Abstract
BACKGROUND:
Bronchiectasis is common in patients with advanced chronic obstructive pulmonary disease (COPD) and adversely affects the patients' clinical condition. This study aimed to investigate the effects of bronchiectasis on exercise capacity, dyspnea perception, disease-specific quality of life, and psychological status in patients with COPD and determine the extent of these adverse effects by the severity of bronchiectasis.
METHODS:
A total of 387 COPD patients (245 patients with only COPD [Group 1] and 142 COPD patients with accompanying bronchiectasis [Group 2]) were included in the study. The patients in Group 2 were divided into three subgroups as mild, moderate, and severe using the Bronchiectasis Severity Index. Six-minute walk distance, dyspnea perception, St. George's Respiratory Questionnaire (SGRQ), and hospital anxiety and depression scores were compared between the groups.
RESULTS:
In Group 2, dyspnea perception, SGRQ total scores, depression score were higher, and walking distance was lower (P = 0.001, P = 0.007, P = 0.001, and P = 0.011, respectively). Group 2 had significantly worse arterial blood gas values. Dyspnea perception increased with the increasing severity in Group 2 (P < 0.001). Walking distance was lower in patients with severe bronchiectasis (P < 0.001). SGRQ total score, anxiety, and depression scores were significantly higher in the severe subgroup (P < 0.001, P = 0.003, and P = 0.002, respectively).
CONCLUSIONS:
In patients with Stage 3 and 4 COPD, the presence of bronchiectasis adversely affects the clinical status of the patients, decreases their exercise capacity, deteriorates their quality of life, and disrupts their psychological status. Investigating the presence of bronchiectasis in COPD patients is crucial for early diagnosis and proper treatment.
Keywords: Bronchiectasis, bronchiectasis severity index, chronic obstructive pulmonary disease, exercise capacity, psychological symptoms, quality of life
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of morbidity and mortality worldwide. It is characterized with partially reversible, persistent airflow restriction associated with chronic airway inflammation and emphysema.[1] Furthermore, it is a complicated and heterogeneous disease with possible different clinical features in patients with pathological and structural anomalies and similar pulmonary functions.[1,2] Bronchiectasis is the persistent and usually progressive enlargement of bronchi. It involves the remodeling and destruction of the bronchial wall due to chronic airway inflammation and recurrent infection.[3,4] Pathophysiologically, there are three different matters to be considered: (1) COPD patients may develop bronchiectasis due to chronic airway inflammation and recurrent infections. (2) Bronchiectasis caused by other etiological factors in nonsmokers may be diagnosed with COPD due to fixed airway obstruction. (3) The presence of COPD and bronchiectasis may be regarded as two different illnesses in the same patient.[5] COPD and bronchiectasis share common traits even though they are two different illnesses. Inflammation and airway obstruction are important for the pathogenesis of both COPD and bronchiectasis. Moreover, pathological mechanisms and clinical characteristics such as productive cough and predisposition to infections are also common and the incidence of both diseases increases with age.[6,7]
Ignored for a long time, bronchiectasis became more important with the increasing awareness in recent years.[8] With the increasing use of high-resolution computed tomography (CT), the prevalence of bronchiectasis has been found to be very high, especially in patients with moderato-to-severe COPD.[9] Being called COPD-bronchiectasis overlap syndrome,[10,11] this condition has been reported to be seen in 20%–69% of the patients in various studies.[12] The question whether bronchiectasis presence in COPD is a phenotype, endotype, or comorbidity remains to be answered.[5] While some publications called bronchiectasis as a comorbidity in addition to COPD,[1,9,11] others considered it as a phenotype of COPD.[2,13,14] In 2014, bronchiectasis was defined as a comorbidity in the GOLD guidelines.[15] In 2015, in the updated guidelines, this definition was changed and it was stated that bronchiectasis is seen as a natural result of COPD.[11]
Early diagnosis and management of bronchiectasis in patients with COPD are important.[6] While studies have shown that the presence of bronchiectasis in patients with COPD is associated with higher mortality and exacerbation frequency, more severe airway obstruction and more pathogenic microorganism isolation,[9] there is insufficient information about its effects on functional capacity, dyspnea perception and quality of life, and the association of these effects with the severity of bronchiectasis. Therefore, this study aimed to: (1) Evaluating the effects of bronchiectasis presence in patients with COPD on exercise capacity, dyspnea perception, arterial blood gas values, emergency admission, and quality of life. (2) Determining the effects of bronchiectasis severity on exercise capacity, quality of life, and psychological symptoms.
Methods
Study design
Our retrospective study included patients with Stage 3 and 4 COPD who admitted to chest diseases outpatient clinic between January 2013 and July 2018. The study was approved by the ethics committee of our hospital (number: 49109414-806.02.02).
COPD was defined as a postbronchodilator ratio of forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) of <0.7. The severity of airflow limitation was defined as described by the 2017 GOLD grading system as Grade 2 (50% ≤FEV1<80%), Grade 3 (30% ≤FEV1<50%), or Grade 4 (FEV1<30%).[16] Since patients with bronchiectasis had low respiratory functions, patients with Stage 3–4 COPD who had bronchiectasis were included in the study to eliminate the consequences of differences in pulmonary function test values. The demographic and clinical characteristics of the patients were recorded. Body mass index (BMI) was calculated by height and weight measurement. Sputum culture tests were studied. The patients underwent respiratory and cardiac system examination. Arterial blood gas analysis and pulmonary functions tests were performed in all patients. Chest X-rays and thorax CTs were examined by the same radiologist. The presence of bronchiectasis was investigated based on the radiological characteristics. Bronchiectasis was defined according to accepted criteria when one or more of the following were fulfilled: (1) an internal diameter of the bronchus greater than that of the adjacent pulmonary artery, (2) a lack of tapering of the bronchial lumen toward the periphery, or (3) visualization of the bronchus within 10 mm of the pleural space. (4) Patients without thorax CT, who underwent lung operation for any reason, and who have pulmonary comorbidities such as sarcoidosis, interstitial lung disease, and lung cancer were excluded from the study. Patients with only COPD were placed into Group 1, and COPD patients with accompanying bronchiectasis into Group 2. Demographic and clinical characteristics of the patients were compared between the two groups. In COPD patients with accompanying bronchiectasis, the severity of bronchiectasis was scored using the bronchiectasis severity index (BSI). A total score was obtained by scoring the age, BMI, expected FEV1 value, the number of hospitalizations within the past 2 years, the number of exacerbations within the last year, Modified Medical Research Council (mMRC) dyspnea scale, colonization, and radiological spread parameters. The patients were evaluated in three individual groups with a score of 0–4 being mild, 5–8 being moderate, and 9 and over being severe.[17] All parameters except the ones in BSI were compared again between these three groups.
Pulmonary functions
Body plethysmography (Zan 500, Germany) and carbon-monoxide diffusion capacity (TLCO) (Zan 300, Germany) were measured and evaluated in all patients.
Dyspnea assessment
Dyspnea severity was determined using the “Modified Medical Research Council” scale in all patients. A score of “1” was considered to be the mildest and a score of “4” to be the most severe dyspnea perception.[18]
Exercise capacity
All patients were requested to walk as fast as they can, and 6-minute-walk test was performed. The test result was recorded in meters.[19]
Quality of life
St. George's Respiratory Questionnaire (SGRQ) was used to assess the disease-specific quality of life. In this questionnaire, high scores indicate worsening quality of life and symptoms.[20]
Psychological symptoms
Hospital Anxiety Depression scale consisting of 14 questions were used to determine the mental status of the patients. For anxiety and depression, a score of 0–7 is normal, 8–11 is borderline, and >11 shows anxiety and depression.[21]
Statistical analysis
Statistical analysis of the data obtained in the study was performed using the “Statistical Package for the Social Science for Windows version 17 (SPSS Inc, Chicago, U.S)” statistics software. Distribution normality of the data was checked by Kolmogorov–Smirnov Test. Since the data were not normally distributed; continuous variables were expressed as median (interquartile range), and categorical variables as percentage (%). Kruskal–Wallis H-test was conducted to compare the data between groups. The Mann–Whitney U-test was performed to test the significance of pairwise differences using Bonferroni correction to adjust for multiple comparisons. The analysis of categorical data was performed using Fisher's exact test. P < 0.05 value is considered as statistically significant.[22]
Posterior power analysis was performed with the PASS 11 package program. When the type I error was taken as 0.05 and the sample size, mean, and standard deviation values of the groups were used, the power of the test was 0.84 to detect a 4 point reduction in the SGRQ total score.
Results
Of 387 COPD patients in this study, 142 (36.7%) were detected to have bronchiectasis. Demographic and clinical characteristics of the groups are shown in Table 1. COPD patients with accompanying bronchiectasis were evaluated based on BSI scores in three different groups as mild, moderate, and severe. The number of male patients increased significantly with increasing bronchiectasis severity (P = 0.031). In terms of smoking, no significant difference was found between the groups (P = 0.893). As for arterial blood gas values, O2 saturation decreased significantly with the increasing bronchiectasis severity in each group (P < 0.001). Partial arterial oxygen pressure (PaO2) value was comparable between the mild and moderate groups; however, significantly lower in the severe group (P < 0.001). Partial arterial carbon-dioxide pressure (PaCO2) value was comparable between the mild and moderate groups, however, significantly higher in the severe group compared to the mild and moderate groups (P < 0.001). TLCO value was comparable between the moderate and severe groups, however, significantly higher in the mild group compared to the moderate and severe groups (P = 0.002, P = 0.002). Six-minute walking distance (6MWD) was comparable between the mild and moderate groups, however, significantly lower in the severe group compared to the mild and moderate groups (P < 0.001). By SGRQ quality of life questionnaire, symptom score was significantly higher in the moderate group compared to the mild group, and in the severe group compared to the moderate group (P = 0.029, P = 0.007). Activity, effect, and total scores were comparable between the mild and moderate groups, however, significantly higher in the severe group compared to the mild and moderate groups (P < 0.05). By anxiety and depression scale, anxiety and depression scores were comparable between the mild and moderate groups, however, significantly higher in the severe group compared to the mild and moderate groups [P < 0.005; Table 2].
Table 1.
Comparison of demographic and clinical features of all patients
| Variables | All patients (n=387) | COPD (Group 1) (n=245) | COPD + bronchiectasis (Group 2) (n=142) | P |
|---|---|---|---|---|
| Age (years) | 63 (56-68) | 62 (56-67) | 64 (57-71) | 0.081 |
| BMI (kg/m2) | 26 (23-31) | 28 (24-31) | 25 (21-30) | <0.001* |
| Males, n (%) | 300 (77.3) | 179 (73.1) | 120 (84.5) | 0.009** |
| Cigarette consumption (pack-year) | 50 (30-80) | 50 (30-75) | 50 (25-90) | 0.727 |
| Previous tuberculosis, n (%) | 51 (13.1) | 21 (8.5) | 30 (21.1) | <0.001** |
| Emergency admission (n/last/year) | 1 (0-4) | 1 (0-3) | 2 (1-5) | <0.001* |
| Hospitalization (n/last 1 year) | 0 (0-1) | 0 (0-1) | 1 (0-2) | <0.001* |
| Positive sputum culture (Pseudomonas aeruginosa), n (%) | 21 (5.42) | 4 (1.63) | 17 (11.9) | <0.001** |
| COPD stage, n (%) | ||||
| Stage 3 | 244 (63.1) | 153 (62.4) | 91 (64.1) | 0.874 |
| Stage 4 | 143 (36.9) | 92 (37.6) | 51 (35.9) | |
| Pulmonary function test | ||||
| FEV1 (%) | 45 (35-49) | 46 (37-50) | 42 (30-46) | 0.062 |
| FEV1/FVC | 61 (52-64) | 62 (58-67) | 57 (47-63) | 0.073 |
| TLCO (%) | 38 (24-52) | 41 (28-53) | 29 (17-49) | <0.001* |
| Blood gas analysis | ||||
| PaO2 (mmHg) | 74 (65-82) | 76 (67-83) | 71 (62-80) | <0.001* |
| PaCO2 (mmHg) | 41 (37-45) | 39 (36-44) | 42 (39-46) | <0.001* |
| SatO2 (mmHg) | 95 (93-96) | 95 (93-97) | 94 (92-96) | 0.001* |
| mMRC | 3 (2-4) | 3 (2-4) | 4 (3-4) | 0.001* |
| 6MWD (m) | 360 (290-420) | 370 (310-430) | 350 (248-410) | 0.009* |
| SGRQ | ||||
| Symptom | 55 (41-70) | 53 (37-67) | 61 (43-76) | 0.015* |
| Activity | 67 (54-80) | 66 (54-79) | 68 (54-86) | 0.048* |
| Impact | 46 (32-64) | 44 (29-60) | 51 (34-70) | 0.003* |
| Total | 55 (41-69) | 53 (41-65) | 58 (43-73) | 0.006* |
| HAD | ||||
| Anxiety | 7 (4-11) | 7 (4-10) | 7 (4-12) | 0.096 |
| Depression | 6 (3-9) | 5 (3-9) | 7 (4-10) | 0.001* |
Data are expressed as median (IQR) or percentile (%). *Mann–Whitney U-test, **Fisher’s exact test. BMI=Body mass index, FEV1=Forced expiratory volume in 1 s, FVC=Forced vital capacity, TLCO=Carbon-monoxide diffusion capacity, PaO2=Partial arterial oxygen pressure, PaCO2=Partial arterial carbon-dioxide pressure, SatO2=Arterial oxygen saturation, mMRC=Medical Research Council Dyspnea Scale, 6MWD=6 min walking distance, SGRQ=St. George’s Respiratory Questionnaire, HAD=Hospital Anxiety and Depression Scale, IQR=Interquartile range, COPD=Chronic obstructive pulmonary disease
Table 2.
Comparison of groups according to the bronchiectasis severity index
| Variables | Mild (n=20) | Moderate (n=46) | Severe (n=77) | P |
|---|---|---|---|---|
| Males, n (%) | 13 (65.0) | 41 (89.1) | 66 (86.80) | 0.032** |
| Cigarette | 50 (23-98) | 55 (25-98) | 50 (30-80) | 0.893 |
| consumption (pack-year) | ||||
| TLCO (%) | 57 (29-67) | 28 (18-46) | 27 (15-43) | 0.004* |
| Blood gas analysis | ||||
| PaO2 (mmHg) | 82 (71-88) | 76 (71-84) | 65 (56-73) | <0.001* |
| PaCO2 (mmHg) | 42 (37-44) | 41 (38-44) | 43 (40-50) | 0.013* |
| SatO2 (mmHg) | 96 (95-97) | 95 (94-96) | 93 (90-95) | <0.001* |
| 6MWD (m) | 395 (350-428) | 400 (320-440) | 290 (200-364) | <0.001* |
| SGRQ | ||||
| Symptom | 41 (22-59) | 55 (40-72) | 68 (54-81) | <0.001* |
| Activity | 57 (42-73) | 55 (48-79) | 80 (67-93) | <0.001* |
| Impact | 41 (32-52) | 43 (29-60) | 62 (45-74) | <0.001* |
| Total | 47 (31-60) | 49 (38-68) | 68 (55-80) | <0.001* |
| HAD | ||||
| Anxiety | 5 (3-8) | 7 (3-11) | 10 (6-13) | 0.004* |
| Depression | 6 (3-9) | 6 (3-9) | 8 (5-11) | 0.002* |
Data are expressed as median (IQR) or percentile (%). *Kruskal–Wallis test, **Fisher’s exact test. TLCO=Carbon-monoxide diffusion capacity, PaO2=Partial arterial oxygen pressure, PaCO2=Partial arterial carbon-dioxide pressure, SatO2=Arterial oxygen saturation, SGRQ=St. George’s Respiratory Questionnaire, HAD=Hospital Anxiety and Depression Scale, IQR=Interquartile range, 6MWD=6-min walking distance
A moderate negative correlation was detected between bronchiectasis severity score and exercise capacity in all bronchiectasis patients [r = −0.508, P < 0.001, Figure 1]. When groups were evaluated individually, no correlation was detected between BSI score and 6MWD in the mild group (P = 0.961), a weak negative correlation in the moderate group (r = −0.305; P = 0.039], and a moderate negative correlation in the severe group (r = −0.404; P < 0.001). A moderate positive correlation was obtained between the BSI score and SGRQ quality of life total score [r = 0.521, P < 0.001, Figure 2].
Figure 1.
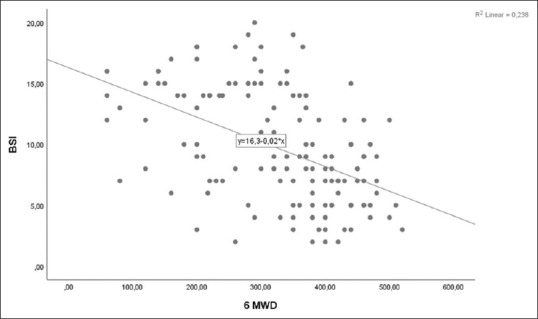
Relationship between 6-min walking distance and Bronchiectasis Severity Score
Figure 2.
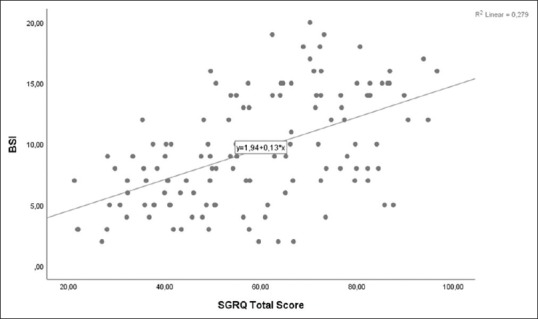
Relationship between St. George's Respiratory Questionnaire Total Score and Bronchiectasis Severity Score
Discussion
In this study, in which we evaluated the effects of presence and severity of bronchiectasis on functional exercise capacity, quality of life, and psychological symptoms in COPD patients; we concluded that the presence of bronchiectasis adversely affects the oxygenation, exercise capacity, dyspnea perception, quality of life, and depression symptoms with these effects worsening even more with the increasing severity of bronchiectasis.
No study evaluating the exercise capacity, quality of life, and psychological symptoms in patients with COPD and bronchiectasis was found in the literature. For this reason, we believe that our study is distinct. Studies have reported a bronchiectasis prevalence between 20% and 69% in COPD patients.[12] In our study, this rate is 36.7%. The shared feature of COPD and bronchiectasis is the fact that both are more frequent in older patients.[7,9,23,24] In our patient, median age of our patients was >60, and no difference was detected between the groups. While bronchiectasis is more common in female patients,[23] bronchiectasis accompanying to COPD is more common in males.[9,24] Similarly, the percentage of male patients was significantly higher in Group 2 in our study. While a previous study has found that the amount of smoking (package/year) was significantly higher in COPD patients with accompanying bronchiectasis[25] our study did not find any difference between the groups as with two other studies.[15,26] Having tuberculosis in moderate and severe COPD patients is among the risk factors for bronchiectasis.[15] In a study evaluating COPD patients in two groups as the ones who had or did not have tuberculosis previously, bronchiectasis presence was found to be significantly higher in the group with the history of tuberculosis.[27] While tuberculosis history did not cause any difference between the groups in a study,[26] bronchiectasis presence in COPD patients (47%) was more in patients with the history of tuberculosis in another study.[15] In our study, the history of tuberculosis was 21% in COPD patients with bronchiectasis and previous history of tuberculosis was significantly higher in Group 2. Studies have shown that bronchiectasis present in COPD patients significantly increases the numbers of exacerbations and hospitalizations.[15,26,28] Another study has detected that bronchiectasis is mostly seen in lower lobes and associated with the frequency of exacerbations in COPD patients.[29] In support of these studies, the numbers of exacerbations and hospitalizations were significantly higher in Group 2 in our study. As with studies which found significantly higher rate of P. Aeruginosa isolation in sputum in COPD patients with accompanying bronchiectasis,[4,7,15,25] it was also higher in Group 2 in our study.
The presence of bronchiectasis in moderate-to-severe COPD patients is over 50%.[4,7,29] Bronchiectasis accompanying to COPD leads to worse pulmonary functions and more rapid progression.[28] Previous studies[7,15,26,28] have found significantly lower FEV1% and FEV1/FVC values in COPD patients with bronchiectasis. In our study, although the difference was not significant, the intensity of Stage 3 and 4 patients and the severity of obstruction in the bronchiectasis group seemed to be higher. TLCO value which was not assessed in those studies was found to be significantly lower in Group 2 in our study. According to a hypothesis, severe forms of COPD might be a risk factor for bronchiectasis development.[30] As with other studies,[7,26] the study has also found that in the arterial blood gas values of COPD patients with accompanying bronchiectasis, PaO2 value was significantly lower and PaCO2 value was higher. As with our study, all studies evaluating the mMRC dyspnea perception[12,15,26,28] have found significantly higher dyspnea score in COPD patients with accompanying bronchiectasis.
Some studies assessed COPD patients in two groups as with and without accompanying bronchiectasis,[7,15,26,28] and others grouped the patients by determining the radiological severity.[4,6] Differently from these studies, in our study, bronchiectasis severity was scored according to BSI and evaluated in three groups as mild, moderate, and severe. While a study[4] has found significantly higher number of male patients with the increasing severity of bronchiectasis, another[6] has not detected a significant relationship between the bronchiectasis severity and the number of male patients. In our study, the number of male patients was found to be significantly higher with the increasing severity of bronchiectasis. The amount of smoking (package/year) was not found to be associated with bronchiectasis severity both in our study and in another study.[6] While diffusion capacity did not differ significantly by bronchiectasis severity,[4] it was found to be higher in the mild group compared to the moderate and severe groups in our study. Evaluation of arterial blood gas, 6MWD, quality of life, and hospitalization anxiety depression score by bronchiectasis severity was not carried out in any of the studies. In our study, PaO2 value and oxygen saturation decreased with the increasing severity of bronchiectasis. 6MWD was found to be lower in the severe group compared to the other two groups. SGRQ symptom score by quality of life worsened with the increasing severity of bronchiectasis. Activity, effect, and total scores were higher in the severe group compared to the mild and moderate groups. Anxiety and depression scores did not differ between the mild and the severe groups, however, were higher in the severe group.
We had some limitations in our study. For example, we had to exclude COPD patients without CT of the thorax. We could not use the cardiopulmonary exercise test which is the gold standard for the exercise capacity and provides detailed information about pulmonary, cardiac and metabolic functions due to technical inadequacies. Finally, since our study was retrospective, we could not examine factors affecting outcome such as muscle strength, fatigue, and nutritional assessment which were not included in our routine evaluations.
Conclusions
In this study, patients with severe and very severe COPD were examined in two separate groups, with and without bronchiectasis; COPD patients with bronchiectasis were found to have higher number of emergency admissions and hospitalizations, and lower oxygenation and diffusion capacity. In addition, dyspnea perception, exercise capacity, quality of life, and psychological symptoms were negatively affected in these patients. As the severity of bronchiectasis increases, the negative effect on patients with COPD increases. Therefore, it is important to investigate the presence of bronchiectasis in terms of early diagnosis and appropriate treatment in COPD patients with advanced stage, low BMI, history of tuberculosis, and increased emergency admissions and hospitalization.
Clinical trial number: NCT03670940
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Du Q, Jin J, Liu X, Sun Y. Bronchiectasis as a comorbidity of chronic obstructive pulmonary disease: A systematic review and meta-analysis. PLoS One. 2016;11:e0150532. doi: 10.1371/journal.pone.0150532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, et al. Chronic obstructive pulmonary disease phenotypes: The future of COPD. Am J Respir Crit Care Med. 2010;182:598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martínez-García MÁ, de Gracia J, Vendrell Relat M, Girón RM, Máiz Carro L, de la Rosa Carrillo D, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: The FACED score. Eur Respir J. 2014;43:1357–67. doi: 10.1183/09031936.00026313. [DOI] [PubMed] [Google Scholar]
- 4.Gatheral T, Kumar N, Sansom B, Lai D, Nair A, Vlahos I, et al. COPD-related bronchiectasis; independent impact on disease course and outcomes. COPD. 2014;11:605–14. doi: 10.3109/15412555.2014.922174. [DOI] [PubMed] [Google Scholar]
- 5.Blasi F, Chalmers JD, Aliberti S. COPD and bronchiectasis: Phenotype, endotype or co-morbidity? COPD. 2014;11:603–4. doi: 10.3109/15412555.2014.974744. [DOI] [PubMed] [Google Scholar]
- 6.Bak SH, Kim S, Hong Y, Heo J, Lim MN, Kim WJ. Quantitative computed tomography features and clinical manifestations associated with the extent of bronchiectasis in patients with moderate-to-severe COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:1421–31. doi: 10.2147/COPD.S157953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martínez-García MÁ, Soler-Cataluña JJ, Donat Sanz Y, Catalán Serra P, Agramunt Lerma M, Ballestín Vicente J, et al. Factors associated with bronchiectasis in patients with COPD. Chest. 2011;140:1130–7. doi: 10.1378/chest.10-1758. [DOI] [PubMed] [Google Scholar]
- 8.Contarini M, Finch S, Chalmers JD. Bronchiectasis: A case-based approach to investigation and management. Eur Respir Rev. 2018;27:180016. doi: 10.1183/16000617.0016-2018. DOI:10.1183/16000617.0016-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YH, Sun YC. Bronchiectasis as a comorbidity of chronic obstructive pulmonary disease: Implications and future research. Chin Med J (Engl) 2016;129:2017–9. doi: 10.4103/0366-6999.189071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalmers JD. Bronchiectasis and COPD overlap: A case of mistaken identity? Chest. 2017;151:1204–6. doi: 10.1016/j.chest.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 11.Hurst JR, Elborn JS, De Soyza A, BRONCH-UK Consortium. COPD-bronchiectasis overlap syndrome. Eur Respir J. 2015;45:310–3. doi: 10.1183/09031936.00170014. [DOI] [PubMed] [Google Scholar]
- 12.Ni Y, Shi G, Yu Y, Hao J, Chen T, Song H. Clinical characteristics of patients with chronic obstructive pulmonary disease with comorbid bronchiectasis: A systemic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2015;10:1465–75. doi: 10.2147/COPD.S83910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stockley RA. Bronchiectasis with chronic obstructive pulmonary disease: Association or a further phenotype? Am J Respir Crit Care Med. 2013;187:786–8. doi: 10.1164/rccm.201302-0203ED. [DOI] [PubMed] [Google Scholar]
- 14.O'Donnell AE. Bronchiectasis in patients with COPD: A distinct COPD phenotype? Chest. 2011;140:1107–8. doi: 10.1378/chest.11-1484. [DOI] [PubMed] [Google Scholar]
- 15.Jin J, Yu W, Li S, Lu L, Liu X, Sun Y. Factors associated with bronchiectasis in patients with moderate-severe chronic obstructive pulmonary disease. Medicine (Baltimore) 2016;95:e4219. doi: 10.1097/MD.0000000000004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogelmeier CF, Criner GJ, Martínez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Arch Bronconeumol. 2017;53:128–49. doi: 10.1016/j.arbres.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Chalmers JD, Goeminne P, Aliberti S, McDonnell MJ, Lonni S, Davidson J, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med. 2014;189:576–85. doi: 10.1164/rccm.201309-1575OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sweer L, Zwillich CW. Dyspnea in the patient with chronic obstructive pulmonary disease. Etiology and management. Clin Chest Med. 1990;11:417–45. [PubMed] [Google Scholar]
- 19.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 20.Polatlı M, Yorgancıoǧlu A, Aydemir Ö, Yılmaz Demirci N, Kırkıl G, Atış Naycı S, et al. Validity and reliability of Turkish version of St. George's respiratory questionnaire. Tuberk Toraks. 2013;61:81–7. doi: 10.5578/tt.5404. [DOI] [PubMed] [Google Scholar]
- 21.Snaith RP, Zigmond AS. The hospital anxiety and depression scale. Br Med J (Clin Res Ed) 1986;292:344. doi: 10.1136/bmj.292.6516.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Released. SPSS Statistics for Windows, Version 17.0. Chicago: SPSS Inc; 2008. SPSS Inc. [Google Scholar]
- 23.Smith MP. Diagnosis and management of bronchiectasis. CMAJ. 2017;189:E828–35. doi: 10.1503/cmaj.160830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tulek B. Co-occurrence of chronic obstructive pulmonary disease and bronchiectasis: Is ıt a new phenotype of chronic obstructive pulmonary disease? Eurasian J Pulmonol. 2014;16:8–12. [Google Scholar]
- 25.Arram EO, Elrakhawy MM. Bronchiectasis in COPD patients. Egypt J Chest Dis Tuberc. 2012;61:307–12. [Google Scholar]
- 26.Martínez-García MA, de la Rosa Carrillo D, Soler-Cataluña JJ, Donat-Sanz Y, Serra PC, Lerma MA, et al. Prognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:823–31. doi: 10.1164/rccm.201208-1518OC. [DOI] [PubMed] [Google Scholar]
- 27.Jin J, Li S, Yu W, Liu X, Sun Y. Emphysema and bronchiectasis in COPD patients with previous pulmonary tuberculosis: Computed tomography features and clinical implications. Int J Chron Obstruct Pulmon Dis. 2018;13:375–84. doi: 10.2147/COPD.S152447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crisafulli E, Guerrero M, Ielpo A, Ceccato A, Huerta A, Gabarrús A, et al. Impact of bronchiectasis on outcomes of hospitalized patients with acute exacerbation of chronic obstructive pulmonary disease: A propensity matched analysis. Sci Rep. 2018;8:9236. doi: 10.1038/s41598-018-27680-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel IS, Vlahos I, Wilkinson TM, Lloyd-Owen SJ, Donaldson GC, Wilks M, et al. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:400–7. doi: 10.1164/rccm.200305-648OC. [DOI] [PubMed] [Google Scholar]
- 30.García MA, Cataluña JJ. Chronic obstructive pulmonary disease and bronchiectasias. Arch Bronconeumol. 2010;46:11–7. doi: 10.1016/S0300-2896(10)70021-1. [DOI] [PubMed] [Google Scholar]


