Abstract
Context:
Thyroid hormones (THs) are critically important for development, homeostasis, and metabolic regulation in mammals. Iodine, one of the constituents of TH, is actively supplied by sodium iodide symporter (NIS) into the thyroid gland. TH is subsequently transported to distant organs where its activation and deactivation is catalyzed by isoforms of deiodinases (DIOs). NIS protein has been known to overexpress in cancer cases of the breast and gastrointestinal organs. Recent studies show a possible role of DIOs in various cancers.
Aims:
In the present investigation, the prognostic significance of NIS and DIO-1, 2 and 3 was studied in gastric cancer using a data mining bioinformatic approach.
Methods:
“The Kaplan–Meier plotter” database was used for direct in silico validation in clinically relevant 876 gastric cancer patients with >15 years of follow-up information. After obtaining KM survival plots, hazard ratio and log-rank P value were calculated.
Results:
Increased expression of NIS and DIO 1-3 is significantly associated with worsen overall survival of gastric cancer patients followed for 20 years. Prognostic roles of NIS and individual DIOs were assessed in different types of gastric cancer classified based on morphologies, human epidermal growth factor receptor-2 receptor status, treatment choices, and different clinicopathological features.
Conclusions:
Based on these analyses, the present study found the indication of prognostic values of these genes. This information will contribute to better understanding of managing complex and heterogeneous gastric cancer. Further, these findings may be beneficial as a companion diagnostic tool predicting more accurate gastric cancer prognosis.
Keywords: Biomarkers, deiodinases, gastric cancer, Kaplan–Meier, Kaplan–Meier plot, survival
Introduction
Globally, gastric cancer is one of the most common causes of cancer-related deaths. Incidence-wise, there are 989,600 new cases/year with 738,000 deaths annually making it the second-most common cause of cancer-related deaths.[1,2] There have been advances in the area of early detection of cancer, improved tumor resection, and target-based therapeutic modalities, leading to improved disease management. However the prognosis of gastric cancer remains dismal with a median overall survival (OS) of 12 months for advanced disease in the Western world.[3]
There is a lack of robust biomarker for gastric cancer other than human epidermal growth factor receptor-2 (HER2) overexpression that is linked to advanced gastric cancer. Overexpression of HER2 has been determined as a negative prognostic factor.[4] There are biomarkers for gastric cancer diagnosis and prognosis that include carcinoembryonic antigen, CA125, CA19-9, CA72-4, and alpha-fetoprotein.[5] To further improve the prognosis of gastric cancer and devising personalized treatment, there is a need to identify the potentially significant prognostic biomarker and novel drug targets.
The sodium iodide symporter (NIS) is a plasma membrane glycoprotein that mediates active iodine transport not only in thyroid, where it is important for thyroid hormone (TH) synthesis but also on other tissues such as salivary gland, lactating breast, and small intestine.[6] It has been shown that NIS is functionally overexpressed in breast cancer.[7] In addition, molecular iodine has been shown to induce apoptotic cell death in breast cancer.[8] There could be an important metabolic role of iodine in extrathyroidal tissue.[9] Expression of NIS is markedly reduced in gastric cancer and intestinal metaplastic mucosa of Barrett's esophagus;[10] however, its significance is not completely understood. Functional analysis and clinical significance of protein expression in gastric cancer have been evaluated providing an insight that NIS expression is linked to worse prognosis in patients with gastric cancer.[11]
Another potential source of local iodine generation is deiodinases (DIOs), a family of enzymes that play an important function in TH activation and homeostasis as well as regulating action and activity of TH.[12] There are three known DIOs, DIO1 and DIO2 are important for the conversion of prohormone thyroxin to triiodothyronine, its most active form. On the other hand, DIO3 inactivates and terminates TH action.[13] There are growing evidence indicating altered expression of DIOs in thyroid and various cancer.[14,15]
These genes related to iodine transport and TH's functional regulation could potentially have a bearing on cancer development and response to therapy. However, there is a lack of understanding about the role of NIS and DIOs in gastric cancer prognosis. The prognostic roles of individual DIO enzymes specifically at the transcription level in the gastric cancer patient remain elusive. Therefore, the present study focused on the prognostic role and significance of NIS and DIOs in human gastric cancer patient by Kaplan–Meier plotter (KM plotter). We utilized KM plotter for the analysis of individual DIO and NIS with clinical results calculating OS and hazard ratio (HR). By using this methodology, we assessed the prognostic value of these genes in gastric cancer.
Methods
An open access (http://kmplot.com/analysis/index.php?p=service&cancer=gastric) online database was used to interrogate and determine the significance of NIS and DIO1-3 mRNA expression in the OS of gastric cancer patients.[16] The KM plot analysis was used to predict the prognostic value of NIS and DIO1-3 from the available gene expression and survival data from up to 876 gastric cancer patients. The present study did not require ethical committee clearance because publicly available microarray data were used for bioinformatics analysis only. The clinical data took consideration of gender, perforation history, Lauren classification, differentiation, stages of cancer, HER2 expression status, and treatment modality.
DIO1-3 and NIS were entered into the database, and after the validation of desired Affymetrix ID, KM survival plots were obtained. KM curves were generated using KM plotter using univariate analysis. This kind of analysis enabled us to directly perform in silico validation of NIS and DIO in gastric cancer in which numbers at risk were indicated below each KM plot. HRs were calculated at autoselected cutoff. Log-rank P values were computed, and P ≤ 0.05 was considered statistically significant.
Results
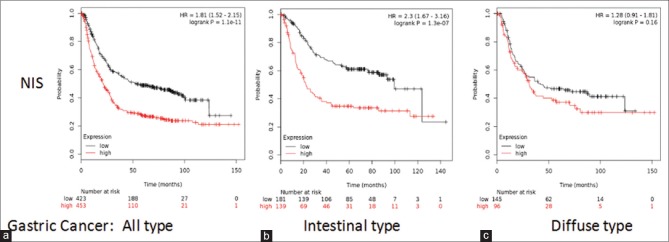
Availability of iodine is predominantly dependent on the expression of NIS in the given tissue. The study started with assessing the prognostic value of NIS in gastric cancer using www. kmplot. com from the dataset of n = 876 [Figure 1a], for intestinal-type cancer patients (n = 320) [Figure 1b] and for diffuse-type cancer patients (n = 241) [Figure 1c]. OS, the length of time starting from the diagnosis of disease or the start of treatments, is an important measure to determine the efficacy of new treatment and prognosis. High-NIS transcript levels were found to be correlated to worsen OS for all gastric cancer patients in the analysis followed for 150 months with HR = 1.81 (1.52–2.15), P = 1.1e-11. NIS high expression was also correlated significantly to worsen OS in intestinal-type cancer patients; HR = 2.3 (1.67–3.16), P = 1.3e-07, although not significantly in diffuse-type cancer patients; HR = 1.28 (0.91–1.81), P = 0.16.
Figure 1.
Expression of sodium iodide symporter associated to worsen overall survival for all gastric cancer patients. Analysis of prognostic value of sodium iodide symporter mRNA expression. (a) All gastric cancer patients, (b) intestinal-type cancer patients, (c) diffuse-type cancer patients
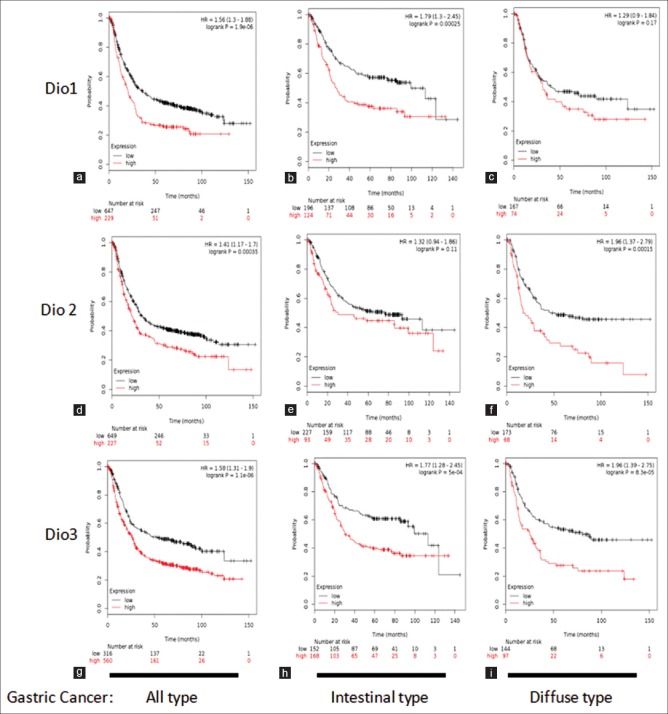
Subsequently, the prognostic value of DIOs was analyzed. DIO1 increased mRNA expression correlated significantly to worsen OS for all gastric cancer patients; HR = 1.56 (1.3–1.88), P = 1.9 e-06 [Figure 2a]. DIO1 high expression was also correlated significantly to worsen OS in intestinal-type cancer patients; HR = 1.79 (1.3–2.45), P = 0.00025 [Figure 2b]. DIO1 was not significantly associated with OS in diffuse-type cancer patients; HR = 1.29 (0.9–1.84), P = 0.17 [Figure 2c]. DIO2 increased mRNA expression correlated significantly to worsen OS for all gastric cancer patients; HR = 1.41 (1.17–1.7), P = 0.00035 [Figure 2d]. It is noteworthy that DIO2 high expression did not correlate significantly to worsen OS in intestinal-type cancer patients; HR = 1.32 (0.94–1.86), P = 0.11 [Figure 2e]. However, DIO2 overexpression correlated significantly to poor survival in diffuse-type cancer patients; HR = 1.96 (1.37–2.79), P = 0.00015 [Figure 2f]. Further, DIO3 increased mRNA expression correlated significantly to worse OS for all gastric cancer including intestinal- and diffuse-type gastric cancer [Figure 2g-i].
Figure 2.
Expression of deiodinase enzymes is associated to worsen overall survival for all gastric cancer patients. Analysis of prognostic value of deiodinase1 mRNA expression (a-c), deiodinase2 mRNA expression (d-f), deiodinase3 mRNA expression (g-i) in kmplot.com. Survival curves are plotted for all gastric cancer patients (a, d and g), gastric cancer intestinal-type cancer patients (b, e and h), and gastric cancer diffuse-type cancer patients (c, f and i)
Various clinical and pathological characteristics of gastric cancer determine the treatment outcome of anticancer treatment regime. Therefore, the correlation of NIS and DIOs with established clinicopathological features was probed. The patients' data were correlated with gender of the patients, clinical stages, HER2 expression status, and choices of treatment in gastric cancer for computation of patient OS and HR. As summarized in Table 1, DIO3 expression and NIS expression correlated significantly to the worsen OS of the gastric cancer patients. The data from Table 1 also show the increased HR with increased DIO3 and NIS expression. In terms of clinical stages of gastric cancer patients' DIO1 expression, DIO3 expression and NIS expression lead to significantly increased HR in the patients belonging to clinical stages 1 and 2.
Table 1.
Correlation and prognostic value of deiodinase/ sodium iodide symporter expression with gender of gastric cancer patients
| DIOs/NIS | Gender | Cases | HR (95% CI) | P |
|---|---|---|---|---|
| NIS | Male | 545 | 1.85 (1.5-2.29) | 9.2e-09 |
| Female | 236 | 1.99 (1.4-2.83) | 9.9e-05 | |
| DIO1 | Male | 545 | 1.42 (1.15-1.76) | 0.0012 |
| Female | 236 | 1.96 (1.36-2.84) | 0.00028 | |
| DIO2 | Male | 545 | 1.25 (0.99-1.59) | 0.065 |
| Female | 236 | 1.96 (1.35-2.84) | 0.00031 | |
| DIO3 | Male | 545 | 1.62 (1.38-2.04) | 4.5e-05 |
| Female | 236 | 2.19 (1.52-3.16) | 1.7e-05 |
NIS: Sodium iodide symporter; CI: Confidence interval; HR: Hazard ratio; DIOs: Deiodinases
Overexpression of HER2 relates to recalcitrant cancer; hence, it is considered as a negative prognostic factor.[5,17] Regardless of HER2 receptor status, NIS correlates to the poor OS, as shown in Table 2. Another significant observation was with DIO3 where overexpression DIO3 and HER2 negative status correlate with the poor OS; however, HER2 positive status does not. Finally, the prognostic value of NIS expression/DIO was correlated with treatment choices of surgery alone and chemotherapy of gastric cancer patients [Table 3]. DIO2 correlated with poor OS in surgery alone as well as 5-fluorouracil based adjuvant.
Table 2.
Correlation and prognostic value of deiodinase/ sodium iodide symporter expression with human epidermal growth factor receptor-2 status of gastric cancer patients
| DIOs/NIS | HER2 status | Cases | HR (95% CI) | P |
|---|---|---|---|---|
| NIS | Negative | 532 | 1.65 (1.32-2.07) | 9.5e-06 |
| Positive | 344 | 2.02 (1.49-2.75) | 3.9e-06 | |
| DIO1 | Negative | 532 | 1.47 (1.17-1.85) | 0.00097 |
| Positive | 344 | 1.42 (1.08-1.85) | 0.01 | |
| DIO2 | Negative | 532 | 1.5 (1.18-1.91) | 0.001 |
| Positive | 344 | 1.42 (1.09-1.85) | 0.0094 | |
| DIO3 | Negative | 532 | 1.69 (1.35-2.11) | 4.5e-06 |
| Positive | 344 | 1.27 (0.99-1.72) | 0.11 |
NIS: Sodium iodide symporter; CI: Confidence interval; HR: Hazard ratio; DIOs: Deiodinases; HER2: Human epidermal growth factor receptor-2
Table 3.
Correlation and prognostic value of deiodinase/ sodium iodide symporter expression with surgery and chemotherapeutic intervention of gastric cancer patients
| DIOs/NIS | Treatment | Cases | HR (95% CI) | P |
|---|---|---|---|---|
| NIS | Surgery alone | 380 | 1.52 (1.13-2.04) | 0.0053 |
| 5-FU-based adjuvant | 153 | 1.41 (0.93-2.15) | 0.1 | |
| DIO1 | Surgery alone | 380 | 1.28 (0.95-1.73) | 0.1 |
| 5-FU-based adjuvant | 153 | 0.62 (0.42-0.9) | 0.011 | |
| DIO2 | Surgery alone | 380 | 1.93 (1.41-2.62) | 2.4e-05 |
| 5-FU-based adjuvant | 153 | 0.52 (0.35-0.75) | 0.00048 | |
| DIO3 | Surgery alone | 380 | 1.77 (1.23-2.54) | 0.0019 |
| 5-FU-based adjuvant | 153 | 1.4 (0.96-2.05) | 0.082 |
NIS: Sodium iodide symporter; CI: Confidence interval; HR: Hazard ratio; DIOs: Deiodinases; 5-FU: 5-fluorouracil
Discussion
In this study, we utilized a data mining approach to assess whether NIS and DIO1-3 are related to the prognosis of gastric cancer patients using KM plot survival analysis. Normal thyroid function depends on the active and adequate supply of iodide that is an essential constituent of THs; therefore, NIS is a key plasma membrane transport protein performing this function. The expression of NIS is naturally observed in thyroid cells. The rat NIS cDNA was cloned by functional expression in Xenopus laevis oocytes from overtly functional FRTL-5 rat thyroid cells.[18] Due to availability of NIS cDNA cloned, it became possible to analyze the expression of NIS in thyroidal and extrathyroidal tissues. It was observed that NIS protein is functionally expressed in extrathyroidal tissues such as salivary gland,[19] gastric mucosa,[20] small intestine,[21] lactating memory gland,[22] and choroid plexus.[23] Apart from the physiological expression of NIS, it is found that NIS is overexpressed in breast cancer[24] and autoimmune Graves' disease.[25] Interestingly, NIS is downregulated in gastric cancer as well as in intestinal metaplasia.[10] This observation suggests that deregulated NIS expression could be a diagnostic and prognostic marker in cancer.[26]
The present investigation showed that NIS overexpression is linked with poor prognosis and decreased OS in gastric cancer patients [Figure 1] regardless of gender and HER2 status [Tables 1 and 2]. NIS overexpression also significantly associated with clinical Stage 3 of the gastric cancer patient. These analyses are in conformation to the earlier findings where NIS expression was linked with gastric cancer.[10,27] Overexpression of NIS significantly reduced the OS of gastric cancer patients. Specifically, increased NIS expression in intestinal-type gastric cancer correlates with poor OS, however, not in diffuse-type gastric cancer. Expression of NIS in thyroid has been exploited to be targeted by β-emitting radioactive iodide for its therapeutic targeting. NIS overexpressing intestinal-type gastric cancer could be explored further to be targeted by radioactive iodine therapy.[28] Interestingly, estrogen receptor, progesterone receptor, and HER2 overexpression have been correlated with altered NIS expression in fibrocystic breast and breast carcinoma.[26] This strong association of NIS expression and gastric cancer warrants further studies to elucidate the role of iodine and its transporter in gastric cancer progression.
Several studies have indicated the role of THs in gastrointestinal cancers. The alterations in TH receptor expression have been reported in human gastric cancer and has been associated with gastric cancer metastasis.[29] Given the association of TH signaling and host metabolism, it could play a role in the occurrence and carcinogenesis in various tissues and organ system related to the gastrointestinal system.[30] As TH's activity is regulated by DIOs, this link needs to be explored in detail. Despite increased amount of evidence about altered DIO expression in cancer,[15] substantial evidence linking DIO expression in cancer is lacking. However, there are still good indications that DIO activation in some cases could be useful marker of the disease.[13,15] The research and studies about the role of DIOs in gastric cancer are limited.[13] The adopted data mining approach utilized in this study show that DIOs increased expression links to worsen OS of gastric cancer patients and worse HR most significantly in the case of DIO3 and gastric cancer. In case of intestinal and diffused gastric cancer, DIO3 overexpression was linked to the poor prognosis of gastric cancer. DIO3 expression also linked regardless of gender, clinical stage, and treatment modality. In selective instances, DIO1 and DIO2 were found to be linked with gastric cancer subgroup based on gender clinical stages HER2 status and treatment regime [Tables 1-4].
Table 4.
Correlation and prognostic value of deiodinase/ sodium iodide symporter expression with clinical stages of gastric cancer patients
| DIOs/NIS | Clinical stages | Cases | HR (95% CI) | P |
|---|---|---|---|---|
| NIS | I | 67 | 372891726.8 (0-inf) | 0.0035 |
| II | 140 | 1.41 (0.74-2.7) | 0.29 | |
| III | 305 | 1.8 (1.35-2.41) | 5.3e-05 | |
| IV | 148 | 1.58 (1.08-2.31) | 0.018 | |
| DIO1 | I | 67 | 4.33 (1.61-11.69) | 0.0016 |
| II | 140 | 3.07 (1.21-7.79) | 0.013 | |
| III | 305 | 1.73 (1.3-2.3) | 0.00016 | |
| IV | 148 | 1.27 (0.86-1.9) | 0.23 | |
| DIO2 | I | 67 | 0.33 (0.07-1.48) | 0.13 |
| II | 140 | 1.69 (0.91-3.15) | 0.093 | |
| III | 305 | 1.2 (0.87-1.66) | 0.26 | |
| IV | 148 | 1.93 (1.29-2.89) | 0.0012 | |
| DIO3 | I | 67 | 2.33 (0.87-6.22) | 0.083 |
| II | 140 | 2.64 (1.42-4.88) | 0.0014 | |
| III | 305 | 1.7 (1.24-2.33) | 0.00087 (S) | |
| IV | 148 | 1.41 (0.96-2.07) | 0.074 |
NIS: Sodium iodide symporter; CI: Confidence interval; HR: Hazard ratio; DIOs: Deiodinases; S: Significant
Thyroid cells are able to iodinate polyunsaturated fatty acids to form iodolactones. α-iodohexadecanal is a major iodolipid which has multiple inhibitory effects on adenylate cyclase, NADPH-oxidase, and thyroid peroxidase.[31,32,33] Iodocompound 6-iodo-5-hydroxy-8, 11, 14-eicosatrienoic acid delta-lactone is known to specifically inhibit signal transduction pathways induced by local growth factors such as epidermal growth factor and basic fibroblast growth factor.[34] These iodocompounds seem to act as mediators of iodine function as an antioxidant and autoregulation of cAMP-independent thyroid cell proliferation. Similarly, the formation of iodocompound due to upregulated NIS and local iodine generation can regulate cell growth and proliferation regulating cancer cell growth.
Conclusion
Cancer is such a heterogeneous disease that treatment choices and outcomes depend on the altered genetic makeup of cancer cells. In the era of precision medicine, there is a need to look at specific genetic alterations in the given cancer. According to the finding of this manuscript, NIS is linked with gastric cancer prognosis. Further, DIO expression in the specific subgroup of gastric cancer patients could be a potential biomarker. These findings may lead to increased interest in elucidating the potential role NIS and DIO may have in gastric cancer as well as new anticancer targets susceptible to new drugs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Oba K, Paoletti X, Alberts S, Bang YJ, Benedetti J, Bleiberg H, et al. Disease-free survival as a surrogate for overall survival in adjuvant trials of gastric cancer: A meta-analysis. J Natl Cancer Inst. 2013;105:1600–7. doi: 10.1093/jnci/djt270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boku N. HER2-positive gastric cancer. Gastric Cancer. 2014;17:1–2. doi: 10.1007/s10120-013-0252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yasui W. Future perspectives of gastric cancer treatment: From bench to bedside. Pathobiology. 2011;78:293–4. doi: 10.1159/000331226. [DOI] [PubMed] [Google Scholar]
- 6.Portulano C, Paroder-Belenitsky M, Carrasco N. The Na+/I- symporter (NIS): Mechanism and medical impact. Endocr Rev. 2014;35:106–49. doi: 10.1210/er.2012-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Upadhyay G, Singh R, Agarwal G, Mishra SK, Pal L, Pradhan PK, et al. Functional expression of sodium iodide symporter (NIS) in human breast cancer tissue. Breast Cancer Res Treat. 2003;77:157–65. doi: 10.1023/a:1021321409159. [DOI] [PubMed] [Google Scholar]
- 8.Shrivastava A, Tiwari M, Sinha RA, Kumar A, Balapure AK, Bajpai VK, et al. Molecular iodine induces caspase-independent apoptosis in human breast carcinoma cells involving the mitochondria-mediated pathway. J Biol Chem. 2006;281:19762–71. doi: 10.1074/jbc.M600746200. [DOI] [PubMed] [Google Scholar]
- 9.Venturi S, Donati FM, Venturi A, Venturi M, Grossi L, Guidi A. Role of iodine in evolution and carcinogenesis of thyroid, breast and stomach. Adv Clin Path. 2000;4:11–7. [PubMed] [Google Scholar]
- 10.Altorjay A, Dohán O, Szilágyi A, Paroder M, Wapnir IL, Carrasco N. Expression of the Na+/I- symporter (NIS) is markedly decreased or absent in gastric cancer and intestinal metaplastic mucosa of Barrett esophagus. BMC Cancer. 2007;7:5. doi: 10.1186/1471-2407-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiozaki A, Ariyoshi Y, Iitaka D, Kosuga T, Shimizu H, Kudou M, et al. Functional analysis and clinical significance of sodium iodide symporter expression in gastric cancer. Gastric Cancer. 2019;22:473–85. doi: 10.1007/s10120-018-0874-2. [DOI] [PubMed] [Google Scholar]
- 12.Dentice M, Salvatore D. Deiodinases: The balance of thyroid hormone: Local impact of thyroid hormone inactivation. J Endocrinol. 2011;209:273–82. doi: 10.1530/JOE-11-0002. [DOI] [PubMed] [Google Scholar]
- 13.Casula S, Bianco AC. Thyroid hormone deiodinases and cancer. Front Endocrinol (Lausanne) 2012;3:74. doi: 10.3389/fendo.2012.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer EL, Wagner MS, Maia AL. Iodothyronine deiodinases expression in thyroid neoplasias. Arq Bras Endocrinol Metabol. 2007;51:690–700. doi: 10.1590/s0004-27302007000500006. [DOI] [PubMed] [Google Scholar]
- 15.Piekiełko-Witkowska A, Nauman A. Iodothyronine deiodinases and cancer. J Endocrinol Invest. 2011;34:716–28. doi: 10.3275/7754. [DOI] [PubMed] [Google Scholar]
- 16.Szász AM, Lánczky A, Nagy Á, Förster S, Hark K, Green JE, et al. Cross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patients. Oncotarget. 2016;7:49322–33. doi: 10.18632/oncotarget.10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Webb A, Scott-Mackie P, Cunningham D, Norman A, Andreyev J, O'Brien M, et al. The prognostic value of serum and immunohistochemical tumour markers in advanced gastric cancer. Eur J Cancer. 1996;32A:63–8. doi: 10.1016/0959-8049(95)00504-8. [DOI] [PubMed] [Google Scholar]
- 18.Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379:458–60. doi: 10.1038/379458a0. [DOI] [PubMed] [Google Scholar]
- 19.Dagogo-Jack S. Dietary iodine affects epidermal growth factor levels in mouse thyroid and submaxillary glands. Endocr Res. 1994;20:247–57. doi: 10.1080/07435809409035862. [DOI] [PubMed] [Google Scholar]
- 20.Carr JA, Murali S, Hu F, Goleman WL, Carr DL, Smith EE, et al. Changes in gastric sodium-iodide symporter (NIS) activity are associated with differences in thyroid gland sensitivity to perchlorate during metamorphosis. Gen Comp Endocrinol. 2015;219:16–23. doi: 10.1016/j.ygcen.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Nicola JP, Basquin C, Portulano C, Reyna-Neyra A, Paroder M, Carrasco N. The Na+/I- symporter mediates active iodide uptake in the intestine. Am J Physiol Cell Physiol. 2009;296:C654–62. doi: 10.1152/ajpcell.00509.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dohán O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M, et al. The sodium/iodide symporter (NIS): Characterization, regulation, and medical significance. Endocr Rev. 2003;24:48–77. doi: 10.1210/er.2001-0029. [DOI] [PubMed] [Google Scholar]
- 23.Wright EM. Active transport of iodide and other anions across the choroid plexus. J Physiol. 1974;240:535–66. doi: 10.1113/jphysiol.1974.sp010622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tandon A, Shrivastava A, Kumar A, Prayaga AK, Sundaram C, Godbole MM. Sodium iodide symporter, estrogen receptor, and progesterone receptor expression in carcinoma breast – an immunohistochemical analysis. Indian J Pathol Microbiol. 2011;54:745–51. doi: 10.4103/0377-4929.91514. [DOI] [PubMed] [Google Scholar]
- 25.Kollecker I, von Wasielewski R, Langner C, Müller JA, Spitzweg C, Kreipe H, et al. Subcellular distribution of the sodium iodide symporter in benign and malignant thyroid tissues. Thyroid. 2012;22:529–35. doi: 10.1089/thy.2011.0311. [DOI] [PubMed] [Google Scholar]
- 26.Rai R, Shrivastava A, Tandon A, Godbole MM, Kumar S, Das V, et al. Human sodium iodide symporter (hNIS) in fibroadenoma breast – A immunohistochemical study. Indian J Exp Biol. 2011;49:113–7. [PubMed] [Google Scholar]
- 27.Shiozaki A, Hikami S, Ichikawa D, Kosuga T, Shimizu H, Kudou M, et al. Anion exchanger 2 suppresses cellular movement and has prognostic significance in esophageal squamous cell carcinoma. Oncotarget. 2018;9:25993–6006. doi: 10.18632/oncotarget.25417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kogai T, Brent GA. The sodium iodide symporter (NIS): Regulation and approaches to targeting for cancer therapeutics. Pharmacol Ther. 2012;135:355–70. doi: 10.1016/j.pharmthera.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kandemir EG, Yonem A, Narin Y. Gastric carcinoma and thyroid status. J Int Med Res. 2005;33:222–7. doi: 10.1177/147323000503300210. [DOI] [PubMed] [Google Scholar]
- 30.Brown AR, Simmen RC, Simmen FA. The role of thyroid hormone signaling in the prevention of digestive system cancers. Int J Mol Sci. 2013;14:16240–57. doi: 10.3390/ijms140816240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dugrillon A. Iodolactones and iodoaldehydes – Mediators of iodine in thyroid autoregulation. Exp Clin Endocrinol Diabetes. 1996;104(Suppl 4):41–5. doi: 10.1055/s-0029-1211700. [DOI] [PubMed] [Google Scholar]
- 32.Langer R, Burzler C, Bechtner G, Gärtner R. Influence of iodide and iodolactones on thyroid apoptosis. Evidence that apoptosis induced by iodide is mediated by iodolactones in intact porcine thyroid follicles. Exp Clin Endocrinol Diabetes. 2003;111:325–9. doi: 10.1055/s-2003-42721. [DOI] [PubMed] [Google Scholar]
- 33.Cann SA, van Netten JP, Glover DW, van Netten C. Iodide accumulation in extrathyroidal tissues. J Clin Endocrinol Metab. 1999;84:821–2. doi: 10.1210/jcem.84.2.5472-4. [DOI] [PubMed] [Google Scholar]
- 34.Dugrillon A, Bechtner G, Uedelhoven WM, Weber PC, Gärtner R. Evidence that an iodolactone mediates the inhibitory effect of iodide on thyroid cell proliferation but not on adenosine 3',5'-monophosphate formation. Endocrinology. 1990;127:337–43. doi: 10.1210/endo-127-1-337. [DOI] [PubMed] [Google Scholar]