Abstract
Introduction
Pirfenidone is an oral antifibrotic agent approved for idiopathic pulmonary fibrosis (IPF). Real-world data on adverse event (AE) management for pirfenidone are limited. Strategies for managing potential antifibrotic therapy AEs were examined in a sample of US pulmonologists.
Methods
An online, self-administered survey was fielded to pulmonologists between April 10 and May 17, 2017. Pulmonologists were included if they spent > 20% of their time in direct patient care and had ≥ 5 patients with IPF on antifibrotic therapy. Participants answered questions regarding initiation of pirfenidone, dose titration, and management of potential AEs.
Results
A total of 169 pulmonologists participated. Gastrointestinal (GI) intolerance was the most important factor in implementing alternative titration schedules for pirfenidone. Approximately three-quarters of pulmonologists recommended the standard titration scheme for starting treatment; however, a range of titration schedules up to 8 weeks were described, with a 4-week schedule being most common. Pulmonologists reported that most patients treated with alternative titration schedules could achieve the full dose of pirfenidone. Pulmonologists who were most effective at mitigating pirfenidone-related GI AEs by advising dosing at mealtimes more frequently recommended taking pirfenidone during a substantial meal than pulmonologists who were less effective. For photosensitivity AEs, pulmonologists recommended sunscreen use, sun avoidance, wearing a hat, and ultraviolet protection factor clothing.
Conclusions
Pulmonologists reported that alternative titration schedules for initiating pirfenidone were common and can aid in maintaining the full dose. Proposed strategies to ameliorate pirfenidone-related GI and photosensitivity AEs included taking pirfenidone during a substantial meal and minimizing sun exposure, respectively.
Funding
F. Hoffmann-La Roche Ltd./Genentech, Inc.
Plain Language Summary
Plain language summary available for this article.
Keywords: Adverse event management, Antifibrotic therapy, Idiopathic pulmonary fibrosis, Pirfenidone, Real-world, Titration
Plain Language Summary
Idiopathic pulmonary fibrosis (IPF) is a deadly lung disease. Pirfenidone is a medication that slows down the disease in patients with IPF. Stomach- and skin-related side effects are common with pirfenidone. There is little information on ways to manage these side effects. In an online survey, US pulmonologists (lung disease specialists) answered questions on how they start patients on pirfenidone, adjust doses, and manage side effects. The use of different dosing schedules when starting pirfenidone was common practice among these pulmonologists and could help patients stay on pirfenidone if they had side effects. Taking pirfenidone during a large meal and staying out of the sun were key recommendations for managing common stomach and skin side effects.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive, irreversible, and fatal fibrotic lung disease [1]. The clinical course of IPF is highly variable and unpredictable in individual patients; the median survival is 2–5 years from diagnosis [2]. IPF typically presents with unexplained dyspnea on exertion or cough for > 3 months and nonspecific bibasilar, inspiratory crackles [1, 2]. Pirfenidone is an oral antifibrotic therapy approved for the treatment of patients with IPF [3–5]. The recommended total daily dose of pirfenidone is 2403 mg/day in three equally divided doses, with food [6].
Three multinational, phase III clinical trials [CAPACITY (Studies 004 and 006; NCT00287716 and NCT00287729) and ASCEND (Study 016; NCT01366209)] investigated the efficacy and safety of pirfenidone in patients with IPF [3, 4]. Pirfenidone slows disease progression by reducing the rate of lung function decline [3, 4]. In a pre-specified pooled analysis of all-cause mortality in ASCEND and CAPACITY, pirfenidone reduced the risk of death at 1 year by 48% compared with placebo [3, 7]. For patients receiving pirfenidone, gastrointestinal (GI)- and skin-related adverse events (AEs) are the most common AEs reported [3–5, 8]. These AEs can affect tolerability in some patients, particularly within the first 6 months of treatment [9]. In the phase III trials, the median time to the first GI AE (in 77.8% of patients), rash AE (35.8%), and photosensitivity AE (9.3%) was 14.0 (range, 5.0–40.0), 82.0 (range, 49.0–132.0), and 90.0 (range, 48.0–149.0) days, respectively [10].
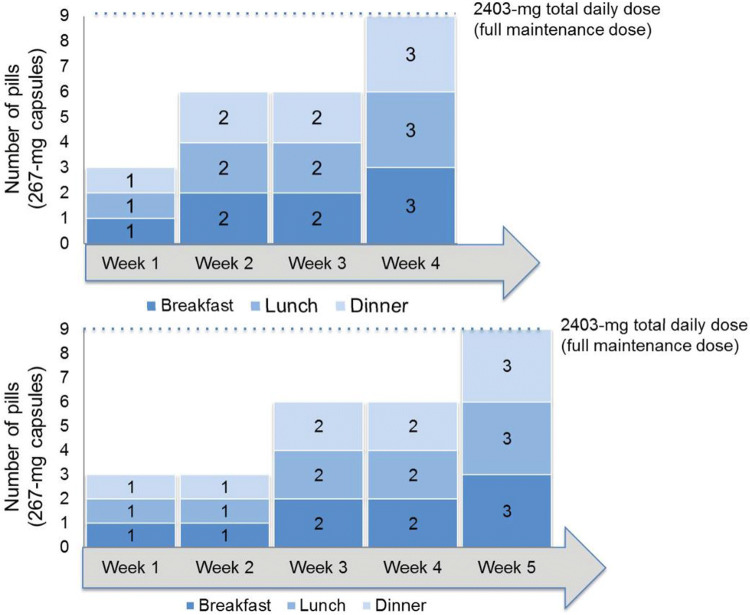
A 2-week titration period is recommended for pirfenidone, with a starting dose of one 267-mg pill (capsule or tablet) three times daily (tid) with food for 1 week, followed by two pills tid (534 mg) with food for 1 week and then three pills (801 mg) tid (maintenance dose) with food [6]. Once maintenance dosing is achieved, patients may continue to take three 267-mg pills tid or switch to one 801-mg pill tid, which reduces the pill burden in patients receiving pirfenidone [11].
One empirical method for preventing pirfenidone-related GI AEs is gradual dose titration upon initiation of pirfenidone [9]. Although an expert panel has recommended strategies for mitigating pirfenidone AEs and management strategies have been proposed on the basis of clinical trial data, real-world data on AE management in patients receiving pirfenidone therapy are limited [9, 12]. Other real-world data analyses have focused on efficacy and tolerability of pirfenidone, physicians’ expected use of IPF therapy based on disease severity in Europe and the United States, and treatment patterns and prescribing practices for antifibrotic therapy in patients with IPF in Europe [13–15]. This real-world study examined practice patterns for treatment with antifibrotic therapy (pirfenidone and/or nintedanib) in patients with IPF in a geographically varied sample of pulmonologists in the United States. In this analysis, we describe strategies for prevention and management of potential AEs in patients with IPF who are receiving pirfenidone.
Methods
Survey Design
An online, self-administered, 30-min survey was developed using experts in the fields of medicine, epidemiology, health economics, and psychometrics. The survey was individually pilot tested by an expert physician in interstitial lung disease (ILD) who advised on the development of the survey so that questions were placed in the proper context for respondents. The survey was additionally pilot tested using a random sample of six ILD experts and four community-based pulmonologists to assess the content, flow, complexity, and timing for completion of the survey. The identity of the company sponsoring the survey, Genentech, Inc., was blinded to respondents, and respondents’ identities were kept confidential to Genentech, Inc. Additionally, to maintain confidentiality, the survey collected information on both approved antifibrotic therapies.
The survey was fielded in the United States between April 10, 2017, and May 17, 2017. More than 400 ILD experts—deemed to be experts in the treatment of pulmonary fibrosis by way of being part of the Pulmonary Fibrosis Foundation’s (PFF) designated PFF Care Center Network—were contacted [16]. The compilation of experts was based on the PFF’s experience in working with leading medical centers around the United States to fund research and improve care [16]. In addition, over 1200 community pulmonologists sourced from an external database of physicians were contacted. For this study, a “community pulmonologist” was defined as a pulmonologist who did not report seeing most of their patients in an ILD center participating in the PFF Care Center Network. As part of the survey, respondents were asked to further categorize their main practice setting (e.g., academic or community). Respondents answered questions regarding initiation of antifibrotic therapy (pirfenidone and/or nintedanib), dose titration, and management of potential AEs. This analysis specifically focused on real-world strategies for pirfenidone. For questions regarding pirfenidone, the survey reflected clinical experience prior to the commercial availability of the 267- or 801-mg pirfenidone tablets, which were launched in the United States in mid-May 2017. Therefore, results reflect use of the 267-mg pirfenidone capsule only.
Pulmonologist Screening Criteria
Healthcare practitioners were eligible to participate in the survey if they were licensed to practice medicine in the United States; were board eligible or board certified in pulmonary disease; completed training (residency/fellowship) > 1 year prior to participation; spent > 20% of their time in direct patient care; treated patients with IPF with antifibrotic therapy (pirfenidone and/or nintedanib); and had ≥ five patients with IPF on antifibrotic therapy.
Statistical Analyses
Survey responses regarding pirfenidone were described using summary statistics, including frequencies and percentages for categorical data and means (standard deviations) for continuous data. For some questions, participants were asked to rank the importance of several factors using a scale of 1–5, where 1 = “not important” and 5 = “extremely important”. Pulmonologists who rated the effectiveness of their specific recommendations for pirfenidone dosing at mealtimes at mitigating GI-related AEs as a 4 or 5 (“often” or “always”) were compared with those who rated the effectiveness as a 1, 2, or 3 (“never”, “rarely”, or “sometimes”, respectively).
When examining advice about sun protection according to climate, survey respondents were grouped into nine climate regions based on the National Centers for Environmental Information, which categorizes climatically consistent regions within the contiguous United States (Central, East North Central, Northeast, Northwest, South, Southeast, Southwest, West, and West North Central) [17]. Because three regions (Northwest, Southwest, West North Central) had fewer than ten respondents each, these regions were grouped based on distance from the equator: Northwest and West North Central were combined with East North Central, and Southwest and South were combined with West, resulting in five regions: Northeast, Southeast, Central, Northwest/West North Central/East North Central, and West/Southwest/South. Puerto Rico was included in the Southeast region.
Results
Respondent Characteristics
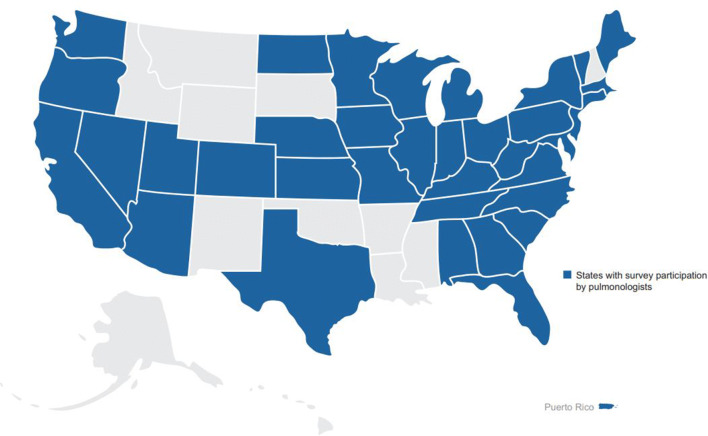
A total of 169 pulmonologists participated in the survey (Table 1). Sixty-nine ILD experts represented referral center practices in 25 states, and 100 community pulmonologists represented practices in 37 states in the United States (Fig. 1). Approximately one-third of pulmonologists [35.5% (n = 60)] had 11–20 years of experience in pulmonary medicine after completing their last year of formal training, and approximately one-quarter [27.8% (n = 47)] had 21–30 years of experience. Over two-thirds of pulmonologists (68.6%) participating in the survey spent 81–100% of their time in direct patient care. By practice setting, 57.4% of pulmonologists primarily practiced in an academic medical center or affiliated teaching hospital, 24.3% in a private practice office, 17.8% in a non-academic community hospital or outpatient clinic, and 0.6% in an “other” practice setting. The mean [standard deviation (SD)] number of patients with IPF in these practices was 66 (109) and ranged from 10 to 1000 patients. The mean (SD) percentage of patients with IPF treated with an approved antifibrotic medication (pirfenidone or nintedanib) was 55.2% (28.8%).
Table 1.
Respondent characteristics
| Characteristic | All respondents (N = 169) |
|---|---|
| Classification, n (%) | |
| ILD expert | 69 (40.8) |
| Community pulmonologist | 100 (59.2) |
| Years practicing pulmonary medicine, n (%) | |
| 1–5 | 22 (13.0) |
| 6–10 | 35 (20.7) |
| 11–20 | 60 (35.5) |
| 21–30 | 47 (27.8) |
| 31–40 | 5 (3.0) |
| Professional time spent in direct patient care, n (%) | |
| 21–40% | 3 (1.8) |
| 41–60% | 14 (8.3) |
| 61–80% | 36 (21.3) |
| 81–100% | 116 (68.6) |
| Primary practice setting, n (%) | |
| Academic | 97 (57.4) |
| Private practice | 41 (24.3) |
| Community | 30 (17.8) |
| Other | 1 (0.6) |
| Patients with IPF in the practice, mean (SD), n | 66 (109) |
| Patients treated with an approved antifibrotic, mean (SD), % | 55.2 (28.8) |
ILD interstitial lung disease, IPF idiopathic pulmonary fibrosis, SD standard deviation
Fig. 1.
Geographical distribution of survey respondents. Shading represents states with survey participation by pulmonologists
Initiating Pirfenidone: Dose Titration Schedules
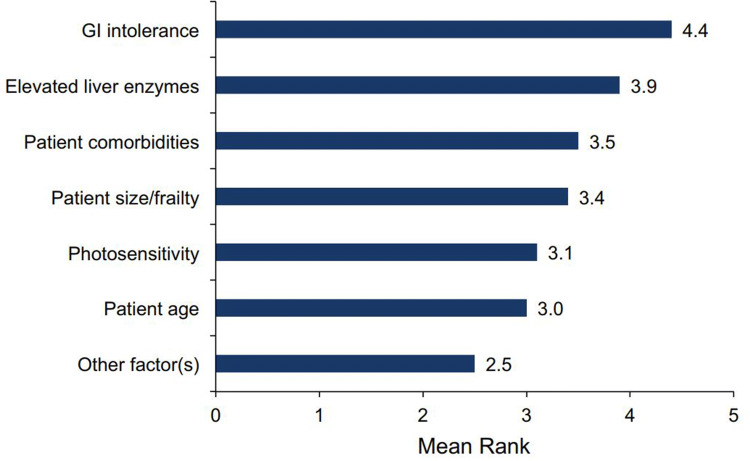
GI intolerance was indicated as the most important factor when deciding to implement an alternative titration schedule for pirfenidone, followed by liver enzyme elevations and patient comorbidities (Fig. 2). When initiating treatment with pirfenidone, 119 pulmonologists (70.4%) commonly recommended the standard titration scheme (Fig. 3). Non-standard titration schedules ranging up to 8 weeks were described, with 3- and 4-week schedules being the most commonly reported non-standard titration schedules (Fig. 4).
Fig. 2.
Importance of factors in implementing alternative titration schedules for pirfenidone. 1 not important, 5 extremely important, GI gastrointestinal
Fig. 3.
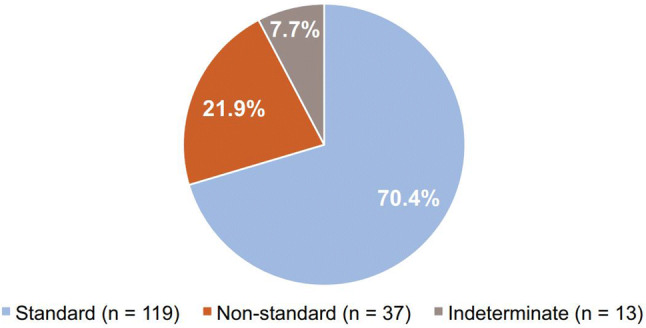
Distribution of common initial titration schedules for pirfenidone. Standard, 2-week titration; non-standard, > 2-week titration; indeterminate, unknown time frame
Fig. 4.
Commonly reported alternative titration schedules for pirfenidone
The same initial titration schedule was typically recommended by 83.2% of pulmonologists (n = 139) for all their patients. These pulmonologists reported that an average of 72.9% of their patients reached the full maintenance dose of pirfenidone using their most commonly recommended titration schedule.
Initiating Pirfenidone: Dosing at Mealtimes
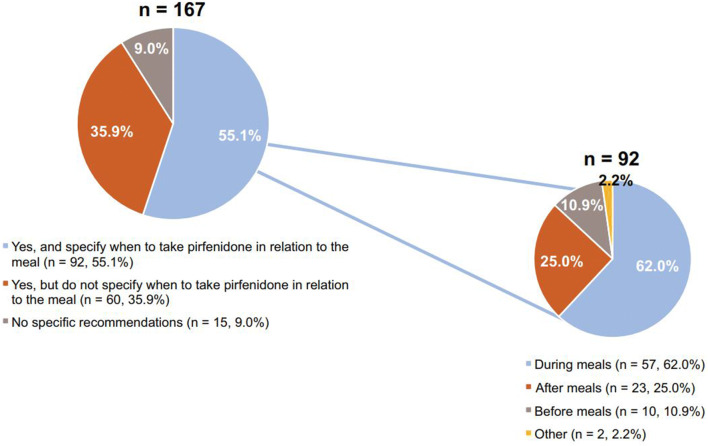
When initiating pirfenidone treatment, 55.1% of pulmonologists (92 of 167) advised their patients to take pirfenidone at mealtimes and specified when to take it in relation to the meal (e.g., before, during, after, or other) (Fig. 5). Of the pulmonologists who made specific recommendations regarding pirfenidone dosing in relation to the meal, 62.0% (57 of 92) counseled their patients to take the dose during the meal (Fig. 5).
Fig. 5.
Initiation of pirfenidone and recommendations for dosing around mealtimes a. a167 pulmonologists reported on the initiation of pirfenidone; of those, 55.1% (n = 92) reported on dosing around mealtimes
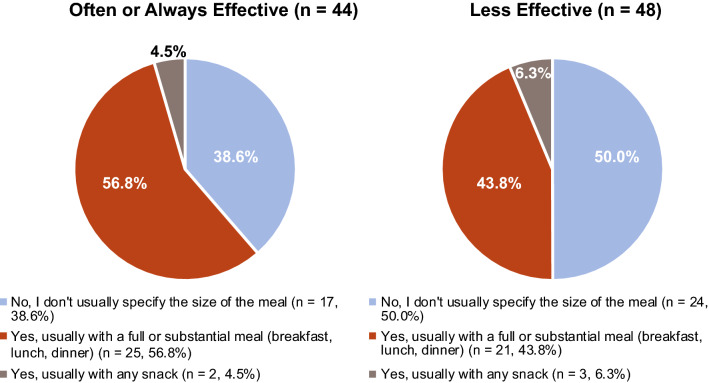
When asked how effective their dosing recommendations at mealtimes were at mitigating pirfenidone-related GI AEs, 47.8% of pulmonologists (44 of 92) rated their advice as being “often” or “always” effective. Of these pulmonologists, 56.8% (25 of 44) were more likely to recommend taking pirfenidone during a “substantial” meal than those who reported their dosing recommendations to be less effective at mitigating pirfenidone-related AEs [43.8% (21 of 48)] (Fig. 6).
Fig. 6.
Perceived effectiveness of dosing recommendations regarding meal size a. aOf 92 pulmonologists reporting
Initiating Pirfenidone: Managing Photosensitivity
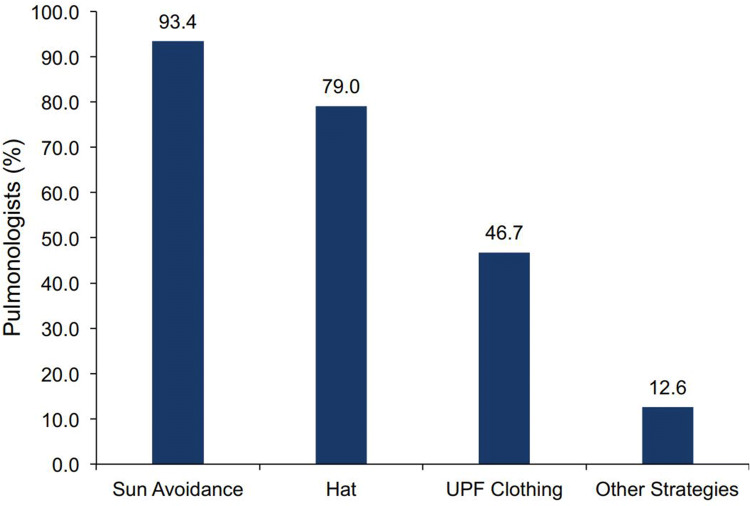
Most pulmonologists [94.0% (157 of 167)] proposed the use of sunscreen to their patients who were initiating pirfenidone and spending time outdoors. However, 24 pulmonologists (14.7%) did not proactively recommend the use of sunscreen to any of their patients or recommended its use to ≤ 50% of their patients. Of the ten pulmonologists who did not recommend the use of sunscreen to any of their patients, eight recommended sun avoidance and two recommended “other strategies”. Among the 167 pulmonologists who responded, strategies other than the use of sunscreen for managing photosensitivity in patients initiating pirfenidone were common, including sun avoidance (93.4%) and wearing a hat (79.0%). Nearly half of pulmonologists (46.7%) advised their patients to wear ultraviolet protection factor (UPF) clothing (Fig. 7).
Fig. 7.
Recommendations other than sunscreen for managing photosensitivity with pirfenidone a. aOf 167 pulmonologists reporting. UPF ultraviolet protection factor
When examining advice regarding sun protection across the five climate regions, pulmonologists in the Northeast and West/Southwest/South regions were more likely to recommend the use of sunscreen in patients who spend time outdoors than pulmonologists in the other regions (Table 2). For other strategies for managing photosensitivity, pulmonologists in the West/Southwest/South were most likely to recommend use of UPF clothing; these pulmonologists and those in the Northwest/West North Central/East North Central region were most likely to recommend wearing a hat compared with the other regions (Table 3). Approximately half of pulmonologists in the Southeast recommended UPF clothing. Sun avoidance was highly recommended across all regions.
Table 2.
Frequency of sunscreen recommendation by geographic region
| Region | Mean percentage of patients who spend time outdoors for whom sunscreen is recommended |
|---|---|
| Northeast, n = 56 | 90.4 |
| Southeast, n = 30 | 81.7 |
| Central, n = 22 | 80.2 |
| Northwest/West North Central/East North Central, n = 24 | 88.3 |
| West/Southwest/South, n = 35 | 91.0 |
Table 3.
Frequency of recommendations for managing photosensitivity by geographic region
| Region | UPF clothing n (%) | Hats n (%) | Sun avoidance n (%) | Other strategies n (%) |
|---|---|---|---|---|
| Northeast | 25 (44.6) | 44 (78.6) | 53 (94.6) | 9 (16.1) |
| Southeast | 14 (46.7) | 22 (73.3) | 28 (93.3) | 2 (6.7) |
| Central | 7 (31.8) | 15 (68.2) | 19 (86.4) | 1 (4.5) |
| Northwest/West North Central/East North Central | 10 (41.7) | 21 (87.5) | 22 (91.7) | 4 (16.7) |
| West/Southwest/South | 22 (62.9) | 30 (85.7) | 34 (97.1) | 5 (14.3) |
Discussion
Randomized, controlled trials have demonstrated that pirfenidone treatment for patients with IPF reduces lung function decline, improves progression-free survival, and significantly reduces the risk of all-cause mortality at 1 year [5]. AE management is critical to helping some patients maintain long-term treatment. This study provides real-world data from a geographically varied sample of pulmonologists in the United States on strategies for prevention and management of potential AEs in patients with IPF who are receiving pirfenidone.
These real-world survey data suggest that alternative titration schedules for initiating pirfenidone are common and may aid in achieving the full maintenance dose in patients with IPF. Non-standard titration schedules ranging up to 8 weeks were described, and 3- and 4-week titration schedules were the most commonly reported. These observations are consistent with the clinical trial experience that is available for alternative titration schedules. An alternative titration schedule was tested in another study with pirfenidone. In LOTUSS (NCT01933334), a phase II trial that investigated pirfenidone in systemic sclerosis ILD (SSc-ILD), a longer titration schedule (4 vs. 2 weeks) was associated with better tolerability of pirfenidone [18]. The 4-week titration schedule was tested with pirfenidone in this specific population because patients with SSc-ILD may be more susceptible to GI, skin, and liver-related AEs due to the nature of their disease [19, 20]. Patients in the 2-week titration group had more dose modifications overall than those in the 4-week titration group during the titration period [18]. Discontinuation rates due to AEs were 15.6 and 3.2% for the 2- and 4-week titration groups, respectively [18]. Patients in the 2- and 4-week groups reported similar AEs, most commonly nausea, headache, and fatigue, similar to patients with IPF; however, more patients in the 2-week group experienced a severe AE, suggesting that more gradual titration periods of > 2 weeks may be associated with a better tolerability profile [18]. In this survey, most pulmonologists implemented the same initial titration schedule for all their patients and reported that most patients could reach the full maintenance dose of pirfenidone.
The timing and amount of food intake with pirfenidone administration appeared to impact pirfenidone-related GI AEs. In this survey, approximately half of the pulmonologists specifically advised their patients to take pirfenidone at mealtimes and provided specific recommendations on timing. A common and reportedly effective strategy used by these pulmonologists to ameliorate pirfenidone-related GI AEs was advising taking pirfenidone during a substantial meal. This strategy aligns with what is recommended in the prescribing information as well as what has been advised by leading experts in the field [6, 9, 12]. Pre-clinical and pharmacokinetic data have also indicated the potential effectiveness of administering pirfenidone with a substantial meal to reduce GI AEs. In a phase I study in healthy volunteers (NCT02525484) receiving pirfenidone 801 mg (as three 267-mg capsules) under fasted or fed conditions, GI AEs were decreased in the fed state [11]. The pharmacokinetic data indicated that food reduced the rate and extent of pirfenidone absorption. In a multivariate statistical model, a higher maximum plasma concentration (Cmax) was associated with GI AEs, and reduction in GI AEs was associated with a lower Cmax. Rat gastric emptying models have also investigated the effects of timing pirfenidone administration and meal intake on GI tolerability. Results indicated that administering pirfenidone with food or dividing the dose over the course of a meal could decrease the Cmax and therefore the impact of pirfenidone on gastric emptying (L Pan et al. Pulm Pharm Ther. Submitted). However, further research is needed to better understand the specific mechanism by which pirfenidone impacts gastric emptying.
Recommendations for managing photosensitivity include use of sunscreen, sun avoidance, and wearing a hat and UPF clothing. These strategies also align with recommendations in the prescribing information as well as those advised by leading experts in the field [6, 9, 12, 21]. Data from this survey suggest that sunscreen use in patients who spend time outdoors may need to be recommended more extensively, particularly in regions such as the Southeast, where sunshine hours are elevated. However, pulmonologists in all regions were highly likely to recommend sun avoidance for managing photosensitivity, which may help explain the lower frequency of recommending UPF clothing in areas such as the Southeast.
Pulmonologists in this survey sample reported that, on average, 55% of patients with IPF were receiving antifibrotic therapy in their practices. Recent analyses of claims data suggest potentially even lower real-world treatment rates. For example, analyses of the Truven Health MarketScan® Commercial and Medicare Supplemental Research Databases from January 1, 2014, to January 31, 2017, indicated that approximately 2% of enrollees (1192 of 50,296) who had ≥ 1 health care claim associated with a diagnosis of IPF (and meeting other criteria) also had ≥ 1 claim for antifibrotic therapy [22]. Notably, ascertaining rates of antifibrotic use in a claims database is challenging due to differences in the case definitions of IPF. Conversely, the current study was constructed specifically to examine antifibrotic use in patients with IPF and may not be representative of a more generalized IPF population. The disparity in treatment patterns between claims data and survey data suggests that additional studies are needed to better understand key factors in treatment decision-making in the real world from both the physician and patient perspectives and to truly capture current practice patterns of pulmonologists who treat patients with IPF.
Limitations of these real-world survey data include the potential for response bias, given that the pulmonologists who were contacted and ultimately participated in the survey may be different than those who did not. Thus, it is possible that the data collected may not be fully representative of common IPF practices. Additionally, the data collected reflect individual physician experiences rather than prospective, objective measures comparing varying management strategies to on-label dosing and administration. Varying interpretations of the questions by participants may have also impacted responses. For example, one physician’s interpretation of “extremely important” may be different from another physician’s interpretation. In collecting data on dose titrations, there were challenges in accurately determining and summarizing the alternative titration schemes due to variations in how they were inputted by the respondents. Despite these limitations, the ease and convenience of this online pre-programmed survey administered to a varied sample of pulmonologists allowed the capture of real-world IPF practices with a broad range of questions and flexibility in the data analysis, with minimal opportunities for error in administration. The data collected provide practical and feasible guidance regarding strategies for prevention and management of potential AEs in patients with IPF who are receiving pirfenidone. This guidance expands on the limited information available in the label and prior expert opinion, reflecting the real-world experiences of physicians in a range of practice settings and broader population of patients with IPF receiving antifibrotics.
Conclusions
These real-world data from pulmonologists provide insight into effective practice patterns for managing common pirfenidone-related AEs to improve tolerability and treatment persistence. These recommended strategies expand upon what has previously been reported, and alternative dose titration schedules may be an effective method for allowing patients to achieve the full dose of pirfenidone.
Acknowledgements
The authors would like to thank all of the survey participants as well as Benjamin L. Trzaskoma, MS, for his input on the data and statistical analysis.
Funding
Sponsorship for this study and article processing charges were funded by F. Hoffmann-La Roche Ltd./Genentech, Inc. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and Editorial Assistance
Third-party writing assistance was provided by Christine Gould, PhD, CMPP, of Health Interactions, Inc. Support for this assistance was funded by F. Hoffmann-La Roche Ltd./Genentech, Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Tmirah Haselkorn is a consultant to Genentech, Inc. Susan L. Limb is an employee of Genentech, Inc. John L. Stauffer is an employee of Genentech, Inc. Karina Raimundo is an employee of Genentech, Inc. Elizabeth Morgenthien is an employee of Genentech, Inc. Mark L. Wencel has been a speaker for and received compensation from Genentech, Inc. Peter P. LaCamera has received personal fees for consulting and advisory boards from Genentech, Inc.
Compliance with Ethics Guidelines
Informed consent was obtained from all survey participants. This article does not contain any interventional or observational studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available, as they were generated by a third-party company sponsored by Genentech, Inc., but are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced digital features
To view enhanced digital features for this article go to 10.6084/m9.figshare.6222455.
References
- 1.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ley B, Collard HR, King TE Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183(4):431–40. [DOI] [PubMed] [Google Scholar]
- 3.King TE Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–92. [DOI] [PubMed] [Google Scholar]
- 4.Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377(9779):1760–9. [DOI] [PubMed] [Google Scholar]
- 5.Noble PW, Albera C, Bradford WZ, et al. Pirfenidone for idiopathic pulmonary fibrosis: analysis of pooled data from three multinational phase 3 trials. Eur Respir J. 2016;47(1):243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esbriet (pirfenidone) capsules and film-coated tablets, for oral use [package insert]. South San Francisco, CA: Genentech, Inc; 2017.
- 7.Nathan SD, Albera C, Bradford WZ, et al. Effect of pirfenidone on mortality: pooled analyses and meta-analyses of clinical trials in idiopathic pulmonary fibrosis. Lancet Respir Med. 2017;5(1):33–41. [DOI] [PubMed] [Google Scholar]
- 8.Lancaster L, Albera C, Bradford WZ, et al. Safety of pirfenidone in patients with idiopathic pulmonary fibrosis: integrated analysis of cumulative data from 5 clinical trials. BMJ Open Respir Res. 2016;3(1):e000105 (2015-000105. eCollection 2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costabel U, Bendstrup E, Cottin V, et al. Pirfenidone in idiopathic pulmonary fibrosis: expert panel discussion on the management of drug-related adverse events. Adv Ther. 2014;31(4):375–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mason WR, Nathan SD, Zibrak JD, et al. Time-to-event analysis of common adverse events with pirfenidone in patients with IPF—a pooled analysis of three Phase III clinical trials. Am J Respir Crit Care Med. 2017;195:A6798. [Google Scholar]
- 11.Pan L, Belloni P, Ding HT, Wang J, Rubino CM, Putnam WS. A pharmacokinetic bioequivalence study comparing pirfenidone tablet and capsule dosage forms in healthy adult volunteers. Adv Ther. 2017;34(9):2071–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lancaster LH, de Andrade JA, Zibrak JD, et al. Pirfenidone safety and adverse event management in idiopathic pulmonary fibrosis. Eur Respir Rev. 2017;26(146):170057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cottin V, Maher T. Long-term clinical and real-world experience with pirfenidone in the treatment of idiopathic pulmonary fibrosis. Eur Respir Rev. 2015;24(135):58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maher TM, Molina-Molina M, Russell AM, et al. Unmet needs in the treatment of idiopathic pulmonary fibrosis—insights from patient chart review in five European countries. BMC Pulm Med. 2017;17(1):124 (017-0468-5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Audibert C, Livoti C, Caze A. Idiopathic pulmonary fibrosis: physicians’ perceptions of patient treatment with recently approved drugs. Contemp Clin Trials Commun. 2016;3:80–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pulmonary Fibrosis Foundation. Find medical care. Available at: http://www.pulmonaryfibrosis.org/life-with-pf/find-medical-care. Accessed Dec 2017.
- 17.US Climate regions. Available at: https://www.ncdc.noaa.gov/monitoring-references/maps/us-climate-regions.php. Accessed April 2018.
- 18.Khanna D, Albera C, Fischer A, et al. An open-label, Phase II study of the safety and tolerability of pirfenidone in patients with scleroderma-associated interstitial lung disease: the LOTUSS Trial. J Rheumatol. 2016;43(9):1672–9. [DOI] [PubMed] [Google Scholar]
- 19.Savarino E, Furnari M, de Bortoli N, et al. Gastrointestinal involvement in systemic sclerosis. Presse Med. 2014;43(10 Pt 2):e279–91. [DOI] [PubMed] [Google Scholar]
- 20.van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013;65(11):2737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cottin V, Crestani B, Valeyre D, et al. Diagnosis and management of idiopathic pulmonary fibrosis: French practical guidelines. Eur Respir Rev. 2014;23(132):193–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raimundo K, Kong A, Gray S, Benloucif S, Limb S. Adherence and persistence to antifibrotic treatments for idiopathic pulmonary fibrosis. Chest. 2017;152(4):A441. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available, as they were generated by a third-party company sponsored by Genentech, Inc., but are available from the corresponding author on reasonable request.