Abstract
Introduction
Persistent symptoms, poor disease control, and reduced quality of life (QoL) are common in patients with asthma and chronic obstructive pulmonary disease (COPD). Current therapies are only partially effective and inhaler misuse contributes to insufficient disease control and poor outcomes. This real-world study aimed to evaluate the effectiveness of budesonide/formoterol fumarate (B/F) Easyhaler® in everyday clinical practice in Hungary.
Methods
Post hoc, subgroup analyses of this 12-week, real-world, multicenter, open-label study were conducted in adults diagnosed with asthma or COPD. Endpoints included the change in patient-reported outcome measures; i.e., symptoms and disease control measured by Asthma Control Test or COPD Assessment Test and health-related (HR)QoL measured by mini-Asthma Quality of Life Questionnaire or modified Medical Research Council dyspnea scale. Changes in lung function and patient satisfaction with B/F Easyhaler versus their previous inhaler were also evaluated. Results were stratified by the inhaler device used at visit 1 (baseline, when patients switched device); comparisons were made with B/F Easyhaler use after 12 weeks, assessed at visit 3.
Results
In total, 398 and 563 patients with asthma and COPD, respectively, were analyzed. Significant improvements (p < 0.0001) in symptoms and disease control, HRQoL, and lung function were reported 12 weeks after switching treatment to B/F Easyhaler from the most commonly used devices (≥ 10% of patients). Significant increases in patient satisfaction were also reported versus comparators.
Conclusions
Patients with asthma or COPD who switched to B/F Easyhaler from their previous inhaler due to lack of disease control achieved significant improvements in symptoms and disease control, HRQoL, and lung function within 12 weeks of real-world use with significant increase in patient satisfaction also observed. Such comparative information may reassure clinicians and patients that may be viewed as an appropriate and potentially beneficial treatment option.
Trial Registration Number
OGYÉI/13942-5/2016 (National Pharmaceutical Institute of Pharmacy and Nutrition of Hungary).
Funding
Orion Corporation, Orion Pharma.
Plain Language Summary
Plain language summary available for this article.
Electronic supplementary material
The online version of this article (10.1007/s41030-019-0097-7) contains supplementary material, which is available to authorized users.
Keywords: Asthma, Asthma control, Budesonide/formoterol Easyhaler®, Chronic obstructive pulmonary disease, Switching, Effectiveness, Health-related quality of life, Patient satisfaction, Real-world evidence
Plain Language Summary
Introduction
Long-lasting symptoms and reduced quality of life are common in patients with asthma and chronic obstructive pulmonary disease (COPD). Current therapies are only partially effective and handling/usage errors can affect how well patients’ disease is controlled and how many patients see an improvement in their condition.
Methods
This study, conducted in clinical practice in Hungary, aimed to evaluate whether 12 weeks’ treatment with budesonide/formoterol fumarate (B/F) Easyhaler® (after switching from a previous inhaler) resulted in (1) reduced asthma or COPD symptoms, (2) improved disease control, (3) improved quality of life, and (4) increased lung function; the first three items were evaluated using validated questionnaires and the fourth by spirometry. Patients also answered questions on how satisfied they were after switching to the Easyhaler from their previous device.
Results
Overall, 398 and 563 patients with asthma and COPD, respectively, were analyzed. The patients’ asthma/COPD symptoms reduced, their disease control and quality of life improved, and lung function increased (all significantly) after 12 weeks’ treatment with B/F Easyhaler, following their treatment switch. In addition, more patients rated the Easyhaler as ‘very good or good’ after switching.
Conclusions
Switching treatment to B/F Easyhaler may be an effective treatment option for patients with asthma or COPD.
Electronic supplementary material
The online version of this article (10.1007/s41030-019-0097-7) contains supplementary material, which is available to authorized users.
Introduction
Asthma treatment guidelines are well established [1], yet many patients with asthma continue to experience persistent symptoms and poor disease control [2, 3]. Poorly controlled asthma is also often associated with lifestyle limitations, negative psychological and social effects, and a reduced quality of life (QoL) [2–4]. Similarly, available therapies for patients with chronic obstructive pulmonary disease (COPD) are often only partially effective, and patients can remain symptomatic and experience a considerable impact of the disease on their daily activities and health-related (HR)QoL [5].
The first-choice route of administering medication to manage asthma [1, 4] and COPD [6, 7] is inhalation. Many types of devices are available for the delivery of inhaled drugs [8]; however, studies have shown that ~ 32–89% of patients do not use their inhaler correctly [9–14]. A similar rate of inhaler technique errors occurs for patients with asthma or COPD [15]. Correct inhaler technique is important because inhaler misuse is associated with insufficient disease control and poor patient outcomes [16], such as an increased risk of hospitalization due to an asthma exacerbation. Patient preference is an important factor when choosing an inhaler device, because a greater level of satisfaction is related to improved outcomes. Conversely, dissatisfaction with an inhaler and difficulties using it can contribute to poor adherence [17].
Randomized controlled trials typically train participants in the optimal use of their inhaler and require demonstration of effective use throughout the trial with close monitoring [18], which may not reflect the situation in real-world clinical practice. Therefore, a need exists for real-world studies in patients with asthma and COPD, to examine how different inhaler devices compare in terms of training, the level of education required for use, ease of use, patient and physician satisfaction, and clinical effectiveness.
Real-world studies can highlight interactions between patient characteristics, preferences, and lifestyle and treatment outcomes that may be missing from randomized controlled trials due to strict exclusion criteria [18]. They also permit exploration of how these may differ between therapies, and also allow evaluation of clinical outcomes that may be underpowered in randomized controlled trials because of their short duration and strict and idealized patient selection criteria [18]. For these reasons, regulatory bodies and other organizations such as the European Medicines Agency, United States Food and Drug Administration, and United Kingdom National Institute for Health and Care Excellence are increasingly recognizing the value of real-world evidence to inform decision-making [19–21] and complement evidence from randomized controlled trials.
Budesonide/formoterol fumarate (B/F) Easyhaler® (Orion Pharma, Espoo, Finland) is a multidose dry powder inhaler containing a combination of the inhaled corticosteroid, budesonide, and the long-acting β2-adrenergic agonist, formoterol fumarate, indicated for the treatment of adults with COPD and/or asthma, and adolescents aged 12–17 years with asthma [22], and approved in several European countries. Two non-randomized, open-label, single-arm studies conducted in Poland and Hungary have confirmed the effectiveness of the B/F Easyhaler for the treatment of asthma [23], COPD, and asthma-COPD overlap (ACO) [24] in everyday clinical practice. In the latter study, 30.4% of patients were inhaler-naïve and 69.6% switched to the B/F Easyhaler from other inhalers; after 12 weeks of treatment, significant improvements in lung function, disease control, and HRQoL (all p ≤ 0.002) were observed in both inhaler-naive patients and switchers [24]. However, neither study stratified by prior treatment, precluding comparisons of the effect of switching from specific inhaler devices to the B/F Easyhaler.
Here, we present subgroup analysis of the Hungarian study reported by Tamási et al. [24]. In the present analyses, patients were stratified by inhaler device used at baseline to confirm the real-world clinical benefits of the B/F Easyhaler in patients with asthma or COPD, and to compare measures of clinical effectiveness, HRQoL, and patient satisfaction associated with the B/F Easyhaler versus the patient’s prior inhaler device.
Methods
Study Design
This was a post hoc subanalysis of a 12-week, real-world, multicenter, open-label, non-randomized, non-interventional, single-arm study, conducted across 200 Hungarian centers between May 1, 2016 and December 31, 2017 (National Pharmaceutical Institute of Pharmacy and Nutrition of Hungary; trial registration number: OGYÉI/13942-5/2016) [24]. The study consisted of three visits and assessed treatment effectiveness in patients diagnosed with asthma or COPD who switched from their current inhaler to B/F Easyhaler. The daily dose and dosing regimen were agreed at visit 1 (baseline, when patients switched to the B/F Easyhaler), in line with the B/F Easyhaler summary of product characteristics [16, 17]. An optional control visit (visit 2) was offered for study participants to provide additional support prior to assessment of endpoints at 12 weeks (visit 3).
Study Participants
Inclusion and exclusion criteria have been previously described [24]. Eligible patients were ≥ 18 years of age with a diagnosis of asthma or COPD (according to Global Initiative for Asthma [GINA] [1] or Global Initiative for Chronic Obstructive Lung Disease therapeutic guidelines [7]), and without an exacerbation in the 4 weeks before enrollment. Patients were excluded if they had a hypersensitivity to the study medication or excipients, or were pregnant or breastfeeding. For the subanalyses, patients with ACO and those who were inhaler-naïve were also excluded in order to focus on those who switched their inhaler. The study was approved by the Medical Research Council, Scientific and Research Ethics Committee of Hungary and all procedures followed their ethical standards, as well as those of the 1964 Declaration of Helsinki and its later amendments. Written informed consent was obtained from all study participants prior to study commencement.
Endpoints
Study endpoints have been described in detail elsewhere [24]. In brief, the primary endpoint was change in patient-reported outcome measures after 12 weeks of B/F Easyhaler treatment, comprising the Asthma Control Test (ACT; asthma categorized according to GINA 2018 guidelines [1] and scored as very poorly controlled (≤ 15), not well controlled [16–19], or well controlled (≥ 20)) [25] and mini-Asthma Quality of Life Questionnaire (mini-AQLQ; a score < 4 indicates very limited daily life due to asthma) [26] for patients with asthma, and the COPD Assessment Test (CAT; a score > 20 indicates a high impact of COPD on daily life) [27] and modified Medical Research Council dyspnea scale (mMRC; a score > 1 indicates difficulty in walking due to breathlessness) [28] for patients with COPD.
Secondary endpoints assessed disease control at each visit (using the ACT or CAT), health-related quality of life (HRQoL; using the mini-AQLQ and mMRC) and forced expiratory volume in 1 s (FEV1; via spirometry according to the American Thoracic Society/European Respiratory Society task force guidelines [29] and expressed as FEV1 % predicted normal). Patient satisfaction with their previous inhaler and the (B/F) Easyhaler was assessed at visits 1 and 3, respectively, using closed questions scored on a six-point scale (where 1 = very good and 6 = unsatisfactory) [24]. The length of time taken to teach the patient how to use the B/F Easyhaler was also assessed at visit 1 by the clinician.
Statistical Analyses
All data were expressed as percentages or mean (standard deviation [SD]). Wilcoxon’s signed rank test was used to compare change from baseline for categorical variables and linear mixed model for continuous variables, with a p value < 0.05 considered statistically significant. To limit the number of inhalers compared, only those used by ≥ 10% of patients at baseline were included in the analyses. All statistical analyses were performed using statistical analysis software (SAS)®, version 9.4 for Windows (SAS Institute Inc., Cary, NC, USA).
Results
Patients
Patient demographics and baseline characteristics are shown in Table 1. In total, 398 and 563 patients with asthma or COPD, respectively, were included. Patients with asthma were younger than those with COPD (mean age: 53.9 versus 64.7 years), and a greater proportion were female (71.0 vs. 51.4%), had received education beyond primary or high school (16.2 vs. 6.0%), had never smoked (74.4 vs. 17.3%), and had better pulmonary function (76.4 vs. 49.9% FEV1% predicted). At baseline, about two-thirds of patients with asthma had poorly controlled disease and impaired daily life, and equally often those with COPD experienced a high-to-medium-high impact on their daily life and difficulty in walking, because of their symptoms.
Table 1.
Patient demographics and baseline characteristics (n = 961)
| Parameter | Patients with asthma (n = 398) | Patients with COPD (n = 563) |
|---|---|---|
| Age (years), mean (SD) | 53.9 (15.8) | 64.7 (9.6) |
| Gender (female), n (%) | 282 (71.0)a | 289 (51.4)b |
| Height (cm), mean (SD) | 165 (13.3) | 165 (11.2) |
| Weight (kg), mean (SD) | 77 (18.3) | 75 (19.9) |
| Education, n (%) | ||
| Primary school | 99 (25.1) | 294 (52.3) |
| High school | 232 (58.7) | 234 (41.6) |
| University or college degree | 64 (16.2) | 34 (6.0) |
| Smoking status, n (%) | ||
| Current smoker | 51 (12.8) | 256 (45.6) |
| Former smoker | 51 (12.8) | 209 (37.2) |
| Never smoked | 296 (74.4) | 97 (17.3) |
| HRQoL | ||
| Mini-AQLQ, mean (SD) | 3.8 (0.9) | n/a |
| mMRC dyspnea scale, mean (SD) | n/a | 2.0 (0.9) |
| Disease control | ||
| ACT score, mean (SD) | 14.2 (4.2) | n/a |
| CAT score, mean (SD) | n/a | 24.4 (5.8) |
| Lung function, mean (SD) | ||
| FEV1 % predicted | 76.4 (19.1) | 49.9 (16.4) |
ACT Asthma Control Test, AQLQ Asthma Quality of Life Questionnaire, CAT COPD Assessment Test, COPD chronic obstructive pulmonary disease, FEV1 forced expiratory volume in one second, HRQoL health-related quality of life, mMRC modified Medical Research Council dyspnea scale, SD standard deviation
a,bPatients with missing data were excluded from percentage calculations
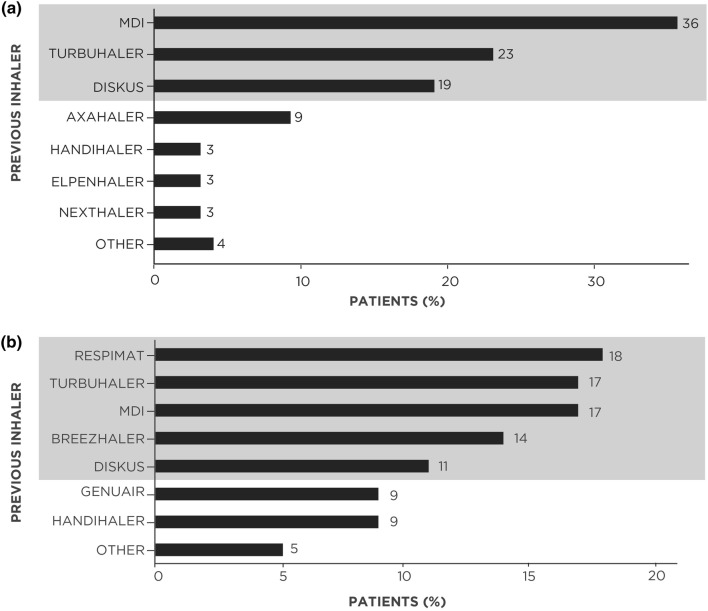
The most commonly used inhalers (reported by ≥ 10% of patients before switching to the B/F Easyhaler) were the metered-dose inhaler (MDI), Symbicort Turbuhaler® (AstraZeneca), and DISKUS® (GlaxoSmithKline) among patients with asthma, and the Respimat® (Boehringer Ingelheim), Symbicort Turbuhaler, MDI, Breezhaler® (Novartis) and DISKUS among patients with COPD (Fig. 1).
Fig. 1.
Prior individual inhaler use in patients with a asthma (n = 398) or b COPD (n = 563) who switched to the B/F Easyhaler. Grey shading denotes the most commonly used inhalers in each disease cohort (those reported by ≥ 10% of patients). B/F budesonide/formoterol fumarate, COPD chronic obstructive pulmonary disease, MDI metered-dose inhaler
Effect on Symptoms, Disease Control, HRQoL, and Pulmonary Function after Switching to the B/F Easyhaler
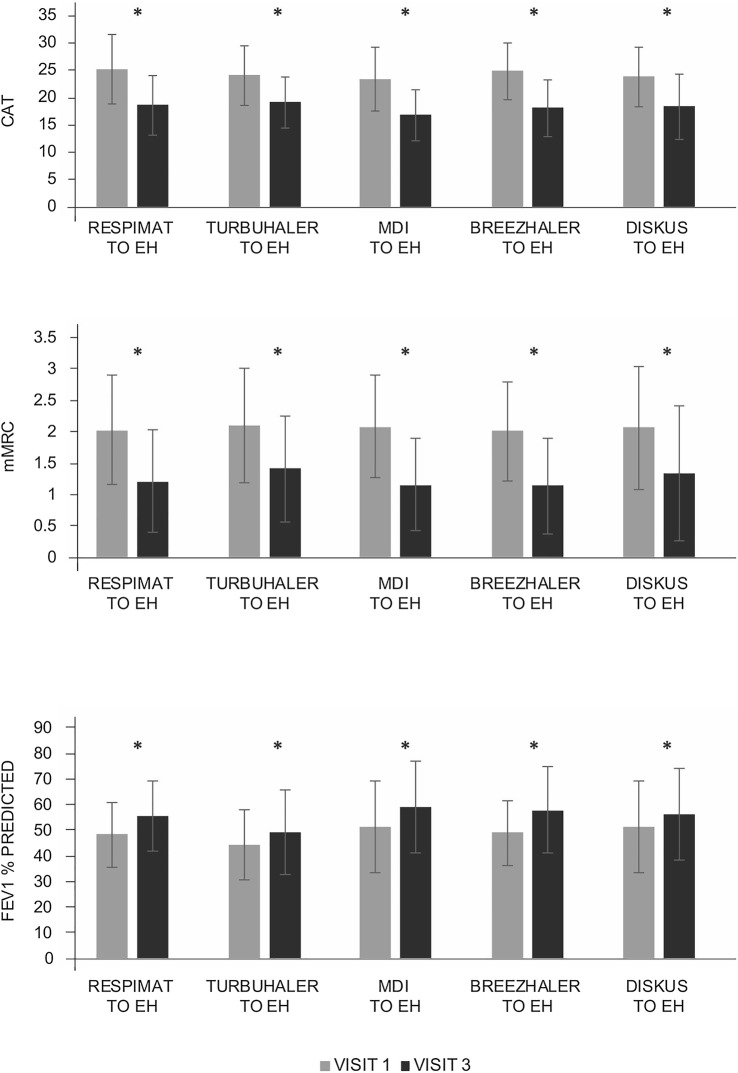
Compared with baseline, patients with asthma and COPD experienced significant improvements in disease symptoms (as measured by ACT and CAT), HRQoL (based on mAQLQ and mMRC) and lung function tests after 12 weeks of B/F Easyhaler treatment, irrespective of prior inhaler type used (p < 0.0001 for all comparisons among the most commonly used inhalers) (Figs. 2 and 3, respectively). Although patient numbers were too low to calculate statistical significance, numerical improvements from baseline in patients’ reported outcomes and FEV1 % predicted were also noted with a switch from all other less frequently used inhaler types (Supplementary Tables S1 and S2). Exceptions were observed for lung function after switching from Breezhaler in patients with asthma, and patient-reported outcomes and lung function after switching from Axahaler® (Laboratoires SMB) in patients with COPD.
Fig. 2.
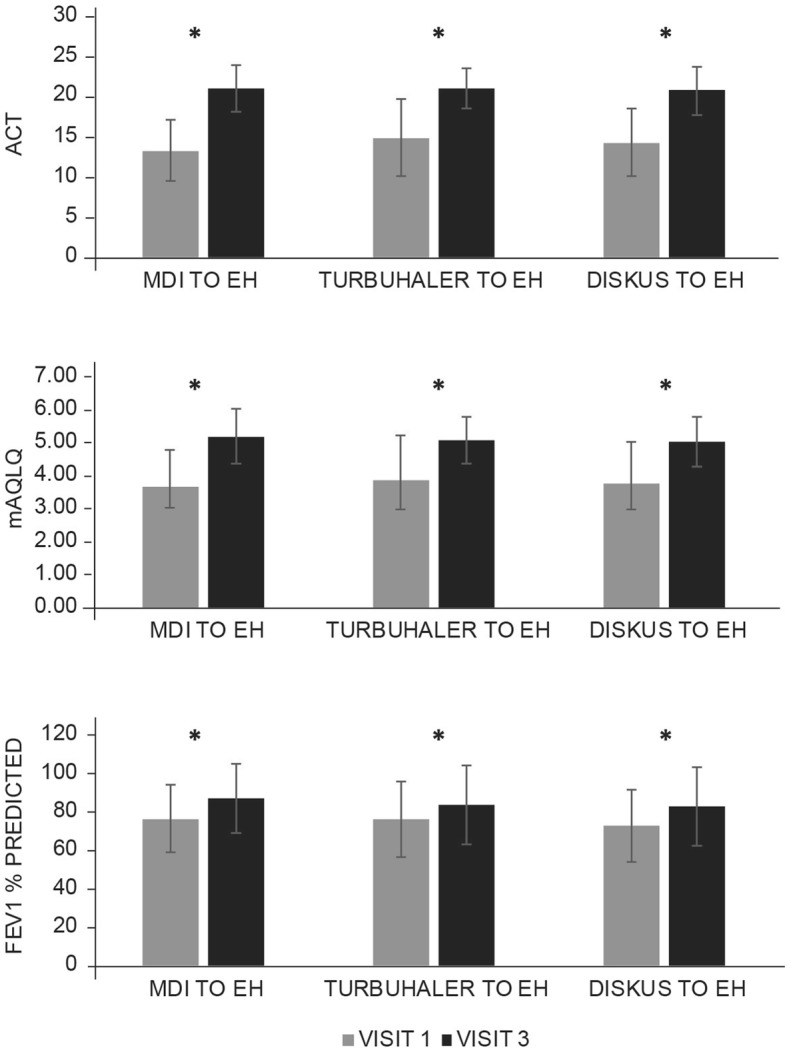
Assessment of switch to B/F Easyhaler combination therapy on changes in patient-reported outcomes and spirometry in patients with asthma (n = 310). *p < 0.0001 visit 1 versus visit 3. All values are mean ± standard deviation. Analyses were performed on the most commonly used inhalers in each disease cohort (those reported by ≥ 10% of patients). ACT Asthma Control Test, B/F budesonide/formoterol fumarate, EH Easyhaler, FEV1 forced expiratory volume in 1 s, mAQLQ mini-Asthma Quality of Life Questionnaire, MDI metered-dose inhaler
Fig. 3.
Assessment of switch to B/F Easyhaler combination therapy on changes in patient-reported outcomes and spirometry in patients with COPD (n = 435). *p < 0.0001 visit 1 versus visit 3. All values are mean ± standard deviation. Analyses were performed on the most commonly used inhalers in each disease cohort (those reported by ≥ 10% of patients). B/F budesonide/formoterol fumarate, CAT COPD Assessment Test, COPD chronic obstructive pulmonary disease, EH Easyhaler, FEV1 forced expiratory volume in 1 s, MDI metered-dose inhaler, mMRC modified Medical Research Council dyspnea scale
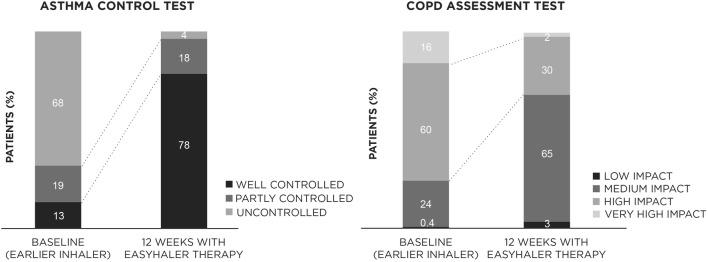
Overall, 12 weeks of treatment with the B/F Easyhaler led to significant improvements in asthma and COPD control (p < 0.0001, respectively) (Fig. 4). At visit 3, over three-quarters (78%) of patients with asthma had well-controlled disease (ACT score ≥ 20) versus 13% at baseline, and over two-thirds of patients (68%) with COPD experienced a medium-to-low impact of the disease (CAT score ≤ 20) on their daily life and physical functioning (versus 24% at baseline).
Fig. 4.
Effect of switching to B/F Easyhaler combination therapy on disease control in patients with asthma (n = 398) or COPD (n = 563), assessed using the ACT or CAT, respectively. ACT Asthma Control Test, B/F budesonide/formoterol fumarate, CAT COPD Assessment Test, COPD chronic obstructive pulmonary disease. p < 0.0001 (visit 1 versus visit 3) for both asthma and COPD comparisons
Patient and Clinician Perspectives of B/F Easyhaler and Prior Inhaler Use
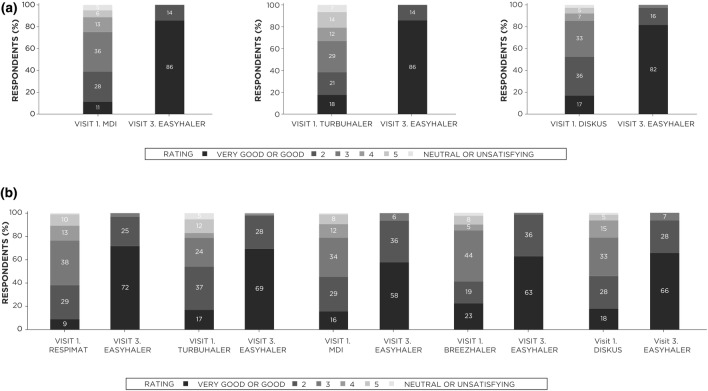
After 12 weeks of treatment, patient satisfaction with the B/F Easyhaler was significantly (p < 0.0001) greater than with their prior inhaler (among the most commonly used inhalers) for both patients with asthma or COPD (Fig. 5). In total, 82–86% of patients with asthma rated their satisfaction with the B/F Easyhaler as good or very good, compared with 11, 17, and 18% when using the MDI, DISKUS, or Turbuhaler devices, respectively. Similarly, good or very good satisfaction rates with the B/F Easyhaler were reported by 58–72% of patients with COPD, compared with 9–23% when using prior inhaler devices.
Fig. 5.
Effect of switching to B/F Easyhaler combination therapy on patient satisfaction in patients with a asthma (n = 310) or b COPD (n = 435). Analyses were performed on the most commonly used inhalers in each disease cohort (those reported by ≥ 10% of patients). p < 0.0001 (visit 1 versus visit 3) in all comparisons. B/F budesonide/formoterol fumarate, COPD chronic obstructive pulmonary disease, MDI metered-dose inhaler
Clinicians were able to teach correct use of the B/F Easyhaler within 5 min to 82.9 and 69.5% patients with asthma and COPD, respectively; within 10 min, 98.7 and 98.4% of patients with asthma and COPD had learned how to use the device (Table 2). The longer time required for mastering the Easyhaler usage among the patients with COPD may have been affected by their older age compared to the patients with asthma.
Table 2.
Clinician-reported length of time required to teach patients with asthma (n = 392) or COPD (n = 561) to use the B/F Easyhaler
| Time required (minutes) | Patients with asthma, n (%) | Patients with COPD, n (%) |
|---|---|---|
| < 5 | 325 (82.9) | 390 (69.5) |
| 5–10 | 62 (15.8) | 162 (28.9) |
| 10–20 | 5 (1.3) | 9 (1.6) |
B/F budesonide/formoterol fumarate, COPD chronic obstructive pulmonary disease
Discussion
In this subgroup analysis, patients with asthma or COPD who switched to the B/F Easyhaler from their previous inhaler device due to lack of disease control achieved significant improvements within 12 weeks of real-world use in symptoms, disease control, HRQoL, lung function, and patient satisfaction. These findings support and build on those of the primary study in patients with asthma, COPD, or ACO [24], which found disease to be well controlled after patients switched to the B/F Easyhaler. In the primary analyses, significant benefits in patient-reported outcomes and lung function, and clear improvements in patient satisfaction were also reported, but the study did not directly compare the B/F Easyhaler with specific prior inhaler devices. Another recent real-world, non-interventional, single-arm study in Swedish patients with asthma who switched from the Turbuhaler to the B/F Easyhaler demonstrated that asthma control and lung function were maintained after switching, with most patients obtaining good or complete asthma control (statistically significant; p ≤ 0.001) over the 12-week treatment period [30]. Such comparative information from the present and Swedish studies can be helpful for guiding prescribing practice and switching of treatment for asthma and COPD, and provides reassurance and confidence to clinicians and patients that switching, for example when disease is poorly controlled, should be viewed as an appropriate and potentially beneficial treatment option.
Aerosol inhalation is considered the primary route of administering medication to manage asthma [1, 4] and other obstructive airway diseases [6, 7]. However, correct inhaler use is often suboptimal in patients with obstructive airway disease, resulting in reduced therapeutic benefit [6, 12]. In particular, patients who are older, have underlying pulmonary symptoms, and/or who have received less education and training regarding correct use of their specific device can experience difficulty with achieving correct inhalation technique [12, 14, 31, 32]. Good inhaler competence appears to be a contributor to the beneficial outcomes and rates of patient satisfaction achieved with the B/F Easyhaler in the present study of older patients with asthma or COPD. As in the primary study, instructing patients on the correct use of the B/F Easyhaler was straightforward, and most clinicians were able to achieve this within 5 min in this patient population who were mainly educated to a primary or high school level. This observation is in line with previous reports supporting that the Easyhaler is simple to teach and use, and associated with high rates of user satisfaction [17, 33, 34]. Suboptimal inhaler use may result in patients being unable to achieve an inspiratory flow rate strong enough to deliver sufficient bronchodilator medication to the lungs, and this is of particular concern in elderly patients, children, and those with severe airflow limitation [35]. A pooled analysis of two randomized, multicenter, crossover, open-label studies in patients aged 6–88 years with asthma (n = 287) or COPD (n = 96) found that by using the Easyhaler, most could achieve or exceed a peak inspiratory flow rate of 30 l/min, which is the minimum required to ensure optimal and consistent dose delivery [36]. Recent dose uniformity analyses also confirmed consistent delivered dose using the Easyhaler under simulated real-world conditions and a variety of inspiratory flow rates [37].
A strength of this real-world study was that it included a representative population of patients with asthma or COPD who switched inhaler therapy in everyday clinical practice; however, it had a number of limitations. Satisfaction rates may have been biased because patients switching to the B/F Easyhaler were likely doing so because of uncontrolled disease, and therefore may have been dissatisfied with their prior inhaler. No information on baseline education on inhaler training and adherence data were available for the specific prior inhalers, to enable comparison with the B/F Easyhaler. Patient adherence may have also been influenced by their participation in a clinical trial and inhaler training given (albeit under real-world conditions). The study was limited to 12-week follow-up without a comparator arm.
Currently, few studies exist that describe real-world patient-reported outcomes data for the B/F Easyhaler, or the effects of inhaler switching in patients with COPD (most studies are conducted in patients with asthma); this highlights the need for further real-world studies in switching and in this specific patient population, to inform patients on the possible benefits and how to achieve better adherence and disease control when switching between inhaler devices.
Conclusions
This subanalysis demonstrated that a switch to the B/F Easyhaler was accompanied by significant benefits in symptoms, disease control, HRQoL, lung function, and patient satisfaction in patients with asthma or COPD in real-world clinical practice. As a next step, these findings should be confirmed in other populations where the B/F Easyhaler is approved for use to investigate whether country-specific differences in patient demographics and characteristics and/or healthcare systems may exist, which might influence inhaler usage and/or the tendency to switch inhalers. Future studies should also assess whether the improved clinical effectiveness, and associated patient adherence and satisfaction, with the B/F Easyhaler is maintained over the long term.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the patients who participated in this study. Mikko Vahteristo, MSc (Stat) of Orion Corporation, provided assistance with statistical analyses.
Funding
The study design, collection, analyses, and interpretation of the data, writing of the manuscript, and the article processing charges were sponsored by Orion Corporation, Orion Pharma. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, had full access to all of the data in this study, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing, Editorial, and Other Assistance
Mikko Vahteristo, MSc (Stat), of Orion Corp. provided assistance with statistical analyses. Medical writing support in the preparation of this article was provided by David Griffiths, Ph.D. of Bioscript Medical, funded by Orion Corporation., Orion Pharma.
Disclosures
Gabriella Gálffy, Maria Szilasi, and Lilla Tamási have nothing to disclose.
Compliance with Ethics Guidelines
All procedures were performed in accordance with the ethical standards of the National Scientific and Research Ethics Committee of Hungary and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all individual participants included in the study, prior to study commencement.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.8851814.
References
- 1.Global Initiative for Asthma (GINA). National Heart, Lung, and Blood Institute, National Institutes of Health. GINA report. Global Strategy for Asthma Management and Prevention (2018 update). https://ginasthma.org/gina-reports/. Accessed Mar 14, 2019.
- 2.Pavord ID, Mathieson N, Scowcroft A, et al. The impact of poor asthma control among asthma patients treated with inhaled corticosteroids plus long-acting β2-agonists in the United Kingdom: a cross-sectional analysis. NPJ Prim Care Respir Med. 2017;27(1):17. doi: 10.1038/s41533-017-0014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters SP, Ferguson G, Deniz Y, et al. Uncontrolled asthma: a review of the prevalence, disease burden and options for treatment. Respir Med. 2006;100(7):1139–1151. doi: 10.1016/j.rmed.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 4.Global Asthma Network. The global asthma report. 2014. http://www.globalasthmareport.org/resources/Global_Asthma_Report_2014.pdf. Accessed Apr 12, 2018.
- 5.Miravitlles M, Ribera A. Understanding the impact of symptoms on the burden of COPD. Respir Res. 2017;18(1):67. doi: 10.1186/s12931-017-0548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broeders ME, Sanchis J, Levy ML, et al. The ADMIT series—issues in inhalation therapy. 2. Improving technique and clinical effectiveness. Prim Care Respir J. 2009;18(2):76–82. doi: 10.4104/pcrj.2009.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease. http://goldcopd.org/. Accessed Mar 14, 2019.
- 8.Laube BL, Janssens HM, de Jongh FH, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. 2011;37(6):1308–1331. doi: 10.1183/09031936.00166410. [DOI] [PubMed] [Google Scholar]
- 9.Carrión Valero F, Maya Martínez M, Fontana Sanchis I, et al. Inhalation technique in patients with chronic respiratory diseases. Arch Bronconeumol. 2000;36(5):236–240. doi: 10.1016/S0300-2896(15)30163-0. [DOI] [PubMed] [Google Scholar]
- 10.Girodet PO, Raherison C, Abouelfath A, et al. Real-life use of inhaler devices for chronic obstructive pulmonary disease in primary care. Therapie. 2003;58(6):499–504. doi: 10.2515/therapie:2003081. [DOI] [PubMed] [Google Scholar]
- 11.Molimard M, Raherison C, Lignot S, et al. Assessment of handling of inhaler devices in real life: an observational study in 3811 patients in primary care. J Aerosol Med. 2003;16(3):249–254. doi: 10.1089/089426803769017613. [DOI] [PubMed] [Google Scholar]
- 12.Ramadan WH, Sarkis AT. Patterns of use of dry powder inhalers versus pressurized metered-dose inhalers devices in adult patients with chronic obstructive pulmonary disease or asthma: an observational comparative study. Chron Respir Dis. 2017;14(3):309–320. doi: 10.1177/1479972316687209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shrestha M, Parupia H, Andrews B, et al. Metered-dose inhaler technique of patients in an urban ED: prevalence of incorrect technique and attempt at education. Am J Emerg Med. 1996;14(4):380–384. doi: 10.1016/S0735-6757(96)90054-6. [DOI] [PubMed] [Google Scholar]
- 14.van Beerendonk I, Mesters I, Mudde AN, et al. Assessment of the inhalation technique in outpatients with asthma or chronic obstructive pulmonary disease using a metered-dose inhaler or dry powder device. J Asthma. 1998;35(3):273–279. doi: 10.3109/02770909809068218. [DOI] [PubMed] [Google Scholar]
- 15.Ocakli B, Ozmen I, Tuncay EA, et al. A comparative analysis of errors in inhaler technique among COPD versus asthma patients. Int J Chron Obstruct Pulmon Dis. 2018;13:2941–2947. doi: 10.2147/COPD.S178951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price DB, Roman-Rodriguez M, McQueen RB, et al. Inhaler errors in the CRITIKAL study: type, frequency, and association with asthma outcomes. J Allergy Clin Immunol Pract. 2017;5(4):1071–1081. doi: 10.1016/j.jaip.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Valero A, Ribo P, Maiz L, et al. Asthma patient satisfaction with different dry powder inhalers. Expert Rev Respir Med. 2019;13(2):133–138. doi: 10.1080/17476348.2019.1567339. [DOI] [PubMed] [Google Scholar]
- 18.Price D, Brusselle G, Roche N, et al. Real-world research and its importance in respiratory medicine. Breathe (Sheff) 2015;11(1):26–38. doi: 10.1183/20734735.015414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bell H, Wailoo AJ, Hernandez M, et al. NICE Decision Support Unit: the use of real-world data for the estimation of treatment effects in NICE decision making. 2016. http://scharr.dept.shef.ac.uk/nicedsu/wp-content/uploads/sites/7/2017/05/RWD-DSU-REPORT-Updated-DECEMBER-2016.pdf. Accessed Mar 14, 2019.
- 20.European Medicines Agency. Regulatory perspective on real-world evidence (RWE) in scientific advice. 2018. https://www.ema.europa.eu/en/documents/presentation/presentation-regulatory-perspective-real-world-evidence-rwe-scientific-advice-emas-pcwp-hcpwp-joint_en.pdf. Accessed Mar 15, 2019.
- 21.Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-world evidence—what is it and what can it tell us? N Engl J Med. 2016;375(23):2293–2297. doi: 10.1056/NEJMsb1609216. [DOI] [PubMed] [Google Scholar]
- 22.Orion Pharma. Bufomix Easyhaler® summary of product characteristics. https://www.medicines.ie/medicines/bufomix-easyhaler-320-micrograms-9-micrograms-31497/. Accessed 14 March 2019.
- 23.Pirożyński M, Hantulik P, Almgren-Rachtan A, et al. Evaluation of the efficiency of single-inhaler combination therapy with budesonide/formoterol fumarate in patients with bronchial asthma in daily clinical practice. Adv Ther. 2017;34(12):2648–2660. doi: 10.1007/s12325-017-0641-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamási L, Szilasi M, Gálffy G. Clinical effectiveness of budesonide/formoterol fumarate Easyhaler® for patients with poorly controlled obstructive airway disease: a real-world study of patient-reported outcomes. Adv Ther. 2018;35(8):1140–1152. doi: 10.1007/s12325-018-0753-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas M, Kay S, Pike J, et al. The Asthma Control Test (ACT) as a predictor of GINA guideline-defined asthma control: analysis of a multinational cross-sectional survey. Prim Care Respir J. 2009;18(1):41–49. doi: 10.4104/pcrj.2009.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juniper EF, Guyatt GH, Cox FM, et al. Development and validation of the mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14(1):32–38. doi: 10.1034/j.1399-3003.1999.14a08.x. [DOI] [PubMed] [Google Scholar]
- 27.Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 28.Hsu KY, Lin JR, Lin MS, et al. The modified Medical Research Council dyspnoea scale is a good indicator of health-related quality of life in patients with chronic obstructive pulmonary disease. Singapore Med J. 2013;54(6):321–327. doi: 10.11622/smedj.2013125. [DOI] [PubMed] [Google Scholar]
- 29.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 30.Rytilä PH, Syk J, Vinge I, et al. Switch from Symbicort Turbuhaler to Bufomix Easyhaler; a real-life prospective study in asthma patients. Eur Respir J. 2018;52(Suppl 62):PA3998. [Google Scholar]
- 31.Sestini P, Cappiello V, Aliani M, et al. Prescription bias and factors associated with improper use of inhalers. J Aerosol Med. 2006;19(2):127–136. doi: 10.1089/jam.2006.19.127. [DOI] [PubMed] [Google Scholar]
- 32.Turan O, Turan PA, Mirici A. Parameters affecting inhalation therapy adherence in elderly patients with chronic obstructive lung disease and asthma. Geriatr Gerontol Int. 2017;17(6):999–1005. doi: 10.1111/ggi.12823. [DOI] [PubMed] [Google Scholar]
- 33.Galffy G, Mezei G, Nemeth G, et al. Inhaler competence and patient satisfaction with Easyhaler®: results of two real-life multicentre studies in asthma and COPD. Drugs R D. 2013;13(3):215–222. doi: 10.1007/s40268-013-0027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malmberg LP, Everard ML, Haikarainen J, et al. Evaluation of in vitro and in vivo flow rate dependency of budesonide/formoterol Easyhaler®. J Aerosol Med Pulm Drug Deliv. 2014;27(5):329–340. doi: 10.1089/jamp.2013.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lavorini F, Magnan A, Dubus JC, et al. Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir Med. 2008;102(4):593–604. doi: 10.1016/j.rmed.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Malmberg PL, Pelkonen A, Lähelmä S, et al. Young patients with asthma and patients with COPD can generate sufficient inspiratory flows via Easyhaler dry powder inhaler. London: British Thoracic Society (BTS) Winter Meeting; 2018. [Google Scholar]
- 37.Haikarainen J, Rytila P, Roos S, et al. Dose uniformity of budesonide Easyhaler® under simulated real-life conditions and with low inspiration flow rates. Chron Respir Dis. 2018;15(3):265–271. doi: 10.1177/1479972317745733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.