Abstract
Background:
Lichen planus is a T-cell-mediated autoimmune disease, in which CD8+ T-cells releases the cytokines such as tumor necrosis factor-alpha and interleukin-12 disrupting basement membrane integrity. Treatment modalities were directed toward the relief in signs and symptoms and preventing recurrences. Zinc activates caspase-3 and DNA fragmentation, resulting in the apoptosis of keratinocytes. Prevention of matrix metalloproteinases1 (MMP1) activation, inhibits the Tcell accumulation in oral lichen planus (OLP) and by inhibiting MMP9, prevents the cleavage of collagen resulting in maintaining the integrity of the basement membrane.
Objectives:
The main objective of the study is to compare the efficacy of oral zinc 50 mg and 0.1% triamcinolone Orabase with 0.1% triamcinolone Orabase alone on the healing process of symptomatic OLP.
Materials and Methods:
A total of forty participants were randomly categorized into two groups: Group A and Group B with 20 patients with OLP and having symptoms of burning sensation. Group A patients had received 0.1% triamcinolone Orabase twice daily application. Group B patients had provided with oral zinc 50 mg and 0.1% triamcinolone Orabase twice daily for 8 weeks. The follow-up period for both the groups was 6 months. Lesional size was measured by Thongprasom scale and burning sensation was assessed by visual analog scale at each visit till the cessation of treatment.
Results:
There was decrease in the burning sensation and lesional size from the first visit to follow-up period which was statistically significant in both groups (P = 0.000).
Conclusion:
Oral zinc therapy was adjunctive in reducing the burning sensation and lesional size in the symptomatic OLP.
Keywords: Oral lichen planus, Thongprasom scale, zinc
Introduction
Zinc is an essential micronutrient with more than 300 metalloenzymes that are needed for the regulation of lipid, protein and nucleic acid metabolism, and gene transcription. It plays an important role in maintaining the immune function and wound repair via regulation of DNA and RNA polymerases, thymidine kinase, and ribonuclease.[1] It maintains macrophage and neutrophil functions, natural killer cell activity, and complement activity.[2] Zinc has anti-inflammatory action by blocking the cytokines, but it also causes induction of apoptosis. Decrease in level of zinc is associated with suppression of the oxidant-antioxidant system.[3] Matrix metalloproteinases (MMPs), which are the family of zinc-containing endoproteinases consists of 20 members, have the main function of proteolytic degradation of connective tissue matrix proteins. On MMP-1 activation, it aggravates the T-cell accumulation in oral lichen planus (OLP). By inhibiting MMP-9, it prevents cleavage of collagen resulting in maintaining the integrity of the basement membrane It was believed that MMPs are also involved in etiopathogenesis of OLP.[4]
Lichen planus is a chronic autoimmune inflammatory disease which involves the skin, mucous membrane, scalp, and nails with a prevalence rate of 0.5%–2.6% in the general population, with no racial predilection.[5]
The definite etiology for this condition is still unknown, but it was believed that factors such as stress, use of systemic medications, dental materials, genetics, immunity and hypersensitivity reactions, and viruses such as human papillomavirus 16 and 18, human herpes virus, and hepatitis contribute for the cause of lichen planus.[6]
It is a T-cell-mediated autoimmune disease, in which CD8+ T-cells are involved for the development of the lesion by the release of various cytokines such as tumor necrosis factor-alpha and interleukin-12 which helps in the disruption of basement membrane integrity.[7]
Clinically, the appearance of Wickham striae with the lesions ranges from white striations, papules, plaques, erythema, or erosions affecting the buccal mucosa, tongue, and gingiva.
Signs and symptoms include roughness of the lining of the mouth, sensitivity of the oral mucosa to hot or spicy foods, painful oral mucosa, red or white patches on the oral mucosa, or oral ulcerations with a history of phases of remission and exacerbation.[8]
Treatment modalities were directed toward the relief in signs and symptoms and to prevent possible recurrences. Although corticosteroid therapy is the main line of treatment, it has many side effects such as insomnia, mood swings, fatigue, fluid retention, nausea, dry mouth, sore throat, thinning of the oral mucosa, and fungal overgrowth in normal oral flora leading to candidiasis.[9] To reduce the adverse effects of steroids, many other therapies such as use of immunosuppressants, immunomodulators, retinoids, cytotoxics, antimicrobials, and other alternative therapies such as lycopene, curcumin, and zinc were used.[10]
The present study was designed with an objective to compare the efficacy of oral zinc 50 mg and 0.1% triamcinolone acetonide Orabase with 0.1% triamcinolone acetonide Orabase alone on the healing process of symptomatic OLP.
Materials and Methods
The study protocol was approved by the Institutional Ethical Review Board (Ethical clearance number: PMVIDSandRC/IEC/OMR/DN/0154-17); Panineeya Mahavidyalaya Institute of Dental Sciences and Research Centre, Hyderabad, Telangana, India.
A total of forty patients were included in the study, who were randomly categorized into two groups: Group A and Group B. It was a single-centered, single-blinded, prospective, double-arm trial. The participants in each group were selected from outpatients attending the Department of Oral Medicine and Radiology by simple random sampling method. Group A (control group) comprised 20 patients and Group B (case group) comprised 20 patients with clinical and histopathologically confirmed cases of OLP with symptoms of burning sensation.
A specially designed pro forma was used for recording demographic details, clinical findings, to assess the burning sensation by visual analog scale, and to assess the size of the lesion by Thongprasom scale.
Group A patients had received 0.1% triamcinolone acetonide Orabase twice daily application after food. Group B patients had provided with oral zinc 50 mg tablets and 0.1% triamcinolone acetonide Orabase twice daily after food for 8 weeks. Steroid Orabase was discontinued after 1-week application, whereas oral zinc 50 mg was continued for all over the treatment period. The follow-up period for both the groups was 6 months.
For both the groups, lesional size was measured using Thongprasom scale and burning sensation was assessed by visual analog scale at the initial visit and weekly visits till the cessation of treatment.
Thongprasom et al., 1992, had given scoring for the clinical assessment of the lesion for both objective and subjective findings which include scores 0 for no lesion, 1 for mild white striae with no erythematous area, 2 for white striae with atrophic area <1 cm2, 3 for white striae with atrophic area more than 1 cm2, 4 for white striae with erosive area <1 cm2, and 5 for white striae with erosive area more than 1 cm2 for the objective findings of the lesion, whereas score 0 for not cured lesions and 1 for cured lesions with no inflammation or erythematous areas, no white striae or very mild striae, and no other symptoms.[11,12]
The scores obtained by the visual analog scale for burning sensation and Thongprasom scale for clinical assessment of size of the lesion of both case and control groups were subjected to statistical analysis.
Data were analyzed by statistical software by IBM SPSS 20.0 version (SPSS inc., Chicago, III., USA).
Results
Demographic results revealed that OLP was most commonly observed in the middle-age group ranging from 30 to 49 years of age with more female predilection at the age of 40–49 years.
Majority of the participants were diagnosed with erosive type of lichen planus followed by reticular and papular type of lichen planus. The most common site of occurrence of the lesion was buccal mucosa followed by the gingiva, tongue, and floor of the mouth [Table 1].
Table 1.
Site and age distribution of patients in two groups
| Lesion site | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buccal mucosa | Gingiva | Tongue | Floor of the tongue | Buccal mucosa and gingiva | ||||||
| Count | n (%) | Count | n (%) | Count | n (%) | Count | n (%) | Count | n (%) | |
| 30-39 | 6 | 27.3% | 3 | 30.0% | 0 | 0.0% | 0 | 0.0% | 2 | 66.7% |
| 40-49 | 10 | 45.5% | 3 | 30.0% | 3 | 75.0% | 1 | 100.0% | 0 | 0.0% |
| 50-59 | 4 | 18.2% | 4 | 40.0% | 1 | 25.0% | 0 | 0.0% | 0 | 0.0% |
| 60-70 | 2 | 9.1% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 1 | 33.3% |
| Total | 22 | 100.0% | 10 | 100.0% | 4 | 100.0% | 1 | 100.0% | 3 | 100.0% |
Assessment of burning sensation in both groups
Group A (control group)
In this group, the mean difference of burning sensation had decreased from the first visit till the follow-up period which was statistically significant (P = 0.001) between the visits [Table 2].
Table 2.
Assessment of visual analog scale scores between the days of visits among the control group (Group A)
| n | Mean | SD | 95% CI for mean | P | ||
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| 1st visit | 20 | 7.60 | 2.010 | 6.66 | 8.54 | 0.001* |
| 4th visit | 20 | 3.90 | 1.252 | 3.31 | 4.49 | |
| 8th visit | 20 | 1.45 | 0.826 | 1.06 | 1.84 | |
| Follow-up | 20 | 0.75 | 0.639 | 0.45 | 1.05 | |
P < 0.005*. SD: Standard deviation, CI: Confidence interval
Group B (study group)
In this group, the mean difference of burning sensation had decreased from the first visit till the follow-up period which was statistically significant (P = 0.001) between the visits [Table 3].
Table 3.
Assessment of visual analog scale scores between the days of visits among the study group (Group B)
| n | Mean | SD | 95% CI for mean | P | ||
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| 1st visit | 20 | 8.70 | 1.949 | 7.79 | 9.61 | 0.001* |
| 4th visit | 20 | 4.75 | 2.124 | 3.76 | 5.74 | |
| 8th visit | 20 | 1.90 | 1.021 | 1.42 | 2.38 | |
| Follow-up | 20 | 0.65 | 0.813 | 0.27 | 1.03 | |
P < 0.005*. SD: Standard deviation, CI: Confidence interval
By comparing the burning sensation in study and control groups, it was found that there was gradual decrease in the burning sensation in both the groups from the first visit till the follow-up period. Although the study group shows the mean higher burning sensation from the initial first visit to follow-up visit than the control group, there was increase in the mean burning sensation in the follow-up period in the control group [Graph 1].
Graph 1.
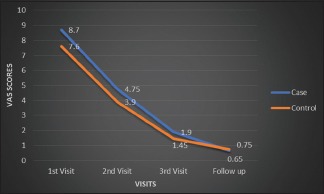
Comparison of visual analog scale regarding burning sensation between zinc and triamcinolone groups
Assessment of size of the lesion in both groups by Chi-square test
Group A (control group)
In this group, there was gradual decrease in the lesional size between the weeks till the follow-up period with (χ = 113.251a) which was statistically significant between the size of lesion and visits of the patients (P = 0.000) [Table 4].
Table 4.
Assessment of size of the lesions scores between the days of visits among the control group (Group A)
| Visits | Total | P | ||||
|---|---|---|---|---|---|---|
| 1st visit | 4th visit | 8th visit | Follow-up | |||
| Thongprasom Scale | ||||||
| No lesion | 0 | 1 | 2 | 15 | 18 | 0.000* |
| Mild white striae, no erythema | 1 | 19 | 18 | 5 | 43 | |
| White striae with atrophic area | 19 | 0 | 0 | 0 | 19 | |
| Total | 20 | 20 | 20 | 20 | 80 | |
P < 0.005*
Group B (study group)
In this group, there was gradual decrease in the lesional size between the weeks till the follow-up period with (χ = 103.873a) which was statistically significant between the size of lesion and visits of the patients (P = 0.000) [Table 5].
Table 5.
Assessment of size of the lesions scores between the days of visits among the study group (Group B)
| Visits | Total | P | ||||
|---|---|---|---|---|---|---|
| 1st visit | 4th visit | 8th visit | Follow-up | |||
| Thongprasom Scale | ||||||
| No lesion | 0 | 0 | 0 | 16 | 16 | 0.000* |
| Mild white striae, no erythema | 1 | 14 | 19 | 2 | 36 | |
| White striae with atrophic area | 19 | 6 | 1 | 2 | 28 | |
| Total | 20 | 20 | 20 | 20 | 80 | |
P < 0.005*
By comparing both the groups, there was gradual decrease in the size of the lesion from the first visit till the follow-up period which was statistically significant in both the groups (P = 0.000). In the study group, there was gradual decrease in the size of the lesion, but in the control group, there was constant range in the lesional size from 4th visit to 8th visit followed by decrease in the lesional size till the follow-up period [Graph 2].
Graph 2.
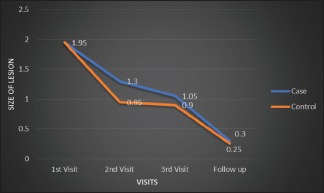
Comparison of size of lesion using Thongprasom scale in both zinc and triamcinolone groups
Discussion
OLP is a chronic, autoimmune mucocutaneous disease and is most commonly seen in the middle-aged women. It can occur concurrently or independently in the skin and genital, anal, esophageal, nasal and laryngeal mucosae. The prevalence rate in general population varies from 0.5% to 2.6%. OLP occurs more frequently than the cutaneous form, and it tends to be more persistent and more resistant to treatment.
The present study revealed that oral zinc 50 mg twice daily was found to be effective in the patients with OLP with decrease in burning sensation and lesion size due to its anti-inflammatory and wound healing properties of zinc which was similar to the studies conducted by Mehdipour et al. and Thomas et al.[10,13]
Mehdipour et al. compared the efficacy of 0.2% zinc mouthwash with fluocinolone and fluocinolone alone in the treatment of OLP and found that 0.2% zinc mouthwash with fluocinolone was effective to decrease in lesion surface area, pain and burning sensation with the effect of zinc in healing the disrupted epithelium.[10]
Thomas et al. compared the efficacy of topical zinc sulfate 2.5% with 0.05% clobetasol propionate cream and only 0.05% clobetasol propionate cream in the treatment of subacute and chronic eczema, lichen planus, and psoriasis and was found zinc sulfate 2.5% was effective in reducing the severity of the lesion by its anti-inflammatory action.[13]
In the above studies, the use of steroids either in the form of mouthwash or in ointment form was continued for 8 weeks all along the treatment period. However, in the present study, the steroid Orabase was discontinued after 1 week mainly to reduce its long-term adverse effects of the steroids in the patients. The steroid Orabase was given for only 1 week in order to reduce the burning sensation in the patients to get the faster relief in the patients.
In this present study, there was a decrease in the mean burning sensation from the first visit to follow-up period in the study group compared with the control group, probably due to its anti-inflammatory and antioxidant action.
Zinc has the action on inflammation by acting on the oxidative stress. It occurs when cellular antioxidant systems prove insufficient to remove increased reactive oxygen species (ROS) levels. ROS plays beneficial role in the immune response to infection; their excess causes, lipid peroxidation, and damage to proteins and nucleic acids. Inflammation generates oxidative stress by increasing ROS, reactive nitrogen species that is generated by phagocytic cells, neutrophils, and macrophages in their host defense mechanism, which is dependent on oxygen, which is also known as oxidative outburst. Free radical chain reactions may be induced by transition metals and in response to many exogenous factors, such as pollutants, ultraviolet radiation, cigarette smoking, alcohol; drugs, such as nonsteroidal anti-inflammatory drugs leading to chronic infections; and immunologic and inflammatory disorders such as atherosclerosis, cancer, and neurodegeneration which also provoke the increased production of free radicals.[14]
In this present study, it was reported that 80% of patients in the study group reported with no lesions till the end of the treatment period. This is due to the action of zinc in wound healing in experimental group.
Animal studies demonstrated that 0.3 mg/cm2 zinc oxide cream promotes epithelialization by enhancing endogenous growth factors and enzymes important for epithelial proliferation and migration.[15] Fibroblasts play an important role by stimulating fibrosis. Fibroblasts release the multifunctional growth factor, i.e., fibroblast growth factor (FGF) for epidermal keratinocytes. Baroni et al. added zinc oxide to human dermal fibroblasts and observed increased secretion of FGF, suggesting that zinc in granulating wounds possibly was capable of upregulating growth factors other than insulin-like growth factor-I.[16]
There are various biological forms of zinc for therapeutic use. Out of all forms, zinc sulfate was the most commonly available form for therapeutics. Nevertheless, it is used sparingly due to many adverse effects such as nausea, stomach upset, heartburn, fever, sore throat, mouth sores, weakness, and fatigue when compared to zinc acetate.
Hence, in the present study, zinc acetate was given to the patients for the treatment of OLP. In overall treatment period, no adverse effects were reported by the patients using zinc acetate.
Limitations
In the present study, serum zinc levels were not monitored in the patients to support the usage of the drug as treatment of OLP. Long-term follow-up should perform in the patients to check any rebound phenomenon with larger sample size is recommended.
Conclusion
From the present study, it could be concluded that oral zinc therapy was found to be adjunctive in reducing the burning sensation and lesion size in the symptomatic OLP. Hence, the regime could be effectively used in treatment of OLP adding to reduced side effects of steroid usage.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gupta M, Mahajan VK, Mehta KS, Chauhan PS. Zinc therapy in dermatology: A review. Dermatol Res Pract. 2014;2014:1–11. doi: 10.1155/2014/709152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prasad AS. Zinc in human health: Effect of zinc on immune cells. Mol Med. 2008;14:353–7. doi: 10.2119/2008-00033.Prasad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nogueira PA, Carneiro S, Ramos-e-Silva M. Oral lichen planus: An update on its pathogenesis. Int J Dermatol. 2015;54:1005–10. doi: 10.1111/ijd.12918. [DOI] [PubMed] [Google Scholar]
- 4.Sugerman PB, Savage NW, Walsh LJ, Zhao ZZ, Zhou XJ, Khan A, et al. The pathogenesis of oral lichen planus. Crit Rev Oral Biol Med. 2002;13:350–65. doi: 10.1177/154411130201300405. [DOI] [PubMed] [Google Scholar]
- 5.Ismail SB, Kumar SK, Zain RB. Oral lichen planus and lichenoid reactions: Etiopathogenesis, diagnosis, management and malignant transformation. J Oral Sci. 2007;49:89–106. doi: 10.2334/josnusd.49.89. [DOI] [PubMed] [Google Scholar]
- 6.Razavi SM, Ghalayani P, Salehi MR, Attarzadeh H, Shahmoradi M. Human papilloma virus as a possible factor in the pathogenesis of oral lichen planus. Dent Res J (Isfahan) 2009;6:82–6. [PMC free article] [PubMed] [Google Scholar]
- 7.Lavanya N, Jayanthi P, Rao UK, Ranganathan K. Oral lichen planus: An update on pathogenesis and treatment. J Oral Maxillofac Pathol. 2011;15:127–32. doi: 10.4103/0973-029X.84474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boorghani M, Gholizadeh N, Taghavi Zenouz A, Vatankhah M, Mehdipour M. Oral lichen planus: Clinical features, etiology, treatment and management; a review of literature. J Dent Res Dent Clin Dent Prospects. 2010;4:3–9. doi: 10.5681/joddd.2010.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thongprasom K, Dhanuthai K. Steriods in the treatment of lichen planus: A review. J Oral Sci. 2008;50:377–85. doi: 10.2334/josnusd.50.377. [DOI] [PubMed] [Google Scholar]
- 10.Mehdipour M, Taghavi Zenouz A, Bahramian A, Yazdani J, Pouralibaba F, Sadr K. Comparison of the effect of mouthwashes with and without zinc and fluocinolone on the healing process of erosive oral lichen planus. J Dent Res Dent Clin Dent Prospects. 2010;4:25–8. doi: 10.5681/joddd.2010.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, van der Waal I. Disease scoring systems for oral lichen planus; a critical appraisal. Med Oral Patol Oral Cir Bucal. 2015;20:e199–204. doi: 10.4317/medoral.20524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piboonniyom SO, Treister N, Pitiphat W, Woo SB. Scoring system for monitoring oral lichenoid lesions: A preliminary study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:696–703. doi: 10.1016/j.tripleo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Thomas J, Kandhari S, Oberoi C, Jayaseelan E, Raj KY. A double-blind randomised multicentre controlled study of topical 005% clobetasol propionate with 25% zinc sulphate preparation. Indian J Dermatol Venereol Leprol. 2001;67:135–7. [PubMed] [Google Scholar]
- 14.Jarosz M, Olbert M, Wyszogrodzka G, Młyniec K, Librowski T. Antioxidant and anti-inflammatory effects of zinc.Zinc-dependent NF-κB signaling. Inflammopharmacology. 2017;25:11–24. doi: 10.1007/s10787-017-0309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magnus S, Hendrick H. Agren zinc oxide augments endogenous expressionof insulin like growth factor I (IGF-I) and activates matrix metalloproteinases (MMPS) in wounds. EMWA J. 2001;1:1–3. [Google Scholar]
- 16.Baroni A, Perfetto B, Buttini G, Catalanotti P, Gorga F, Tufano MA. Topical amikacin formulation induces fibroblast growth factor and cytokine release from human dermal fibroblasts. Arch Dermatol Res. 1999;291:296–9. doi: 10.1007/s004030050411. [DOI] [PubMed] [Google Scholar]


