Abstract
Background
Plants possess a sophisticated immune system to defend from herbivores. These defence responses are regulated by plant hormones including salicylic acid (SA) and jasmonic acid (JA). Sometimes, plant defences can be complemented by the presence of symbiotic microorganisms. A remarkable example of this are grasses establishing symbiotic associations with Epichloë fungal endophytes. We studied the level of resistance provided by the grass’ defence hormones, and that provided by Epichloë fungal endophytes, against an introduced herbivore aphid. These fungi protect their hosts against herbivores by producing bioactive alkaloids. We hypothesized that either the presence of fungal endophytes or the induction of the plant salicylic acid (SA) defence pathway would enhance the level of resistance of the grass to the aphid.
Methods
Lolium multiflorum plants, with and without the fungal endophyte Epichloë occultans, were subjected to an exogenous application of SA followed by a challenge with the aphid, Sipha maydis.
Results
Our results indicate that neither the presence of E. occultans nor the induction of the plant’s SA pathway regulate S. maydis populations. However, endophyte-symbiotic plants may have been more tolerant to the aphid feeding because these plants produced more aboveground biomass. We suggest that this insect insensitivity could be explained by a combination between the ineffectiveness of the specific alkaloids produced by E. occultans in controlling S. maydis aphids and the capacity of this herbivore to deal with hormone-dependent defences of L. multiflorum.
Keywords: Alkaloids, Beneficial microorganisms, Endophyte symbiosis, Epichloë fungalendophytes, Plant defences, Salicylic acid, Plant-herbivore interaction
Introduction
To defend from herbivore attacks, plants harbour a sophisticated immune system in which hormone pathways including salicylic acid (SA) and jasmonic acid (JA) mediate defence responses. These hormone pathways are known to be differentially involved in the response to distinct natural enemies. While the plant SA-dependent defence pathway is generally induced by sap-sucking insect herbivores and biotrophic pathogens, the JA-dependent defence pathway acts in response to chewing insect herbivores and necrotrophic pathogens (Thaler, Humphrey & Whiteman, 2012; Ballaré, 2014). In some cases, the plant immune system can be complemented by hormone-independent mechanisms of defences. A particularly striking example of this is provided by grasses establishing symbiotic associations with Epichloë fungal endophytes (Clay, 1988; Bastias et al., 2017a; Bastías et al., 2017b). These fungi protect their host grasses against herbivores by the production of bioactive alkaloids (Panaccione, Beaulieu & Cook, 2014).
Some grasses establish symbiotic associations with asexual Epichloë fungal species that are strictly vertically-transmitted. These grass-Epichloë endophyte associations are usually mutualistic, since plants provide the fungus a place to live (the fungus is an obligate symbiont) and the fungus provides the grass with beneficial traits such as anti-herbivore defences (Gundel, Rudgers & Ghersa, 2011; Saikkonen, Saari & Helander, 2010; Schardl et al., 2013a; Schardl et al., 2013b). Epichloë fungal endophyte species can collectively synthesize a vast number of secondary metabolites and this diversity of bioactive compounds provides their host grasses defences against a range of herbivore species (Saikkonen, Saari & Helander, 2010; Schardl et al., 2013a; Schardl et al., 2013b). Four classes of Epichloë-derived alkaloids have been well-studied: pyrrolizidines (e.g., lolines), peramine, indole-diterpenes (e.g., lolitrem B, terpendoles), and ergot alkaloids (e.g., ergovaline) (Panaccione, Beaulieu & Cook, 2014; Saikkonen, Gundel & Helander, 2013; Schardl et al., 2013a; Schardl et al., 2013b). The alkaloid profile produced depends on the fungal species/strain, with some species/strains producing only one type of alkaloid (Schardl et al., 2013a; Schardl et al., 2013b). Moreover, the effectiveness of a given alkaloid type depends on its concentration and the herbivore species (Fuchs et al., 2017b; Bastias et al., 2017a; Bastías et al., 2017b; Bultman et al., 2018). Alkaloid production is dependent on the fungal biomass, herbivory level, plant ontogenic stage and nutritional status, plant tissue type, and some abiotic conditions (e.g., temperature, CO2 levels) (Justus, Witte & Hartmann, 1997; Hunt et al., 2005; Rasmussen et al., 2007; Ryan et al., 2014; Fuchs et al., 2017a; Fuchs et al., 2017b; Gundel et al., 2018; Bultman et al., 2018).
The presence of biotrophic pathogens within plants is usually controlled by SA-dependent responses (Thaler, Humphrey & Whiteman, 2012). Recent research shows that the same pathway can also regulate the interaction with beneficial symbionts. For instance, the plant induction of the SA pathway inhibits the development and establishment of rhizobacterial and mycorrhizal symbionts in plant tissues (Cao et al., 2017; Bedini et al., 2018). Moreover, plant SA immune responses can also affect the activities performed by plant beneficial symbionts. For example, alkaloid production and nitrogen fixation performed by beneficial symbionts is reduced by the induction of the SA pathway in grasses, legumes, and ferns (Hayat et al., 2010; Bastías et al., 2018a; De Vries et al., 2018). Independently of the control exerted by plants on their beneficial symbionts, these symbionts can, in turn, modulate the SA pathway (Johnson et al., 2003; Stacey et al., 2006; Navarro-Meléndez & Heil, 2014; Dupont et al., 2015; Moreira, Abdala-Roberts & Castagneyrol, 2018; Ramos et al., 2018). For instance, genes encoding proteins pertaining to SA biosynthesis and signalling were downregulated by the presence of the endophyte E. festucae Fl1 in Lolium perenne plants (Dupont et al., 2015). The symbiont’s suppression of SA-dependent responses might be a mechanism used by these microorganisms to facilitate growth into plant tissues (Jung et al., 2012; Bastías et al., 2018a).
Here, we studied the level of resistance mediated by plant hormones and provided by an Epichloë endophyte fungus against an introduced aphid species in grasses. We hypothesized that either the presence of fungal endophytes or the induction of the plant SA pathway would enhance the level of resistance of the grass against the aphid. We subjected Italian ryegrass plants (Lolium multiflorum), symbiotic and non-symbiotic with the endophyte Epichloë occultans (Moon et al., 2000), to an exogenous SA application followed by a challenge with the hedgehog grain aphid (Sipha maydis). This aphid species is native to Eurasia, and individuals feed on cereals and other grasses (Skvarla et al., 2017). In Argentina, where this work was conducted, S. maydis was first discovered in 2002 and has since spread throughout temperate grasslands and cropping regions of the country (Corrales et al., 2007). Corrales et al. (2007) reported for this aphid species, average densities from 18 to 98 individuals per plant depending of the season and the geographic location. In temperate grasslands, the first populations were found in 2003 in the south-east of the Buenos Aires province (34°55′S, 57°57′W) (Corrales et al., 2007). In these grasslands, Sipha maydis is commonly found feeding on L. multiflorum plants with populations reaching sizes of around 250 individuals per plant (Chaneton & Omacini, 2007). The ryegrass L. multiflorum is a naturalised and abundant species in the Argentinian temperate grasslands. This species is native to the European Mediterranean region and was introduced in Argentina more than a century ago (Uchitel, Omacini & Chaneton, 2011). While many different alkaloids are produced across the genus Epichloë, E. occultans produces only loline alkaloids (i.e., N-formylloline (NFL) and N-acetylnorloline (NANL)) (Sugawara et al., 2006; Moore et al., 2015; Bastias et al., 2017a; Bastías et al., 2017b). It has been shown that both the presence of E. occultans (Omacini et al., 2001; Miranda, Marina & Chaneton, 2011; Gundel et al., 2012; Ueno et al., 2015; Bastias et al., 2017a; Bastías et al., 2017b), and the loline alkaloids produced by this and other endophyte species provide the plant with protection from aphids (Johnson et al., 1985; Eichenseer, Dahlman & Bush, 1991; Wilkinson et al., 2000; Panaccione, Beaulieu & Cook, 2014).
We predicted that the presence of Epichloë fungal endophytes within their host plants would enhance the level of plant resistance against the aphid and consequently reduce the aphid’s performance (i.e., aphid individual metabolic rates and population sizes). In addition, the stimulation of the SA-dependent defence response, by the exogenous application of the hormone, would increase the resistance in non-symbiotic plants, affecting negatively the aphid performance. However, we expected that the SA treatment would affect the endophyte-conferred plant resistance against aphids. Specifically, since Epichloë fungal endophytes are biotrophic microorganisms, the exogenously applied SA would impair the Epichloë, subsequently reducing its alkaloid production, and thus decreasing the resistance level and consequently, increasing the metabolic rates and population sizes of the aphids.
Material and Methods
Plant and aphid stocks
We worked with the annual grass plants of Lolium multiflorum both symbiotic (E+) and non-symbiotic (E-) with its common fungal endophyte E. occultans (Moon et al., 2000). More than a decade ago, seeds of L. multiflorum with high percentages of endophyte infection were hand-collected from a naturalised population in the Pampean grassland (Argentina) (36°00′S, 61°5′W) (Gundel et al., 2009). Immediately after collection, a proportion of these seeds were treated with the systemic fungicide Triadimenol (150 g kg−1; Baytan®) in order to obtain endophyte-free individuals. Since then, plants of these two biotypes (i.e., E+ and E-) have been cultivated annually in a common garden (recall that E. occultans is strictly vertically transmitted), multiplying fresh seeds for experimentation [IFEVA - CONICET, Universidad de Buenos Aires, Argentina (34°35′S, 58°28′W)]. Genetic segregation between plant biotypes has been prevented by allowing individual plants to freely exchange pollen during flowering (Gundel et al., 2012). Each late spring–early summer, ripe seeds produced by each plant biotype are harvested and evaluated for endophyte presence; after confirming the level of endophytes in each biotype, the seeds are stored in a 4 °C freezer. The endophyte detection is carried out by looking for fungal hypha in stained individual seeds following the “seed squash technique” (Bacon & White, 1994; Card et al., 2011). For this, 100 seeds from each seed lot (E+ and E-), are incubated in NaOH (5%), stained with rose bengal and examined under a light microscope at 40X power. The seeds produced in 2014 were examined for endophyte infection frequency (E+: 99%, and E-: 1%; n = 100 each) and stored in cold and dry conditions until use (2015).
In early-spring 2015, individual aphids S. maydis (Passerini) were collected from the local extant vegetation dominated by cereals and wild grasses. Starting with approximately 150 apterous adults, an aphid population was established within a growth chamber [21 °C (±1) constant, radiation 150 µmol m−2 s−1, and photoperiod L16:D8 h] on wheat plants (Var. Cronox; Don Mario). Wheat plants were replaced periodically to provide fresh material for the aphid population. After 6 weeks, the aphid population was large enough to provide the required number of individual adult aphids for the experiment (see next section).
Experimental description
In 2015 during the normal growing season for L. multiflorum (autumn-winter-spring), 50 E+ and 50 E- plants were grown in 1.5 L pots, filled with a mix of soil, sand, and peat in equal proportions. Plants were watered to field capacity, as needed, to avoid water deficits. In early-spring, 28 E+ and 28 E- healthy plants were selected and transferred to a growth chamber with the same environmental conditions as described earlier. At that time, the plants averaged 48 tillers (range: 25–73) and were starting the reproductive stage (spike appearance). After careful examination to ensure there were no invertebrates present on the L. multiflorum plants, each plant was individually enclosed within a white cotton voile fabric bag supported by a tubular plastic net. Before the application of the hormone treatment (see below), the plants were acclimated to the growth chamber conditions for one week.
The experiment comprised a 2 × 2 full factorial design, with endophyte (E+, E-) and salicylic acid (SA+, SA-) as the experimental treatments. Fourteen plants from each biotype were sprayed with 10 ml of SA solution (0.5 mM; Biopack®, Argentina). The same procedure was done on the other 14 E+ and 14 E- plants but sprayed with 10 ml of distilled water. Three days later, each plant was challenged with 5 apterous adult aphids. This number of aphids as starting population was chosen based on field observations, where the colonization of L. multiflorum plants by S. maydis aphids is usually carried out by only few individuals (Chaneton & Omacini, 2007). The 3-days period between the application of SA and the aphid challenge was previously identified as enough time for the plants to develop a defence response prior to contact with aphids (Bastías et al., 2018a).
The aphid populations on the L. multiflorum plants developed over the next 24 days. We counted the number of insects on each plant (aphid population size) at days 7 and 14 (corresponding to days 10 and 17 since the SA application, respectively). At day 24 (27th since SA application), a group of approximately 35 randomly chosen aphids were sampled from a subset of 5 individual plants per treatment to measure the mass-specific standard metabolic rate (SMR) by means of open-flow respirometry.
Two serial samples of plant tissues were taken to measure the physiological concentrations of plant defence hormones and fungal alkaloids. The tissues were sampled from a subset of 8 plants per treatment, selected at random. The first sample was taken on day 3, just prior to the introduction of the aphids. Two leaf-blades were excised from one tiller per plant just before the aphid challenge (day 3 post SA application) to evaluate the concentrations of SA and JA hormones. Since the recognized role of JA-signalling pathway responses in plant defences (Thaler, Humphrey & Whiteman, 2012), JA concentration levels were also measured in response to the endophyte presence and the SA treatment. The second harvest comprised the removal of one tiller per E+ plant 7 days after the aphid challenge (10 days post SA application). We used the pseudostem from this tiller to evaluate the concentration of loline alkaloids [note that the fungal endophyte E. occultans only produces this type of alkaloid (Bastias et al., 2017a; Bastías et al., 2017b)]. We selected tillers in visibly good conditions but with symptoms of aphids feeding activities. Even though E- plants are incapable of producing loline alkaloids, E- were subjected to the same sampling procedure as E+ plants to avoid any manipulation-dependent effects (Cahill Jr, Castelli & Casper, 2002). We estimated that the total tissue removed for hormone and alkaloid assessments represented around 1% of the aboveground plant biomass. At day 27 post SA application, the total aboveground biomass of the plants was harvested, and immediately dried in an oven (2 d at 60 °C) to evaluate the individual plant dry weight (Analytical scale, ± 0.01 g, Mettler Toledo).
Quantification of SA and JA hormones
Starting from freeze-dried and ground leaf material, subsamples of 50–100 mg were extracted with 100% Acetonitrile containing 100 ng of d6-SA and d5-JA as internal standards. The extracts were dried, derivatized with N-Methyl-N-(trimethylsilyl)trifluoroacetamide, and injected into an Agilent DB-5MS column (30 m, 0.25 mm inner diameter, 0.25 µm film thickness with a 10 m guard column). The column effluent was added into the ion source of a Scion TQ GC-MS/MS (Bruker Daltonics Inc.). The mass spectrometer was operated in positive ionization mode with multiple reactions monitoring (MRM) as previously described in Bastías et al. (2018a). SA and JA hormones were quantified relative to the peak area of their corresponding internal standards.
Quantification of loline alkaloids
Lolines were extracted from 50 mg of freeze-dried, ground plant samples using a solution of 40% methanol/5% ammonia and 1,2-dichloroethane containing 54.8 ng mL−1 4-phenylmorpholine as internal standard. Plant extracts were centrifugated, and supernatants transferred to glass GC vials via a 20 µm filter for analysis. The analysis was conducted using a GC flame ionization detector (GC2010Plus, Shimadzu Corporation, Japan) and separation was achieved on a ZB-5 capillary column (30 m × 0.32 mm × 0.25 µm film). More information can be found in Bastías et al. (2018a). The detection limit was 25 µg g−1 DW.
Measurements of standard metabolic rate (SMR)
The SMR quantifies the energy budget required for insects to maintain homeostasis, thus this variable represents a measure of the general physiological status of insects (Nespolo, Roff & Fairbairn, 2008). Aphid maximum annual growth rates (rm) are negatively correlated with SMR. Aphids with high metabolic rates (i.e., high levels of maintenance costs) might have less energy available for reproduction thus negatively impacting their population sizes (Castañeda, Figueroa & Nespolo, 2010). The SMR was measured by the production of CO2 (VCO2) of aphid groups placed within of an open-flow respirometry system (LI-6400; Li-Cor, Lincoln, USA). We estimated the mass-specific SMR that is, the amount of CO2 produced per mass unit of aphid per hour (i.e., µL of VCO2 per mg of aphid per hour). Aphids were obtained from a subset of 5 randomly chosen plants per treatment. From each of these plants, 35 adult and non-winged aphids were carefully removed and placed in Eppendorf tubes. We considered each group of aphids from one individual plant to be a replicate (i.e., 5 replicates per treatment). All the aphid groups were weighted (±0.01 mg, analytical balance, Mettler Toledo), and kept for one hour without food before the metabolic measurements. The VCO2 of each aphid group was registered every second during a period of 10 min at 24 °C (±0.5). For this, the insects were placed in 10 mL-metabolic chamber that received CO2-scrubbed air at a constant rate of 70 mL min−1 and connected to a sensor of CO2 (LI-6400; Li-Cor, Lincoln, USA). The mass-specific SMR was obtained averaging the 2-mins continuous and most stable VCO2 values (from the 10-mins register) and dividing this averaged value by the insect group weight. Aphids were discarded after the SMR measurements.
Statistical analyses
The effects of the plant symbiotic status and SA application on the concentrations of SA and JA hormones, and on the plant above-ground biomass were analysed separately with linear effects models, using the function gls from the nlme package in R software (R Core Team, 2013), and assuming independent, identically distributed normal random errors (Pinheiro et al., 2009). The models included the plant’s symbiotic status (E+, E-) and SA treatment (SA+, SA-) as categorical factors. VarIdent variance structures were used on the SA treatments to accommodate deviations in the variance homogeneity in the SA and JA concentrations response variables (Zuur et al., 2009). After this procedure, all the ANOVA assumptions were met.
Similarly, the effects of the SA treatment on the concentrations of alkaloids (total lolines, NFL, and NANL) were analysed separately with lineal effect models, using the same software package mentioned earlier, and assuming independent, identically distributed normal random errors. The models included the SA treatment (SA+, SA-) as a categorical factor. When required, we used the function VarIdent on the SA treatment to accommodate deviations in the variance homogeneity (Zuur et al., 2009). ANOVA assumptions were then met.
The effects of plant symbiotic status and SA treatment on the population size of aphids (number of individuals) were analysed with linear mixed-effects models using the package glmmADMB in R software, and assuming that random errors were distributed independently and following a negative binomial distribution (Fournier et al., 2012). The model included plant symbiotic status (E+, E-), SA treatment (SA+, SA-), and time (7d and 14d since the aphid challenge) as categorical factors, and the random effect included the time nested in pot. Temporal autocorrelation between the repeated measurements was not observed.
The effects of the plant symbiotic status and the SA treatment on mass-specific SMR of aphids were analysed with linear effects models using the same R package and assuming the same random error distribution that for hormone concentration variables. The model included the plant symbiotic status (E+, E-) and SA treatment (SA+, SA-) as categorical factors. All the ANOVA assumptions were met. We performed post-hoc analyses between treatments when significant interactions were detected using the package lsmeans in R (Lenth, 2016). All the presented values in the result section are means ± standard errors (S.E.M). All data obtained in the present study are available in Table S1.
Results
Effects of plant endophyte presence and SA on hormone levels and plant growth
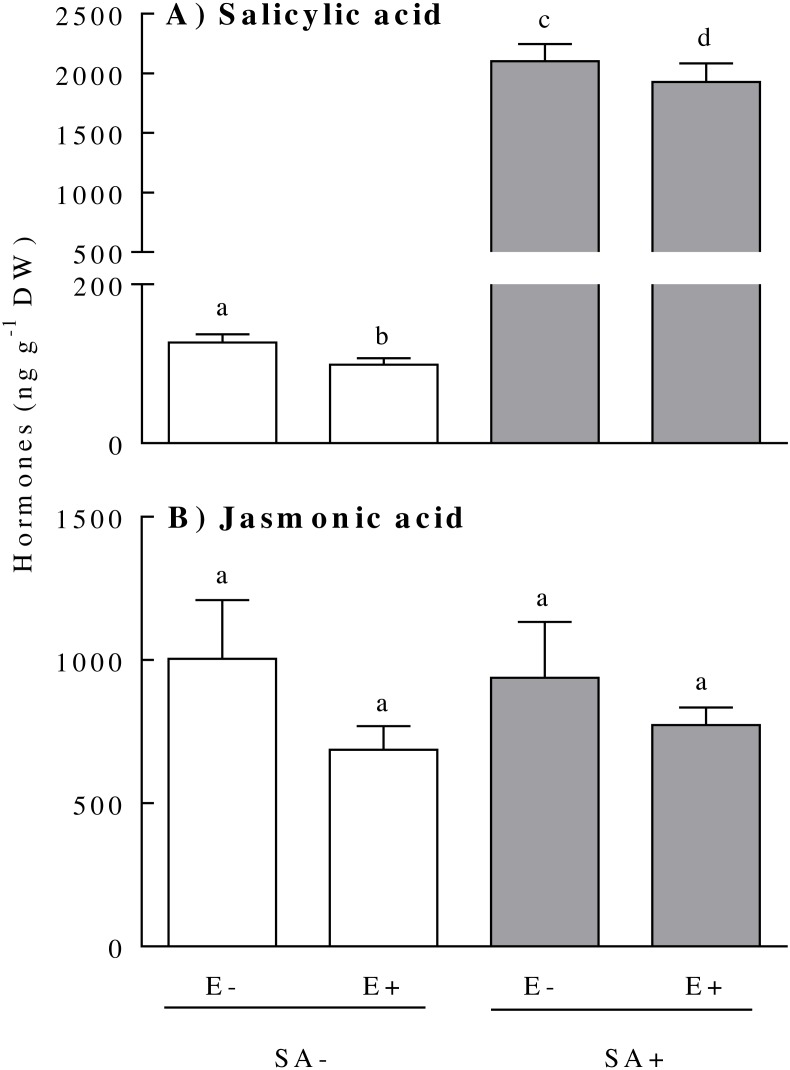
The concentration of the hormones SA and JA within the plants responded differentially to the endophyte presence and the exogenous application of the SA. The plant SA concentration was independently affected by the plant endophyte status and the hormonal treatment (Table 1). The presence of the endophyte in the plant reduced the SA by ca. 10% [E-: 1114.00 ± 264.50, and E+: 1014.00 ± 248.00 (ng SA g−1 DW)]. In addition, both plant biotypes (i.e., endophyte-symbiotic and non-symbiotic) showed increased SA concentrations of about 19-fold 3 days after the exogenous application of the hormone (Fig. 1A). Although the interaction effect between the treatments was not significant (Table 1), the reduction in SA concentration due to the endophyte presence was much more evident in plants not exposed to SA (the mean SA concentration difference between E+ and E- plants in the SA- treatment was around 22%) (Fig. 1A). The concentration of JA was not significantly modified by either the plant endophyte status or by the SA treatment (Fig. 1B) (Table 1).
Table 1. Effects of plant symbiotic status (E+, E−) and the exposure to the hormone salicylic acid (SA+, SA−) on different response variables of Lolium multiflorum plants symbiotic with the endophyte fungus Epichloë occultans.
Note that for lolines, only the effect of SA is evaluated since endophyte-free plants do not contain these alkaloids. NFL and NANL mean N-formylloline and N-acetylnorloline alkaloids, respectively. Statistically significant effects are highlighted in bold. Mean values, S.E.M, and post hoc statistical differences are shown in Figures 1 and 2.
| Response variable | Treatment | df | F | P-value |
|---|---|---|---|---|
| Salicylic acid (ng g−1 DW) (n = 8) | ||||
| Symbiosis | 1,28 | 4.70 | 0.038 | |
| SA | 1,28 | 316.87 | <0.001 | |
| Symbiosis × SA | 1,28 | 0.45 | 0.506 | |
| Jasmonic acid (ng g−1 DW) (n = 8) | ||||
| Symbiosis | 1,28 | 2.57 | 0.120 | |
| SA | 1,28 | 0.49 | 0.487 | |
| Symbiosis × SA | 1,28 | 0.25 | 0.614 | |
| Above-ground plant biomass (g) (n = 14) | ||||
| Symbiosis | 1,108 | 4.41 | 0.038 | |
| SA | 1,108 | 1.08 | 0.300 | |
| Symbiosis × SA | 1,108 | 3.86 | 0.052 | |
| Lolines (µg g−1 DW) (n = 8) | ||||
| Total | SA | 1,14 | 0.25 | 0.626 |
| NFL | SA | 1,14 | 0.36 | 0.555 |
| NANL | SA | 1,14 | 0.06 | 0.809 |
Figure 1. Physiological concentration of salicylic acid (panel A) and jasmonic acid (panel B) of Lolium multiflorum plants symbiotic with the endophyte fungus Epichloë occultans.
Concentrations were measured three days after the salicylic acid application [treated: SA+ (shaded bars), and untreated: SA− (unshaded bars)] on L. multiflorum plants with (E+) and without (E−) the endophyte fungus. Different letters indicate significant differences at P < 0.05. Bars represent mean values ± S.E.M. (n = 8).
The above-ground plant tissues at the end of the aphid challenge (day 27 from the application of SA) was 11% higher in endophyte-symbiotic than in non-symbiotic plants (E-: 4.42 ± 0.28 g, and E+: 4.99 ± 0.39 g). This effect was, however, not affected by the treatment with SA (Table 1).
Effects of SA on fungal loline concentrations
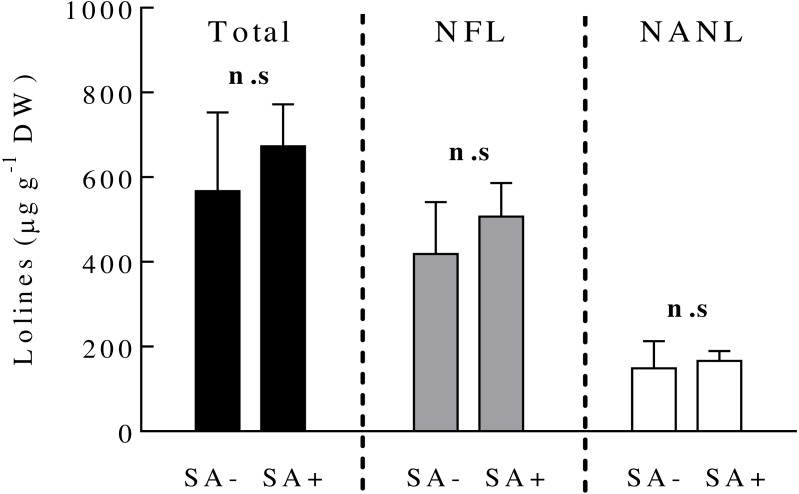
Ten days after plant exposure to the SA hormone, the concentration of loline alkaloids (total and the derivatives NFL and NANL) in endophyte-symbiotic plants did not vary among SA-treated and SA-untreated plants (Fig. 2) (Table 1).
Figure 2. Concentrations of loline alkaloids produced by the fungal endophyte Epichloë occultans in Lolium multiflorum plants.
Loline alkaloids were measured ten days after plants were exposed to salicylic acid (treated: SA+, untreated: SA−). Total lolines (black bars) are the sum of N-formylloline (NFL, grey bars) and N-acetylnorloline (NANL, white bars) derivatives. Non-symbiotic plants do not produce loline alkaloids. Each loline compound was analysed separately (see ‘Material and Method’ section). n.s. means non-significant differences between treatments. The bars represent mean values ± S.E.M. (n = 8).
Effects of plant endophyte presence and SA on S. maydis populations
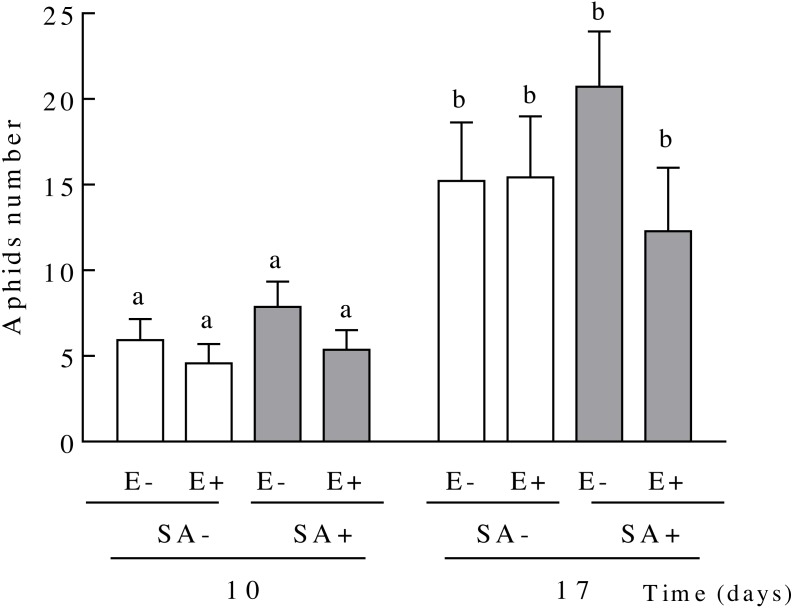
The aphid population size on L. multiflorum plants increased over time but this increase was independent of both the endophyte presence or the plant exposure to salicylic acid (Table 2 and Fig. 3). On average, the aphid population size increased 2.60 fold in 7 days (from days 10 to 17 since plants exposure to SA, 5.96 ± 0.63 and 15.91 ± 1.74, respectively) (Fig. 3).
Table 2. Effects of plant symbiotic status (E+, E−) and the exposure salicylic acid (SA+, SA-) on aphids number and standard metabolic rate of Sipha maydis aphids grown on Lolium multiflorum plants with the endophyte fungus Epichloë occultans.
Aphids number were measured at days 10 and 17 post salicylic acid application. Specific statistical differences for ‘aphids number’ response variable are shown in Figure 3. The volume of CO2 produced by aphids is abbreviated as ‘VCO2’. No significant differences were observed in standard metabolic rate (SMR) values. Replicate numbers are indicated in parenthesis. Values are mean ± S.E.M.
| Response variable | Treatment | df | χ2 or F | P-value | SA− | SA+ | ||
|---|---|---|---|---|---|---|---|---|
| E− | E+ | E− | E+ | |||||
| Aphids number (n = 14) | ||||||||
| Symbiosis | 1,52 | 2.30 | 0.127 | – | – | – | – | |
| SA | 1,52 | 0.01 | 0.922 | – | – | – | – | |
| Time | 1,52 | 7.26 | 0.007 | – | – | – | – | |
| Symbiosis × SA | 1,52 | 0.32 | 0.567 | – | – | – | – | |
| Symbiosis × Time | 1,52 | 0.76 | 0.382 | – | – | – | – | |
| SA× Time | 1,52 | 1.52 | 0.217 | – | – | – | – | |
| Symbiosis × SA× Time | 1,52 | 1.37 | 0.241 | – | – | – | – | |
| Aphid mass-specific SMR (µL VCO2 h−1 mg−1) (n = 5) | ||||||||
| Symbiosis | 1,16 | 0.28 | 0.601 | 5.53 ± 0.91 | 5.13 ± 0.56 | 4.98 ± 0.89 | 4.38 ± 0.59 | |
| SA | 1,16 | 0.52 | 0.479 | |||||
| Symbiosis × SA | 1,16 | 0.07 | 0.794 | |||||
Figure 3. Population sizes of Sipha maydis aphids grown on Lolium multiflorum plants exposed to the salicylic acid hormone and symbiotic with the endophyte fungus Epichloë occultans.
Aphids number were measured at days 10 and 17 post salicylic acid application [treated: SA+ (shaded bars), and untreated: SA− (unshaded bars)] on L. multiflorum plants with (E+) and without (E−) the endophyte fungus. Different letters indicate significant differences at P < 0.05. The bars represent mean values ± S.E.M. (n = 14).
Effects of plant endophyte presence and SA on S. maydis metabolic rate
The aphid mass-specific SMR, evaluated at day 24 since the insects were placed on the plants, was unaffected by the endophytic fungus, the SA hormone, or the interaction between them (Table 2).
Discussion
Since Epichloë fungi produce anti-herbivore alkaloids (Schardl et al., 2013a; Schardl et al., 2013b), we expected that endophytes would provide protection to host plants against the aphid S. maydis. However, we found that neither populations nor individuals of this aphid species were affected by the endophyte presence in plants. Despite the fact that aphids are usually controlled by the SA-dependent defence pathway (Thaler, Humphrey & Whiteman, 2012), here S. maydis aphids were not sensitive to the plant hormone exposure. Consistent with previous reports involving the plant interaction with beneficial microorganisms, the concentration of SA was lower in presence of the Epichloë endophyte fungus (Bastías et al., 2018a; Bastías et al., 2018b). However, the concentrations of alkaloids produced by endophytes was not affected by the plant exposure to SA.
The protection that each endophyte alkaloid type confers to host plants depends on, among other factors, the herbivore species (Bastias et al., 2017a; Bastías et al., 2017b). For example, loline fungal alkaloids confer effective protection against Rhopalosiphum padi aphids (Wilkinson et al., 2000), but the same alkaloids were ineffective in controlling Heteronychus arator beetles (Ball, Miles & Prestidge, 1997). In the present study, the presence of E. occultans, a loline producing endophyte fungus, did not confer protection to host L. multiflorum plants against S. maydis aphids. Similar findings were obtained in previous experiments studying the growth of S. maydis populations on the same plant-endophyte species system under field and laboratory conditions (Chaneton & Omacini, 2007; Miranda, Marina & Chaneton, 2011). In meadow fescue grass (Festuca pratensis) the presence of E. uncinatum, well-known to produce high concentrations of loline alkaloids, did not affect the population growth of S. maydis (Sabzalian, Hatami & Mirlohi, 2004). In that same study, however, the endophyte E. coenophiala (formerly Neotyphodium coenophialum) in tall fescue (Schedonorus arundinacea; formerly F. arundinacea) did effectively control S. maydis populations (Sabzalian, Hatami & Mirlohi, 2004). The difference between these two grass-endophyte symbioses, in terms of alkaloid profiles, is that while E. uncinatum only produces lolines, E. coenophiala synthesises ergopeptine and peramine alkaloids (Schardl et al., 2013a; Schardl et al., 2013b). Peramine and ergopeptine alkaloids produced by Epichloë endophytes are known for producing bioactivity against insects (Rowan, Hunt & Gaynor, 1986; Fleetwood et al., 2008). Thus, it is possible that the inefficiency of E. occultans endophytes in controlling S. maydis aphids in L. multiflorum is due, in part, to the particular profile of alkaloids produced by this endophyte species (Siegel et al., 1990).
The SA-dependent defence pathway is usually involved in plant responses to aphid attacks (Thaler, Humphrey & Whiteman, 2012; Ballaré, 2014). In the present study, the aphid S. maydis was not affected by the induction of the SA pathway (triggered by the plant exposure to the hormone). This insect insensitivity to plant SA-dependent defences has been reported for other aphids species (Bastías et al., 2018a; Bastías et al., 2018b; Onkokesung et al., 2016; Selig et al., 2016). The S. maydis insensitivity to SA-dependent defences could be explained by the potential capability of this insect species in detoxify L. multiflorum anti-herbivore metabolites. Detoxification of plant toxins is performed by enzymes that can deactivate or neutralize these metabolites (Després, David & Gallet, 2007). The synthesis of these detoxification enzymes is however, generally costly for insects, and these costs can be captured by metabolic measurements (e.g., Castañeda et al., 2010). In our study, however, S. maydis aphids grown on SA-treated L. multiflorum plants did not show any changes in their standard metabolic rates. To our knowledge, efficient mechanisms of detoxification of plant’s toxins have not been described for S. maydis aphids, which would be consistent with our findings for SMR. Adjustments in the feeding behaviour is another strategy that S. maydis aphids could have used to cope with plant defences (Walling, 2008). For instance, the aphid Sitobion avenae can reduce the time spent sucking phloem when feeding on SA-treated Triticum aestivum plants (Cao, Wang & Liu, 2014). In addition, it is also possible that an effective defence against S. maydis aphids in L. multiflorum requires more complex responses than just SA induction. For example, it has been documented that defence responses to aphids in Arabidopsis thaliana, Glycine max, and Sorghum bicolor plant species involves several hormone pathways that are sequentially induced during attacks by aphids (e.g., JA, SA, ethylene) (Moran & Thompson, 2001; Zhu-Salzman et al., 2004; De Vos et al., 2005; Li et al., 2008), and more recently that non-hormonal pathways can also be involved in these plant responses (i.e., methyl-D-erythritol-4-phosphate pathway) (Onkokesung et al., 2019).
Despite the fact that the fungal endophyte did not increase the resistance level of host plants against S. maydis aphids, endophyte-symbiotic plants may have been more tolerant to the aphid feeding due to the higher biomass they produced compared to their non-symbiotic counterparts. This growth promotion of L. multiflorum plants in presence of fungal endophytes has also been documented in other studies (Vila-Aiub, Gundel & Ghersa, 2005; Ueno et al., 2015; Bastías et al., 2018b). Since we had no aphid-free treatments, we cannot discard the possibility that the growth promotion documented in the present study had been a plant response to the aphid feeding more than a response to the endophyte presence (or a combination of both). However, findings from other studies suggest that this growth promotion might be a plant response to the endophyte presence. For example, another study using a similar experimental set-up to that used in the present work showed that endophyte-symbiotic L. multiflorum plants had a higher biomass than endophyte-free plants, and that this growth enhance was independent of the herbivory by R. padi aphids (Ueno et al., 2015).
It has been proposed that beneficial plant symbionts may regulate the SA pathway of the host to facilitate their own growth within plant tissues (Pozo & Azcón-Aguilar, 2007; Bastias et al., 2017a; Bastías et al., 2017b). This hypothesis has emerged from studies showing that the SA pathway can regulate the growth of these symbionts (Khaosaad et al., 2007; López-Ráez et al., 2010). In support of this, we observed that the plant SA concentration was reduced in presence of Epichloë fungal endophytes (see also Bastías et al., 2018a). In addition to regulating the growth of symbionts within plant tissues, the SA pathway can also modulate the functioning of beneficial symbiotic microorganisms (Hayat et al., 2010; Bastías et al., 2018a; De Vries et al., 2018). For example, the nitrogen fixation gene NifE in the beneficial cyanobiont, Nostoc azollae, was downregulated when Azolla filiculoides host plants were treated exogenously with methyl salicylate (De Vries et al., 2018). Based on these previous studies, we expected that symbiotic plants exposed to SA would show reduced levels of fungal alkaloids compared to untreated plants. Nevertheless, 10-days after the plants were exposed to the hormone, the concentration of loline alkaloids was similar between SA-treated and SA-untreated symbiotic plants. This result does not support our previous work which showed that alkaloid concentrations of endophyte-symbiotic plants were indeed reduced by SA treatment (Bastías et al., 2018a). It may be the case that in the present study, after an initial drop caused by the plant exposure to SA, fungal alkaloids were able to recover to their pre-treatment concentrations. Rapid increases in fungal alkaloids concentrations have been previously reported in plants symbiotic with Epichloë fungal endophytes. For example, we previously found that alkaloid concentrations took less than 7-days to respond to aphid herbivory (Bastías et al., 2018a).
Conclusions
The present study indicates that the aphid Sipha maydis is insensitive to the anti-herbivore defences of L. multiflorum in symbiosis with Epichloë occultans. Our results indicate that neither the presence of E. occultans endophytes nor the induction of plant SA pathway regulate S. maydis populations. However, endophyte-symbiotic plants may have been more tolerant to the aphid feeding because these plants produced more aboveground biomass. We suggest that this insect insensitivity could be explained by a combination between the ineffectiveness of loline alkaloids (produced by E. occultans) in controlling S. maydis aphids and the capacity of this herbivore to tolerate the hormone-dependent defences of L. multiflorum.
Supplemental Information
Plant: Lolium multiflorum; fungal endophyte: Epichloë occultans; aphid: Sipha maydis.
Acknowledgments
We gratefully acknowledge Dr. Armen Charchoglyan (University of Guelph) for assistance with GC-MS/MS analysis and Dr. Edmundo L. Ploschuk (Universidad de Buenos Aires) for assistance with metabolic measurements.
Funding Statement
Daniel A. Bastías was supported by a fellowship from the Agencia Nacional de Promoción Científica and CONICET, and from the Canadian Emerging Leaders in the Americas Program (ELAP). Funding to support this research was provided by the Agencia Nacional de Promoción Científica FONCYT (PICT-2355), Universidad de Buenos Aires UBA (UBACYT 2014-30BA) to M. Alejandra Martínez-Ghersa, and Canadian Natural Science and Engineering Research Council to Jonathan A. Newman. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Daniel A. Bastías, Stuart D. Card and Wade J. Mace are employed by Forage Science, AgResearch Limited.
Author Contributions
Daniel A. Bastías conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Maria Alejandra Martínez-Ghersa conceived and designed the experiments, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Jonathan A. Newman conceived and designed the experiments, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Stuart D. Card and Wade J. Mace performed the experiments, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Pedro E. Gundel conceived and designed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability: The raw data are available on figshare: Bastías, Daniel A; Martínez-Ghersa, Maria Alejandra; Newman, Jonathan A; Card, Stuart D.; Mace, Wade J.; Gundel, Pedro E. (2019): Table S1_Data.xlsx. figshare. Dataset. https://doi.org/10.6084/m9.figshare.9345524.v1.
References
- Bacon & White (1994).Bacon C, White J. Stains, media and procedures for analyzing endophytes. In: Bacon C, White J, editors. Biotechnology of endophytic fungi of grasses. CRC Press; Boca Raton: 1994. [Google Scholar]
- Ball, Miles & Prestidge (1997).Ball OJP, Miles CO, Prestidge RA. Ergopeptine alkaloids and Neotyphodium lolii-mediated resistance in perennial ryegrass against adult Heteronychus arator (Coleoptera: Scarabaeidae) Journal of Economic Entomology. 1997;90:1382–1391. doi: 10.1093/jee/90.5.1382. [DOI] [Google Scholar]
- Ballaré (2014).Ballaré CL. Light regulation of plant defense. Annual Review of Plant Biology. 2014;65:335–363. doi: 10.1146/annurev-arplant-050213-040145. [DOI] [PubMed] [Google Scholar]
- Bastias et al. (2017a).Bastias DA, Martínez-Ghersa MA, Ballaré CL, Gundel PE. Epichloë fungal endophytes and plant defenses: not just alkaloids. Trends in Plant Science. 2017a;22:939–948. doi: 10.1016/j.tplants.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Bastías et al. (2018a).Bastías DA, Martínez-Ghersa MA, Newman JA, Card SD, Mace WJ, Gundel PE. The plant hormone salicylic acid interacts with the mechanism of anti-herbivory conferred by fungal endophytes in grasses. Plant, Cell & Environment. 2018a;41:395–405. doi: 10.1111/pce.13102. [DOI] [PubMed] [Google Scholar]
- Bastías et al. (2018b).Bastías DA, Martínez-Ghersa MA, Newman JA, Card SD, Mace WJ, Gundel PE. Jasmonic acid regulation of the anti-herbivory mechanism conferred by fungal endophytes in grasses. Journal of Ecology. 2018b;106:2365–2379. doi: 10.1111/1365-2745.12990. [DOI] [Google Scholar]
- Bastías et al. (2017b).Bastías DA, Ueno AC, Machado Assefh CR, Alvarez AE, Young CA, Gundel PE. Metabolism or behavior: explaining the performance of aphids on alkaloid-producing fungal endophytes in annual ryegrass (Lolium multiflorum) Oecologia. 2017b;185:245–256. doi: 10.1007/s00442-017-3940-2. [DOI] [PubMed] [Google Scholar]
- Bedini et al. (2018).Bedini A, Mercy L, Schneider C, Franken P, Lucic-Mercy E. Unraveling the initial plant hormone signaling, metabolic mechanisms and plant defense triggering the endomycorrhizal symbiosis behavior. Frontiers in Plant Science. 2018;9:1800–1800. doi: 10.3389/fpls.2018.01800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultman et al. (2018).Bultman TL, McNeill MR, Krueger K, De Nicolo G, Popay AJ, Hume DE, Mace WJ, Fletcher LR, Koh YM, Sullivan TJ. Complex interactions among sheep, insects, grass, and fungi in a simple New Zealand grazing system. Journal of Chemical Ecology. 2018;44:957–964. doi: 10.1007/s10886-018-0993-6. [DOI] [PubMed] [Google Scholar]
- Cahill Jr, Castelli & Casper (2002).Cahill Jr JF, Castelli JP, Casper BB. Separate effects of human visitation and touch on plant growth and herbivory in an old-field community. American Journal of Botany. 2002;89:1401–1409. doi: 10.3732/ajb.89.9.1401. [DOI] [PubMed] [Google Scholar]
- Cao, Wang & Liu (2014).Cao HH, Wang SH, Liu TX. Jasmonate- and salicylate-induced defenses in wheat affect host preference and probing behavior but not performance of the grain aphid, Sitobion avenae. Insect Science. 2014;21:47–55. doi: 10.1111/1744-7917.12023. [DOI] [PubMed] [Google Scholar]
- Cao et al. (2017).Cao Y, Halane MK, Gassmann W, Stacey G. The role of plant innate immunity in the legume-Rhizobium symbiosis. Annual Review of Plant Biology. 2017;68:535–561. doi: 10.1146/annurev-arplant-042916-041030. [DOI] [PubMed] [Google Scholar]
- Card et al. (2011).Card SD, Rolston MP, Park Z, Cox N, Hume DE. Fungal endophyte detection in pasture grass seed utilising the infection layer and comparison to other detection techniques. Seed Science and Technology. 2011;39:581–592. doi: 10.15258/sst.2011.39.3.05. [DOI] [Google Scholar]
- Castañeda et al. (2010).Castañeda LE, Figueroa CC, Fuentes-Contreras E, Niemeyer HM, Nespolo RF. Physiological approach to explain the ecological success of ‘superclones’ in aphids: interplay between detoxification enzymes, metabolism and fitness. Journal of Insect Physiology. 2010;56:1058–1064. doi: 10.1016/j.jinsphys.2010.02.019. [DOI] [PubMed] [Google Scholar]
- Castañeda, Figueroa & Nespolo (2010).Castañeda LE, Figueroa CC, Nespolo RF. Do insect pests perform better on highly defended plants? Costs and benefits of induced detoxification defences in the aphid Sitobion avenae. Journal of Evolutionary Biology. 2010;23:2474–2483. doi: 10.1111/j.1420-9101.2010.02112.x. [DOI] [PubMed] [Google Scholar]
- Chaneton & Omacini (2007).Chaneton EJ, Omacini M. Bottom–up cascades induced by fungal endophytes in multitrophic systems. In: Price PW, Ohgushi T, Craig TP, editors. Ecological communities: plant mediation in indirect interaction webs. Cambridge University Press; Cambridge: 2007. pp. 164–187. [DOI] [Google Scholar]
- Clay (1988).Clay K. Fungal endophytes of grasses: a defensive mutualism between plants and fungi. Ecology. 1988;69:10–16. doi: 10.2307/1943155. [DOI] [Google Scholar]
- Corrales et al. (2007).Corrales CE, Castro AM, Ricci M, Dixon AFG. Sipha maydis: distribution and host range of a new aphid pest of winter cereals in Argentina. Journal of Economic Entomology. 2007;100:1781–1788. doi: 10.1093/jee/100.6.1781. [DOI] [PubMed] [Google Scholar]
- De Vos et al. (2005).De Vos M, Van Oosten VR, Van Poecke RMP, Van Pelt JA, Pozo MJ, Mueller MJ, Buchala AJ, Métraux J-P, Van Loon LC, Dicke M, Pieterse CMJ. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Molecular Plant-Microbe Interactions. 2005;18:923–937. doi: 10.1094/MPMI-18-0923. [DOI] [PubMed] [Google Scholar]
- De Vries et al. (2018).De Vries S, De Vries J, Teschke H, Von Dahlen JK, Rose LE, Gould SB. Jasmonic and salicylic acid response in the fern Azolla filiculoides and its cyanobiont. Plant, Cell & Environment. 2018;41:2530–2548. doi: 10.1111/pce.13131. [DOI] [PubMed] [Google Scholar]
- Després, David & Gallet (2007).Després L, David J-P, Gallet C. The evolutionary ecology of insect resistance to plant chemicals. Trends in Ecology & Evolution. 2007;22:298–307. doi: 10.1016/j.tree.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Dupont et al. (2015).Dupont P, Eaton CJ, Wargent JJ, Fechtner S, Solomon P, Schmid J, Day RC, Scott B, Cox MP. Fungal endophyte infection of ryegrass reprograms host metabolism and alters development. New Phytologist. 2015;208:1227–1240. doi: 10.1111/nph.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenseer, Dahlman & Bush (1991).Eichenseer H, Dahlman DL, Bush LP. Influence of endophyte infection, plant age and harvest interval on Rhopalosiphum padi survival and its relation to quantity of N-formyl and N-acetyl loline in tall fescue. Entomologia Experimentalis et Applicata. 1991;60:29–38. doi: 10.1111/j.1570-7458.1991.tb01519.x. [DOI] [Google Scholar]
- Fleetwood et al. (2008).Fleetwood DJ, Scott B, Voisey CR, Johnson RD. Insights into the molecular biology of Epichloë endophyte alkaloid biosynthesis. Proceedings of the New Zealand Grassland Association. 2008;70:217–220. [Google Scholar]
- Fournier et al. (2012).Fournier DA, Skaug HJ, Ancheta J, Ianelli J, Magnusson A, Maunder MN, Nielsen A, Sibert J. AD model builder: using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optimization Methods and Software. 2012;27:233–249. doi: 10.1080/10556788.2011.597854. [DOI] [Google Scholar]
- Fuchs et al. (2017a).Fuchs B, Krischke M, Mueller MJ, Krauss J. Herbivore-specific induction of defence metabolites in a grass—endophyte association. Functional Ecology. 2017a;31:318–324. doi: 10.1111/1365-2435.12755. [DOI] [Google Scholar]
- Fuchs et al. (2017b).Fuchs B, Krischke M, Mueller MJ, Krauss J. Plant age and seasonal timing determine endophyte growth and alkaloid biosynthesis. Fungal Ecology. 2017b;29:52–58. doi: 10.1016/j.funeco.2017.06.003. [DOI] [Google Scholar]
- Gundel et al. (2009).Gundel PE, Garibaldi L, Tognetti P, Aragón R, Ghersa C, Omacini M. Imperfect vertical transmission of the endophyte Neotyphodium in exotic grasses in grasslands of the flooding pampa. Microbial Ecology. 2009;57:740–748. doi: 10.1007/s00248-008-9447. [DOI] [PubMed] [Google Scholar]
- Gundel et al. (2012).Gundel PE, Martínez-Ghersa MA, Omacini M, Cuyeu R, Pagano E, Ríos R, Ghersa CM. Mutualism effectiveness and vertical transmission of symbiotic fungal endophytes in response to host genetic background. Evolutionary Applications. 2012;5:838–849. doi: 10.1111/j.1752-4571.2012.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundel, Rudgers & Ghersa (2011).Gundel PE, Rudgers JA, Ghersa CM. Incorporating the process of vertical transmission into understanding of host–symbiont dynamics. Oikos. 2011;120:1121–1128. doi: 10.1111/j.1600-0706.2011.19299.x. [DOI] [Google Scholar]
- Gundel et al. (2018).Gundel PE, Seal CE, Biganzoli F, Molina-Montenegro MA, Vázquez-de Aldana BR, Zabalgogeazcoa I, Bush LP, Martínez-Ghersa MA, Ghersa CM. Occurrence of alkaloids in grass seeds symbiotic with vertically-transmitted Epichloë fungal endophytes and its relationship with antioxidants. Frontiers in Ecology and Evolution. 2018;6 doi: 10.3389/fevo.2018.00211. Article 211. [DOI] [Google Scholar]
- Hayat et al. (2010).Hayat Q, Hayat S, Irfan M, Ahmad A. Effect of exogenous salicylic acid under changing environment: a review. Environmental and Experimental Botany. 2010;68:14–25. doi: 10.1016/j.envexpbot.2009.08.005. [DOI] [Google Scholar]
- Hunt et al. (2005).Hunt MG, Rasmussen S, Newton PCD, Parsons AJ, Newman JA. Near-term impacts of elevated CO2, nitrogen and fungal endophyte-infection on Lolium perenne L. growth, chemical composition and alkaloid production. Plant, Cell & Environment. 2005;28:1345–1354. doi: 10.1111/j.1365-3040.2005.01367.x. [DOI] [Google Scholar]
- Johnson et al. (2003).Johnson LJ, Johnson RD, Schardl CL, Panaccione DG. Identification of differentially expressed genes in the mutualistic association of tall fescue with Neotyphodium coenophialum. Physiological and Molecular Plant Pathology. 2003;63:305–317. doi: 10.1016/j.pmpp.2004.04.001. [DOI] [Google Scholar]
- Johnson et al. (1985).Johnson MC, Dahlman DL, Siegel MR, Bush LP, Latch GCM, Potter DA, Varney DR. Insect feeding deterrents in endophyte-infected tall fescue. Applied and Environmental Microbiology. 1985;49:568–571. doi: 10.1128/aem.49.3.568-571.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung et al. (2012).Jung S, Martinez-Medina A, Lopez-Raez J, Pozo M. Mycorrhiza-induced resistance and priming of plant defenses. Journal of Chemical Ecology. 2012;38:651–664. doi: 10.1007/s10886-012-0134-6. [DOI] [PubMed] [Google Scholar]
- Justus, Witte & Hartmann (1997).Justus M, Witte L, Hartmann T. Levels and tissue distribution of loline alkaloids in endophyte-infected Festuca pratensis. Phytochemistry. 1997;44:51–57. doi: 10.1016/S0031-9422(96)00535-3. [DOI] [Google Scholar]
- Khaosaad et al. (2007).Khaosaad T, García-Garrido JM, Steinkellner S, Vierheilig H. Take-all disease is systemically reduced in roots of mycorrhizal barley plants. Soil Biology and Biochemistry. 2007;39:727–734. doi: 10.1016/j.soilbio.2006.09.014. [DOI] [Google Scholar]
- Lenth (2016).Lenth RV. Least-squares means: the R package lsmeans. Journal of Statistical Software. 2016;69:1–33. doi: 10.18637/jss.v069.i01. [DOI] [Google Scholar]
- Li et al. (2008).Li Y, Zou J, Li M, Bilgin DD, Vodkin LO, Hartman GL, Clough SJ. Soybean defense responses to the soybean aphid. New Phytologist. 2008;179:185–195. doi: 10.1111/j.1469-8137.2008.02443.x. [DOI] [PubMed] [Google Scholar]
- López-Ráez et al. (2010).López-Ráez JA, Verhage A, Fernández I, García JM, Azcón-Aguilar C, Flors V, Pozo MJ. Hormonal and transcriptional profiles highlight common and differential host responses to arbuscular mycorrhizal fungi and the regulation of the oxylipin pathway. Journal of Experimental Botany. 2010;61:2589–2601. doi: 10.1093/jxb/erq089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda, Marina & Chaneton (2011).Miranda MI, Omacini M, Chaneton EJ. Environmental context of endophyte symbioses: interacting effects of water stress and insect herbivory. International Journal of Plant Sciences. 2011;172:499–508. doi: 10.1086/658921. [DOI] [Google Scholar]
- Moon et al. (2000).Moon CD, Scott B, Schardl CL, Christensen MJ. The evolutionary origins of Epichloë endophytes from annual ryegrasses. Mycologia. 2000;92:1103–1118. doi: 10.2307/3761478. [DOI] [Google Scholar]
- Moore et al. (2015).Moore JR, Pratley JE, Mace WJ, Weston LA. Variation in alkaloid production from genetically diverse Lolium accessions infected with Epichloë species. Journal of Agricultural and Food Chemistry. 2015;63:10355–10365. doi: 10.1021/acs.jafc.5b03089. [DOI] [PubMed] [Google Scholar]
- Moran & Thompson (2001).Moran PJ, Thompson GA. Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiology. 2001;125:1074–1085. doi: 10.1104/pp.125.2.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira, Abdala-Roberts & Castagneyrol (2018).Moreira X, Abdala-Roberts L, Castagneyrol B. Interactions between plant defence signalling pathways: evidence from bioassays with insect herbivores and plant pathogens. Journal of Ecology. 2018;106:2353–2364. doi: 10.1111/1365-2745.12987. [DOI] [Google Scholar]
- Navarro-Meléndez & Heil (2014).Navarro-Meléndez AL, Heil M. Symptomless endophytic fungi suppress endogenous levels of salicylic acid and interact with the jasmonate-dependent indirect defense traits of their host, lima bean (Phaseolus lunatus) Journal of Chemical Ecology. 2014;40:816–825. doi: 10.1007/s10886-014-0477-2. [DOI] [PubMed] [Google Scholar]
- Nespolo, Roff & Fairbairn (2008).Nespolo RF, Roff DA, Fairbairn DJ. Energetic trade-off between maintenance costs and flight capacity in the sand cricket (Gryllus firmus) Functional Ecology. 2008;22:624–631. doi: 10.1111/j.1365-2435.2008.01394.x. [DOI] [Google Scholar]
- Omacini et al. (2001).Omacini M, Chaneton EJ, Ghersa CM, Muller CB. Symbiotic fungal endophytes control insect host-parasite interaction webs. Nature. 2001;409:78–81. doi: 10.1038/35051070. [DOI] [PubMed] [Google Scholar]
- Onkokesung et al. (2016).Onkokesung N, Reichelt M, Van Doorn A, Schuurink RC, Dicke M. Differential costs of two distinct resistance mechanisms induced by different herbivore species in Arabidopsis. Plant Physiology. 2016;170:891–906. doi: 10.1104/pp.15.01780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onkokesung et al. (2019).Onkokesung N, Reichelt M, Wright LP, Phillips MA, Gershenzon J, Dicke M. The plastidial metabolite 2-C-methyl-D-erythritol-2, 4-cyclodiphosphate modulates defense responses against aphids. Plant, Cell & Environment. 2019;42:2309–2323. doi: 10.1111/pce.13538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaccione, Beaulieu & Cook (2014).Panaccione DG, Beaulieu WT, Cook D. Bioactive alkaloids in vertically transmitted fungal endophytes. Functional Ecology. 2014;28:299–314. doi: 10.1111/1365-2435.12076. [DOI] [Google Scholar]
- Pinheiro et al. (2009).Pinheiro J, Bates D, DebRoy S, Sarkar D. Vienna: R Foundation for Statistical Computing; 2009. [Google Scholar]
- Pozo & Azcón-Aguilar (2007).Pozo MJ, Azcón-Aguilar C. Unraveling mycorrhiza-induced resistance. Current Opinion in Plant Biology. 2007;10:393–398. doi: 10.1016/j.pbi.2007.05.004. [DOI] [PubMed] [Google Scholar]
- R Core Team (2013).R Core Team . Vienna: R Foundation for Statistical Computing; 2013. [Google Scholar]
- Ramos et al. (2018).Ramos P, Rivas N, Pollmann S, Casati P, Molina-Montenegro MA. Hormonal and physiological changes driven by fungal endophytes increase Antarctic plant performance under UV-B radiation. Fungal Ecology. 2018;34:76–82. doi: 10.1016/j.funeco.2018.05.006. [DOI] [Google Scholar]
- Rasmussen et al. (2007).Rasmussen S, Parsons AJ, Bassett S, Christensen MJ, Hume DE, Johnson LJ, Johnson RD, Simpson WR, Stacke C, Voisey CR, Xue H, Newman JA. High nitrogen supply and carbohydrate content reduce fungal endophyte and alkaloid concentration in Lolium perenne. New Phytologist. 2007;173:787–797. doi: 10.1111/j.1469-8137.2006.01960.x. [DOI] [PubMed] [Google Scholar]
- Rowan, Hunt & Gaynor (1986).Rowan DD, Hunt MB, Gaynor DL. Peramine, a novel insect feeding deterrent from ryegrass infected with the endophyte Acremonium loliae. Journal of the Chemical Society, Chemical Communications. 1986;142:935–936. doi: 10.1039/C39860000935. [DOI] [PubMed] [Google Scholar]
- Ryan et al. (2014).Ryan GD, Rasmussen S, Xue H, Parsons AJ, Newman JA. Metabolite analysis of the effects of elevated CO2 and nitrogen fertilization on the association between tall fescue (Schedonorus arundinaceus) and its fungal symbiont Neotyphodium coenophialum. Plant, Cell & Environment. 2014;37:204–212. doi: 10.1111/pce.12146. [DOI] [PubMed] [Google Scholar]
- Sabzalian, Hatami & Mirlohi (2004).Sabzalian MR, Hatami B, Mirlohi A. Mealybug, Phenococcus solani, and barley aphid, Sipha maydis, response to endophyte-infected tall and meadow fescues. Entomologia Experimentalis et Applicata. 2004;113:205–209. doi: 10.1111/j.0013-8703.2004.00227. [DOI] [Google Scholar]
- Saikkonen, Gundel & Helander (2013).Saikkonen K, Gundel P, Helander M. Chemical ecology mediated by fungal endophytes in grasses. Journal of Chemical Ecology. 2013;39:962–968. doi: 10.1007/s10886-013-0310-3. [DOI] [PubMed] [Google Scholar]
- Saikkonen, Saari & Helander (2010).Saikkonen K, Saari S, Helander M. Defensive mutualism between plants and endophytic fungi? Fungal Diversity. 2010;41:101–113. doi: 10.1007/s13225-010-0023-7. [DOI] [Google Scholar]
- Schardl et al. (2013a).Schardl CL, Florea S, Pan J, Nagabhyru P, Bec S, Calie PJ. The Epichloae: alkaloid diversity and roles in symbiosis with grasses. Current Opinion in Plant Biology. 2013a;16:480–488. doi: 10.1016/j.pbi.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardl et al. (2013b).Schardl CL, Young C, Pan J, Florea S, Takach J, Panaccione D, Farman M, Webb J, Jaromczyk J, Charlton N, Nagabhyru P, Chen L, Shi C, Leuchtmann A. Currencies of mutualisms: sources of alkaloid genes in vertically transmitted Epichloae. Toxins. 2013b;5:1064–1088. doi: 10.3390/toxins5061064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selig et al. (2016).Selig P, Keough S, Nalam VJ, Nachappa P. Jasmonate-dependent plant defenses mediate soybean thrips and soybean aphid performance on soybean. Arthropod-Plant Interactions. 2016;10:273–282. doi: 10.1007/s11829-016-9437-9. [DOI] [Google Scholar]
- Siegel et al. (1990).Siegel MR, Latch GCM, Bush LP, Fannin FF, Rowan DD, Tapper BA, Bacon CW, Johnson MC. Fungal endophyte-infected grasses: alkaloid accumulation and aphid response. Journal of Chemical Ecology. 1990;16:3301–3315. doi: 10.1007/BF00982100. [DOI] [PubMed] [Google Scholar]
- Skvarla et al. (2017).Skvarla MJ, Halbert SE, Foottit RG, Jensen AS, Maw E, Miller GL. An update to the adventive aphids (Hemiptera: Aphidoidea) of America north of Mexico, with notes on intercepted species. Proceedings of the Entomological Society of Washington. 2017;119:90–112. doi: 10.4289/0013-8797.119.1.90. [DOI] [Google Scholar]
- Stacey et al. (2006).Stacey G, McAlvin CB, Kim S-Y, Olivares J, Soto MJ. Effects of endogenous salicylic acid on nodulation in the model legumes Lotus japonicus and Medicago truncatula. Plant Physiology. 2006;141:1473–1481. doi: 10.1104/pp.106.080986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara et al. (2006).Sugawara K, Inoue T, Yamashita M, Ohkubo H. Distribution of the endophytic fungus, Neotyphodium occultans in naturalized Italian ryegrass in western Japan and its production of bioactive alkaloids known to repel insect pests. Grassland Science. 2006;52:147–154. doi: 10.1111/j.1744-697X.2006.00060.x. [DOI] [Google Scholar]
- Thaler, Humphrey & Whiteman (2012).Thaler JS, Humphrey PT, Whiteman NK. Evolution of jasmonate and salicylate signal crosstalk. Trends in Plant Science. 2012;17:260–270. doi: 10.1016/j.tplants.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Uchitel, Omacini & Chaneton (2011).Uchitel A, Omacini M, Chaneton EJ. Inherited fungal symbionts enhance establishment of an invasive annual grass across successional habitats. Oecologia. 2011;165:465–475. doi: 10.1007/s00442-010-1740-z. [DOI] [PubMed] [Google Scholar]
- Ueno et al. (2015).Ueno AC, Gundel PE, Omacini M, Ghersa CM, Bush LP, Martínez-Ghersa MA. Mutualism effectiveness of a fungal endophyte in an annual grass is impaired by ozone. Functional Ecology. 2015;30:226–232. doi: 10.1111/1365-2435.12519. [DOI] [Google Scholar]
- Vila-Aiub, Gundel & Ghersa (2005).Vila-Aiub MM, Gundel PE, Ghersa CM. Fungal endophyte infection changes growth attributes in Lolium multiflorum Lam. Austral Ecology. 2005;30:49–57. doi: 10.1111/j.1442-9993.2005.01423.x. [DOI] [Google Scholar]
- Walling (2008).Walling LL. Avoiding effective defenses: strategies employed by phloem-feeding insects. Plant Physiology. 2008;146:859–866. doi: 10.1104/pp.107.113142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson et al. (2000).Wilkinson HH, Siegel MR, Blankenship JD, Mallory AC, Bush LP, Schardl CL. Contribution of fungal loline alkaloids to protection from aphids in a grass-endophyte mutualism. Molecular Plant-Microbe Interactions. 2000;13:1027–1033. doi: 10.1094/MPMI.2000.13.10.1027. [DOI] [PubMed] [Google Scholar]
- Zhu-Salzman et al. (2004).Zhu-Salzman K, Salzman RA, Ahn J-E, Koiwa H. Transcriptional regulation of Sorghum defense determinants against a phloem-feeding aphid. Plant Physiology. 2004;134:420–431. doi: 10.1104/pp.103.028324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuur et al. (2009).Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smit GM. Mixed effects models and extensions in ecology with R. Springer Sciences; New York: 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plant: Lolium multiflorum; fungal endophyte: Epichloë occultans; aphid: Sipha maydis.
Data Availability Statement
The following information was supplied regarding data availability: The raw data are available on figshare: Bastías, Daniel A; Martínez-Ghersa, Maria Alejandra; Newman, Jonathan A; Card, Stuart D.; Mace, Wade J.; Gundel, Pedro E. (2019): Table S1_Data.xlsx. figshare. Dataset. https://doi.org/10.6084/m9.figshare.9345524.v1.