Abstract
The rapid clearance of ciprofloxacin hydrochloride from the lungs following administration as an aerosol leads to poor efficacy in the treatment of pulmonary infections. The development of formulations capable of sustaining ciprofloxacin concentrations in the lungs has the potential to significantly improve antibacterial activity. The present review compares two approaches for sustaining levels of ciprofloxacin in the lungs, a liposomal formulation where ciprofloxacin is encapsulated in small unilamellar vesicles, and a dry powder formulation of the practically insoluble zwitterionic form of the drug. These two formulations recently completed large multicenter, phase 3 clinical studies in bronchiectasis patients. As such, they present a unique opportunity to examine the chemistry, manufacturing, and control of the dosage forms in addition to their tolerability and efficacy in more than 1000 bronchiectasis patients. Both formulations were generally well tolerated with most adverse events found to be mild to moderate in intensity. While the formulations were effective in reducing and/or eradicating infections, this did not lead to reductions in pulmonary exacerbations, the primary endpoint. The failures speak more to the heterogeneous nature of the disease and the difficulty in identifying bronchiectasis patients likely to exacerbate, rather than an inherent limitation of the formulations. While the formulations are similar in many respects, they also present some interesting differences. This review explores the implications of these differences on the treatment of respiratory infections.
Keywords: Ciprofloxacin DPI, Linhaliq®, Liposomes, PulmoSphere™, Sustained release, Tolerability
Key Summary Points
| This review explores the impact of the presentation of ciprofloxacin in phospholipid-based particles (i.e., liposomes and spray-dried dry powder formulations) on drug delivery, safety, and tolerability in bronchiectasis patients. |
| Despite the differences in formulation design, the two formulations deliver comparable ciprofloxacin doses to the lungs, and have similar aerodynamic particle size distributions and kinetics of drug clearance from the lungs. |
| Both formulations are generally well tolerated with most adverse events mild to moderate in intensity. A significant difference does exist, however, in the reported incidence of treatment emergent adverse events in favor of the dry powder formulation. |
| Significant differences also exist in design features that may impact adherence to treatment, with once daily delivery favoring the liposomal formulation, and factors related to daily treatment burden, device portability, and tolerability favoring the dry powder. |
Introduction
In the last decade, numerous phospholipid-based formulations have been advanced into the clinic for the pulmonary administration of anti-infectives. These include two formulations that have achieved marketing authorization: TOBI® Podhaler™, a spray-dried dry powder formulation of tobramycin sulfate for the treatment of chronic Pseudomonas aeruginosa infections in cystic fibrosis (CF) patients [1–5], and Arikayce®, a liposomal dispersion of amikacin sulfate for the treatment of non-tuberculosis mycobacterial (NTM) infections [6–10].
More recently, clinical studies of dry powder and liposomal formulations of ciprofloxacin were completed in large, multicenter, placebo-controlled phase 3 trials in non-CF bronchiectasis (BE) patients [11–13]. The concurrent trials in the same patient population present a unique opportunity to examine the impact of the ‘presentation’ of drug in phospholipid-based particles on drug delivery, safety, and tolerability in the BE patient population.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by the author.
Bronchiectasis
Bronchiectasis is a severe, debilitating, chronic respiratory disease with a heterogeneous etiology [14, 15]. In BE patients, repeated cycles of bacterial infection and inflammation cause irreversible dilation of the bronchi with thickening of the bronchial wall. Furthermore, pooled mucus in the airways provides an ideal environment for bacterial growth. Patients with BE suffer from debilitating symptoms including excessive sputum production, persistent chronic cough, hemoptysis, fatigue, and increased anxiety and depression. Episodic pulmonary exacerbations (PE) are an important driver of patient morbidity and mortality and are strongly associated with disease progression. Forty percent of BE patients reportedly suffer from three or more exacerbations per year [15]. Unfortunately, there are no approved therapies that reduce or delay pulmonary exacerbations.
The Drug Substance
Ciprofloxacin is a second-generation fluoroquinolone antibiotic with broad-spectrum activity against both Gram-negative and Gram-positive bacteria [16]. Ciprofloxacin interferes with DNA replication and transcription by inhibition of DNA-gyrase and topoisomerase IV enzymes.
Ciprofloxacin contains two ionizable groups: a carboxylic acid with a pKa of 6.2, and a secondary amine with a pKa of 8.6 (Fig. 1).
Fig. 1.
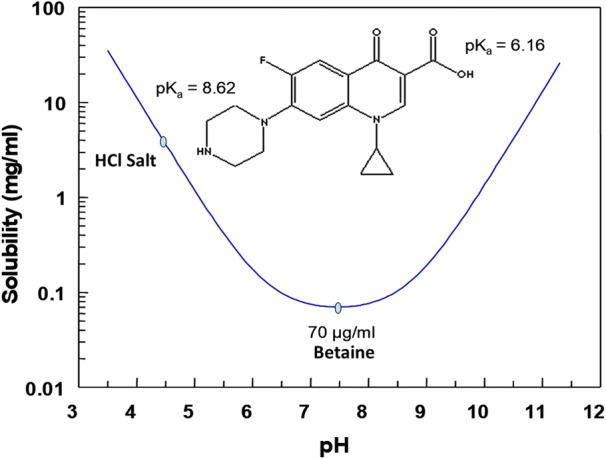
Solubility of ciprofloxacin as a function of pH
At neutral pH, the carboxylic acid group is largely deprotonated and the amine group protonated, resulting in a neutral zwitterionic form that is sometimes referred to as ciprofloxacin betaine. Ciprofloxacin betaine is practically insoluble in water with a solubility of ~ 70 μg/ml (Fig. 1) [17]. At low pH, the carboxylic acid group is protonated, and the molecule becomes positively charged. Formation of the hydrochloride salt with the positively charged amine group increases the aqueous solubility of the drug by more than 250-fold (> 20 mg/ml at pH 3.2) (Fig. 1) [18].
In terms of its antibacterial activity, ciprofloxacin displays concentration-dependent killing, with the best pharmacokinetic/pharmacodynamic (PK/PD) correlates being the area under the curve (AUC) divided by the minimum inhibitory concentration (MIC), i.e., the AUC/MIC ratio, or the peak concentration to the MIC, i.e., peak/MIC ratio [19]. Investigators have suggested that AUC/MIC ratios ≥ 125, or peak/MIC ratios > 10 are required for microbiological and clinical success [19]. Unfortunately, the soluble hydrochloride salt exhibits poor targeting to the lungs following oral inhalation. It is rapidly absorbed into the systemic circulation with a half-life in the lungs of < 1 h [20]. For optimal antibacterial activity, it is critical to sustain therapeutic levels of ciprofloxacin in the lungs.
Maintaining drug levels within the lungs above the MIC can be challenging due to the multiple drug clearance pathways (e.g., mucociliary clearance, macrophages clearance, systemic absorption) [21–24], and limited toolbox of approved excipients for pulmonary sustained release [25, 26]. Indeed, many of the excipients utilized to sustain drug levels following oral administration are off-limits for inhaled therapeutics (e.g., many higher molecular weight polymers), due to the potential for excipient accumulation in the lungs following multiple dose administration [25].
Phospholipid-Based Delivery Systems
Two formulation strategies have been advanced in clinical development for sustaining levels of ciprofloxacin in the lungs: a dispersion of the drug encapsulated in liposomes [18, 20, 21, 27–32], and a dry powder formulation comprising the poorly soluble betaine form of the drug substance [17, 33–41]. A detailed comparison of the two formulations is presented in Table 1, with electron microscopy images of the two types of particles presented in Fig. 2.
Table 1.
Comparison of a liposomal dispersion (CDI) [18, 30], and a spray-dried dry powder formulation (CIP) [17, 33] of ciprofloxacin
| Metric | Ciprofloxacin dispersion for inhalation (CDI) | Ciprofloxacin inhalation powder (CIP) |
|---|---|---|
| Name | Pulmaquin®/Linhaliq® | Ciprofloxacin DPI |
| Delivery device | Jet nebulizer (e.g., PARI LC® Sprint, with TurboBoy S compressor) | T-326 dry powder inhaler |
| Form of drug product | Mixture of drug encapsulated in liposomes (CFI) and free drug in solution (FCI) | Spray-dried dry powder (suspension-based PulmoSphere™): 50 mg filled in size 2 HPMC capsule) |
| Form of drug substance in drug product | Hydrochloride salt | Mostly the neutral, zwitterionic form (betaine, 3.5 hydrate) |
| Nominal dose (ND) (as ciprofloxacin) | 189 mg/day (135 mg encapsulated, 54 mg free, in separate 3-ml vials) | 65 mg/day (2 × 32.5 mg) |
| Treatment regimen | Once daily | Twice daily |
| Excipients |
Encapsulated: HSPCa (70.6 mg/ml), cholesterol (29.4 mg/ml), 145 mM NaCl, 25 mM histidine buffer (pH 6.0) Free drug: 10 mM sodium acetate buffer (pH 3.2) |
2:1 mol/mol ratio of DSPC/CaCl2 |
| Lipid dose | Lipids: 211.8 (HSPC), 88.2 (Chol) = 300 mg/day; lipid/drug = 2.22 w/w | Lipids: 21.4 (DSPC) = 21.4 mg/day; lipid/drug = 0.33 w/w |
| Salts/buffers dose | NaCl (25.4 mg/day), histidine (11.7 mg/day), sodium acetate (2.5 mg/day) = 39.6 mg/day | CaCl2 (1.5 mg/day) |
| Total excipient | 339.6 mg/day | 22.9 mg/day |
| pH | ~ 6.0 (encapsulated)/3.2 (free); pH 4 to 5 after mixing | ~ 7.0 |
| Osmolarity | 300 mOsm/kg (encapsulated); ~ 128 mOsm/kg (free); after mixing the dispersion is hypoosmotic | Based on drug solubility of 70 μg/ml, the incremental increase in osmolarity in airway surface liquid for CIP is ~ 0.7 mOsm/kg |
| Storage | Refrigerated | Room temperature |
| Sterile | Yes | No |
aHSPC (hydrogenated soy phosphatidylcholine) comprises approximately 89% disteroylphosphatidylcholine (DSPC) and 11% dipalmitoylphosphatidylcholine (DPPC) [43]
Fig. 2.
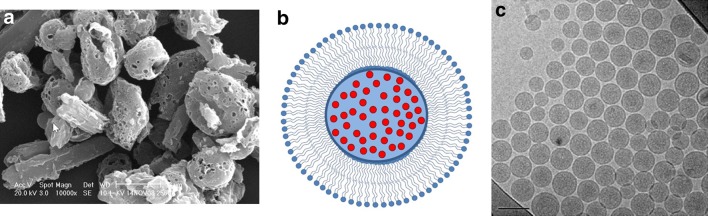
Electron microscopy images of phospholipid-based particles of ciprofloxacin. a SEM image of spray-dried particles (CIP) comprising ciprofloxacin betaine crystals coated with a porous layer of phospholipid [33]. The bar represents a length of 2 µm. Reproduced with permission from Respiratory Drug Delivery 2016, Virginia Commonwealth University; b schematic of small unilamellar vesicles loaded with ciprofloxacin, showing a single bilayer of phospholipid, with drug (red circles) encapsulated within the aqueous core [80]; c Cryo-TEM image of small unilamellar vesicles containing ciprofloxacin. The bar represents a length of 100 nm [80]. Reproduced with permission from Elsevier
Ciprofloxacin Dispersion for Inhalation (CDI)
CDI (Aradigm, Hayward, CA, USA) is comprised of ~ 70% slow-release ciprofloxacin encapsulated in liposomes, i.e., small unilamellar vesicles, SUVs with a mean diameter of ~ 80–100 nm (ciprofloxacin for inhalation, CFI) (Fig. 2b, c), and ~ 30% immediately available ciprofloxacin hydrochloride (free ciprofloxacin for inhalation, FCI) [18, 30]. The ‘free’ and ‘encapsulated’ forms of the drug are packaged in separate vials. The nominal dose of ciprofloxacin hydrochloride is 210 mg, of which 150 mg is encapsulated within the liposomes at pH 6.0, and 60 mg of free drug (20 mg/ml) is present in solution at pH 3.2 (Fig. 1) [18, 30]. When expressed in terms of ciprofloxacin, the 6-ml dosage form contains 189 mg of ciprofloxacin, comprising about 135 mg of encapsulated drug and 54 mg of free drug. It was hypothesized that the combination of free and encapsulated drug may lead to enhanced antibacterial activity, with the liposomal component enhancing bactericidal activity against the parent strain via increases in the AUC/MIC ratio, and the high concentration of free drug suppressing the development of resistant subpopulations through a high peak/MIC ratio [42].
The liposomes are stabilized at a lipid/drug ratio of 2.0 (2.22, as ciprofloxacin), with a mixture of hydrogenated soy phosphatidylcholine (HSPC) and cholesterol [18, 30]. HSPC contains approximately 89% distearoylphosphatidylcholine (DSPC) and 11% dipalmitoylphosphatidylcholine (DPPC) by weight [43]. The liposomal dispersion is iso-osmotic with the concentrations of NaCl (145 mM) and histidine buffer (25 mM) providing an osmolarity of ~ 300 mOsm/kg [18, 30].
In contrast, the osmolarity of the solution of free drug in 10 mM sodium acetate buffer at pH 3.2 is not reported. An estimate based on the reported formulation composition suggests that the solution is hypoosmotic with an osmolarity of ~ 128 mOsm/kg. Addition of NaCl to the free drug solution to increase the osmolarity was likely avoided, as chloride ions would reduce the solubility of the hydrochloride salt based on the common ion effect [44].
The pH of the liposomal dispersion (pH = 6.0) was selected to minimize acid- or base-catalyzed hydrolysis of the phospholipid acyl chain to form lyso-phosphatidylcholine and free fatty acid [45]. Indeed, acceptable physical and chemical stability of the drug substance and the phospholipid excipient in the liposomal dispersion was observed over 24-month storage at refrigerated temperatures [18, 30].
The liposomes are prepared in a multi-step manufacturing process that involves multiple ultrafiltration and diafiltration steps [18, 30], viz: (a) dissolution of the lipids in a solvent comprising mixtures of tert-butanol:ethanol:water in a 49:49:2 weight ratio; (b) mixing of the solution with methylamine sulfate buffer (10% v/v) to form multilamellar vesicles (MLVs); (c) extrusion of the MLVs through polycarbonate filters to form SUVs with a mean diameter between 80 and 100 nm; (d) ultrafiltration to concentrate the liposomes to a concentration of ~ 55 mg/ml; (e) diafiltration against isosmotic histidine buffer to remove the ethanol and generate a transmembrane pH gradient; (f) loading of powdered ciprofloxacin into the liposomes at elevated temperature; (g) diafiltration steps to remove unencapsulated drug; (h) ultrafiltration to the desired ciprofloxacin concentration of ~ 50 mg/ml, and; (h) sterile filtration through a 0.2-μm sterilizing grade filter.
The drug product comprising both free and encapsulated drug formulations has been referred to by various names in the literature ranging from ARD-3150 and DRCFI, to Pulmaquin® and Linhaliq®.
Ciprofloxacin Inhalation Powder (CIP)
CIP (Bayer Healthcare) is a dry powder formulation of ciprofloxacin administered twice daily with a portable capsule-based dry powder inhaler (i.e., the T-326 inhaler) [17, 33]. It is formulated using the suspension-based PulmoSphere™ technology [34, 35]. The PulmoSphere technology creates small porous microparticles by spray-drying a liquid feed comprising nano-emulsion droplets of a volatile oil [34, 35]. The nanoemulsion droplets are stabilized by a 2:1 mol:mol ratio of DSPC to calcium chloride. The PulmoSphere technology utilizes multiple formats for the introduction of the drug substance into the liquid feedstock [34]. In the suspension-based format, the drug substance is present as micronized crystalline drug particles suspended within the continuous phase of the oil-in-water emulsion [34, 35]. The spray-drying process leads to coating of the micronized drug crystals with a porous layer of phospholipid (Fig. 2a) [17, 33]. The low solubility of ciprofloxacin betaine ensures that the crystalline form of the drug substance is maintained throughout the spray-drying process, while minimizing the formation of amorphous domains within the particles [34, 35].
In contrast to CDI, ciprofloxacin is not ‘encapsulated’ and the phospholipid coating does not provide a barrier to release of a drug. The sustained levels of drug within the lungs for CIP is controlled solely by the inherently slow dissolution of the drug crystals in airway surface liquid (ASL). The lipid/drug (w/w) ratio in the non-retentive particles in CIP is 6.7-fold lower than in the liposomal formulation (Table 1).
Each dose of CIP contains 32.5 mg of ciprofloxacin betaine, 10.7 mg DSPC, 0.8 mg calcium chloride, with the remainder of the 50-mg fill mass comprising water associated with the drug substance, which is present as the 3.5 hydrate [17, 33]. Owing to the low solubility of the drug and phospholipid excipient, dissolution of CIP particles within ASL is not expected to significantly alter osmolarity, contributing just 0.7 mOsm/kg, due primarily to the calcium chloride.
CIP exhibits excellent long-term stability at room temperature. No chemical degradation products exceeding 0.1% w/w were observed following storage at room temperature (25°C/60% RH) over a period of 5 years [17, 33]. In addition to the excellent chemical stability, no significant changes in physical form of the drug substance or in aerosol performance were observed [17, 33].
Aerosol Delivery
The aerosol performance of liposomal and dry powder formulations of ciprofloxacin are detailed in Table 2 [17, 27, 33]. The key differences lie in the nature of the delivery systems (jet nebulizer versus dry powder inhaler), and in the dosing frequency (once daily for the liposomal formulation versus twice daily for the dry powder).
Table 2.
Comparison of aerosols of liposomal dispersion (CFI) and spray-dried dry powder formulation (CIP) of ciprofloxacin [17, 27, 33, 38]
| Metric | Ciprofloxacin for inhalation (CFI) (liposomes only) | Ciprofloxacin inhalation powder (CIP) |
|---|---|---|
| Treatment regimen | Once daily | Twice daily |
| Delivery device | Jet nebulizer (e.g., PARI LC® Sprint, with TurboBoy S compressor) | T-326 dry powder inhaler |
| Particle size (liposomes or drug crystals) | Small unilamellar vesicles (mean diameter = 80–100 nm) | Not disclosed |
| Particle size (nebulized droplets or dry powder) | VMD = 3.6 μm; GSD = 2.3 | x50 ~ 2.5 µm |
| Nominal dose (ND) | 150 mg (135 mg as base) | 32.5 mg (65 mg/day as base) |
| Emitted dose (ED) | 52.4% | ~ 94% |
| Fine particle dose < 5 μm | 64% of ED | 53% of ED |
| Mass median aerodynamic diameter (MMAD) | ~ 3.6 μm | 3.4–3.9 µm |
| Total lung dose (%ND) | ~ 16.7%: 25 mg (22.5 mg as base) | 51-53% (ca., 33.8 mg/day) |
| Administration time | ~ 8 min/day for 3 ml | < 4 min/day |
| Q index | Not determined | − 12.2% |
| Portable inhaler? | No | Yes |
| Power source required? | Yes | No |
| Cleaning required? | Yes | No |
ED emitted dose, GSD geometric standard deviation, MMAD mass median aerodynamic diameter, ND nominal dose, Q index [49]: Measure of flow rate dependence, VMD volume-weighted mean diameter, x50 volume-weighted median diameter
As mentioned, the CDI drug product comprises two 3-ml vials, one containing drug encapsulated in liposomes (CFI), and the other with free drug in solution (FCI). The contents of the two vials are added together in the nebulizer cup immediately prior to use, with the pH of the resulting CDI mixture between pH 4 and pH 5 [30]. The admixture is administered with the PARI LC Sprint jet nebulizer equipped with a Turboboy S compressor [27]. Vibrating mesh nebulizers were assessed but not adopted for administration of CDI, as the mesh became clogged during use, resulting in inconsistent drug delivery [27]. Other liposomal dispersions comprising amikacin or cyclosporine seemingly do not have clogging issues when using vibrating mesh nebulizers such as the PARI eFlow®. One possible explanation for this observation is that the free drug component of CDI is precipitating at the higher pH observed following mixing of the two formulations (Fig. 1).
Detailed information regarding the aerosol performance of CDI has not been publicly disclosed. Bruinenberg et al. did publish aerosol performance results for Lipoquin®(CFI), the liposomal component of CDI [27]. These results are presented in Table 2. The total lung dose (16.7% of the nominal dose) was not determined experimentally but was instead estimated from the in vitro results. The TLD is estimated from the product of the emitted dose and fine particle dose divided by two, to account for aerosol lost during exhalation. The reported value (ca. 16.7% delivery) is consistent with gamma scintigraphy studies with other antibiotic formulations delivered with PARI jet nebulizers [46, 47].
If one assumes that the aerosol performance for CFI and FCI mixtures in CDI is comparable to CFI alone, then the TLD is about 31.6 mg, of which 22.6 mg of ciprofloxacin (as base) is encapsulated in liposomes, and 9.0 mg is free drug. The mass of ciprofloxacin depositing in the upper respiratory tract (URT) is about 17.9 mg, along with 39.7 mg of excipients. The total administration time for CDI would be about 16 min.
In contrast, CIP is administered twice daily with a portable dry powder inhaler (i.e., the T-326 DPI, aka the Podhaler in TOBI Podhaler) [48]. The administration time for CIP is less than 2 min per dose administration, and less than 4 min/day [17, 33]. The TLD, as determined by gamma scintigraphy, was ~ 51–53% of the nominal dose, or about 33.8 mg/day [38]. Deposition in the URT is 27.3 mg/day with 9.0 mg/day excipients. For a single dose administration, URT deposition is 13.7 mg ciprofloxacin and 4.5 mg excipients.
The TLD observed with CIP was comparable for healthy volunteers and for patients with chronic obstructive pulmonary disease (COPD) or BE, although a more central lung deposition was observed in COPD and BE patients [38]. Drug delivery to the lungs with CIP is largely independent of inspiratory flow rate, with a Q index of - 12.2% [35]. The Q index represents the percentage difference in TLD between pressure drops of 1 and 6 kPa [49]. Values less than 15% are consistent with low flow rate dependence, values between 15 and 40%, medium flow rate dependence, and values > 40% high flow rate dependence [49].
Significant differences exist between the delivery devices in terms of their portability, power requirements, cleaning requirements, administration time, and treatment regimen. These differences may impact the daily treatment burden and/or convenience of treatment, ultimately influencing patient adherence.
Pharmacokinetics
Improved Lung Targeting
Superior targeting of drug to the site of the infection in the airways can be achieved with inhaled sustained release formulations of ciprofloxacin, compared to current marketed oral and parenteral formulations.
For example, inhalation of 32.5 mg of CIP by healthy volunteers results in a mean peak sputum concentration of 149.7 mg/l [17, 50]. This is about 1000-fold higher than sputum levels achieved with oral ciprofloxacin, which range from 0.11 to 0.21 mg/l following treatment with doses of 500, 750, and 1000 mg [51]. The sputum concentrations achieved with CIP are more than 100-fold higher than the MIC of ciprofloxacin susceptible (≤ 1 mg/l) Pseudomonas aeruginosa, Hemophilus influenzae, Staphylococcus aureus, and Streptococcus pneumoniae strains [52]. This helps to ensure that the required concentration for bactericidal action is achieved, while limiting the development of ciprofloxacin-resistant strains.
The high levels of ciprofloxacin in sputum observed for CIP are achieved without corresponding high levels of drug in the systemic circulation. Indeed, the peak plasma levels for CIP are 0.151 mg/l [50], versus 3.59 mg/l for a 750-mg oral dose [51]. The ratio of sputum/plasma ciprofloxacin concentrations provides a measure of lung targeting (i.e., the ratio of drug targeted to the site of the infection in the airways relative to off-target concentrations in the systemic circulation). The sputum/plasma ratio for CIP is 25,000-fold greater than the ratio observed for the 750-mg oral dose.
Inhalation of a 270-mg dose of CFI also leads to excellent lung targeting, with peak ciprofloxacin levels in sputum of 240 mg/l and plasma levels of just 0.105 mg/l [18].
Animal Studies Demonstrating Impact of Improved Lung Targeting
The pioneering work of Wong and colleagues demonstrated that encapsulation of ciprofloxacin in liposomes improves lung targeting relative to inhalation of free ciprofloxacin hydrochloride, leading to dramatic improvements in efficacy in animal models of lung infection [20, 21]. In their experiments, mice were infected by intranasal administration of a lethal dose of Francisella tularensis (i.e., 10 × the LD50). At 24-h post-infection, the mice were treated with a single dose of either aerosolized free ciprofloxacin or aerosolized liposome-encapsulated ciprofloxacin. The survival rates were assessed until day 14. Untreated control animals exhibited 100% mortality by day 9. Mice treated with free ciprofloxacin also exhibited 100% mortality by day 9. In stark contrast, mice treated with liposomal ciprofloxacin exhibited 100% survival at day 14. The CFU of F. tularensis in lung, spleen, and liver tissue at day 14 in the untreated controls were > 4 × 106 CFU, while organs from the animals treated with liposomal ciprofloxacin were devoid of F. tularensis.
The potential for CFI to provide post-exposure prophylaxis or effective treatment of biodefense pathogens including F. tularensis, Yersinia pestis (pneumonic plague), and Coxiella burnetti (Q fever) has been conclusively demonstrated in multiple animal studies [18, and references therein].
Dramatic improvements in bacterial killing were also observed for rats infected with P. aeruginosa following intratracheal administration of a 7.5 mg/kg dose of insoluble ciprofloxacin betaine versus an equivalent dose of soluble ciprofloxacin hydrochloride [36]. Ciprofloxacin betaine increased the half-life in the lungs to 13.5 h, while also increasing the AUC by 40-fold relative to ciprofloxacin hydrochloride. The improved lung targeting led to a 10-log reduction in P. aeruginosa CFU counts in the lungs compared to only a 2.8-log reduction for the hydrochloride salt.
Systemic Pharmacokinetics
Systemic levels of drugs following oral inhalation contain contributions from drugs that are absorbed from the lungs, and drug that is deposited in the oropharynx, swallowed, and subsequently absorbed from the gastrointestinal tract. Given the high oral bioavailability observed for ciprofloxacin (~ 78%) [53], the contribution of gastrointestinal absorption to systemic drug levels can be significant. For fluoroquinolones like ciprofloxacin, the situation is further complicated by enteric recirculation, where drug is continuously excreted from the blood back into the gastrointestinal tract, whereupon it gets reabsorbed [38, 54].
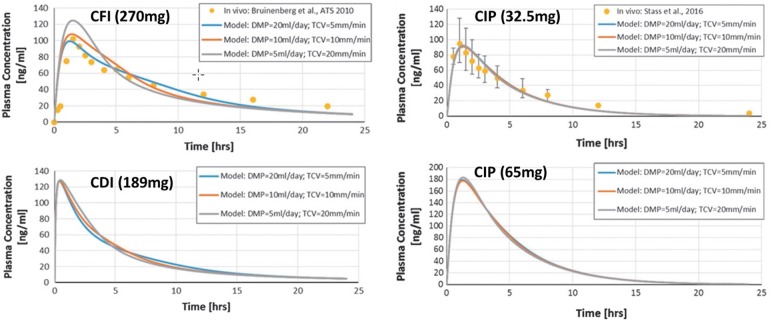
Both liposome-encapsulated ciprofloxacin and the practically insoluble betaine form of ciprofloxacin exhibit flip-flop kinetics, where the rate of absorption from the lungs is significantly slower than its rate of clearance from the body. The systemic clearance half-life for ciprofloxacin following oral or parenteral administration varies between about 3.6 and 7.0 h, depending on the dose [17]. In contrast, the reported terminal half-life in plasma for CFI (i.e., the liposomal component of CDI) in healthy volunteers is 10.5 h (Fig. 3, points on modeled curves) [27], while that for CIP is 9.5 h (Fig. 3, points on modeled curves) [39]. For CIP, the total clearance from plasma was 91.7 l/h, and the apparent volume of distribution was 1262 l [39].
Fig. 3.
Systemic pharmacokinetics of phospholipid-based formulations following single dose administration of various ciprofloxacin formulations [27, 39, 55]. CFI (ciprofloxacin for inhalation) represents the liposomal component of the dual-release CDI (ciprofloxacin dispersion for inhalation) comprising a combination of liposomal and free drug. CIP (ciprofloxacin inhalation powder) is a dry powder formulation of ciprofloxacin. Note that the dose of liposomal ciprofloxacin administered in CFI is two times higher than that in CDI. The points presented above were determined experimentally [27, 39], while the curves represent fits to a compartmental pharmacokinetic model [55]. Reproduced with permission from Mary Ann Liebert, Inc. [55]
Martin and Finlay [55] utilized a compartmental disposition model to fit the pharmacokinetic profiles of the phospholipid-based formulations of ciprofloxacin. ASL volumes in each tracheobronchial generation were determined using a model based on specified values of daily mucous production (DMP) and tracheal clearance velocity (TCV) [55]. Three different combinations of DMP and TCV were used, yielding low, intermediate, and high estimates of initial ciprofloxacin ASL concentrations (Fig. 3). The model provides reasonable fits to the experimental data. The model was then used to predict plasma concentrations following administration of CDI and a higher dose (65 mg) of CIP.
As discussed, maintaining the concentration of ciprofloxacin above the MIC is expected to improve efficacy of the drug with respect to bacterial killing of the parent strain, while achieving high peak concentrations of free drug may suppress development of resistant sub-populations. Doubling the dose of CIP effectively doubled the peak plasma concentration. In CDI, the tmax was shifted to earlier times relative to CFI, and the Cmax to higher concentrations, despite the lower ciprofloxacin dose. This reflects the rapid absorption of the free drug portion of the dual-release delivery system into the systemic circulation. The dual-release CDI drug product also has a lower apparent half-life in the lungs than was observed for CFI.
Despite the similar sputum drug concentrations and systemic clearance profiles, the liposomal CDI formulation is administered once daily, while the dry powder CIP formulation is administered twice daily. Based on the data detailed herein, the choice of QD vs. BID dosing seems to be more driven by sponsor preference than by significant differences in pharmacokinetics.
One of the challenges with sustained release systems in the lungs is the difficulty in assessing the percentage of free drug available to elicit a pharmacodynamic effect. Martin and Finlay [55] took their work one step further and modeled the time that ‘free’ ciprofloxacin concentrations in the ASL remained above an MIC of 4 μg/ml (Table 3). The calculations utilized established computational fluid dynamics models for assessing regional deposition and the ASL volumes described above to evaluate the disposition of drug resulting from dissolution or release, absorption, and mucociliary clearance within the lungs. They extended these calculations to various regions within the respiratory tract (trachea, generation 5, and generation 12).
Table 3.
Model predictions of peak concentration of free ciprofloxacin and time above the MIC of P. aeruginosa for four different inhaled ciprofloxacin formulations administered once daily [55]
| Drug product | Peak concentration of free cipro (µg/ml) | Time above the MIC (h) | ||||
|---|---|---|---|---|---|---|
| Trachea | Generation 5 | Generation 12 | Trachea | Generation 5 | Generation 12 | |
| CFI (270 mg) | 82.9 | 66.5 | 49.9 | 6.20 | 5.15 | 2.75 |
| CDI (189 mg) | 1323.8 | 1457.5 | 1043.4 | 5.65 | 4.65 | 2.35 |
| CIP (32.5 mg) | 52.1 | 49.9 | 40.4 | 4.90 | 4.34 | 2.84 |
| CIP (65 mg) | 57.7 | 56.3 | 48.5 | 5.69 | 5.01 | 3.32 |
| CIP (32.5 mg BID) | – | – | – | 9.80 | 8.68 | 5.68 |
Results shown for daily mucous production (DMP) of 10 ml/day and a tracheal clearance velocity (TCV) of 10 mm/min; Assumes an MIC for P. aeruginosa of 4 µg/ml
The modeled ASL concentrations in the tail of the pharmacokinetic profile are generally below the MIC of the pathogen. As a result, the predicted times above the MIC were comparable following once daily administration for CFI, CDI, and CIP (Table 3). When administered according to its twice daily dosing regimen, CIP is predicted to double the time above MIC relative to CDI.
The peak concentrations of free ciprofloxacin in the ASL were dramatically higher for CDI (Table 3), a feature that may be critical for suppressing resistant sub-populations. In contrast, increasing the dose of CIP from 32.5 mg to 65 mg does not significantly increase the ASL concentration of free drug, as ASL concentrations are limited by the solubility of the drug. Hence, there is no benefit in increasing the drug concentration with CIP beyond the current 32.5-mg dose. An alternative dry powder formulation comprising mixtures of ciprofloxacin betaine and ciprofloxacin hydrochloride could provide a dual release formulation, like CDI, if desired.
The results in Table 3 also indicate that drug concentrations are likely to vary throughout the respiratory tract. This is related to a variety of factors that are patient specific, such as anatomical features associated with their respiratory tract, the extent of their disease and its impact on air flow during inhalation, and their inspiratory flow profile during inhalation of the drug. Bos et al. [56] used functional respiratory imaging to demonstrate that some CF patients nebulizing aztreonam received subtherapeutic levels of drug in regions of the lungs where airflow was obstructed due to advanced bronchiectasis. Additional tools and studies are needed to better understand the impact of lung disease, patient anatomical features and inspiratory flow profiles on regional deposition of antibiotics in the lungs.
No significant differences in microbiological performance or in the development of resistant strains were observed between CDI and CIP in phase 3 studies [11–13, 28]. Hence, it remains inconclusive as to whether the presence of free drug improves the microbiological or clinical effect.
The uncertainty in DMP and TCV values can have a significant effect on estimates of time above MIC. Hence, the time above MIC values could be higher for each of these formulations than the model predicts [55].
Phase 3 Trials
CDI
Phase 3 studies with CDI (ORBIT-3 and ORBIT-4) were multinational, randomized (2:1), 48-week, double-blind, placebo-controlled trials in BE patients with chronic P. aeruginosa infection [11, 57]. The decision to restrict enrollment to those patients with P. aeruginosa infections was intended to focus the study on a more homogeneous and sicker patient population with an increased likelihood to exacerbate. Drug was administered in 28-day on and 28-day off-drug cycles over the 48-week treatment period. The primary endpoint was the time to first PE from baseline to week 48. Secondary endpoints included: the number of PE events per subject from baseline to week 48, the number of severe PEs per subject from baseline to week 48, and changes in the Respiratory Symptoms Scale score of the Quality of Life Questionnaire–Bronchiectasis (QoL-B) from baseline to week 48.
Inclusion criteria specified that subjects should have had two or more PE in the previous year. In the phase 3 trials, a PE was defined as the presence of specific abnormal respiratory signs or symptoms with an onset date and an end date [57]. An abnormality was defined as a change from a subject’s baseline in at least four of the following nine symptoms, signs, or laboratory findings: (1) change in sputum production (consistency, color, volume, or hemoptysis); (2) increased dyspnea (chest congestion or shortness of breath); (3) increased cough; (4) fever (≥ 38 °C); (5) increased wheezing; (6) decreased exercise tolerance, malaise, fatigue, or lethargy; (7) FEV1 or FVC decreased ≥ 10% from a previously recorded value; (8) radiographic changes indicative of a new pulmonary process; (9) changes in chest sounds.
CIP
Phase 3 studies (RESPIRE 1 and RESPIRE 2) for CIP were replicate, randomized, controlled studies with four treatment arms over 48 weeks of treatment [12, 13, 58]. Treatment groups were compared to placebo, with placebo defined as the placebo inhaler plus standard of care active treatment. Eligibility criteria were selected to represent BE patients expected to be treated with long-term inhaled antibiotic therapy in the real world, including an array of pathogens found in these patients (i.e., not just P. aeruginosa). As with CDI, the primary efficacy measure was time to first exacerbation, with the frequency of exacerbations being the first secondary endpoint. Patients were to have had two or more PE in the previous year. A qualifying exacerbation was defined as one that required systemic antibiotic treatment and was associated with the presence of fever or malaise, fatigue, and worsening of at least three signs/symptoms [i.e., dyspnea, wheezing, coughing, sputum volume, and sputum purulence (color)]. The acute worsening of signs and symptoms was required to be beyond daily variations. Two active treatment groups/schedules were studied: 28-day on–off treatment cycles, and 14-day on–off treatment cycles.
Both CDI and CIP had one positive trial and one trial that failed to meet statistical significance in the primary endpoint [11–13, 57, 58]. As a result, both failed to receive marketing authorization from the FDA.
In a recent Editorial concerning the RESPIRE studies with CIP, Chotirmall and Chalmers [59] posed the question: “does the drug actually work?” Their belief was, “yes the drug works, and is most likely to be of benefit in selected patients with poorly controlled disease and very frequent exacerbations (e.g., > 3 PE per year).” While the RESPIRE studies aimed at enrolling patients who had experienced a minimum of two PE in the previous year, the number of PE events observed in the placebo groups during the RESPIRE trials was significantly fewer than this number. Indeed, 62.2% of patients in RESPIRE 2 and 52.0% of patients in RESPIRE 1 did not have a single PE over the duration of the 48-week trial [12, 13], and in RESPIRE 2, patients averaged just 0.6 PE over the course of the study [13]. Hence, a challenge remains in identifying those BE patients, from a heterogeneous patient population, who are at risk of an exacerbation due to an underlying infection, as these patients would be most likely to benefit from treatment. The lack of a consensus definition of exacerbation amongst treating physicians also hampered enrolling the ‘right patients’ and in properly powering the phase 3 studies. Finally, it was also proposed that the frequency of PE events may be a better endpoint than time to a PE.
Adverse events
The adverse event profiles observed in phase 3 for the two phospholipid-based formulations are presented in Tables 4 and 5 [11–13, 57, 58]. In Table 5, the incidence rates are coded by preferred term and system organ class (SOC) as per the most updated version of the Medical Dictionary for Regulatory Activities (MedDRA). Investigators also summarized the Treatment Emergent Adverse Events (TEAE) in terms of intensity (mild, moderate, severe) and causality (i.e., is it drug related). Both formulations were generally well tolerated, with most adverse events mild to moderate in intensity.
Table 4.
Summary of treatment emergent adverse events (TEAE) reported for CDI and CIP in phase 3 clinical studies in BE patients [11–13, 57, 58]
| CDI (28-d) (N = 389) N (%) | CDI Placebo (N = 193) N (%) | CIP (14-d) (N = 310) N (%) | CIP (28-d) (N = 312) N (%) | CIP Placebo (N = 311) N (%) | |
|---|---|---|---|---|---|
| Adverse events (AE) | 343 (88.2) | 182 (94.3) | 239 (77.1) | 204 (65.4) | 230 (74.0) |
| Drug-related AEs | 136 (35.0) | 66 (34.2) | 61 (19.7) | 54 (17.3) | 60 (19.3) |
| Cough | 42 (10.8) | 26 (13.5) | 4 (1.3) | 6 (1.9) | 11 (3.5) |
| Dyspnea | 25 (6.4) | 13 (6.7) | 7 (2.3) | 6 (1.9) | 5 (1.6) |
| Wheezing | 20 (5.1) | 10 (5.2) | NR | NR | NR |
| Serious AEs (SAE) | 91 (23.4) | 52 (26.9) | 68 (21.9) | 56 (18.0) | 73 (23.5) |
| Drug-related SAE | 7 (1.8) | 2 (1.0) | 2 (0.6) | 4 (1.3) | 1 (0.3) |
| Severe AEs | 85 (21.9) | 47 (24.4) | 48 (15.5) | 38 (12.2) | 51 (16.4) |
| AEs Leading to discontinuation | 34 (8.7) | 16 (8.3) | 32 (10.3) | 26 (8.3) | 19 (2.0) |
| AEs Leading to death | 6 (1.5) | 5 (2.6) | 4 (1.3) | 6 (1.9) | 5 (1.6) |
NR not reported (less than 2% threshold)
Table 5.
Comparison of treatment-emergent adverse events (TEAEs) for inhaled ciprofloxacin formulations in BE patients [11–13, 57, 58]
| Adverse event | CDI (28-d) (N = 389) | CDI Placebo (N = 193) | CIP (14-d) (N = 310) | CIP (28-d) (N = 312) | CIP Placebo (N = 311) |
|---|---|---|---|---|---|
| TEAEs | 343 (88.2%) | 182 (94.3%) | 239 (77.1%) | 204 (65.4%) | 230 (74.0%) |
| Respiratory, thoracic, mediastinal disorders | 295 (75.8%) | 158 (81.9%) | 134 (43.2%) | 104 (33.3%) | 127 (40.8%) |
| Cough | 251 (64.5%) | 126 (65.3%) | 20 (6.5%) | 20 (6.4%) | 20 (6.4%) |
| Dyspnea | 211 (54.2%) | 103 (53.4%) | 26 (8.4%) | 20 (6.4%) | 12 (3.9%) |
| Sputum increased | 181 (46.5%) | 108 (56.0%) | NR | NR | NR |
| Wheezing | 153 (39.3%) | 84 (43.5%) | NR | NR | NR |
| Increased viscosity of bronchial secretion | 66 (17.0%) | 37 (19.2%) | NR | NR | NR |
| Hemoptysis | 58 (14.9%) | 27 (14.0%) | 33 (10.6%) | 27 (8.7%) | 32 (10.3%) |
| Oropharyngeal pain | 19 (4.9%) | 20 (10.4%) | NR | NR | NR |
| Rhinorrhea | 14 (3.6%) | 15 (7.8%) | NR | NR | NR |
| Discolored sputum | 13 (3.3%) | 11 (5.7%) | NR | NR | NR |
| Abnormal sputum | 46 (11.8%) | 27 (14.8%) | NR | NR | NR |
| Bronchiectasis | NR | NR | 32 (10.3%) | 33 (10.6%) | 38 (12.2%) |
| Abnormal breath sounds | 103 (26.5%) | 45 (23.3%) | NR | NR | NR |
| Bronchospasm | 5 (1.3%) | 2 (1.0%) | 14 (4.5%) | 10 (3.2%) | 19 (6.1%) |
| General disorders | 237 (60.9%) | 133 (68.9%) | 47 (15.2%) | 39 (12.5%) | 29 (9.3%) |
| Fatigue | 142 (36.5%) | 89 (46.1%) | 14 (4.5%) | 8 (2.6%) | 5 (1.6%) |
| Exercise tolerance decreased | 98 (25.2%) | 55 (28.5%) | NR | NR | NR |
| Pyrexia | 90 (23.1%) | 56 (29.0%) | NR | NR | NR |
| Malaise | 52 (13.4%) | 29 (15.0%) | 7 (2.3%) | 7 (2.2%) | 1 (0.3%) |
| Chest pain | 23 (5.9%) | 9 (4.7%) | NR | NR | NR |
| Chest discomfort | 19 (4.9%) | 10 (5.2%) | NR | NR | NR |
| Infections and infestations | 191 (49.1%) | 111 (57.5%) | 99 (31.9%) | 114 (36.5%) | 106 (34.1%) |
| Sputum purulent | 86 (22.1%) | 50 (25.9%) | NR | NR | NR |
| Nasopharyngitis | 21 (5.4%) | 11 (5.7%) | 32 (10.3%) | 25 (8.0%) | 24 (7.7%) |
| Pneumonia | 20 (5.1%) | 7 (3.6%) | NR | NR | NR |
| URT infection | NR | NR | 17 (5.5%) | 14 (4.5%) | 15 (4.8%) |
| Nervous system disorders | 153 (39.3%) | 72 (37.3%) | 59 (19.0%) | 58 (18.6%) | 30 (9.6%) |
| Lethargy | 88 (22.6%) | 38 (19.7%) | NR | NR | NR |
| Headache | 44 (11.3%) | 25 (13.0%) | 24 (7.7%) | 21 (6.7%) | 9 (2.9%) |
| Dysguesia | 32 (8.2%) | 13 (6.7%) | 14 (4.5%) | 18 (5.8%) | 4 (1.9%) |
| Dizziness | 28 (7.2%) | 9 (4.7%) | 11 (3.6%) | 3 (1.0%) | 3 (1.0%) |
| Gastrointestinal disorders | 92 (23.7%) | 58 (30.1%) | 70 (22.6%) | 56 (17.9%) | 62 (19.9%) |
| Nausea | 30 (7.7%) | 11 (5.7%) | NR | NR | NR |
| Diarrhea | 21 (5.4%) | 19 (9.8%) | 16 (5.2%) | 8 (2.6%) | 10 (3.2%) |
| Musculoskeletal/connective tissue disorders | 93 (23.9%) | 44 (22.8%) | 46 (14.8%) | 43 (13.8%) | 28 (9.0%) |
| Arthralgia | 23 (5.9%) | 9 (4.7%) | 6 (1.9%) | 7 (2.2%) | 3 (1.0%) |
| Back pain | 21 (5.4%) | 6 (3.1%) | NR | NR | NR |
Note that some values less than 5% for CIP were found in the FDA briefing books, and included in the table for comparative purposes
NR not reported (less than 5% threshold)
CDI
In their FDA Advisory Committee briefing book, the Sponsor concluded that results from the two phase 3 studies indicated that 48 weeks of treatment with CDI (i.e., six 28-day on-treatment periods and six 28-day off-treatment periods) was well tolerated [57]. Their summary further stated that there were no clinically meaningful differences in safety between the CDI and placebo groups (Tables 4, 5).
Pre-specified adverse events of special interest (AESI) were focused on airway irritation. The AESIs (e.g., cough, dyspnea, wheezing, throat irritation, bronchospasm, respiratory tract irritation, laryngitis, pharyngitis) were reported at a similar overall rate between the CDI and placebo groups, with the Sponsor concluding that inhalation of CDI did not lead to increases in airway irritation. Additionally, no SOC or individual preferred term was reported more frequently in the CDI group versus the placebo group. Finally, there was no evidence of an increased frequency in any of the commonly known fluoroquinolone class effects in patients receiving CDI [57].
CIP
Similarly, in their FDA Advisory Committee briefing book for CIP, the Sponsor concluded that there were no clinically meaningful differences between the two active treatment groups, or between the two active treatment groups and pooled placebo with respect to TEAE, SAE, deaths, and TEAEs requiring study discontinuation [58]. Most of the reported TEAEs were mild to moderate in intensity.
The incidence of respiratory AESIs as defined above, and adverse events related to the fluoroquinolone class were small (Table 5) [58]. The numerical group differences were not indicative of an increased risk of local irritation, hypersensitivity, or fluoroquinolone class effects for either treatment group in comparison with the placebo.
Comparison of Formulations
While these large multicenter phase 3 trials were run concurrently in the BE patient population, the fact that head-to-head comparisons between formulations were not made within the same trial leaves the data open to bias in how the AEs were reported. This is especially true given that many of the AEs that emerged during the conduct of the studies are also symptoms present in the underlying disease that could be magnified during an exacerbation. Prior to initiation of treatment, the FEV1 % predicted was similar (about 60%) across the various treatment groups in the two trials.
The restriction to BE patients with P. aeruginosa infections in the CDI studies as opposed to the broader range of pathogens in the CIP studies (ca. 60% with P. aeruginosa infection), likely resulted in inclusion of sicker, more symptomatic patients who are more likely to exacerbate. Indeed, the incidence of PEs was higher in the CDI trials. The median time to a PE for CDI was 230 days, versus 430 days for CIP [11–13].
Little difference was observed between CDI and CIP in the reported incidence of serious AEs, drug-related serious AEs, severe AEs, AEs leading to discontinuation, and AEs leading to death (Table 4) [11–13]. Nonetheless, there are apparent differences in the incidence of mild and moderate AEs between the two formulations (Table 5, Fig. 4). Of interest are the large differences in respiratory AEs related to cough (64.5% in CDI vs. 6.5% in CIP), dyspnea (54.2% in CDI vs. 7.4% in CIP), and wheezing (39.3% in CDI vs. < 5% in CIP). Decreases in lung function (FEV1 and FVC) were also observed in up to 30% of patients in the CDI trials, while no decreases in lung function were reported in the CIP studies. The large differences extend beyond respiratory TEAEs, with fatigue (36.5% in CDI vs. 3.6% in CIP), pyrexia (23.1% in CDI vs. not reported in CIP), and malaise (13.4% in CDI vs. 2.3% in CIP) and numerous other AEs also reported at a higher incidence in CDI. The incidence of bronchospasm (3.8% in CIP vs. 1.7% in CDI) and bronchiectasis (10.5% in CIP vs. < 5% in CDI) were reported at a higher incidence in the CIP group.
Fig. 4.
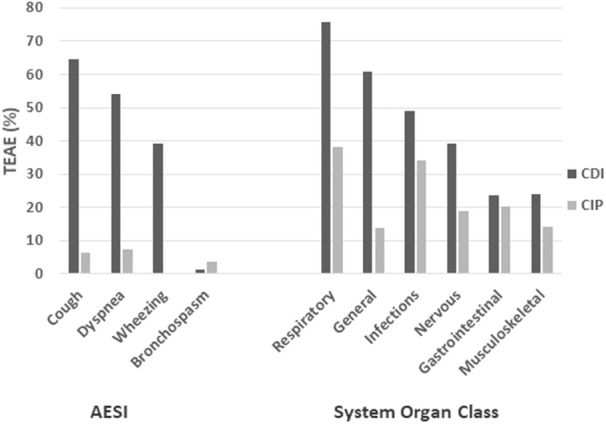
Comparison of reported treatment emergent adverse events (TEAE) between CDI and CIP. The left part of the graph displays differences in respiratory adverse events of special interest (AESI), while the right part compares the differences in TEAE based on system organ class (SOC) [11–13]
While it is customary to compare adverse events relative to placebo, such a practice assumes that the placebo does not contribute significantly to said adverse events. The data suggest that this might not be the case with the liposomal particles comprising the CDI placebo. Overall, 94.3% of CDI placebo patients had a TEAE, versus 74.0% of CIP placebo patients (Table 5). Increases in TEAEs were observed for CDI placebo versus CIP placebo in: (a) respiratory, thoracic and mediastinal disorders (81.9 vs. 40.8%); (b) general disorders (68.9 vs. 9.3%); (c) infections and infestations (57.5 vs. 34.1%); (d) nervous system disorders (37.3 vs. 9.6%); (e) gastrointestinal disorders (30.1 vs. 19.9%), and; (f) musculoskeletal and connective tissue disorders (22.8 vs. less than 9.0%).
Following parenteral administration of phospholipid-based formulations (e.g., emulsions or liposomes), phagocytosis of the particles by cells of the reticuloendothelial system leads to characteristic, predictable, and reversible biological effects that are a consequence of a normal host-defense mechanism [60]. This mechanism is characterized by dose-related stimulation of macrophages and subsequent release of intracellular products (particularly metabolites of the arachidonic acid cascade and cytokines). Particulate clearance can result in a flu-like syndrome characterized by cutaneous flushing and fever at lower doses, and macrophage hypertrophy and recruitment at higher doses. These biological effects are generally reversible and do not result in any permanent tissue alteration, even with prolonged exposure at relatively high doses. The magnitude of the immune response depends on the nature of the particulates with size, shape, and the ‘tastiness’ of the particle surface being critical.
In a review article directed to induced alveolar macrophages responses, Forbes et al. [61] stated that: “Alveolar macrophages (AM) responses are commonly induced in inhalation toxicology studies, typically being observed as an increase in number or a vacuolated ‘foamy’ morphology. Discriminating between adaptive AM responses and adverse events during nonclinical and clinical development is a major scientific challenge.” The authors went on to suggest that the physicochemical properties of the particulates are closely linked with the macrophage responses, although the mechanisms by which macrophages recognize different particulates is still poorly understood. Indeed, Forbes et al. concluded that: “an improved understanding of induced AM responses to inhaled pharmaceuticals is required.” This is particularly critical for sustained release formulations, where the particles are present within the lungs for an extended period.
Arikayce (liposomal amikacin) was the first pulmonary sustained release formulation to receive marketing authorization. The Arikayce formulation differs significantly from the CDI formulation [7]. Arikayce comprises multilamellar liposomes with a larger diameter (250–300 nm vs. 80–100 nm in CDI). The formulation utilizes a mixture of dipalmitoylphosphatidylcholine (DPPC) and cholesterol with a lipid/drug ratio of just 0.70 (vs. 2.22 in CDI). The formulation is nebulized with a vibrating mesh nebulizer (eFlow®). About 30% of the encapsulated drug is subject to ‘burst’ during nebulization, resulting in a comparable combination of free and encapsulated drug [8, 10]. The reported MMAD of the droplets is 4.7 vs. 3.6 μm for CDI [7]. The significant differences in physicochemical properties between the two liposomal formulations suggests that the phagocytosis process for Arikayce may differ from the process with CDI liposomes.
In the NTM patient population, Arikayce is associated with an increased risk of respiratory adverse events, including hypersensitivity pneumonitis (lung inflammation), hemoptysis, bronchospasm, and exacerbation of underlying pulmonary disease that leads to hospitalization in some cases [62]. In their Phase 3 clinical trial, the safety and effectiveness of Arikayce was assessed relative to standard of care, with no liposomal placebo formulation administered in the control group [62]. If the AEs detailed above are related to phagocytosis of the liposomes by alveolar macrophages, then the lack of a liposomal placebo in the control group may have helped to highlight the irritant effects of the liposomes in the Arikayce study (i.e., the claim that there was no difference in AEs between the drug and placebo formulations could not be made).
According to Szebeni et al. [63], bronchopulmonary symptoms of acute hypersensitivity reactions comprise many of the symptoms reported at a greater incidence with the liposomal CDI formulation as compared to the CIP formulation (e.g., cough, dyspnea, wheezing, chest pain, chest discomfort, back pain, headache, pyrexia, and nausea).
The incidence of cough, dyspnea, wheezing, fatigue and pyrexia are higher in CDI than reported for Arikayce. While the incidence of bronchospasm (acute 15% drop in FEV1) was only 1.3% in CDI, bronchospasm was more broadly defined in the Arikayce study, where the following AEs were grouped: asthma, bronchial hyperreactivity, bronchospasm, dyspnea, dyspnea exertional, prolonged expiration, throat tightness, and wheezing. When defined in the same manner, the incidence of bronchospasm would be markedly higher in CDI (> 65%) vs. Arikayce (29%). Hence, it is not possible to rule out that a mild hypersensitivity pneumonitis is present in CDI patients following CDI administration.
The low incidence of cough observed with the high dose administration of the CIP dry powder may seem counterintuitive. It has been hypothesized that inhalation of high doses of a dry powder formulation will inherently result in airway irritation and cough (i.e., a direct effect of delivery of a large powder mass). The low incidence of post-inhalation cough with CIP has now been observed in several patient populations (healthy volunteers, CF patients, COPD patients, BE patients) across multiple clinical studies. In a large phase 2 study in 286 CF patients, the incidence of post-inhalation cough was 2.7% and bronchospasm just 0.5% [41].
Increased cough with high-dose powder formulations is not likely due to a direct powder effect, but rather to an irritant effect driven largely by variations in either proton or ion concentrations in ASL. CIP is formulated at neutral pH, so has no impact on the proton concentration in the ASL. The low solubilities of the long-chain phospholipid and the neutral ciprofloxacin drug substance poses only a low osmotic stress to the ASL (increase of just 0.7 mOsm/kg). Hence, CIP does not lead to irritation of the epithelium and, as a result, does not result in significant post-inhalation cough or other irritant effects in the airways. In contrast, in TOBI Podhaler, the high dose and large number of ionized species associated with the sulfate salt form lead to a significant osmotic stress to the ASL, ultimately resulting in a higher incidence of post-inhalation cough [64].
The differences in cough observed between CDI and CIP could also be the results of differences in regional deposition within the respiratory tract, given that cough receptors are concentrated in the larger airways. However, the differences in regional deposition of ciprofloxacin in the URT and lungs is not anticipated to be significant for an administered dose of the two medications (see earlier discussion on aerosol delivery). Moreover, the aerodynamic particle size distributions do not differ significantly for the two formulations. Hence, differences in regional deposition within the respiratory tract is not likely the cause for the differences in cough observed, unless the differences in excipient delivery are contributory.
The low incidence of AEs reported with the coated crystals versus the liposomal particles is interesting, and points to our poor understanding of induced AM responses, as espoused by Forbes et al. [61].
Patient Adherence
The burden of treatment for BE patients is high. Treatment burden is a combination of several factors, including the number of therapies required on a daily basis, the frequency and complexity of such therapies, the amount of time needed to complete a treatment, and how the patient perceives the treatment (e.g., cost, convenience, and tolerability issues).
Despite having no approved inhaled antibiotic option, BE patients are prescribed 12 ± 5 therapies per day [65]. Patient adherence to off-label nebulized antibiotic treatments is poor (ca. 53% adherence in one study), due in part to the high incidence of adverse events (e.g., bronchospasm and cough) associated with the administration of off-label inhaled antibiotics [65]. These products also do not provide sustained levels of antibiotic in the lungs, likely decreasing their therapeutic effect relative to the formulations described herein.
It has been suggested that reducing the frequency of antibiotic dose administration to once daily with CDI will increase adherence to therapy, with the potential to improve patient outcomes [66]. It has been further suggested that liposomal ciprofloxacin may: “blunt the irritation or side effects associated with drugs which cause cough or have unpleasant taste” [18, 66]. While these purported attributes of CDI would be expected to positively impact inhaled antibiotic delivery, it remains unclear whether patients will ultimately prefer these features relative to the convenience of a portable, maintenance-free dry powder inhaler, as is used in CIP.
Data from the phase 3 trials did not demonstrate improved tolerability for CDI relative to CIP. Indeed, the incidence of dysgeusia reported was 8.7% for CDI and 4.5–5.5% for CIP [11–13, 57, 58]. The incidence of cough was 64.5% for CDI versus 6.5% for CIP [11–13, 57, 58]. Drug-related cough was reported to be 10.8% for CDI vs. 1.3–1.9% for CIP [58, 67]. It is unclear if the presence of free drug in the ‘dual release’ CDI formulations reduces the benefits of encapsulating ciprofloxacin on tolerability (e.g., the unpleasant taste).
The ‘daily treatment burden’ considers not only the time to administer the drug, but also the time for set-up, breakdown, cleaning, and disinfection of the delivery device [68]. Conservatively, these additional activities require an additional 10 min/day for CDI, leading to a daily treatment burden of about 26 min/day. The compliance with cleaning nebulizers is typically poor, and this can lead to contamination of the nebulizer with bacteria, possibly increasing the risk of administration of new, more virulent pathogens to at-risk BE patients during treatment [1, 69].
The T-326 inhaler does not require cleaning or disinfection. It takes less than 2 min to administer a CIP dose, resulting in a daily treatment burden of less than 4 min/day. In this regard, it remains unclear whether the benefit of once-daily administration of CDI outweighs the 20 min of additional treatment burden. The portable dry powder inhaler has the added advantage that it does not require a power source and is easy to carry in a pocket or a purse, enabling discreet use outside the home [1].
The transition from a nebulized treatment to a dry powder treatment with tobramycin inhalation powder (TIP) vs. tobramycin inhalation solution (TIS) led to a dramatic decrease in administration time and daily treatment burden [1, 64, 68]. This translated into a high preference for the TIP treatment among CF patients, and improvements in adherence [64, 70, 71]. CIP has the added benefit over TIP that a dose requires only a single capsule (versus four capsules). Moreover, CIP has a markedly lower incidence of cough relative to TIP. Nonetheless, additional studies are needed to better understand which of the various attributes described above are more important in driving improved adherence in the BE patient population.
Conclusions
The two phospholipid-based aerosol formulations described within this review utilize the same principal phospholipid (DSPC), have similar mass median aerodynamic particle sizes (~ 3.5 μm), deliver a comparable ciprofloxacin dose to the lungs daily (~ 33 mg/day), exhibit sustained release of ciprofloxacin in the lungs, and have a similar rate of clearance from plasma (terminal half-life ~ 10 h). The two formulations differ primarily in their dosing regimen (once daily vs. twice daily), in the presence of free drug in the CDI formulation, in the mass of excipients administered, and in the nature of the delivery system. The CDI formulation may also be able to more effectively target delivery of ciprofloxacin into biofilms or into pulmonary macrophages [6, 16, 66].
There are currently no approved treatments that reduce or delay pulmonary exacerbations in BE patients. Studies with nebulized tobramycin, aztreonam, and colistin failed to provide clinically meaningful improvements in outcomes in BE patients [72–76]. These therapies were poorly tolerated, with a high incidence of respiratory symptoms including cough and bronchospasm. This led Rubin and others to suggest that the overall profile of adverse respiratory symptoms observed with inhaled antibiotics in BE patients may outweigh the benefits of the antibacterial activity [77]. Cipolla et al. hypothesized that inhaled antibiotics may be beneficial in BE patients if an antibiotic formulation and dosing regimen can be identified that is well tolerated and leads to improved adherence [66].
More than 1000 BE patients were administered the CDI and CIP formulations in Phase 3. Both sustained release ciprofloxacin formulations were generally well tolerated, with most AEs mild to moderate in severity. Both formulations also have the potential to improve patient adherence to treatment, due to reduced respiratory symptoms and a reduction in dosing frequency (CDI), or via reductions in daily treatment burden and improved convenience (CIP). Unfortunately, like their predecessors, both formulations failed to meet their primary endpoints in phase 3 clinical studies.
Clinical Development in BE
While the probability is high that both CDI and CIP are effective in treating PEs in BE patients having three or more exacerbations per year, a challenge remains in identifying and enrolling those patients. The lack of a standardized definition of a PE had a negative impact on the outcomes in the recently completed phase 3 studies, as comparisons of PE incidence over time are only useful if we can be certain that the subjective threshold for intervention has not changed [78]. This was obviously not the case. Although patients were required to have had at least two exacerbations in the previous year, the incidence in the four clinical trials was far less than this. Hence, the trials were significantly underpowered for the patients who were enrolled. Given the heterogeneity of the BE patient population, patient selection may also need to explore phenotypic, geographic, and microbiome differences, to more accurately identify those patients who are likely to exacerbate [59].
Recently, a consensus definition of an exacerbation in BE patients has been proposed [79]. The proposed definition is based on the recognition that the change in symptoms should be significant enough to warrant an ‘intervention’. The consensus definition comprises a deterioration in three or more key symptoms (cough, sputum volume and/or consistency, sputum purulence, breathlessness and/or exercise intolerance, fatigue and/or malaise, hemoptysis) for at least 48 h, and that this worsening leads to a change in BE treatment by the clinician.
Formulation Considerations
The late-stage development of two phospholipid-based formulations for sustaining concentrations of antibiotics in the lungs has increased our understanding of formulation attributes that may be important. Given the limited clinical data associated with sustained release formulations in the lungs, it is important to highlight the differences observed, as this may help to guide future development of pulmonary sustained release formulations.
The particulate-based side effects observed with liposomes in the lungs with Arikayce and possibly CDI are consistent with parenteral-based particulate delivery systems (e.g., liposomes, emulsions) that are cleared by the reticuloendothelial system. While liposomes comprise materials that are endogenous to the lungs and have natural clearance pathways, this does not mean that they are biologically inert. They have the potential to elicit an immune response that can increase airway irritation or inflammation. Indeed, the ability to target liposomes to pulmonary macrophages to treat intracellular infections is a double-edged sword, with the potential to dramatically improve treatment of these difficult-to-treat infections (e.g., NTM) while also potentiating an immune response as macrophages release cytokines to signal the need to clear the liposomal particles.
While retentive particulate systems may be needed to effectively target alveolar macrophages in the treatment of NTM infections, these features may not be as important for the treatment of infections in the airway lumen. Indeed, there may be advantages to formulating ‘non-retentive’ particles for infections in the airways.
Moreover, just because less irritation was observed for drug crystals in CIP versus CDI liposomes, this is likely not a generalizable rule. There is still much to be learned about phagocytosis of particles in the lungs and what particle characteristics are important for minimizing airway irritation and inflammation.
Post-inhalation cough is not an inherent AE associated with high-dose delivery of dry powder formulations. It is likely that post-inhalation cough can be minimized for high powder doses by administering the poorly soluble, neutral form of drugs (e.g., ciprofloxacin betaine), and by also utilizing poorly soluble excipients. Particulates that remain largely insoluble cannot negatively impact the osmolarity of ASL. Critical process parameters associated with suspension-based spray drying to formulate neutral forms of multiple anti-infective drugs have recently been described [35].
While both CDI and CIP formulations failed to achieve FDA approval, the lessons learned from these large multicenter trials have advanced our understanding of the important features required in pulmonary sustained release formulations, and in clinical trial design. The hope is that these advances will ultimately lead to FDA approval of therapies that reduce or delay pulmonary exacerbations in BE patients.
Acknowledgements
The author would like to thank Dan Miller and Andy Clark for providing input on the manuscript.
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
The author meets the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, had full access to all the data in this study, take responsibility for the integrity of the work as a whole, and have given their approval for this work to be published.
Disclosures
Jeffry Weers, Ph.D. is an employee of Respira Therapeutics, Inc. and receives salary, stock options and other forms of compensation. Respira was not involved with the work reviewed. Jeff is a co-inventor of the PulmoSphere™ technology and led CMC development of Ciprofloxacin DPI in collaboration with colleagues from Bayer. Jeff also oversaw early development of the liposomal Arykace® at Transave (now Insmed).
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by the author.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.10050230.
References
- 1.Geller D, Weers J, Heuerding S. Development of a dry powder formulation of tobramycin using PulmoSphere™ technology. J Aerosol Med Pulm Drug Deliv. 2011;24:175–182. doi: 10.1089/jamp.2010.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarara T, Weers J, Venthoye G. Pulmonary delivery of aminoglycosides. US Patent 7,368,102, 2008.
- 3.Miller DP, Tan T, Tarara T, Nakamura J, Malcolmson R, Weers J. Physical characterization of tobramycin inhalation powder I: rational design of a stable engineered-particle formulation for delivery to the lungs. Mol Pharm. 2015;12:2582–2593. doi: 10.1021/acs.molpharmaceut.5b00147. [DOI] [PubMed] [Google Scholar]
- 4.Miller D, Tan T, Nakamura J, Malcolmson R, Tarara T, Weers J. Physical characterization of tobramycin inhalation powder. II State diagram of an amorphous engineered particle formulation. Mol Pharm. 2017;14:1950–1960. doi: 10.1021/acs.molpharmaceut.7b00036. [DOI] [PubMed] [Google Scholar]
- 5.Haynes A, Geller D, Weers J, Ament B, Pavkov R, Malcolmson R, Debonnett L, Mastoridis P, Yadao T, Heuerding S. Inhalation of tobramycin using simulated cystic fibrosis patient profiles. Pediatr Pulmonol. 2016;51:1159–1167. doi: 10.1002/ppul.23451. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Leifer F, Rose S, Chun DY, Theisz J, Herr T, Nashed M, Joseph J, Perkins WR, DiPetrillo K. Amikacin liposome inhalation suspension (ALIS) penetrates non-tuberculosis mycobacterial biofilms and enhances amikacin uptake into macrophages. Front Microbiol. 2018;9:915. doi: 10.3389/fmicb.2018.00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z, Zhang Y, Wurtz W, Lee JK, Malinin VS, Durwas-Krishnan S, Meers P, Perkins WR. Characterization of nebulized liposomal amikacin (Arikace™) as a function of droplet size. J Aerosol Med Pulm Drug Deliv. 2008;21:245–253. doi: 10.1089/jamp.2008.0686. [DOI] [PubMed] [Google Scholar]
- 8.Weers J, Metzheiser B, Meers P, Taylor G, Warren S, Saiman L, Perkins W. A gamma scintigraphy study to investigate lung deposition and clearance of inhaled amikacin-loaded liposomes in healthy male volunteers. J Aerosol Med Pulm Drug Deliv. 2009;22:131–138. doi: 10.1089/jamp.2008.0693. [DOI] [PubMed] [Google Scholar]
- 9.Boni LT, Miller BS, Malinin V, Li X. Sustained release of anti-infectives. US Patent 8,802,137, 2014.
- 10.Weers JG. Lipid-based compositions of anti-infectives for treating pulmonary infections and methods of use thereof. US Patent 8,226,975, 2012.
- 11.Haworth CS, Bilton D, Chalmers JD, Davis AM, Froehlich J, Gonda I, Thompson B, Wanner A, O’Donnell AE. Inhaled liposomal ciprofloxacin in patients with non-cystic fibrosis bronchiectasis and chronic lung infection with Pseudomonas aeruginosa (ORBIT-3 and ORBIT-4): two phase 3, randomized controlled trials. The Lancet. 2019;7:213–226. doi: 10.1016/S2213-2600(18)30427-2. [DOI] [PubMed] [Google Scholar]
- 12.De Soyza A, Aksamit T, Bandel T-J, Criollo M, Elborn JS, Operschall E, Polverino E, Roth K, Winthrop KL, Wilson R. RESPIRE 1: a phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur Respir J. 2018;51(1):1702052. doi: 10.1183/13993003.02052-2017. [DOI] [PubMed] [Google Scholar]
- 13.Aksamit T, de Soyza A, Bandel T-J, Criollo M, Elborn JS, Operschall E, Polverino E, Roth K, Winthrop KL, Wilson R. RESPIRE 2: a phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur Respir J. 2018;51(1):1702053. doi: 10.1183/13993003.02053-2017. [DOI] [PubMed] [Google Scholar]
- 14.Grimwood K, Bell SC, Chang AB. Antimicrobial treatment of non-cystic fibrosis bronchiectasis. Expert Rev Anti Infect Ther. 2014;12:1277–1296. doi: 10.1586/14787210.2014.952282. [DOI] [PubMed] [Google Scholar]
- 15.De Soyza A, Aksamit T. Ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Expert Opin Orphan Drug. 2016;4:875–884. [Google Scholar]
- 16.Thadepalli H, Bansal MB, Rao B, See R, Chuah SK, Marshall R, Dhawan VK. Ciprofloxacin: in vitro, experimental, and clinical evaluation. Rev Infect Dis. 1988;10:505–515. doi: 10.1093/clinids/10.3.505. [DOI] [PubMed] [Google Scholar]
- 17.McShane PJ, Weers JG, Tarara TE, Haynes A, Durbha P, Miller DP, Mundry T, Operschall E, Elborn JS. Ciprofloxacin dry powder for inhalation (CIP): technical design and features of an efficient drug-device combination. Pulm Pharmacol Ther. 2018;50:72–79. doi: 10.1016/j.pupt.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Cipolla D, Blanchard J, Gonda I. Development of liposomal ciprofloxacin to treat lung infections. Pharmaceutics. 2016;8:6. doi: 10.3390/pharmaceutics8010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright DH, Brown GH, Peterson ML, Rotschafer JC. Application of fluoroquinolone pharmacodynamics. J Antimicrob Chemother. 2000;46:669–683. doi: 10.1093/jac/46.5.669. [DOI] [PubMed] [Google Scholar]
- 20.Wong JP, Yang H, Blasetti KI, Schnell G, Conley J, Schofield LN. Liposome delivery of ciprofloxacin against intracellular Francisella tularensis infection. J Control Release. 2003;92:265–273. doi: 10.1016/s0168-3659(03)00358-4. [DOI] [PubMed] [Google Scholar]
- 21.Wong JP, Di Ninno VL, Cherwonogrodzky JW. Liposome-encapsulated ciprofloxacin. Eur Patent Appl EP. 1995;0652008:A1. [Google Scholar]
- 22.Controlled Pulmonary Drug Delivery (HDC Smyth, AJ Hickey, Eds.) Springer Nature, Switzerland AG, 2011.
- 23.Loira-Pastoriza C, Todoroff J, VanBever R. Delivery strategies for sustained drug release in the lungs. Adv Drug Deliv Rev. 2014;75:81–91. doi: 10.1016/j.addr.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Salama SR, Traini D, Chan HK, Young PM. Recent advances in controlled release pulmonary therapy. Curr Drug Deliv. 2009;6:404–414. doi: 10.2174/156720109789000546. [DOI] [PubMed] [Google Scholar]
- 25.Weers JG, Miller DP. Formulation of dry powders for inhalation. J Pharm Sci. 2015;104:3259–3288. doi: 10.1002/jps.24574. [DOI] [PubMed] [Google Scholar]
- 26.Pilcer G, Amighi K. Formulation strategy and use of excipients in pulmonary drug delivery. Int J Pharm. 2010;392:1–19. doi: 10.1016/j.ijpharm.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Bruinenberg P, Blanchard JD, Cipolla DC, Dayton F, Mudumba S, Gonda I. Inhaled liposomal ciprofloxacin: once a day management of respiratory infections. Proc Respir Drug Deliv. 2010;2010(1):73–81. [Google Scholar]
- 28.VanDevanter DR, Gonda I, Dahms J, Cipolla D, Davis AM, Chalmers JD, Froelich J. Microbiological changes observed over 48 weeks of treatment with inhaled liposomal ciprofloxacin in individuals with non-cystic fibrosis bronchiectasis and chronic Pseudomonas aeruginosa lung infection. Clin Microbiol Infect. 2019 doi: 10.1016/j.cmi.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 29.Cipolla DC, Blanchard J. Dual action, inhaled formulations providing both an immediate and sustained release profile. US Patent 8,268,347, 2012.
- 30.Gonda I, Blanchard J, Cipolla D, Bermudez LEM. Liposomal ciprofloxacin formulations with activity against non-tuberculosis mycobacteria. US Patent 9,532,986, 2017.
- 31.Weers J. Lipid-based compositions of anti-infectives for treating pulmonary infections and methods of use thereof. US Patent 9,402,845, 2016.
- 32.Serisier DJ, Bilton D, De Soyza A, Thompson PJ, Kolbe J, Greville HW, Cipolla D, Bruinenberg P, Gonda I. Inhaled, dual release liposomal ciprofloxacin in non-cystic fibrosis bronchiectasis (ORBIT-2): a randomized, double-blind, placebo-controlled trial. Thorax. 2013;68:812–817. doi: 10.1136/thoraxjnl-2013-203207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haynes A, Mundry T, Durbha P, Miller DP, Tarara T, Malcolmson R, Weers J. Design of ciprofloxacin dry powder for inhalation. Proc Respir Drug Deliv. 2016;2016(3):455–458. [Google Scholar]
- 34.Weers JG, Tarara TE. The PulmoSphere™ platform for pulmonary drug delivery. Ther Deliv. 2014;5:277–295. doi: 10.4155/tde.14.3. [DOI] [PubMed] [Google Scholar]
- 35.Weers JG, Miller DP, Tarara TE. Spray-dried formulations for inhalation comprising crystalline drug powders. AAPS PharmSci Tech. 2019;20(3):103. doi: 10.1208/s12249-018-1280-0. [DOI] [PubMed] [Google Scholar]
- 36.Endermann H, Labischinski C, Ladel U, Petersen B, Newton B: Treatment of bacterial diseases of the respiratory organs, US Patent 8,034,817, 2011.
- 37.Weers J, Tarara T. Pulmonary delivery of a fluoroquinolone. US Patent 8,834,930, 2014.
- 38.Stass H, Nagelschmitz J, Kappeler D, Sommerer K, Kietzig C, Weimann B. Ciprofloxacin dry powder for inhalation in patients with non-cystic fibrosis bronchiectasis or chronic obstructive pulmonary disease, and in healthy volunteers. J Aerosol Med Pulm Drug Deliv. 2017;30:53–63. doi: 10.1089/jamp.2015.1282. [DOI] [PubMed] [Google Scholar]
- 39.Stass H, Nagelschmitz J, Willmann S, Delesen H, Gupta A, Baumann S. Inhalation of a dry powder ciprofloxacin formulation in healthy subjects: a Phase 1 study. Clin Drug Invest. 2013;33:419–427. doi: 10.1007/s40261-013-0082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson R, Welte T, Polverino E, De Soyza A, Greville H, O’Donnell A, Adler J, Reimnitz P, Hampel B. Ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis: a phase II randomized study. Eur Respir J. 2013;41:1107–1115. doi: 10.1183/09031936.00071312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dorkin HL, Staab D, Operschall E, Alder J, Criollo M. Ciprofloxacin DPI: a randomised, placebo-controlled, phase IIb efficacy and safety study on cystic fibrosis. BMJ Open Respir Res. 2015;2:e000100. doi: 10.1136/bmjresp-2015-000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drusano G, Labro MT, Cars O, Mendes P, Shah P, Sörgel F, Weber W. Pharmacokinetics and pharmacodynamics of fluoroquinolones. Clin Microbiol Infect. 1994;4:2S27–2S41. [PubMed] [Google Scholar]
- 43.Hydrogenated Soy PC Product 840058. https://avantilipids.com/product/840058. Accessed 02 Sep 2019.
- 44.Elder DP, Holm R, de Diego HL. Use of pharmaceutical salts and cocrystals to address the issue of poor solubility. Int J Pharm. 2013;453:88–100. doi: 10.1016/j.ijpharm.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 45.Grit M, de Smidt JH, Struijke A, Crommelin DJA. Hydrolysis of phosphatidylcholine in aqueous liposome dispersions. Int J Pharm. 1989;50:1–6. [Google Scholar]
- 46.Lenney W, Edenborough F, Kho P, Kovarik JM. Lung deposition of inhaled tobramycin with eFlow rapid/LC plus jet nebulizer in healthy and cystic fibrosis subjects. J Cyst Fibros. 2011;10:9–14. doi: 10.1016/j.jcf.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 47.Coates AL, Dinh L, MacNeish CF, Rollin T, Gagnon S, Ho SL, Lands LC. Accounting for radioactivity before and after nebulization of tobramycin to ensure accuracy of quantitation of lung deposition. J Aerosol Med. 2000;13:169–178. doi: 10.1089/jam.2000.13.169. [DOI] [PubMed] [Google Scholar]
- 48.Maltz D, Paboojian SJ. Device engineering insights into TOBI Podhaler: a development case study of high efficiency powder delivery to cystic fibrosis patients. Proc Respir Drug Deliv Eur. 2011;1:55–66. [Google Scholar]
- 49.Weers JG, Clark AR. The impact of inspiratory flow rate on drug delivery to the lungs with dry powder inhalers. Pharm Res. 2017;34:507–528. doi: 10.1007/s11095-016-2050-x. [DOI] [PubMed] [Google Scholar]
- 50.Stass H, Weimann B, Nagelschmitz J, Rolinck-Werninghaus C, Staab D. Tolerability and pharmacokinetic properties of ciprofloxacin dry powder for inhalation in patients with cystic fibrosis: a Phase I, randomized, dose-escalation study. Clin Ther. 2013;10:1571–1581. doi: 10.1016/j.clinthera.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 51.Goldfarb J, Wormser GP, Inchiosa MA, Guideri G, Diaz M, Gandhi R, Goltzman C, Mascia AV. Single-dose pharmacokinetics of oral ciprofloxacin in patients with cystic fibrosis. J Clin Pharmacol. 1986;26:222–226. doi: 10.1002/j.1552-4604.1986.tb02938.x. [DOI] [PubMed] [Google Scholar]
- 52.Cipro® (ciprofloxacin hydrochloride tablets) US Label: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/019537s073,020780s030lbl.pdf Accessed 24 Sep 2019.
- 53.Lettieri JT, Rogge MC, Kaiser L, Echols RM, Heller AH. Pharmacokinetic profiles of ciprofloxacin after intravenous and oral doses. Antimicrob Agents Chemother. 1992;36:993–996. doi: 10.1128/aac.36.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stass H, Kubitza D, Moller JG, Delesen H. Influence of activated charcoal on the pharmacokinetics of moxifloxacin following intravenous and oral administration of a 400 mg single dose to healthy males. Br J Clin Pharmacol. 2005;59:536–541. doi: 10.1111/j.1365-2125.2005.02357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin AR, Finlay WH. Model calculations of regional deposition and disposition for single doses of inhaled liposomal and dry powder ciprofloxacin. J Aerosol Med Pulm Drug Deliv. 2018;31:49–60. doi: 10.1089/jamp.2017.1377. [DOI] [PubMed] [Google Scholar]
- 56.Bos AC, van Holsbeke C, de Backer JW, van Westreenen M, Jannsens HM, Vos WG, Tiddens HAWM. Patient-specific modelling of regional antibiotic concentration levels in airways of patients with cystic fibrosis: are we dosing enough? PLoS One. 2015;10(3):e0118454. doi: 10.1371/journal.pone.0118454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.US FDA: Sponsor Briefing Document; Linhaliq for the treatment of non-cystic fibrosis bronchiectasis patients with chronic lung infections with Pseudomonas aeruginosa- Briefing document for the Antimicrobial Drugs Advisory Committee, Meeting date:11-Jan-2018. www.fda.gov/media/110089/download. Accessed 03 Sep 2019.
- 58.US.FDA: Sponsor Briefing Document; Ciprofloxacin DPI (BAY q3939): Briefing document for FDA Advisory Committee Meeting on 16-Nov-2017 www.fda.gov/media/109200/download. Accessed 03 Sep 2019.
- 59.Chotirmall SH, Chalmers JD. RESPIRE: breathing new life into bronchiectasis. Eur Respir J. 2018;51:1702444. doi: 10.1183/13993003.02444-2017. [DOI] [PubMed] [Google Scholar]
- 60.Flaim SF. Pharmacokinetics and side effects of perfluorocarbon-based blood substitutes. Artif Cells Blood Sub Immob Biotech. 1994;22:1043–1054. doi: 10.3109/10731199409138801. [DOI] [PubMed] [Google Scholar]
- 61.Forbes B, O’Lone R, Allen PP, Cahn A, Clarke C, Collinge M, Dailey LA, Donnelly LE, Dybowski J, Hassall D, Hildebrand D, Jones R, Kilgour J, Klapwijk J, Maier CC, McGovern T, Nikula K, Parry JD, Reed MD, Robinson I, Tomlinson L, Wolfreys A. Challenges of inhaled drug discovery and development: induced alveolar macrophages responses. Adv Drug Deliv Rev. 2014;71:15–33. doi: 10.1016/j.addr.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 62.Arikayce package insert. Available from: www://arikayce.com/pdf/full-prescribing-information.pdf. Accessed September 3, 2019.
- 63.Szebeni J, Barenholz Y. Complement activation, immunogenicity, and immune suppression as potential side effects of liposomes. In: Handbook of Harnessing Biomaterials in Nanomedicine. Singapore: Pan Stanford Publishing Pte. Ltd., 2011. pp. 309–334. 10.4032/9789814364270
- 64.Konstan M, Flume PA, Kappler M, Chiron R, Higgins M, Brockhaus F, Zhang J, Angyalosi G, He E, Geller DE. safety, efficacy, and convenience of tobramycin inhalation powder in cystic fibrosis patients: the EAGER trial. J Cyst Fibros. 2011;10:54–61. doi: 10.1016/j.jcf.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCullough AR, Tunney MM, Quittner AL, Elborn JS, Bradley JM, Hughes CM. Treatment adherence and health outcomes in patients with bronchiectasis. BMC Pulm Med. 2014;14:107. doi: 10.1186/1471-2466-14-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cipolla D, Froehlich J, Gonda I. Emerging opportunities for inhaled antibiotic therapy. J Antimicrob. 2015;1:104. doi: 10.4172/Antimicro.1000104. [DOI] [Google Scholar]
- 67.Haworth C, Wanner A, Froelich J, O’Neal T, Davis A, Gonda I, O’Donnell A: Inhaled liposomal ciprofloxacin in patients with non-cystic fibrosis bronchiectasis and chronic Pseudomonas aeruginosa lung infection. Presented at the International Society for Aerosols in Medicine (ISAM), poster P192, June 3-7, 2017, Santa Fe.
- 68.Weers J. Inhaled antimicrobial therapy barriers to effective treatment. Adv Drug Deliv Rev. 2015;85:24–43. doi: 10.1016/j.addr.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 69.Rottier BL, van Erp CJP, Sluyter TS, Heijerman HG, Frijlink HW, DeBoer AH. Changes in performance of the PARI eFlow rapid and PARI LC Plus during 6 months use by CF patients. J Aerosol Med Pulm Drug Deliv. 2009;22:263–269. doi: 10.1089/jamp.2008.0712. [DOI] [PubMed] [Google Scholar]
- 70.Hamed K, Conti V, Tian H, Loefroth E. Adherence to tobramycin inhaled powder vs inhaled solution in patients with cystic fibrosis: analysis of US insurance claims data. Patient Prefer Adher. 2017;11:831–838. doi: 10.2147/PPA.S134759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harrison MJ, McCarthy M, Fleming C, Hickey C, Shortt C, Eustace JA, Murphy DM, Plant BJ. Inhaled versus nebulized tobramycin: a real-world comparison in adult cystic fibrosis (CF) J Cyst Fibros. 2014;13:692–698. doi: 10.1016/j.jcf.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 72.Bilton D, Henig N, Morrissey B, Gotfried M. Addition of inhaled tobramycin to ciprofloxacin for acute exacerbations of Pseudomonas aeruginosa infection in adult bronchiectasis. Chest. 2006;130:1503–1510. doi: 10.1378/chest.130.5.1503. [DOI] [PubMed] [Google Scholar]
- 73.Barker AF, O’Donnell AE, Flume P, Thompson PJ, Ruzi JD, de Gracia J, Boersma WG, De Soyza A, Shao L, Zhang J, Haas L, Lewis SA, Leitzinger S, Montgomery AB, McKevitt MT, Gossage D, Quittner AL, O’Riordan TG. Aztreonam for inhalation solution in patients with non-cystic fibrosis bronchiectasis (AIR-BX1 and AIR-BX2): two randomized double-blind, placebo-controlled phase 3 trials. Lancet Respir Med. 2014;2:738–749. doi: 10.1016/S2213-2600(14)70165-1. [DOI] [PubMed] [Google Scholar]
- 74.Grimwood K, Bell SC, Chang AB. Antimicrobial treatment of non-cystic fibrosis bronchiectasis. Expert Rev Infect Ther. 2014;12:1277–1296. doi: 10.1586/14787210.2014.952282. [DOI] [PubMed] [Google Scholar]
- 75.Chalmers JD, Smith MP, McHugh BJ, Doherty C, Govan JR, Hill AT. Short and long-term antibiotic treatment reduces airway and systemic inflammation in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2012;186:657–665. doi: 10.1164/rccm.201203-0487OC. [DOI] [PubMed] [Google Scholar]
- 76.Haworth CS, Foweraker JE, Wilkinson P, Kenyon RF, Bilton D. Inhaled colistin in patients with bronchiectasis and chronic Pseudomonas aeruginosa infection. Am J Repsir Crit Care Med. 2014;189:975–982. doi: 10.1164/rccm.201312-2208OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rubin BK. Aerosolized antibiotics for non-cystic fibrosis bronchiectasis. J Aerosol Med Pulm Drug Deliv. 2008;21:71–76. doi: 10.1089/jamp.2007.0652. [DOI] [PubMed] [Google Scholar]
- 78.Weers J. Reply to the comment by Cipolla et al on: inhaled antimicrobial therapy barriers to effective treatment. Adv Drug Deliv Rev. 2015 doi: 10.1016/j.addr.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 79.Haworth CS, Aliberti S, Barker A, Blasi F, Boersma W, Chalmers JD, De Soyza A, Dimakou K, Elborn JS, Feldman C, Flume P, Goeminne PC, Loebinger MR, Menendez R, Morgan L, Murris L, Polverino E, Quittner A, Ringshausen FC, Tino G, Torres A, Vendrell M, Welte T, Wilson R, Wong C, O’Donnell A, Aksamit T. Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research. Eur Respir J. 2017;49:170051. doi: 10.1183/13993003.00051-2017. [DOI] [PubMed] [Google Scholar]
- 80.Cipolla D, Shekunov B, Blanchard J, Hickey A. Lipid-based carriers for pulmonary products: preclinical development and case studies in humans. Adv Drug Deliv Rev. 2014;75:53–80. doi: 10.1016/j.addr.2014.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary information files.