosis questionnaires and record linkage. Occurrence of breast cancer was derived from data from follow-up questionnaires and, for five studies, through linkage to cancer registries. Information on vital status was obtained from municipal or death registries, medical records, or family members.
Distributions of dates of breast cancer diagnosis and DNA testing are shown in Additional file 1: Table S2.
Statistical analysis
We used Cox proportional hazards regression models to assess the association with risk of breast cancer. Follow-up started either at completion of baseline questionnaire or mutation testing, whichever was latest. The primary endpoint was breast cancer (invasive or in situ). Follow-up was censored at the earliest of RRM, diagnosis of breast cancer, ovarian cancer or any other cancer, treatment with chemotherapy or radiotherapy in the absence of information about cancer, reaching age 80 years, or death. For studies that used record linkage, follow-up was stopped at the date on which record linkage was conducted or considered complete. For GENEPSO, there was no linkage to cancer registries and women were censored at age at last questionnaire. Women diagnosed with breast cancer within 2 months of the start of follow-up were excluded from all analyses. RRM occurring within 1 year of breast cancer diagnosis were ignored. To investigate the association of RRSO with breast cancer risk in premenopausal women, women were also censored at natural menopause, hysterectomy, or reaching age 60 years. The association of RRSO with breast cancer risk after natural menopause was investigated by starting follow-up at the age of natural menopause. The association between age at natural menopause and breast cancer was investigated by also censoring at RRSO. For hormone replacement therapy (HRT) analyses, women were eligible if they had never used HRT before baseline and further censored at start of HRT.
A potential bias arises if completion of a subsequent questionnaire is related to RRSO uptake or cancer diagnosis. In order to address this possibility, sensitivity analyses were carried out in which RRSO status was changed at the date of the questionnaire in which the information on RRSO occurrence was reported, rather than the reported age at RRSO (except for the HEBON study, for which RRSO status was determined through record linkage). We also carried out sensitivity analysis excluding women with missing information on age or reason for menopause in the baseline questionnaire, even if this information was provided during follow-up (n = 514). Finally, we examined the effect of excluding women with prevalent RRSO at the start of follow-up (n = 403) (Additional file 1: Table S3).
Natural menopause and RRSO were coded as time-dependent covariates in a Cox regression model. In order to investigate the influence of age at RRSO on breast cancer risk, analyses were carried out separately for women experiencing RRSO before or after age 45 years. Analyses were also carried out estimating the hazard ratio for developing breast cancer for different time intervals following RRSO compared with no RRSO. The trend in HR by time since RRSO was evaluated by categorising the time following RRSO as < 2 years, 2–5 years, and > 5 years and fitting a time-varying parameter for this ordinal covariate (coded 0, 1, 2). We conducted separate analyses for BRCA1 and BRCA2 mutation carriers. We stratified for birth cohort and study (in six categories: EMBRACE, GENEPSO, HEBON, kConFab, BCFR, and other studies (Table 1)) and used robust variance estimation to account for familial clustering. We also assessed associations by birth cohort (1920–1960 or 1961–1992) and study and adjusted for potential confounders including family history of breast cancer in first- and second-degree relatives (collected either from the baseline questionnaire or from pedigrees provided by the genetics centres, and coded as unknown, none, one, or two or more breast cancers), family history of ovarian cancer (similarly defined), body mass index (BMI) at baseline (derived from self-reported height and weight), age at first birth (nulliparous, < 30 and ≥ 30), parity (nulliparous, 1, 2 or 3, and ≥ 4 full-term pregnancies), and HRT use (ever vs never, any formulation). The distribution of potential confounders in study subjects is shown in Additional file 1: Table S4. To test the heterogeneity between studies, fixed effect meta-analysis was carried out. Statistical analyses were performed using STATA v13 (StataCorp, College Station, TX). Statistical tests were considered significant based on two-sided hypothesis tests with p < 0.05.
Results
Cohort characteristics
Among 2272 BRCA1 and 1605 BRCA2 mutation carriers without a previous diagnosis of cancer or RRM, 269 BRCA1 and 157 BRCA2 mutation carriers were diagnosed with breast cancer during follow-up (mean follow-up time 5.4 and 4.9 years for BRCA1 and BRCA2, respectively; Tables 1 and 2). In total, 836 (37%) BRCA1 and 497 (31%) BRCA2 mutation carriers reported RRSO, and 226 (10%) BRCA1 and 221 (14%) BRCA2 mutation carriers went through natural menopause, prior to censoring. Baseline demographics of the cohort are shown in Table 2 and Additional file 1: Table S4.
Association between RRSO and breast cancer risk
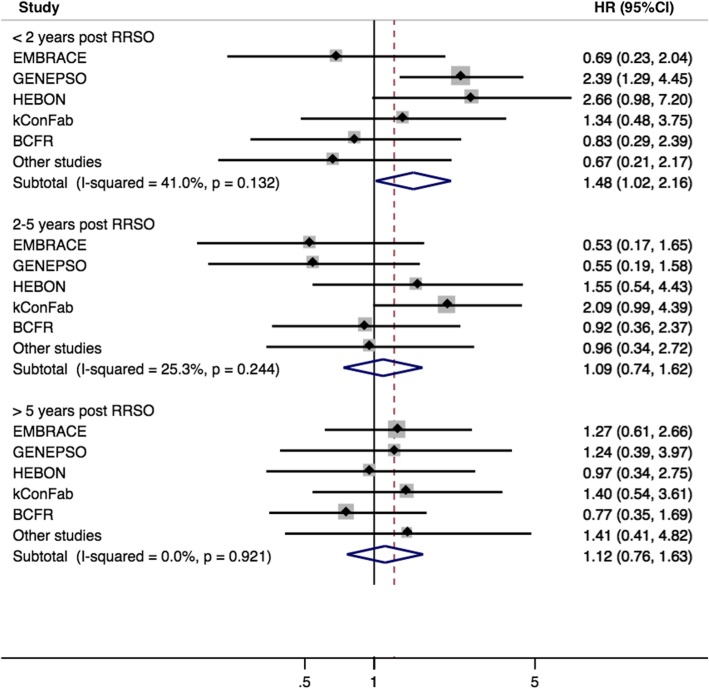
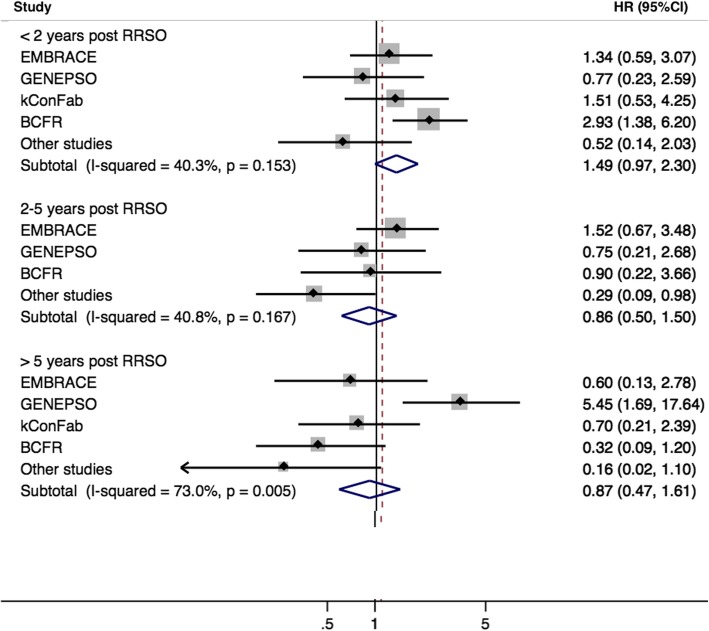
In the primary analysis, the hazard ratio (HR) for the association between RRSO and breast cancer risk was 1.23 (95% CI 0.94–1.61) for BRCA1 and 0.88 (95% CI 0.62–1.24) for BRCA2 mutation carriers (Table 3). For BRCA2 mutation carriers, the HR estimates were 0.68 (95% CI 0.40–1.15) and 1.07 (95% CI 0.69–1.64) for RRSO carried out before and after age 45 years, respectively. For BRCA1 mutation carriers, the estimated HRs were close to 1 across varying times since RSSO (Table 3, Fig. 1), while for BRCA2 mutation carriers, there was some evidence that the HR decreased with increasing time since RRSO (p-trend = 0.011) (Table 3). The HR estimates of greater than 1.0 less than 2 years after RRSO could reflect some inaccuracies in reporting the date of surgery. A protective association was observed for BRCA2 mutation carriers 5 years after RRSO (HR = 0.51 (95% CI 0.26–0.99), p = 0.046, mean time between RRSO and end of follow-up, 9.5 years) (Table 3), although there were differences across studies (p value for heterogeneity = 0.005) (Fig. 2). The HR estimates were slightly lower for premenopausal BRCA2 mutation carriers (Additional file 1: Table S5). There was no significant association between RRSO and breast cancer risk after natural menopause; however, only 221 BRCA1 and 213 BRCA2 mutation carriers were included in these analyses.
Table 3.
Association between RRSO and breast cancer risk
| BRCA1 mutation carriers | BRCA2 mutation carriers | |||||||
|---|---|---|---|---|---|---|---|---|
| Person-years | BC | HR | 95% CI | Person-years | BC | HR | 95% CI | |
| No RRSO | 8353 | 154a | 1.00 | – | 5769 | 106b | 1.00 | – |
| RRSO at any age (years) | 3988 | 115a | 1.23 | 0.94–1.61 | 2117 | 51b | 0.88 | 0.62–1.24 |
| ≤ 45 | 2205 | 64 | 1.19 | 0.88–1.61 | 964 | 17 | 0.68 | 0.40–1.15 |
| > 45 | 1783 | 51 | 1.34 | 0.89–2.02 | 1153 | 34 | 1.07 | 0.69–1.64 |
| Time since RRSO (years) | ||||||||
| < 2 | 1111 | 40 | 1.43 | 1.01–2.03 | 694 | 24 | 1.29 | 0.82–2.02 |
| 2–5 | 1261 | 32 | 1.06 | 0.71–1.57 | 722 | 17 | 0.82 | 0.48–1.38 |
| > 5 | 1616 | 43 | 1.18 | 0.81–1.71 | 701 | 10 | 0.51 | 0.26–0.99 |
A Cox regression model was used adjusting for country, stratified by year of birth (≤ 1960, ≥ 1961) and with robust standard errors (clustering by family)
BC breast cancer, RRSO risk-reducing salpingo-oophorectomy, HR hazard ratio
aAmong BRCA1 mutation carriers, tumour pathology was unknown for 5 women without RRSO and 9 following RRSO
bAmong BRCA2 mutation carriers, tumour pathology was unknown for 12 women without RRSO and 7 following RRSO
Fig. 1.
Association between risk-reducing salpingo-oophorectomy and breast cancer risk for BRCA1 mutation carriers in each study centre category
Fig. 2.
Association between risk-reducing salpingo-oophorectomy and breast cancer risk for BRCA2 mutation carriers in each study centre category. HEBON and, for the 2–5-year category, kConFab were included in the “Other studies” category due to small numbers
The results of the sensitivity analyses were broadly similar to the main analyses (Additional file 1: Tables S6-S8).
Analyses were also adjusted for potential confounders: parity, BMI, age at first birth, and family history of breast or ovarian cancer. Association between breast cancer risk factors and uptake of RRSO are shown in Additional file 1: Tables S9 and S10. In the analyses adjusted for these covariates, the estimated effect sizes were similar to those in the unadjusted analyses (Additional file 1: Table S11). Effect estimates for the analyses carried out among women who had never taken HRT were similar to those in the primary analyses (Additional file 1: Tables S12 and S13).
Discussion
Reliable estimation of the association between uptake and timing of RRSO and breast cancer risk is critical for informing counselling and clinical management of BRCA1 and BRCA2 mutation carriers. Our study of 3877 mutation carriers with 426 incident breast cancer cases is the largest prospective cohort to date and the first prospective study investigating breast cancer risk after RRSO for BRCA1 and BRCA2 mutation carriers in the context of menopausal status.
We found no significant association between RRSO and breast cancer risk for BRCA1 or BRCA2 mutation carriers, although the point estimate for the association for BRCA2 mutation carriers was less than 1 (HR = 0.88 (95% CI 0.62–1.24)) and lower when RRSO was carried out before the age of 45 (HR = 0.68 (95% CI 0.40–1.15) vs 1.07 (95% CI 0.69–1.64) after age 45). Our overall results are inconsistent with previous reports of ~ 50% reduction in breast cancer risk for BRCA1 mutation carriers [3, 6] but more consistent with a study by Kotsopolous et al. reporting risk reduction only for younger BRCA2 mutation carriers [16]. The latter study was prospective, but its results were based on only 3 breast cancers in women aged under 50 years; our study included more than twice as many BRCA2 mutation carriers overall, and the analyses were based on 31 incident breast cancers in premenopausal BRCA2 mutation carriers. In addition, we investigated associations by time since RRSO. For BRCA2 mutation carriers, we observed a decreasing trend in HR with increasing time since RRSO; relative to women who did not have an RSSO, the estimated HR > 5 years following RSSO was 0.51. In contrast, for BRCA1 mutation carriers, the HR was close to 1 at all times since RRSO.
While this is the largest prospective cohort of mutation carriers to date, the number of breast cancer cases was still limited, and hence, the confidence limits for the HR estimates were wide. Additional data would be needed to determine whether or not there is a modest protective effect of RRSO for BRCA1 mutation carriers and whether the suggested protective effect in BRCA2 mutation carriers is real.
There was some suggestion of differences in estimated effect size among studies for BRCA1 mutation carriers in the < 2-year and ‘2–5-year’ post-RRSO groups (Fig. 1), but the heterogeneity was not statistically significant. For BRCA2 mutation carriers, there was statistically significant heterogeneity in the RRSO > 5 years group (Fig. 2); this appeared to be driven by a large effect size in GENEPSO, based on only two breast cancers. Studies differed in methodology (including frequency of questionnaires, assessment of breast cancers or RRSO, loss to follow-up, and mean follow-up time). EMBRACE, GENEPSO, and HEBON ascertained participants through cancer genetics clinics, while BCFR used both clinic- and population-based recruitment. There was also some geographical variation in the uptake and age at RRSO (Additional file 1: Table S3). However, the cohorts were recruited and followed up over broadly similar periods (Additional file 1: Table S2).
The strength of this study is its prospective design. Many of the biases identified in previous reports were addressed [7, 9, 17, 18]. We avoided cancer testing-induced bias by starting follow-up after mutation testing. Women were not selected for inclusion in the study on the basis of RRSO status, and time-dependent covariates were used to examine the effect of RRSO on breast cancer risk. While it is impossible to rule out bias due to unmeasured confounders in an observational study, adjustment for potential confounders (family history of breast and ovarian cancer, parity, age at first birth, and BMI) did not materially influence the results.
In the general population, HRT use is associated with an increased risk of breast cancer. HRT use after RRSO may therefore attenuate the risk reduction due to RRSO. Our preliminary analyses restricted to the subset of women not reporting HRT use gave broadly similar results (Additional file 1: Table S13), but the effects of HRT post-RRSO will need to be further investigated in larger cohorts and studies that consider the type, formulation, and duration of HRT use.
While often considered the ‘gold standard’ for investigating exposure-disease associations, prospective cohort studies are still prone to biases resulting from missing data, loss to follow-up, and informative censoring. In particular, there are gaps in data collection between questionnaires and between the last questionnaire and censoring, during which risk factors can change. We carried out sensitivity analyses in which risk factors were scored according to the most recent questionnaire, thus treating equally women who reached a particular questionnaire follow-up and those who dropped out before reaching this time point. This analysis avoids differential scoring of risk factors between those who developed breast cancer and those who did not develop breast cancer but would be expected to result in loss of power. We also carried out sensitivity analyses excluding two studies, kConFab and BCFR, as these studies were included in a recent analysis of RRSO in women with a family history of breast cancer (Additional file 1: Table S14) [19]. The results of these analyses were almost identical to those from the primary analyses. Reporting of natural menopause is also subject to recall bias and measurement error, and for about half of women reporting premenopausal status, the questionnaires did not cover the entire follow-up period.
A potential bias in the estimate of the RRSO association could arise if the timing of uptake of RRSO was related to the imminent transition to menopause. If there was a protective effect of early natural menopause on cancer risk for mutation carriers, this could result in an overestimation of the RRSO effect in the overall analysis. However, we found no evidence for a strong association between age at natural menopause and breast cancer risk (Additional file 1: Table S15), so any such bias is likely to be small.
Recent genome-wide association analyses have shown that age at natural menopause is partially determined by variants in DNA repair genes, including common coding variants in BRCA1 [20]. Some studies have suggested that natural menopause occurs at a younger age for BRCA1 and BRCA2 mutation carriers compared with women from the general population [21–24] and that BRCA1 mutation carriers have reduced ovarian reserve, and consequently a shortened reproductive lifespan, compared with non-carriers [25]. BRCA1 mutation carriers have also been found to be more likely to have occult ovarian insufficiency [21]. The effect of menopause on breast cancer risk might therefore differ in mutation carriers compared with the general population.
It is plausible that oophorectomy may reduce breast cancer risk in BRCA2 mutation carriers but not in BRCA1 mutation carriers. Breast cancer incidence peaks or plateaus at a younger age (early 40s) in BRCA1 than BRCA2 mutation carriers [2], perhaps suggesting that much of the carcinogenic process in BRCA1 mutation carriers takes place before women typically have RRSO and could influence disease incidence. In addition, BRCA2-related tumours are mainly oestrogen receptor (ER)-positive, and BRCA1-related tumours are mainly ER-negative. Previous analyses have suggested that in the general population, the association of early menopause with reduced breast cancer risk is larger for ER-positive disease [26]. Future analyses stratified by molecular subtype of breast cancer should help delineate mechanisms underlying this difference.
Optimum timing of RRSO should take into account reported age-specific incidences of ovarian cancer among BRCA1 and BRCA2 mutation carriers [2]. National Comprehensive Cancer Network (NCCN) guidelines for example recommend RRSO for BRCA1 mutation carriers, typically between 35 and 40 years of age and upon completion of child-bearing; for BRCA2 mutation carriers, these guidelines suggest that it is reasonable to delay RRSO until age 40–45 years [27]. Cancer Australia clinical guidelines recommend RRSO in confirmed mutation carriers around age 40 years, while considering individual risk and circumstances [28]. Adverse effects of RRSO at a young age, including reduced quality of life, cardiovascular disease, and osteoporosis, should also be taken into consideration. The results of our study indicate that caution should be exercised in conveying information on the risk of breast cancer after RRSO, and emphasise the need for continued surveillance for breast cancer following RRSO for women who do not opt for risk-reducing mastectomy,
The results of our analyses further suggest that continued follow-up of prospective cohorts of mutation carriers, with linkage to end-point and risk factor data, are required. These findings need replication in larger studies of BRCA1 and BRCA2 mutation carriers, particularly including more women in whom RRSO was carried out at a young age. More complete data on factors such as a family history of breast or ovarian cancer would be valuable. Prospective studies with long-term follow-up will also be important for analysing the association between HRT use and breast cancer risk following RRSO, as limited data have been available to date. In addition, RRSO has been reported to reduce mortality from breast cancer [29–31], and there is some evidence that breast cancers arising after RRSO are more indolent than those arising without RRSO [32]. Prospective studies of survival after RRSO would further inform counselling and management of BRCA1 and BRCA2 mutation carriers.
Conclusions
While the primary purpose of RRSO is the prevention of ovarian cancer, information on the effect of RRSO on breast cancer risk is essential for clinical decision-making, including the decision to undergo a risk-reducing mastectomy. Our results suggest that a protective effect of RRSO for BRCA2 mutation carriers may manifest five or more years after surgery. While we cannot rule out an effect of RRSO on breast cancer risk for BRCA1 mutation carriers, this effect is unlikely to be as large.
Supplementary information
Additional file 1 :Table S1. Studies and samples included in the prospective cohort of BRCA1 and BRCA2 mutation carriers. Table S2. Distributions of dates of breast cancer diagnosis, DNA test and start of follow-up in the prospective cohort. Table S3. Characteristics of reported Risk-Reducing Salpingo-oophorectomy. Table S4. Characteristics of cohort of BRCA1 and BRCA2 mutation carriers. Table S5. Association between RRSO and breast cancer by menopausal status. Table S6. Association between RRSO and breast cancer (sensitivity analysis with RRSO status changing at the age at the questionnaire with information on RRSO status changes (all studies except HEBON)). Table S7. Association between RRSO and breast cancer (sensitivity analysis dropping individuals with missing information at baseline). Table S8. Association between RRSO and breast cancer among BRCA1 and BRCA2 mutation carriers (sensitivity analysis excluding women with RRSO before baseline). Table S9. Association between family history of breast cancer and family history of ovarian cancer and RRSO uptake. Table S10. Association between parity, age at first birth, and body mass index and RRSO uptake. Table S11. Association between RRSO and breast cancer adjusting for Body Mass Index, family history of breast cancer, family history of ovarian cancer, parity and age at first birth. Table S12. Hormone replacement therapy use among women in the cohort. Table S13. Association between RRSO and breast cancer among women not exposed to hormone replacement therapy. Table S14. Association between RRSO and breast cancer (excluding kConFab/BCFR). Table S15. Association between natural menopause and breast cancer (censoring at RRSO). Ethics Committee Approvals
Acknowledgements
Study-specific acknowledgments:
We acknowledge the EMBRACE Centres; the Coordinating Centre: University of Cambridge and the Collaborating Centres; Guy’s and St. Thomas’ NHS Foundation Trust, London; Central Manchester University Hospitals NHS Foundation Trust, Manchester: Chapel Allerton Hospital, Leeds; The Institute of Cancer Research and Royal Marsden NHS Foundation Trust, Sutton; Birmingham Women’s Hospital Healthcare NHS Trust, Birmingham; South Glasgow University Hospitals, Glasgow; Addenbrooke’s Hospital, Cambridge; St. Georges, London Royal Devon & Exeter Hospital, Exeter; Southampton University Hospitals NHS Trust, Southampton; Sheffield Children’s Hospital, Sheffield; Newcastle Upon Tyne Hospitals NHS Trust, Newcastle; Great Ormond Street Hospital for Children NHS Trust, London; Churchill Hospital, Oxford; Western General Hospital, Edinburgh; St Michael’s Hospital, Bristol; Belfast City Hospital, Belfast; Nottingham University Hospitals NHS Trust, Nottingham; University Hospital of Wales, Cardiff; Alder Hey Hospital, Liverpool; Kennedy Galton Centre, Harrow; Trinity College Dublin and St James’s Hospital, Dublin; University Hospitals of Leicester NHS Trust, Leicester; NHS Grampian & University of Aberdeen, Aberdeen; Glan Clwyd Hospital, Rhyl; and Singleton Hospital, Swansea. We also wish to thank Steve Ellis (data manager on the EMBRACE study 2010-2014).
BCFR thanks the members and participants in the Breast Cancer Family Registry from the New York, Northern California, Ontario, Philadelphia, Utah, and Australia sites for their contributions to the study.
CNIO thanks the staff for their assistance.
We acknowledge the GENEPSO Centers: the Coordinating Center: Institut Paoli-Calmettes, Marseille, France: Catherine Noguès, Lilian Laborde, Emmanuel Breysse who contributed by centralising, managing the data, and organising BRCA1 and BRCA2 mutation carriers follow-up and the Collaborating Centers which contributed to the mutation carriers recruitment and follow-up: Dominique Stoppa-Lyonnet, PhD, MD, Institut Curie, Paris; Marion Gauthier-Villars, MD, Institut Curie, Paris; Bruno Buecher, MD, Institut Curie, Paris; Olivier Caron, MD, Institut Gustave Roussy, Villejuif; Emmanuelle Fourme-Mouret, MD, Hôpital René Huguenin/Institut Curie, Saint Cloud; Jean-Pierre Fricker, MD, Centre Paul Strauss, Strasbourg; Christine Lasset, MD, Centre Léon Bérard, Lyon; Valérie Bonadona, PhD, MD, Centre Léon Bérard, Lyon; Pascaline Berthet, MD, Centre François Baclesse, Caen; Laurence Faivre, MD, Hôpital d’Enfants CHU and Centre Georges François Leclerc, Dijon; Elisabeth Luporsi, PhD, MD, CHR Metz-Thionville, Hôpital de Mercy, Metz, France; Véronique Mari, MD, Centre Antoine Lacassagne, Nice; Laurence Gladieff, MD, Institut Claudius Regaud, Toulouse; Paul Gesta, MD, Réseau Oncogénétique Poitou Charente, Niort; Hagay Sobol, PhD, MD, Institut Paoli-Calmettes, Marseille; François Eisinger, MD, Institut Paoli-Calmettes, Marseille; Catherine Noguès,MD, Institut Paoli-Calmettes, Marseille; Michel Longy, PhD, MD Institut Bergonié, Bordeaux; Catherine Dugast†, MD, Centre Eugène Marquis, Rennes;Chrystelle Colas, MD, GH Pitié Salpétrière, Paris; Isabelle Coupier, MD, CHU Arnaud de Villeneuve, Montpellier; Pascal Pujol, MD, CHU Arnaud de Villeneuve, Montpellier; Carole Corsini, MD, CHU Arnaud de Villeneuve, Montpellier; Alain Lortholary, MD, Centres Paul Papin, and Catherine de Sienne, Angers, Nantes; Philippe Vennin†,MD, Centre Oscar Lambret, Lille; Claude Adenis, MD, Centre Oscar Lambret, Lille; Tan Dat Nguyen, MD, Institut Jean Godinot, Reims; Capucine Delnatte, MD, Centre René Gauducheau, Nantes; Julie Tinat, MD, Centre Henri Becquerel, Rouen; Isabelle Tennevet, MD, Centre Henri Becquerel, Rouen; Jean-Marc Limacher, MD, Hôpital Civil, Strasbourg; Christine Maugard, PhD, Hôpital Civil, Strasbourg; Yves-Jean Bignon, MD, Centre Jean Perrin, Clermont-Ferrand; Liliane Demange†, MD, Polyclinique Courlancy, Reims; Clotilde Penet, MD, Polyclinique Courlancy, Reims; Hélène Dreyfus, MD, Clinique Sainte Catherine, Avignon; Odile Cohen-Haguenauer, MD, Hôpital Saint-Louis, Paris; Laurence Venat-Bouvet, MD, CHRU Dupuytren, Limoges; Dominique Leroux, MD, Couple-Enfant-CHU de Grenoble; Hélène Dreyfus, MD, Couple-Enfant-CHU de Grenoble; Hélène Zattara-Cannoni, MD, Hôpital de la Timone, Marseille; Sandra Fert-Ferrer, MD, Hôtel Dieu - Centre Hospitalier, Chambery; and Odile Bera, MD, CHU Fort de France, Fort de France. †Deceased.
HCSC acknowledge the staff for their technical assistance.
The Hereditary Breast and Ovarian Cancer Research Group Netherlands (HEBON) consists of the following Collaborating Centers: Netherlands Cancer Institute (coordinating centre), Amsterdam, NL: F.B.L. Hogervorst; Erasmus Medical Center, Rotterdam, NL: J.M. Collée; Leiden University Medical Center, NL: C.J. van Asperen; Radboud University Nijmegen Medical Center, NL: A.R. Mensenkamp; University Medical Center Utrecht, NL: M.G.E.M. Ausems; Amsterdam Medical Center, NL: H.E.J. Meijers-Heijboer; VU University Medical Center, Amsterdam, NL: K. van Engelen; Maastricht University Medical Center, NL: M.J. Blok; University of Groningen, NL: J.C. Oosterwijk; The Netherlands Comprehensive Cancer Organisation (IKNL): J.Verloop; and the nationwide network and registry of histo- and cytopathology in The Netherlands (PALGA): E. van den Broek. HEBON thanks the study participants and the registration teams of IKNL and PALGA for part of the data collection.
INHERIT would like to thank the staff for the sample management and skilful assistance.
We thank Heather Thorne, Eveline Niedermayr and all the kConFab research nurses and staff, the heads and staff of the Family Cancer Clinics, and the many families who contribute to kConFab for their contributions to this resource.
Czech Republic, MMCI, Brno—for the data collection and management.
We wish to thank the Hungarian Breast and Ovarian Cancer Study Group members; the Department of Molecular Genetics, National Institute of Oncology, Budapest, Hungary; and the clinicians and patients for their contributions to this study.
Swedish scientists participating as SWE-BRCA collaborators from the Lund University and University Hospital and from Stockholm and Karolinska University Hospital.
Abbreviations
- BMI
Body mass index
- EMBRACE
Epidemiological Study of Familial Breast Cancer
- GENEPSO
Gene Etude Prospective Sein Ovaire
- HEBON
Hereditary Breast and Ovarian cancer study Netherlands
- HRT
Hormone replacement therapy
- IBCCS
International BRCA1/2 Carrier Cohort Study
- kConFab
Kathleen Cuningham Foundation Consortium for Research Into Familial Breast Cancer
- RRM
Risk-reducing mastectomy
- RRSO
Risk-reducing salpingo-oophorectomy
Authors’ contributions
Concept and design was done by DFE, NM, NA ACA, and MAR. Statistical analysis was done by NM, NA, and DFE. Drafting of the manuscript was done by NM, DFE, NA, ACA, MAR, FEL, CE, KK, DEG, M-BT, K-AP, and RLM. Administrative, technical, or material support was done by TM, DB, and DF. All authors contributed to the acquisition, analysis, and interpretation of the data and revision of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Cancer Research UK grants C1287/A17523, C1287/23382, C1287/A16563, C12292/A20861, and C12292/A11174.
Study-specific funding:
The BCFR was supported by grant UM1 CA164920 from the National Cancer Institute. The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centres in the Breast Cancer Family Registry (BCFR), nor does mention of trade names, commercial products, or organisations imply endorsement by the US Government or the BCFR.
CNIO was partially supported by the Spanish Ministry of Economy and Competitiveness (MINECO) SAF2014-57680-R and the Spanish Research Network on Rare Diseases (CIBERER). CNIO was also partially supported by FISPI16/00440.
INHERIT was supported by the Canadian Institutes of Health Research for the ‘CIHR Team in Familial Risks of Breast Cancer’ program—grant # CRN-87521—and the Ministry of Economic Development, Innovation and Export Trade—grant # PSR-SIIRI-701. The PERSPECTIVE project was supported by the Government of Canada through Genome Canada and the Canadian Institutes of Health Research (GPH-129344), the Ministère de l’Économie, de la Science et de l’ Innovation du Québec through Genome Québec, and The Quebec Breast Cancer Foundation.
Jacques Simard is a Chairholder of the Canada Research Chair in Oncogenetics.
EMBRACE is supported by the Cancer Research UK grants C1287/A23382 and C1287/A16563.
D. Gareth Evans is supported by an NIHR grant to the Biomedical Research Centre, Manchester (IS-BRC-1215-20007). The investigators at The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust are supported by an NIHR grant to the Biomedical Research Centre at The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust. Ros Eeles and Elizabeth Bancroft are supported by the Cancer Research UK grant C5047/A8385. Ros Eeles is also supported by NIHR support to the Biomedical Research Centre at The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust. Antonis C. Antoniou is funded by Cancer Research UK grants C12292/A20861 and C12292/A11174. MT is funded by the European Union Seventh Framework Program (2007–2013)/European Research Council (310018).
The German Consortium for Hereditary Breast and Ovarian Cancer (GC-HBOC) is supported by the German Cancer Aid (grant no 110837, Rita K. Schmutzler).
The national French cohort, GENEPSO, had been supported by a grant from the Fondation de France and the Ligue Nationale Contre le Cancer and is being supported by a grant from INCa as part of the European program ERA-NET on Translational Cancer Research (TRANSCAN-JTC2012, no. 2014-008).
HCSC was supported by CIBERONC 161200301 from ISCIII (Spain), partially supported by European Regional Development FEDER funds.
The HEBON study is supported by the Dutch Cancer Society grants NKI1998-1854, NKI2004-3088, NKI2007-3756, the Netherlands Organisation of Scientific Research grant NWO 91109024, the Pink Ribbon grants 110005 and 2014-187.WO76, the BBMRI grant NWO 184.021.007/CP46, and the Transcan grant JTC 2012 Cancer 12-054.
The IHCC was supported by Grant PBZ_KBN_122/P05/2004 and ERA-NET TRANSAN JTC 2012 Cancer 12-054 (ERA-NET-TRANSCAN/07/2014).
This work was supported by grants to kConFab and the kConFab Follow-Up Study from Cancer Australia (809195); the Australian National Breast Cancer Foundation (IF 17); the National Health and Medical Research Council (454508, 288704, 145684); the National Institute of Health U.S.A. (1RO1CA159868); the Queensland Cancer Fund; the Cancer Councils of New South Wales, Victoria, Tasmania and South Australia; and the Cancer Foundation of Western Australia. KAP is an Australian National Breast Cancer Foundation fellow.
MODSQUAD—Czech Republic, Brno, was supported by MH CZ - DRO (MMCI, 00209805) and by MEYS - NPS I - LO1413 to LF and MN.
The Hungarian Breast and Ovarian Cancer Study was supported by Hungarian Research Grants KTIA-OTKA CK-80745, NKFI OTKA K-112228, and the Norwegian EEA Financial Mechanism HU0115/NA/2008-3/ÖP-9.
Lund-BRCA collaborators are supported by the Swedish Cancer Society, Lund Hospital Funds, and European Research Council Advanced Grant ERC-2011-294576. Stockholm-BRCA collaborators are supported by the Swedish Cancer Society.
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Availability of data and materials
The dataset supporting the conclusions of this article are available upon reasonable request. Requests should be made to Dr. M Rookus (NKI, Amsterdam, NL; m.rookus@nki.nl).
Ethics approval and consent to participate
Participants provided written informed consent, and studies were approved by a relevant ethics committee.
Consent for publication
Not applicable
Competing interests
Wendy Chung reports potential conflict of interest from Regeneron and Biogen; Olivier Caron from AstraZeneca and IPSEN; Pascal Pujol, AstraZeneca, Genomic Health and Roche; D Gareth Evans, AstraZeneca and AmGen; Ros Eeles, Janssen-Cilag; Diane Eccles, Pierre Fabre, AstraZeneca; Karin Kast, Roche Pharma AG; and David Goldgar, University of Utah Foundation and Ambry Genetics. Anne-Marie Gerdes participated in an Advisory Board Meeting London in 2016, sponsored by Astra Zeneca about BRCA-testing in ovarian cancer.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nadine Andrieu and Douglas F Easton are joint senior authors.
Change history
2/26/2020
After publication of the original article [1], we were notified that columns in Table 2 were erroneously displayed.
Contributor Information
Nasim Mavaddat, Email: nm274@medschl.cam.ac.uk.
GENEPSO, Email: noguesc@ipc.unicancer.fr.
EMBRACE, Email: Embrace@medschl.cam.ac.uk.
HEBON, Email: hebon@nki.nl
on behalf of IBCCS, Email: m.rookus@nki.nl.
kConFab, Email: heather.thorne@petermac.org.
BCFR, Email: mt146@cumc.columbia.edu.
GENEPSO:
Catherine Noguès, Lilian Laborde, Emmanuel Breysse, Dominique Stoppa-Lyonnet, Marion Gauthier-Villars, Bruno Buecher, Olivier Caron, Emmanuelle Fourme-Mouret, Jean-Pierre Fricker, Christine Lasset, Valérie Bonadona, Pascaline Berthet, Laurence Faivre, Elisabeth Luporsi, Véronique Mari, Laurence Gladieff, Paul Gesta, Hagay Sobol, François Eisinger, Catherine Noguès, Michel Longy, Catherine Dugast, Chrystelle Colas, Isabelle Coupier, Pascal Pujol, Carole Corsini, Alain Lortholary, Philippe Vennin, Claude Adenis, Tan Dat Nguyen, Capucine Delnatte, Julie Tinat, Isabelle Tennevet, Jean-Marc Limacher, Christine Maugard, Yves-Jean Bignon, Liliane Demange, Clotilde Penet, Hélène Dreyfus, Odile Cohen-Haguenauer, Laurence Venat-Bouvet, Dominique Leroux, Hélène Dreyfus, Hélène Zattara-Cannoni, Sandra Fert-Ferrer, and Odile Bera
EMBRACE:
HEBON:
F. B. L. Hogervorst, J. M. Collée, C. J. van Asperen, A. R. Mensenkamp, M. G. E. M. Ausems, H. E. J. Meijers-Heijboer, K. van Engelen, M. J. Blok, J. C. Oosterwijk, J. Verloop, and E. van den Broek
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13058-020-1247-4.
References
- 1.Mavaddat N, Peock S, Frost D, Ellis S, Platte R, Fineberg E, et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst. 2013;105(11):812–822. doi: 10.1093/jnci/djt095. [DOI] [PubMed] [Google Scholar]
- 2.Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2402–2416. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 3.Kauff ND, Domchek SM, Friebel TM, Robson ME, Lee J, Garber JE, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: a multicenter, prospective study. J Clin Oncol. 2008;26(8):1331–1337. doi: 10.1200/JCO.2007.13.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101(2):80–87. doi: 10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisen A, Lubinski J, Klijn J, Moller P, Lynch HT, Offit K, et al. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers: an international case-control study. J Clin Oncol. 2005;23(30):7491–7496. doi: 10.1200/JCO.2004.00.7138. [DOI] [PubMed] [Google Scholar]
- 6.Domchek SM, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304(9):967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klaren HM, van’t Veer LJ, van Leeuwen FE, Rookus MA. Potential for bias in studies on efficacy of prophylactic surgery for BRCA1 and BRCA2 mutation. J Natl Cancer Inst. 2003;95(13):941–947. doi: 10.1093/jnci/95.13.941. [DOI] [PubMed] [Google Scholar]
- 8.Fakkert IE, Mourits MJ, Jansen L, van der Kolk DM, Meijer K, Oosterwijk JC, et al. Breast cancer incidence after risk-reducing salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers. Cancer Prev Res. 2012;5(11):1291–1297. doi: 10.1158/1940-6207.CAPR-12-0190. [DOI] [PubMed] [Google Scholar]
- 9.Heemskerk-Gerritsen BA, Seynaeve C, van Asperen CJ, Ausems MG, Collee JM, van Doorn HC, et al. Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. J Natl Cancer Inst. 2015;107(5). [DOI] [PubMed]
- 10.Heemskerk-Gerritsen BA, Hooning MJ, Rookus MA. Response. J Natl Cancer Inst. 2015;107(9). [DOI] [PMC free article] [PubMed]
- 11.Phillips KA, Butow PN, Stewart AE, Chang JH, Weideman PC, Price MA, et al. Predictors of participation in clinical and psychosocial follow-up of the kConFab breast cancer family cohort. Familial Cancer. 2005;4(2):105–113. doi: 10.1007/s10689-004-6129-x. [DOI] [PubMed] [Google Scholar]
- 12.Thorne H, Mitchell G, Fox S. kConFab: a familial breast cancer consortium facilitating research and translational oncology. J Natl Cancer Inst Monogr. 2011;43:79–81. doi: 10.1093/jncimonographs/lgr042. [DOI] [PubMed] [Google Scholar]
- 13.Goldgar D, Bonnardel C, Renard H. The international BRCA1/2 carrier cohort study: purpose, rationale, and study design. Breast Cancer Res. 2000;2E10. 10.1186/bcr93.
- 14.John EM, Hopper JL, Beck JC, Knight JA, Neuhausen SL, Senie RT, et al. The Breast Cancer Family Registry: an infrastructure for cooperative multinational, interdisciplinary and translational studies of the genetic epidemiology of breast cancer. Breast Cancer Res. 2004;6(4):R375–R389. doi: 10.1186/bcr801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terry MB, Phillips KA, Daly MB, John EM, Andrulis IL, Buys SS, et al. Cohort profile: The Breast Cancer Prospective Family Study Cohort (ProF-SC) Int J Epidemiol. 2016;45(3):683–692. doi: 10.1093/ije/dyv118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotsopoulos J, Huzarski T, Gronwald J, Kim-Sing C, Neuhausen S, Demsky R, et al. Bilateral oophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2017;109(1). [DOI] [PMC free article] [PubMed]
- 17.Rebbeck TR, Friebel T, Wagner T, Lynch HT, Garber JE, Daly MB, et al. Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE study group. J Clin Oncol. 2005;23(31):7804–7810. doi: 10.1200/JCO.2004.00.8151. [DOI] [PubMed] [Google Scholar]
- 18.Kotsopoulos Joanne, Gronwald Jacek, Karlan Beth Y., Huzarski Tomasz, Tung Nadine, Moller Pal, Armel Susan, Lynch Henry T., Senter Leigha, Eisen Andrea, Singer Christian F., Foulkes William D., Jacobson Michelle R., Sun Ping, Lubinski Jan, Narod Steven A. Hormone Replacement Therapy After Oophorectomy and Breast Cancer Risk Among BRCA1 Mutation Carriers. JAMA Oncology. 2018;4(8):1059. doi: 10.1001/jamaoncol.2018.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terry Mary Beth, Daly Mary B, Phillips Kelly Anne, Ma Xinran, Zeinomar Nur, Leoce Nicole, Dite Gillian S, MacInnis Robert J, Chung Wendy K, Knight Julia A, Southey Melissa C, Milne Roger L, Goldgar David, Giles Graham G, Weideman Prue C, Glendon Gord, Buchsbaum Richard, Andrulis Irene L, John Esther M, Buys Saundra S, Hopper John L. Risk-Reducing Oophorectomy and Breast Cancer Risk Across the Spectrum of Familial Risk. JNCI: Journal of the National Cancer Institute. 2018;111(3):331–334. doi: 10.1093/jnci/djy182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day FR, Ruth KS, Thompson DJ, Lunetta KL, Pervjakova N, Chasman DI, et al. Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair. Nat Genet. 2015;47(11):1294–1303. doi: 10.1038/ng.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins IM, Milne RL, McLachlan SA, Friedlander M, Hickey M, Weideman PC, et al. Do BRCA1 and BRCA2 mutation carriers have earlier natural menopause than their noncarrier relatives? Results from the Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer. J Clin Oncol. 2013;31(31):3920–3925. doi: 10.1200/JCO.2013.49.3007. [DOI] [PubMed] [Google Scholar]
- 22.Oktay K, Kim JY, Barad D, Babayev SN. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol. 2010;28(2):240–244. doi: 10.1200/JCO.2009.24.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finch A, Valentini A, Greenblatt E, Lynch HT, Ghadirian P, Armel S, et al. Frequency of premature menopause in women who carry a BRCA1 or BRCA2 mutation. Fertil Steril. 2013;99(6):1724–1728. doi: 10.1016/j.fertnstert.2013.01.109. [DOI] [PubMed] [Google Scholar]
- 24.van Tilborg TC, Broekmans FJ, Pijpe A, Schrijver LH, Mooij TM, Oosterwijk JC, et al. Do BRCA1/2 mutation carriers have an earlier onset of natural menopause? Menopause. 2016;23(8):903–910. doi: 10.1097/GME.0000000000000633. [DOI] [PubMed] [Google Scholar]
- 25.Phillips KA, Collins IM, Milne RL, McLachlan SA, Friedlander M, Hickey M, et al. Anti-Mullerian hormone serum concentrations of women with germline BRCA1 or BRCA2 mutations. Hum Reprod. 2016;31(5):1126–1132. doi: 10.1093/humrep/dew044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13(11):1141–1151. [DOI] [PMC free article] [PubMed]
- 27.National Comprehensive Cancer Network (NCCN) clinical practice guidelines in Oncology. https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf Accessed 25 Oct 2019
- 28.Cancer Australia clinical recommendations for management of women at high risk of ovarian cancer. https://guidelines.canceraustralia.gov.au/guidelines/high_risk_ovarian/ch01s03.php Accessed Oct 25 2019.
- 29.Domchek SM, Friebel TM, Neuhausen SL, Wagner T, Evans G, Isaacs C, et al. Mortality after bilateral salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Lancet Oncol. 2006;7(3):223–229. doi: 10.1016/S1470-2045(06)70585-X. [DOI] [PubMed] [Google Scholar]
- 30.Metcalfe K, Lynch HT, Foulkes WD, Tung N, Kim-Sing C, Olopade OI, et al. Effect of oophorectomy on survival after breast cancer in BRCA1 and BRCA2 mutation carriers. JAMA Oncol. 2015;1(3):306–313. doi: 10.1001/jamaoncol.2015.0658. [DOI] [PubMed] [Google Scholar]
- 31.Huzarski T, Byrski T, Gronwald J, Cybulski C, Oszurek O, Szwiec M, et al. The impact of oophorectomy on survival after breast cancer in BRCA1-positive breast cancer patients. Breast Cancer Res Treat. 2016;156(2):371–378. doi: 10.1007/s10549-016-3749-4. [DOI] [PubMed] [Google Scholar]
- 32.van Verschuer VM, Heemskerk-Gerritsen BA, van Deurzen CH, Obdeijn IM, Tilanus-Linthorst MM, Verhoef C, et al. Lower mitotic activity in BRCA1/2-associated primary breast cancers occurring after risk-reducing salpingo-oophorectomy. Cancer Biol Ther. 2014;15(4):371–379. doi: 10.4161/cbt.27628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1 :Table S1. Studies and samples included in the prospective cohort of BRCA1 and BRCA2 mutation carriers. Table S2. Distributions of dates of breast cancer diagnosis, DNA test and start of follow-up in the prospective cohort. Table S3. Characteristics of reported Risk-Reducing Salpingo-oophorectomy. Table S4. Characteristics of cohort of BRCA1 and BRCA2 mutation carriers. Table S5. Association between RRSO and breast cancer by menopausal status. Table S6. Association between RRSO and breast cancer (sensitivity analysis with RRSO status changing at the age at the questionnaire with information on RRSO status changes (all studies except HEBON)). Table S7. Association between RRSO and breast cancer (sensitivity analysis dropping individuals with missing information at baseline). Table S8. Association between RRSO and breast cancer among BRCA1 and BRCA2 mutation carriers (sensitivity analysis excluding women with RRSO before baseline). Table S9. Association between family history of breast cancer and family history of ovarian cancer and RRSO uptake. Table S10. Association between parity, age at first birth, and body mass index and RRSO uptake. Table S11. Association between RRSO and breast cancer adjusting for Body Mass Index, family history of breast cancer, family history of ovarian cancer, parity and age at first birth. Table S12. Hormone replacement therapy use among women in the cohort. Table S13. Association between RRSO and breast cancer among women not exposed to hormone replacement therapy. Table S14. Association between RRSO and breast cancer (excluding kConFab/BCFR). Table S15. Association between natural menopause and breast cancer (censoring at RRSO). Ethics Committee Approvals
Data Availability Statement
The dataset supporting the conclusions of this article are available upon reasonable request. Requests should be made to Dr. M Rookus (NKI, Amsterdam, NL; m.rookus@nki.nl).