Abstract
Objective
The purpose of the present study was to evaluate the effectiveness of probiotics on type II diabetes mellitus (T2DM).
Methods
We performed a comprehensive search on PubMed, Web of Science, China National Knowledge Infrastructure, Chinese Scientific Journal Databases, Wan Fang database and China biology medicine disc for relevant studies published before June 2019. Glycated hemoglobin A1c (HbA1c), homeostasis model assessment of insulin resistance (HOMA-IR) and fasting blood glucose (FBG) were used as indicators for T2DM. Inverse-variance weighted mean difference (WMD) with 95% confidence interval (CI) was calculated for the mean HbA1c, FBG and HOMA-IR changes from baseline.
Results
15 randomized controlled trials (RCT) with a total of 902 participants were included into the meta-analysis. Considering the clinical heterogeneity caused by variation of dosage and duration of probiotic treatment, random-effects model was used to estimate the pooled WMD. Significantly greater reduction in HbA1c% (WMD = − 0.24, 95% CI [− 0.44, − 0.04], p = 0.02), FBG (WMD = − 0.44 mmol/L, 95% CI [− 0.74, − 0.15], p = 0.003) and HOMA-IR (WMD = − 1.07, 95% CI [− 1.58, − 0.56], p < 0.00001) were observed in probiotics treated group. Further sensitivity analysis verified the reliability and stability of our results.
Conclusion
The results of our meta-analysis indicated that probiotics treatment may reduce HbA1c, FBG and insulin resistance level in T2DM patients. More clinical data and research into the mechanism of probiotics are needed to clarify the role of probiotics in T2DM.
Keywords: Probiotic, Gut microbiota, Type II diabetes mellitus, Meta-analysis
Background
Type II diabetes mellitus (T2DM) is a prevalent metabolic disease that have attracted wide attention because of its increasing incidence and multiple complications. T2DM is characterized by the increase of fasting blood glucose (FBG) and glycosylated hemoglobin (HbA1c), which indicate the disorder of glucose metabolism [1]. There are also a range of gastrointestinal dysfunction symptoms that occur in T2DM patients, such as delayed gastric or esophageal emptying, diabetic gastroparesis, constipation, diarrhea and obesity [2–4]. The involvement of gastrointestinal dysfunction is therefore considered as a stage in the development of T2DM [5].
The pathogenesis of T2DM is complicated and remains largely known. T2DM is considered to be a chronic inflammation [6]. Insulin resistance (IR) caused by inflammation is a characteristic feature of most patients with T2DM. T2DM is believed to be caused by a series of multiple risk factors such as genetic liability, age, overweight or obesity, and an unhealthy lifestyle. Recently, accumulated evidence suggests that the risk of developing T2DM may also involve factors from the gut microbiota. T2DM patients had a moderate degree of gut microbial dysbiosis, and the decrease of intestinal Roseburia and F. prausnitzii was detected in their stool samples [7]. As well taking the gastrointestinal symptoms of T2DM into consideration, gut microbiota is suspected to involve in the pathogenesis of T2DM.
Data from animal studies revealed that altered microbiota may contribute to the pathogenesis of IR and thereby T2DM by several mechanisms [8]. Gut microbiota mainly carry out proximal digestion of carbohydrates and ferment indigestible oligosaccharides, synthesizing short-chain fatty acids (SCFA), such as butyrate, propionate and acetate [9]. At present, the discussion on the role of SCFA is mainly considered that SCFA promotes secretion of glucagon-like peptide-1 (GLP-1) and peptide YY gastrin regulator by endocrine cells in the colon mucosa. These hormones have an influence on the gastrointestinal tract, such as inhibiting the secretion of gastric juice and gastrointestinal peristalsis, delaying the emptying of gastric contents and stimulating the hypothalamus in the central nervous system to increases the sense of satiety and appetite. Therefore, fasting blood glucose level, weight and other T2DM related indexes can be reduced [10].
Probiotics are defined as live microorganisms that, when administered in adequate amounts, confer a health benefit on the host. The data from animal studies suggested that probiotics beneficial effects can influence on glucose metabolism and improve insulin sensitivity [11]. However the effects of probiotics on human T2DM are inconsistent. Some studies showed that probiotics treatment reduced HbA1c, FBG or IR significantly in T2DM patients [12, 13], while other studies did not find significant difference between patients with probiotics and placebo [14, 15]. To evaluate the role of probiotics in T2DM patients comprehensively, and thus provide a theoretical basis for the extensive clinical application of probiotics in the treatment of T2DM, we performed a meta-analysis to evaluate the effects of probiotics on three indicators of T2DM, including HbA1c, FBG and homeostasis model assessment of IR (HOMA-IR).
Materials and methods
Identification of relevant studies
To identify studies with information on the effects of probiotics on T2DM, we conducted a comprehensive search of literatures published before June 2019 in PubMed, Web of Science (SCI), China National Knowledge Infrastructure (CNKI) database, Chinese Scientific Journal Databases (VIP) database, Wan Fang database and China biology medicine disc. English search terms and search patterns include: ① probiotics”, “symbiotic”, “lactobacillus”, the above search terms are linked by “OR”; ② “type 2 diabetes mellitus”, “T2DM”, or “non-insulin-dependent diabetes mellitus”; connect ① and ② with “AND”. Chinese search terms and search pattern include: ① “yishengjun”, “heshengsu”, “rusuanganjun”, the above search terms are linked with “OR”; ② “2xingtangniaobing”, “T2DM”, connect ① and ② with “AND”. Additional studies were also identified by a hand search of all the references of retrieved articles.
Inclusion and exclusion criteria
Studies were considered eligible only if they: (1) were human clinical randomized controlled trials; (2) only included T2DM patients; (3) the intervention was probiotic, and placebo was applied as comparison to the intervention; (4) reported change from baseline to endpoint for at least one of the following outcomes: FBG, HbA1C and HOMA-IR, or could calculate by formula. Trials were excluded if: (1) subjects had recently used the probiotics or antibiotics; (2) Crucial data are incomplete; (3) studies that probably used relevant samples.
Two system evaluators browse through the literature independently and then exclude the irrelevant literature to the topic (including disease type, interventions, etc.). Read the relevant abstracts to obtain the full RCT text that might meet the inclusion criteria. The two system evaluators will check the included literature and any inconsistencies will be discussed again, or the third system evaluator will decide whether to include or not.
Data extraction
The detailed characteristics of each trial were extracted by two system evaluators independently. The following information was extracted from each study: first author’s name, year of publication, country, dosage and duration of probiotic treatment, sample size of treatment and control groups, and effects on metabolic profiles. The data were compared and the disagreements were resolved by a third author.
Literature quality evaluation
Trials meeting the eligibility criteria were evaluated for their quality by two researchers independently according to the Cochrane manual, which included the following six aspects: (1) generation of random distribution schemes; (2) covert grouping; (3) implementation of the blind method; (4) Integrity of the data; (5) non-selective reporting of results; (6) other sources of bias. There are three bias assessment criteria “Low”, “High”, “Unclear” for each point.
Statistical analysis
The mean difference (MD) of change from baseline between the treatment group and control group was measured as effect size. The effect sizes and pooled estimates of the effects across the trials were calculated with RevMan 5.0 software. If the original study only provide means and standard deviations (SD) for baseline and final in each group, then the mean for change from baseline was obtained by subtracting the final mean from the baseline mean. And SD for changes from baseline was imputed using the following equation, where R is the correlation coefficient.
The heterogeneity effects across trials was assessed using the Cochran Q statistic and the I2 statistic. A significance level of p < 0.1 or I2> 50% suggest the existence of significant heterogeneity.
The publication bias was assessed by Egger’s regression asymmetry test. A two-tailed p value < 0.05 was considered statistically significant. Sensitivity analysis was performed by removing one study at a time and recalculating the pooled effect size. Egger’s regression and sensitivity analysis was tested with Stata 15.0 software.
Results
Literature selection
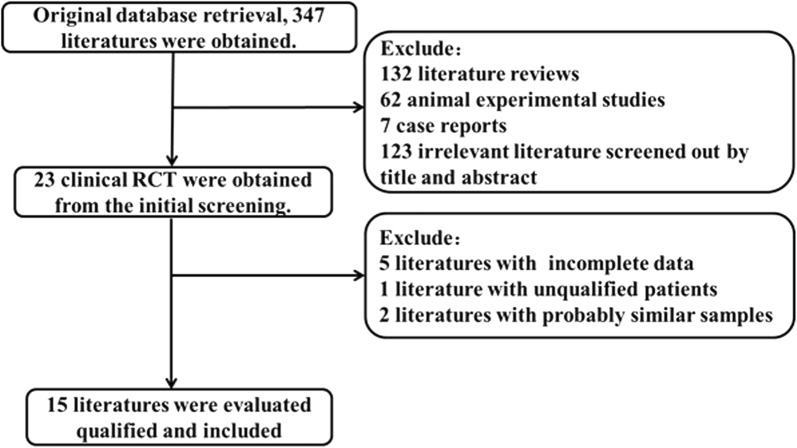
We initially retrieved 347 relevant publications from PubMed, Web of Science, CNKI database, VIP database, Wan Fang database and China biology medicine disc. The majority of the publications were excluded because they were irrelevant studies, case reports, or reviews. According to the inclusion and exclusion criteria, 15 publications (14 in English and 1 in Chinese) with a total of 902 patients were finally included [12, 13, 15–27]. A flow chart showing the workflow for identifying and screening studies is presented in Fig. 1.
Fig. 1.
Flow chart of literature inclusion
Study characteristics
The basic characteristics of the included studies are shown in Table 1. The sample size of studies ranged from 29 to 108. The duration of treatment ranged from 6 to 12 weeks. 6 trials reported the change from baseline for HbA1c, FBG or HOMA-IR directly. While the other 9 trials only provided the mean and SD value for baseline and final, respectively. The mean and SD for change from baseline in these 9 studies were imputed with a correlation coefficient estimate of 0.5.
Table 1.
Basic characteristics of 15 randomized controlled trials included in the meta-analysis
| Study | Country | No. of cases (treatment/control) | Treatment group | Control group | Duration of treatment (week) |
|---|---|---|---|---|---|
| Razmpoosh E 2019 | Iran | 30/30 | Lactobacillus acidophilus 2 × 109 CFU + Lactobacillus casei 7 × 109 CFU + Lactobacillus rhamnosus 1.5 × 109 CFU + Lactobacillus bulgaricus 2 × 108 CFU + Bifidobacterium breve 3 × 1010 CFU + Bifidobacterium longum 7 × 109 CFU + Streptococcus thermophilus 1.5 × 109 CFU 4 times/day | Placebo | 6 |
| Raygan F 2018 | Iran | 30/30 | Bifidobacterium bifidum 2 × 109 CFU + Lactobacillus casei 2 × 109 CFU + Lactobacillus acidophilus 2 × 109 CFU/day | Placebo | 12 |
| Ejtahed HS 2012 | Iran | 30/30 | Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12 4 × 109 CFU/day | Conventional yogurt | 6 |
| Shakeri H 2014 | Iran | 26/26 | Lactobacillus sporogenes 1 × 108 CFU/1 g 40 g 3 times/day | Bread without probiotic bacteria and prebiotic inulin | 8 |
| Khalili L 2019 | Iran | 20/20 | Lactobacillus casei 1 × 108 CFU/day | Placebo | 8 |
| Tajadadi-Ebrahimi M 2016 | Iran | 30/30 | Lactobacillus acidophilus 2 × 109 + Lactobacillus casei 2 × 109 + Bifidobacterium bifidum 2 × 109 CFU/g | Placebo | 12 |
| Mohamadshahi M 2014 | Iran | 22/22 | Lactobacillus acidophilus 3.7 × 106 CFU/g and B. lactic 3.7 × 106 CFU/g 300 g/day | Conventional yogurt | 8 |
| Tajadadi-Ebrahimi M 2014 | Iran | 27/27 | Lactobacillus sporogenes 1 × 108 CFUs/g 3 times/day | Control bread | 8 |
| Kobyliak N 2018 | Ukraine | 31/22 | Lactobacillus + Lactococcus 6 × 1010 CFU/g + Bifidobacterium 1 × 1010 CFU/g + Propionibacterium 3 × 1010 CFU/g + Acetobacter 1 × 106 CFU/g 10 g/day | Placebo | 8 |
| Mobini R 2016 | Swedish | 14/15 | Lactobacillus reuteri DSM 17938 1 × 1010 CFU/day | Placebo | 12 |
| Firouzi S 2017 | Malaysia | 48/53 | Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus lactis 5 × 109 CFU + Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus lactis 5 × 109 CFU 2 times/day | Placebo | 12 |
| Tonucci LB 2017 | Brazil | 23/22 | Lactobacillus acidophilus La-5 1 × 109 CFU/day + Bifidobacterium animalis subsp. lactis BB-12 1 × 109 CFU/day | Conventional fermented milk | 6 |
| Hui Y 2018 | China | 53/55 | Lactobacillus paracasei N1115 1 × 108 CFU/mL 200 g 2 times/day | Placebo | 8 |
| Asemi Z 2016 | Iran | 51/51 | Lactobacillus sporogenes 1 × 107 CFU + 0.05 g beta-carotene 3 times/day | Placebo | 6 |
| Mazloom Z 2013 | Iran | 16/18 | Lactobacillus acidophilus + Lactobacillus bulgaricus + Lactobacillus bifidum + Lactobacillus casei | Placebo | 6 |
Literature quality evaluation
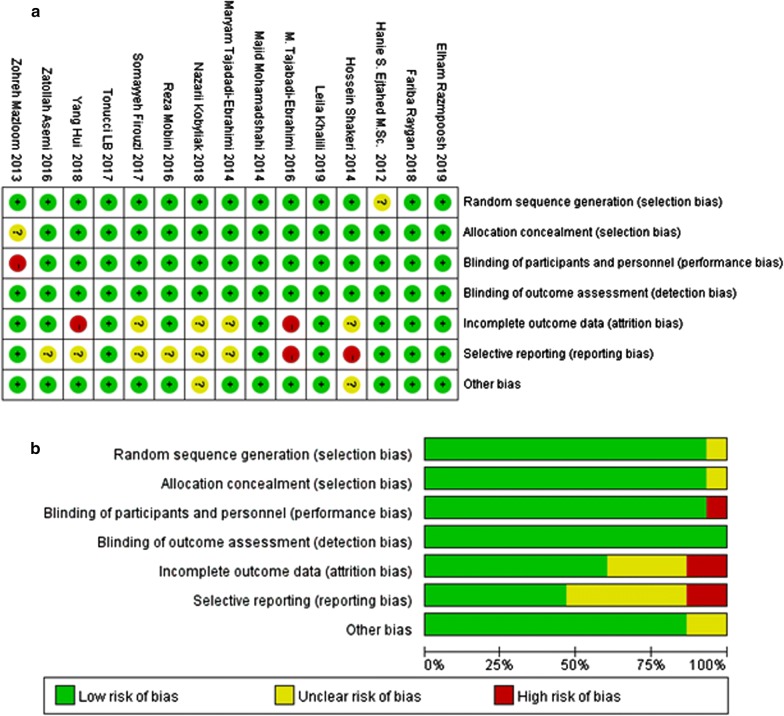
According to the bias risk assessment method in the Cochrane manual, all the 15 trials mentioned random sequence generation. 14 trials [12, 13, 15–23, 25–27] were double-blind clinical studies and 1 trial [24] was a single-blind study. 2 trials [20, 21] reported selecting bias. No other risk of bias was found in the 15 trials, as shown in Fig. 2. No studies were excluded according to the result of this evaluation.
Fig. 2.
Risk of bias assessment for included studies. a Risk of Bias Graph. Literature quality was evaluated according to the following six points: ①generation of random distribution schemes; ②covert grouping; ③implementation of the blind method; ④Integrity of the data; ⑤non-selective reporting of results; ⑥other sources of bias. There are three bias assessment criteria “Low”, “High” and “Unclear” for each point, shown as the color green, yellow and red, respectively. b Risk of bias summary. The quality assessment of each literature has been shown. The color green, yellow and red represent low, high and unclear risk of bias, respectively
Meta-analysis
The tests for heterogeneity across the trials were performed before the trials were pooled for meta-analysis. No statistically significant heterogeneity was observed for effects of probiotics on HbA1C, FBG, and HOMA-IR (p = 0.22, 0.48 and 0.29, I2 = 27%, 0%, and 18%, respectively). While considering true heterogeneity caused by clinical variation, e.g. variation of treatment, dosage and duration, a random effects meta-analysis model was used to estimate the effects of probiotics on T2DM. As random-effects meta-analysis is more robust to heterogeneity effects.
Effects of probiotics on HbA1c%
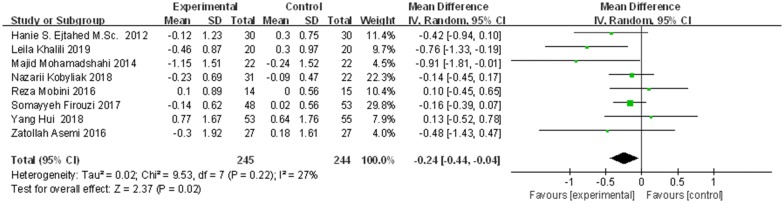
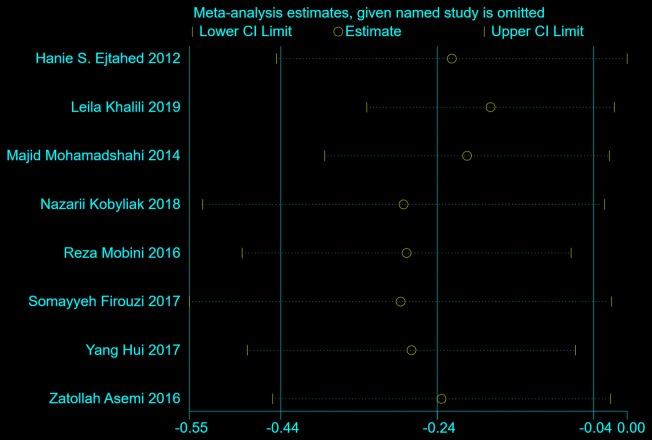
Eight studies, with 489 participants, reported the effects of probiotics on HbA1c. The decrease in HbA1c (%) from baseline to endpoint in patients taking probiotics was statistically greater than that in control groups (inverse-variance weighted MD (WMD) = − 0.24, 95% CI [− 0.44, − 0.04], p = 0.02), as shown in Fig. 3. To explore the stability of this results, sensitivity analysis was performed by removing one study at a time and recalculating the pooled WMD. The results did not find substantial modification of the estimates of change in HbA1c after exclusion of any individual study, as shown in Fig. 4.
Fig. 3.
The effect of probiotics on HbA1c (%) in T2DM patients. Mean differences of change from baseline and 95% confidence intervals (CIs) are shown. Pooled estimates were calculated by the inverse-variance weighted random-effects meta-analysis. The squares indicate the effect of probiotics in a particular trial and the horizontal lines represent the corresponding 95% CIs. The diamond indicated the pooled effect size
Fig. 4.
Sensitivity analysis of the effects of probiotics on HbA1c (%) in T2DM patients. Results were computed by omitting each trial in turn. Random-effects meta-analysis were used to estimate the pooled effect size. The two ends of the dotted lines represent the 95% CIs
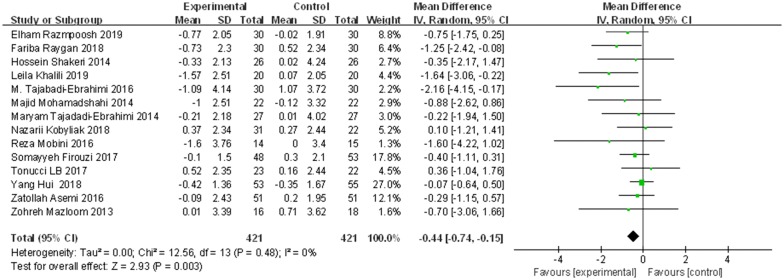
Effects of probiotics on FBG
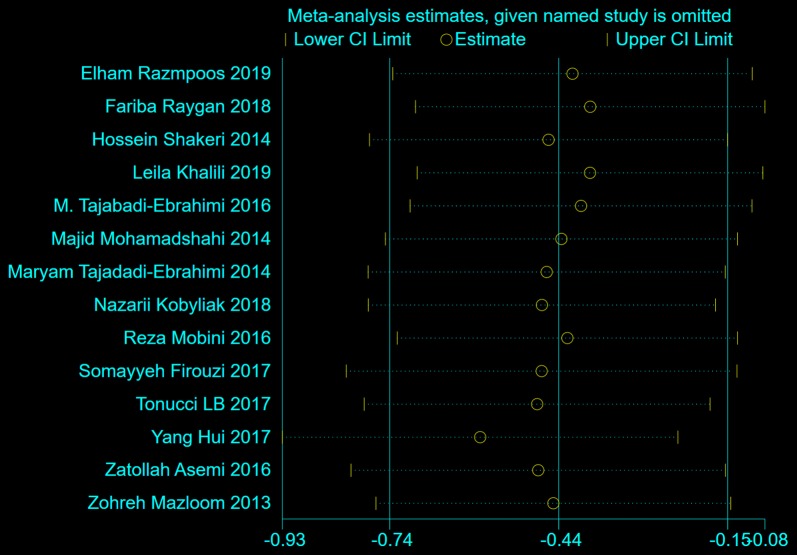
The effects of probiotics on FBG was evaluated in 842 participants from fourteen trials. The results of random-effects meta-analysis in Fig. 5 showed that the difference in FBG reduction (from baseline to endpoint) between probiotics-treated groups and control groups was significant (WMD = − 0.44, 95% CI [− 0.74, − 0.15], p = 0.003). Results of sensitivity analysis demonstrated that the effects of probiotics on FBG remained consistent after removing the trials one by one, as shown in Fig. 6.
Fig. 5.
The effect of probiotics on FBG (mmol/L) in T2DM patitents. Mean differences of change from baseline and 95% confidence intervals (CIs) are shown. Pooled estimates were calculated by the inverse-variance weighted random-effects meta-analysis. The squares indicate the effect of probiotics in a particular trial and the horizontal lines represent the corresponding 95% CIs. The diamond indicated the pooled effect size
Fig. 6.
Sensitivity analysis of the effects of probiotics on FBG (mmol/L) in T2DM patients. Results were computed by omitting each trial in turn. Random-effects meta-analysis were used to estimate the pooled effect size. The two ends of the dotted lines represent the 95% CIs
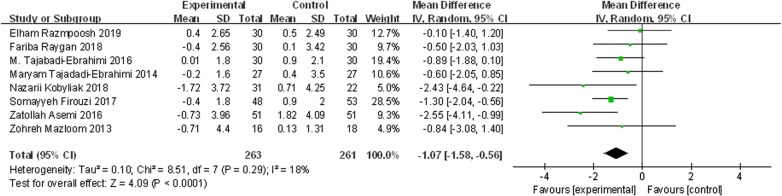
Effects of probiotics on HOMA-IR
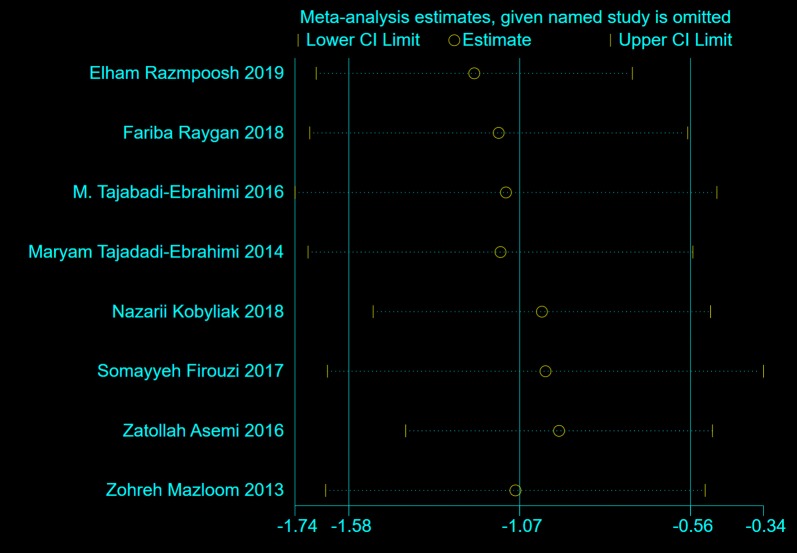
Eight trials, with 524 participants, reported the effects of probiotics on HOMA-IR. The results of the random-effects model, as shown in Fig. 7, demonstrate that the reduction in HOMA-IR (from baseline to end point) in patients taking probiotics was statistically significantly greater than that in control groups (WMD = − 1.07, 95% CI [− 1.58, − 0.56], p < 0.00001). Results of sensitivity analysis demonstrated that the effects of probiotics on HOMA-IR remained consistent after removing the trials one by one, as shown in Fig. 8.
Fig. 7.
The effect of probiotics on HOMA-IR in T2DM patients. Mean differences of change from baseline and 95% confidence intervals (CIs) are shown. Pooled estimates were calculated by the inverse-variance weighted random-effects meta-analysis. The squares indicate the effect of probiotics in a particular trial and the horizontal lines represent the corresponding 95% CIs. The diamond indicated the pooled effect size
Fig. 8.
Sensitivity analysis of the effects of probiotics on HOMA-IR in T2DM patients. Results were computed by omitting each trial in turn. Random-effects meta-analysis were used to estimate the pooled effect size. The two ends of the dotted lines represent the 95% CIs
Publication bias
According to the publication bias tests, the effects of bias on reports of HbA1c (Fig. 9), FBG (Fig. 10) and HOMA-IR (Fig. 11) were not significant, as illustrated by P-values for Egger’s regression asymmetry test were 0.29, 0.86 and 0.07, respectively.
Fig. 9.
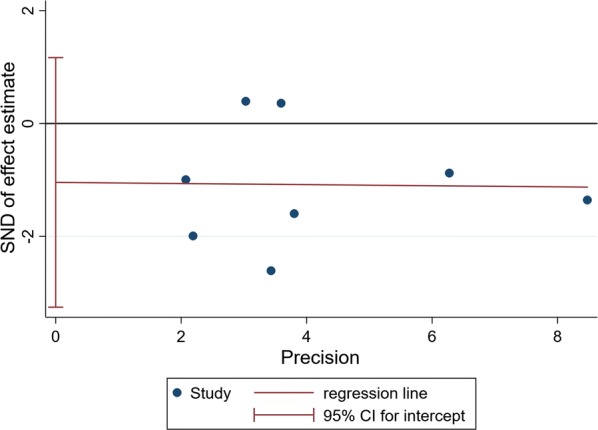
Egger’s regression asymmetry test for effects of probiotics on HbA1c% in T2DM patients. Individual studies are represented by blue dot. Success of the CI for the intercept to include zero indicates symmetry in the funnel plot and give evidence of no publication bias
Fig. 10.
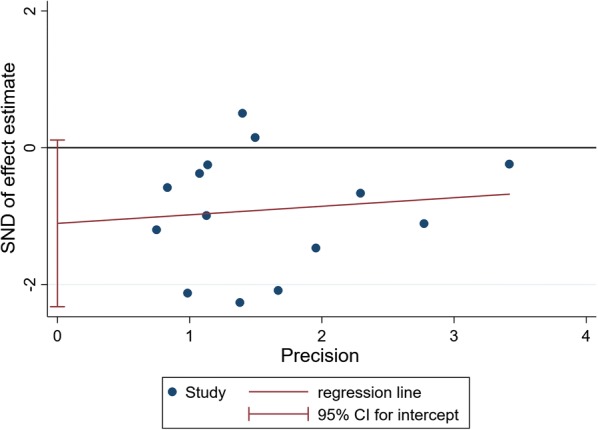
Egger’s regression asymmetry test for effects of probiotics on FBG in T2DM patients. Individual studies are represented by blue dot. Success of the CI for the intercept to include zero indicates symmetry in the funnel plot and give evidence of no publication bias
Fig. 11.
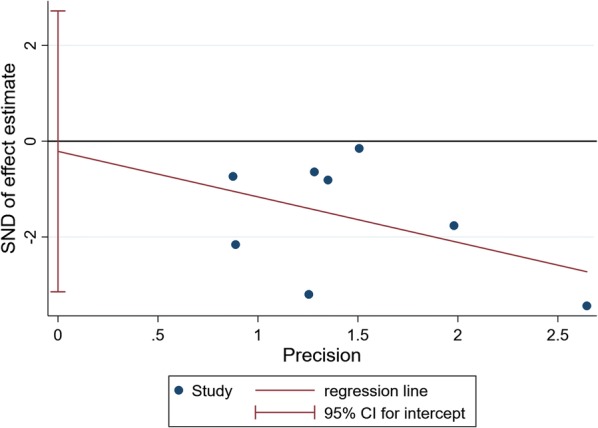
Egger’s regression asymmetry test for effects of probiotics on HOMA-IR in T2DM patients. Individual studies are represented by blue dot. Success of the CI for the intercept to include zero indicates symmetry in the funnel plot and give evidence of no publication bias
Discussion
In the present study, we performed a systematic assessment regarding the effects of probiotics on HbA1c, FBG and HOMA-IR in T2DM patients. A total of 15 RCTs involving 902 patients were finally included into the meta-analysis. The results showed that probiotics may reduce HbA1c, FBG, and HOMA-IR levels from baseline.
The level of HbA1c in T2DM patients can precisely reflect the control of patients’ blood glucose. Both fasting and postprandial blood glucose in patients decide HbA1c level and the latter contributes significantly more to HbA1c [28]. In our meta-analysis, the effect of probiotics on HbA1c % was not as significant as that on FBG. It may be due to the unknown postprandial blood glucose level which was not included in evaluation because of insufficient clinical data. Similar findings were reported in a meta-analysis by Zhang [29].
Although probiotics improved blood sugar, accumulated evidence [12, 18] suggests that probiotics do not improve inflammation. The occurrence of insulin resistance in T2DM patients is related to obesity, however, interestingly, probiotics improved insulin resistance without changing body mass index (BMI) [12, 18]. Of note, although BMI of obese people is higher, it cannot commendably reflect the amount of body fat and inflammation in adipocytes. In Anne Sofie Andreasen’s study [30], subjects accepted Escherichia coli LPS (0.3 ng/kg) intravenous injection in 2 days. Before the intervention, the baseline concentrations of plasma TNF, IL-6, IL-1ra and C-reactive protein in the two groups were basically the same. However, after 4 weeks, neither probiotic treatment nor placebo treatment affected the levels of these inflammatory cytokines, suggesting that there was no direct anti-inflammatory effect of probiotics. Therefore, we speculate that the effect of probiotics on improving insulin resistance in vivo was related to the improvement of gut microbiota and the reduction of LPS translocating into blood. Moreover, the decrease of FBG and HbA1c% may result from the improvement of insulin resistance. For these reasons, we speculate that the disorder of gut microbiota in patients was not the cause of T2DM. Instead, it is more likely the product of abnormal blood glucose metabolism. Therefore, T2DM may not be cured simply relying on the treatment of probiotics.
By performing meta-analysis for RCTs, we increased the sample size and the statistical power. However, there are still some limitations. First, the number of studies included is a little small, so the validity of the results was limited. Second, our meta-analysis included several kinds of probiotics at different dosages and the therapy duration ranged from 6 to 12 weeks. Although the statistical heterogeneity across trials was not significant, true heterogeneity caused by clinical variation did exist. Third, we were unable to assess the effect of probiotics on the complications of T2DM. Thus, more RCTs with large samples are needed to confirm the effect of probiotic on glucose metabolism in T2DM patients. And experimental investigations are also urgently needed to uncover underlying mechanism of probiotics.
In summary, by meta-analyzing the effects of probiotics on HbA1c, FBG and HOMA-IR, we found that probiotics treatment can significantly improve blood glucose of T2DM patients, as reductions in all three measures were observed. Therefore, our results can provide a more comprehensive theoretical basis for the probiotic medicine using in the improvement of T2DM.
Conclusion
In the present study, we performed a systematic assessment regarding the effects of probiotics treatment on the T2DM. A total of 15 RCT and 902 patients were included in this study. The results of our meta-analysis indicated that probiotics treatment can reduce HbA1c, FBG and insulin resistance levels in T2DM patients. The result of sensitivity analysis showed that the above results were quite robust and stable. More clinical data and research into the mechanism of probiotics are needed to clarify the role of probiotics in T2DM.
Acknowledgements
Not applicable.
Abbreviations
- T2DM
type II diabetes mellitus
- HbA1c
glycated hemoglobin A1c
- HOMA-IR
homeostasis model assessment of insulin resistance
- FBG
fasting blood glucose
- WMD
weighted mean difference
- CI
confidence interval
- SCFA
short-chain fatty acids
- GLP-1
glucagon-like peptide-1
- LPS
lipopolysaccharide
- BMI
body mass index
Authors’ contributions
YLG and XQM extracted the data and assessed the quality of the literature. YWT analyzed the data and wrote the paper. LZ and YFP designed the study and revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was partially supported by the support from the National Natural Science Foundation of China (31771417, 31571291) and a project funded by the Priority Academic Program Development (PAPD) of Jiangsu higher education institutions.
Availability of data and materials
The data we used can be found in the references listed.
Ethics approval and consent to participate
Not applicable.
Consent for publication
The manuscript is approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lei Zhang, Email: lzhang6@suda.edu.cn.
Yu-Fang Pei, Email: ypei@suda.edu.cn.
References
- 1.DeFronzo AR. Pathogenesis of type 2 diabetes mellitus. Med Clin N Am. 2004;88:787–835. doi: 10.1016/j.mcna.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Horowitz M, Harding PE, Maddox AF, Wishart JM, Akkermans LMA, Chatterton BE, Shearman DJC. Gastric and oesophageal emptying in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1989;32:151–159. doi: 10.1007/BF00265086. [DOI] [PubMed] [Google Scholar]
- 3.Chang J, Rayner CK, Jones KL, Horowitz M. Diabetic gastroparesis—backwards and forwards. J Gastroenterol Hepatol. 2011;26:46–57. doi: 10.1111/j.1440-1746.2010.06573.x. [DOI] [PubMed] [Google Scholar]
- 4.Azpiroz F, Malagelada C. Diabetic neuropathy in the gut: pathogenesis and diagnosis. Diabetologia. 2016;59:404–408. doi: 10.1007/s00125-015-3831-1. [DOI] [PubMed] [Google Scholar]
- 5.Wu SL. Staging of type 2 diabetes mellitus. Genet Mol Res. 2015;14:2118. doi: 10.4238/2015.March.20.22. [DOI] [PubMed] [Google Scholar]
- 6.Herder C, Brunner EJ, Rathmann W, Strassburger K, Tabák AG, Schloot NC, Witte DR. Elevated levels of the anti-inflammatory interleukin-1 receptor antagonist precede the onset of type 2 diabetes the whitehall II study. Diabetes Care. 2009;32:421–423. doi: 10.2337/dc08-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 8.Kobyliak N, Virchenko O, Falalyeyeva T. Pathophysiological role of host microbiota in the development of obesity. Nutr J. 2016;15:43. doi: 10.1186/s12937-016-0166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Combettes MM. GLP-1 and type 2 diabetes: physiology and new clinical advances. Curr Opin Pharmacol. 2006;6:598–605. doi: 10.1016/j.coph.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Kobyliak N, Conte C, Cammarota G, Haley AP, Styriak I, Gaspar L, Fusek J, Rodrigo L, Kruzliak P. Probiotics in prevention and treatment of obesity: a critical view. Nutr Metab. 2016;13:14. doi: 10.1186/s12986-016-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Firouzi S, Majid HA, Ismail A, Kamaruddin NA, Barakatun-Nisak MY. Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: a randomized controlled trial. Eur J Nutr. 2017;56:1535–1550. doi: 10.1007/s00394-016-1199-8. [DOI] [PubMed] [Google Scholar]
- 13.Khalili L, Alipour B, Asghari Jafar-Abadi M, Faraji I, Hassanalilou T, Mesgari Abbasi M, Vaghef-Mehrabany E, Alizadeh Sani M. The effects of lactobacillus casei on glycemic response, serum sirtuin1 and fetuin-A levels in patients with type 2 diabetes mellitus: a randomized controlled trial. Iran Biomed J. 2019;23:68–77. doi: 10.29252/ibj.23.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mobini R, Tremaroli V, Stahlman M, Karlsson F, Levin M, Ljungberg M, Sohlin M, Berteus Forslund H, Perkins R, Backhed F, Jansson PA. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. 2017;19:579–589. doi: 10.1111/dom.12861. [DOI] [PubMed] [Google Scholar]
- 15.Razmpoosh E, Javadi A, Ejtahed HS, Mirmiran P, Javadi M, Yousefinejad A. The effect of probiotic supplementation on glycemic control and lipid profile in patients with type 2 diabetes: a randomized placebo controlled trial. Diabetes Metab Syndr. 2019;13:175–182. doi: 10.1016/j.dsx.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Yang H, Yang H, Hao HW, Zeng Q, Guo FF, Wang N, Wang BH, Ma XX. Effect on blood glucose of yogurt supplemented with probiotics N1115 in type 2 diabetes mellitus. Food Sci Technol. 2018;43:14–19. [Google Scholar]
- 17.Asemi Z, Alizadeh SA, Ahmad K, Goli M, Esmaillzadeh A. Effects of beta-carotene fortified synbiotic food on metabolic control of patients with type 2 diabetes mellitus: a double-blind randomized cross-over controlled clinical trial. Clin Nutr. 2016;35:819–825. doi: 10.1016/j.clnu.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Tonucci LB, de Santos KM, de Oliveira LL, Ribeiro SM, Martino HS. Clinical application of probiotics in type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study. Clin Nutr. 2017;36:85–92. doi: 10.1016/j.clnu.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Tajadadiebrahimi M, Bahmani F, Shakeri H, Hadaegh H, Hijijafari M, Abedi F, Asemi Z. Effects of daily consumption of synbiotic bread on insulin metabolism and serum high-sensitivity C-reactive protein among diabetic patients: a double-blind, randomized, controlled clinical trial. Ann Nutr Metab. 2014;65:34–41. doi: 10.1159/000365153. [DOI] [PubMed] [Google Scholar]
- 20.Tajabadi-Ebrahimi M, Sharifi N, Farrokhian A, Raygan F, Karamali F, Razzaghi R, Taheri S, Asemi Z. A randomized controlled clinical trial investigating the effect of synbiotic administration on markers of insulin metabolism and lipid profiles in overweight type 2 diabetic patients with coronary heart disease. Exp Clin Endocrinol Diabetes. 2017;125:21–27. doi: 10.1055/s-0042-105441. [DOI] [PubMed] [Google Scholar]
- 21.Shakeri H, Hadaegh H, Abedi F, Tajabadiebrahimi M, Mazroii N, Ghandi Y, Asemi Z. Consumption of synbiotic bread decreases triacylglycerol and VLDL levels while increasing HDL levels in serum from patients with type-2 diabetes. Lipids. 2014;49:695–701. doi: 10.1007/s11745-014-3901-z. [DOI] [PubMed] [Google Scholar]
- 22.Mohamadshahi M, Veissi M, Haidari F, Shahbazian H, Kaydani GA, Mohammadi F. Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. Bioimpacts. 2014;4:83–88. doi: 10.5681/bi.2014.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mobini R, Tremaroli V, Ståhlman M, Karlsson F, PhD MLM, Ljungberg M, Sohlin M, Forslund HB, Perkins R, Bäckhed F. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. 2016;19:579. doi: 10.1111/dom.12861. [DOI] [PubMed] [Google Scholar]
- 24.Mazloom Z, Yousefinejad A, Dabbaghmanesh MH. Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflammatory markers in patients with type 2 diabetes: a clinical trial. Iran J Med Sci. 2013;38:38–43. [PMC free article] [PubMed] [Google Scholar]
- 25.Kobyliak N, Falalyeyeva T, Mykhalchyshyn G, Kyriienko D, Komissarenko I. Effect of alive probiotic on insulin resistance in type 2 diabetes patients: randomized clinical trial. Diabetes Metab Syndr Clin Res Rev. 2018;12:S1871402118301061. doi: 10.1016/j.dsx.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 26.Fariba R, Zohreh R, Fereshteh B, Vahidreza O, Ali MM, Maryam T-E, Shokoofeh B, Zatollah A. The effects of probiotic supplementation on metabolic status in type 2 diabetic patients with coronary heart disease. Diabetol Metab Syndr. 2018;10:51. doi: 10.1186/s13098-018-0353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 2012;28:539–543. doi: 10.1016/j.nut.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Zhou J, Martin RJ, Tulley RT, Raggio AM, Mccutcheon KL, Shen L, Danna SC, Tripathy S, Hegsted M, Keenan MJ. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab. 2008;295:1160–1166. doi: 10.1152/ajpendo.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Q, Wu Y, Fei X. Effect of probiotics on glucose metabolism in patients with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Medicina. 2016;52:28–34. doi: 10.1016/j.medici.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Andreasen AS, Larsen N, Pedersenskovsgaard T, Berg RMG, Møller K, Svendsen KD, Jakobsen M, Pedersen BK. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br J Nutr. 2010;104:1831–1838. doi: 10.1017/S0007114510002874. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data we used can be found in the references listed.