Abstract
Background and Aims:
Intense bleeding during general anaesthesia (GA) is the major limitation during functional endoscopic sinus surgery (FESS). This study was aimed to compare the efficacy of dexmedetomidine and magnesium sulphate (MgSO4) for controlled hypotension in FESS.
Methods:
Sixty eight patients undergoing FESS were randomised to receive either dexmedetomidine 1 μg/kg over 10 min followed by infusion at 0.2 to 0.7 μg/kg/h (Group D) or MgSO4 40 mg/kg over 10 min followed by an infusion at 10 to 15 mg/kg/h (Group M). Anaesthesia and infusion rates for study drugs were maintained with sevoflurane to keep MAP between 60–70 mmHg throughout the surgery. The time to reach the target MAP, the number of patients requiring a minimum and maximum infusion doses of study drugs were noted.
Results:
The mean time to achieve target mean arterial pressure (MAP) was less in group D (10.59 ± 2.04) as compared with (21.32 ± 4.65 min) group M (P < 0.001). The target MAP was achieved between 5–15 min in 73.52% patients (Group D) with an infusion dose of 0.2–0.4 μg/kg/h of dexmedetomidine without the use of sevoflurane, while 82.35% patients in group M required 4% sevoflurane along with >12–15 mg/kg/hr infusion of MgSO4 to achieve target MAP in 10–20 min.
Conclusion:
Dexmedetomidine is superior to MgSO4 in achieving target MAP in lesser time with minimum infusion dose.
Key words: Controlled hypotension, dexmedetomidine, FESS, magnesium sulphate
INTRODUCTION
Intentional hypotension to limit intraoperative blood loss has dramatically improved surgical dissection during the functional endoscopic sinus surgery (FESS). A bloodless surgical field provides better visibility of the operative field, with reduced risk of injury to adjoining structures.[1]
Numerous pharmacological agents have been used effectively for controlled hypotension by reducing the baseline mean arterial pressure (MAP) by 30% or maintaining MAP at 60–70 mmHg. Sodium nitroprusside and nitroglycerine (NTG) precisely control the MAP because of their rapid onset and short duration of action, but disadvantages like resistance to vasodilators, tachyphylaxis and cyanide toxicity pose many challenges.[2]
Inhalational anaesthetics (isoflurane and sevoflurane); vasodilators (e.g., sodium nitroprusside and nitroglycerine); beta (β)-adrenoceptor blocker (e.g., esmolol); opioids (e.g., remifentanil); alpha 2 (α2) adrenergic agonists (clonidine and dexmedetomidine) and magnesium sulphate (MgSO4)[3,4,5,6] are increasingly being used for controlled hypotension during general anaesthesia (GA).
Ideally, a hypotensive agent should be easy to administer, has a short onset time, effects that disappear quickly when the administration is discontinued, rapid elimination without toxic metabolites, negligible effects on vital organs and predictable dose-dependent effects.[3] Dexmedetomidine, a highly selective α2 adrenoreceptor agonist has sedative, analgesic and anaesthesia sparing effects. It has a dose-dependent decrease in arterial blood pressure, heart rate (HR) and cardiac output because of central sympatholysis. MgSO4 has also been found to reduce the arterial pressure and HR by inhibiting the release of norepinephrine. Both the drugs have been studied previously, and they had a proven efficacy as a hypotensive agent, but the ideal hypotensive agent should have a shorter onset time. So, the aim of this study was to compare the two drugs in terms of time to achieve the target MAP and infusion dose required for the same. We aimed to compare the efficacy of dexmedetomidine and MgSO4 for achieving controlled hypotension during FESS. The primary objective of the study was to compare the time to achieve the target MAP with infusion doses of study drugs. The secondary objectives of the study were to assess the quality of the surgical field, adverse effects and recovery characteristics.
METHODS
Institutional ethical committee approval was obtained (dated 27th February 2017; approval number GU/HREC/EC/2017/1425) and a prospective randomised, double-blind, clinical study was conducted in a tertiary care hospital from March2017 to August 2018. Sixty-eight patients belonging to the American Society of Anesthesiologists' Physical Status (ASA-PS) grades I and II of either sex and age group between 18–60 years, who were scheduled to undergo FESS under GA were included in the study. Patients who were hypersensitive to study drugs, having chronic hypertension, sinus bradycardia, hypotension, anticipated difficult airway, general haematological and neuromuscular disorder, neuropathy, coexisting severe cardio-respiratory, hepatorenal diseases and on long-term opioids were excluded from the study. Patients were randomly assigned into two groups of 34 patients each (Group D and Group M) according to computer-generated randomisation table.
A thorough pre-anaesthetic evaluation was performed in all the study patients by the investigator a day prior to surgery and the patients received nil per oral instructions as per the standard protocol in the night. On the day of the surgery, through an intravenous (IV) line with 18 gauge (G) cannula, 10 ml/kg ringer lactate was started. For topical vasoconstriction and further reduction in bleeding, 10 min before the start of the surgery, local anaesthesia (LA) and 1:1000 epinephrine soaked cotton wool pledgets were applied by the surgeons via the nasal cavity for 5 to 10 min. After attaching the monitors (electrocardiogram [ECG], pulse oximetry, non-invasive blood pressure [NIBP], capnography and neuromuscular monitor) baseline vitals were recorded. The envelope was opened by the anaesthesiologist (primary investigator) just before the administration of GA, and according to the group allotted to the patient, drug bolus and infusion were prepared by her. Another anaesthesiologist (secondary investigator) performing GA was unaware of the constituent of the drug given as a bolus and infused via syringe pump and allotment of the group. The drugs infusions were prepared in the dose concentration that was infused at the same rate in ml/hr. The same anaesthesiologist recorded the data throughout the perioperative period. The primary investigator was not involved in the process of administering the drug, performing GA and recording data; hence, blinding was achieved throughout the procedure. Study drugs were infused as follows:
Group D- Dexmedetomidine 1 μg/kg diluted in 100 ml of 0.9% normal saline administered over 10 min was given as a loading dose followed by infusion at 0.2 to 0.7 μg/kg/h via an infusion pump (L and T SP102). For infusion, 100 μg (1 ampoule) of the drug was diluted in 49 ml of 0.9% normal saline NS to make a final volume of 50 ml and final concentration of 2 μg/ml
Group M- MgSO4 40 mg/kg diluted in 100 ml of 0.9% normal saline was given as a loading dose over 10 min followed by an infusion at 10 to 15 mg/kg/h via an infusion pump (L and T SP102). For infusion 5 gm (5 ampoules i.e., 10 ml) were diluted in 40 ml of 0.9% NS to make a final volume of 50 ml and final concentration of 100 mg/ml.
All the patients were pre-medicated with IV ondansetron 0.15 mg/kg, IV glycopyrrolate 5 μg/kg and IV fentanyl 2 μg/kg. After pre-oxygenation with 100% oxygen, induction was done with IV propofol 2 mg/kg and tracheal intubation was facilitated with IV cisatracurium 0.15 mg/kg. Subsequently, anaesthesia was maintained with oxygen in the air (50:50) and sevoflurane (1–3%). The train of four (TOF) ratio was kept between 0–0.1 throughout the surgery using a continuous infusion of cisatracurium at 3 μg/kg/min.
The MAP was maintained between 60–70 mmHg throughout the surgery. The time needed to reach the target MAP with the infusion of the study drug was noted. Infusions were first started at minimum doses for both the drugs (0.2-0.4 μg/kg/hr and 10-12 mg/kg/hr for dexmedetomidine and MgSO4 respectively). If the MAP was >70 mmHg, then the infusion of the study drug was gradually increased to maximum doses (0.4-0.7 μg/kg/hr and 12-15 mg/kg/hr for dexmedetomidine and MgSO4 respectively). The infusion rate for both the drugs was adjusted according to the MAP readings throughout the surgery. After adjusting the rate, we waited for 5 min and if the MAP did not reach the target range the infusion rate was readjusted. This procedure was repeated until the MAP fell within the target range. The number of patients requiring the minimum or maximum doses of infusion were noted and recorded in both the groups. If the MAP did not fall in the target range even after achieving maximum recommended infusion rate then the concentration of sevoflurane was increased to 4% and IV boluses of esmolol 0.5mg/kg were administered. The time at which the target MAP reading was first achieved was noted as the time to achieve target MAP. Hypotension was defined when MAP was <55 mmHg. But, whenever the MAP went below 60 mmHg, it was managed with rapid IV fluids (250 ml–500 ml) and IV mephentermine 5–10 mg, and the rate of infusion and sevoflurane dial concentration were lowered immediately. The HR <50/min was termed bradycardia and managed with IV atropine 0.6 mg.
The HR, systolic blood pressure (SBP), diastolic blood pressure (DBP), MAP, oxygen saturation and end-tidal carbon dioxide (EtCO2) were measured at specific time intervals, i.e., baseline, after pre-medication, after study drug, after induction, after intubation and at every 5 min for the first 15 min. Once the target MAP was achieved, the recording was continued at 5 min interval, till the end of the surgery and after extubation. Adverse events like bradycardia, hypotension and hypertension during the perioperative period were managed accordingly and noted. The subjective assessment of the surgical field for bleeding was carried out by the surgeon. The bleeding score was assessed by the Boezaart scale[7] (0–5). The surgeon satisfaction score was assessed by the surgeon at the end of surgery as 1 = Poor, 2 = Moderate, 3 = Good, 4 = Excellent.
All the infusions (study drug and cisatracurium) and sevoflurane were stopped 5 min before the end of surgery. Neostigmine 0.05 mg/kg with 0.01 mg/kg glycopyrrolate IV was used for the reversal of neuromuscular blockade as per the assessment of the TOF stimulation (ratio >0.7) and patients were extubated when the TOF ratio >0.9 was achieved. The extubation time (time from the end of anaesthesia to extubation of trachea) was noted and compared in both the groups. After extubation, all patients were transferred to the post-anaesthesia care unit (PACU). In the PACU, the patients were monitored for postoperative recovery score (modified Aldrete score 0–10)[8] and sedation scores (Ramsay sedation score [RSS] 1–6),[9] which were observed and noted at 5 min, 30 min and 60 min after tracheal extubation. Patients were considered ready for discharge from the PACU when the Aldrete score was ≥9. Any post-operative adverse effects like dryness of mouth, shivering, nausea and vomiting were observed and recorded.
Based on clinical experience and a pilot study of 10 patients, a 30% difference in time proportion was observed between dexmedetomidine and MgSO4 to achieve the target MAP (60–70 mmHg), with a power of 80% and α error 5%. The sample size was calculated using the Epi InfoTM7 software. The calculated sample size was 58 patients. Considering exclusions and to reject the null hypothesis, a total of 76 patients were evaluated. Out of them, 68 patients who met the inclusion criteria were randomised into two groups (34 in each group). Data were statistically described in terms of mean ± standard deviation (SD), or frequencies (number of cases), and 95% confidence of interval of mean, median and percentages when appropriate. Comparison of numerical variables between the study groups was done using the Student's t-test for independent samples. For comparing categorical data, a Chi-square test was performed. Pearson's correlation coefficient was applied wherever necessary. Alternatively, when the expected frequency was less than five, the exact test was used. P <0.05 was considered statistically significant. All statistical calculations were done using the Statistical Package for the Social Sciences (SPSS) software (SPSS Inc., Chicago, IL, USA) version 15 for Microsoft Windows.
RESULTS
Figure 1 shows the CONSORT diagram for the flow of patients at each stage of the study. All patients who were randomised completed the study. Both the groups were comparable with respect to age, gender and ASA classification. When the duration of surgery, anaesthesia and controlled hypotension were compared between the groups, they were found to be significantly lower in group D as compared with group M (P < 0.05) Table 1. The target MAP 60–70 mmHg was achieved significantly earlier in group D as compared with group M(P < 0.00) [Table 1]. The maximum number of patients achieved target MAP in 10 min or less in group D (n = 13 in group D v/s n = 6 in group M) [Table 2]. Most of the patients in group D achieved target MAP with a minimum infusion of study drug as compared with group M, where all the patients required >12–15 mg/kg/hr MgSO4 to achieve a target MAP [Table 2]. The maximum number of patients in group D had bleeding score of 2 or less (n = 26) as compared with group M, where most of the patients had bleeding score >2 (n = 32) (P < 0.001) [Table 2]. We observed that those patients who had less bleeding score had a better surgeon satisfaction score in both the groups Table 2. Requirement of additional esmolol to maintain targeted MAP were more in group M (n = 1, total dose 30 mg) as compared with group D ([n = 12, total dose 360 mg] [Table 3] [P < 0.001]). Group M patients had a significantly prolonged duration of surgery; 60–90 min in 19 patients and >90 min in 15 patients. Whereas, in group D, the surgery got over in <60 min in 5 patients and took between 60–90 min in 23 patients (P < 0.0001)to get over. The extubation time was also more in group M; 10.78–19 min v/s 6.3–7.5 min in group D (P < 0.001). The mean recovery score was comparable in both the groups (P = 0.85), but group D showed significantly longer time to achieve an Aldrete score of 9 or greater and discharge the patients from the PACU as compared with group M (P < 0.001) [Table 4]. Post-extubation RSS was significantly low and patients were more sedated in group D at 5 and 30 min (P = 0.000), but the RSS was comparable at 60 min in both the groups (P = 1.000) Table 4. Intra- and post-operative adverse effects were not significant in both the groups (P > 0.05) [Table 4].
Figure 1.
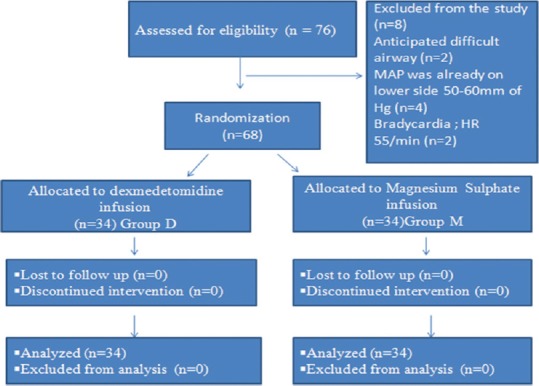
Diagram for the flow of participants through each stage of the present study
Table 1.
Comparison of demographic data in both groups
| Parameters | Group D (Mean±SD) | Group M (Mean±SD) | P |
|---|---|---|---|
| Age (years) | 39.26±15.44 | 38.77±18.72 | 0.894 (NS) |
| Sex (M:F) | 28:6 | 24:10 | |
| Weight (kg) | 58.24±7.96 | 60.06±13.08 | 0.490 (NS) |
| ASA-PS grade I/II | 22/2 | 22/2 | 1.000 (NS) |
| Duration of anaesthesia (min) | 92.94±26 | 112.21±31.12 | 0.008 (S) |
| Duration of surgery (min) | 77.65±28.61 | 92.65±31.62 | 0.044 (S) |
| Duration of controlled | 76.18±29.85 | 91.47±32.27 | 0.047 (S) |
| Hypotension (min) | |||
| Mean time (min) to achieve target MAP | 10.59±2.04 | 21.32±4.65 | <0.001** |
*P<0.05, **P<0.001, SD – Standard deviation, S – Significant, HS – Highly significant, NS – Non-significant, ASA-PS – American Society of Anesthesiology Physical Status, MAP – Mean arterial pressure
Table 2.
Comparison of various parameters between the two groups
| Variables | Range | Group D (n=34) | Group M (n=34) | Chi-square value, P |
|---|---|---|---|---|
| Dose of infusions | Minimum | 27 | 0 | 4.91, 0.026* |
| Maximum | 4 | 6 | ||
| Maximum with sevoflurane | 3 | 28 | ||
| Time in which the target | 10 min or less | 13 | 6 | 3.57, 0.05* |
| MAP was achieved | >10 min | 21 | 28 | |
| Bleeding Score | 2 or less | 26 | 2 | 34.9, <0.001** |
| >2 | 8 | 32 | ||
| Satisfaction score | 2 or less | 5 | 33 | 46.76, <0.001** |
| >2 | 29 | 1 |
Chi-square test was applied, *P<0.05 significant, **P<0.01 highly significant
Table 3.
Comparison esmolol requirement between two groups
| Group D (mean±SD) | Group M (mean±SD) | P | |
|---|---|---|---|
| No. of patients | 1 | 12 | |
| Mean total dose of additional esmolol | 30 mg | 360 mg | 0.001 |
**P<0.01 highly significant, SD – Standard deviation, No. – Number
Table 4.
Post-operative recovery score characteristics, sedation score and adverse effects in both the groups
| Group D (n=34) (Mean±SD) | Group M (n=34) (Mean±SD) | P | |
|---|---|---|---|
| Recovery score characteristics | |||
| Mean recovery Score | 12.97±0.30 | 12.82±0.39 | 0.085 |
| Time to achieve recovery score >9 (min) | 22.64±4.47 | 10±6.5 | <0.001** |
| RSS | |||
| At 5 min | 3.94±0.74 | 2.62±0.49 | 0.000** |
| 30 min | 2.59±0.50 | 2.00±0.00 | 0.000** |
| 60 min | 1.59±0.50 | 1.59±0.50 | 1.000 |
| Side Effects | |||
| Nausea | 3 (8.82%) | 6 (17.64%) | 0.2 |
| Vomiting | 1 (2.94%) | 3 (8.82%) | 0.306 |
| Shivering | 4 (11.76%) | 5 (14.70%) | 0.722 |
**P<0.01 highly significant, SD – Standard deviation, RSS – Ramsay sedation score
DISCUSSION
Controlled hypotension has a definitive role in FESS as it reduces bleeding during surgery and improves visibility of the surgical field; hence, it decreases the time taken for surgery and the duration of GA. We compared the efficacy of dexmedetomidine and MgSO4 for achieving controlled hypotension during FESS in terms of time to achieve target MAP and infusions doses. The total requirement of sevoflurane or additional hypotensive agents to maintain MAP, duration of surgery, duration of anaesthesia and the total duration of controlled hypotension were also noted. The RSS, bleeding score, recovery characteristics and adverse effects of the two drugs were assessed and analysed statistically. In our study, the demographic data were comparable between the groups.
Although we could achieve the target MAP in all the patients in both the groups, it was achieved significantly earlier in group D as compared with group M (10.59 ± 2.04 min in group D as compared with 21.32 ± 4.65 min in group M). Similarly, Khalifa et al. achieved a target MAP of 55–65 mmHg at 10 min following study drugs (dexmedetomidine, MgSO4 and NTG) in FESS.[10] In the current study, 27 patients in group D achieved the target MAP with a minimum infusion of study drug while none of the patients in group M could achieve the target MAP at minimum infusions. Four patients in group D compared with six in group M required maximum infusion of the study drug. The results of the present study indicate that the use of dexmedetomidine infusion reduced the consumption of sevoflurane as we observed more volatile anaesthetic consumption in group M (mean dial concentration up to 4% in group M as compared with group D (P = 0.000). Only three patients required additional sevoflurane to achieve target MAP along with maximum infusion in group D whereas in group M most of the patients (n = 28) required sevoflurane with the maximum infusion of the study drug. Khan et al. found a 35% to 50% reduction in isoflurane requirements in patients treated with either low or high doses of dexmedetomidine.[11] Fragen et al. conducted a study to determine the effect of two target dexmedetomidine infusions (0.3 ng/ml and 0.6 ng/ml) on the minimal alveolar concentration (MAC) of sevoflurane in elderly patients undergoing elective surgery and found administration of dexmedetomidine was associated with a 17% decrease n sevoflurane requirements for the maintenance of anaesthesia.[12] While Bayram et al. compared dexmedetomidine and MgSO4 in controlled hypotension during FESS and found that the requirement of fentanyl and NTG was more in group D as compared with group M.[13] In our study haemodynamics were maintained in both the groups throughout the surgery without any significant episodes of bradycardia and hypotension. Extubation time i.e. the duration from end of anaesthesia until the patients become fully awake and removal of endotracheal tube, was significantly more in group M (10.78 ± 3.44 -19 ± 5.41 min v/s 6.30 ± 2.7 -7.5 ± 4.18 min in group D) (P < 0.001)which could be explained by the potentiation of effect of nondepolarizing muscle relaxants by MgSO4. Soliman et al. also found significantly prolonged extubation time in group M (13.2 ± 1.75) as compared with group D ([9.28 ± 1.51] [P < 0.05])[14] but Bayram et al.[10] found the extubation time to be comparable in both the groups (P > 0.05).[10] Though mean recovery score was comparable in both the groups but group D showed significantly longer time (22.64 ± 4.47) as compared with group M (10 ± 6.5) to achieve an Aldrete score of 9 or greater and to discharge the patients from the PACU. Khalifa et al. 2015 also showed a significantly longer time to achieve a modified Aldrete score of 9 or greater with dexmedetomidine.[10] In our study, we found that in group D, surgeons were satisfied with surgical conditions because of better visibility of surgical field (surgeon satisfaction score >2) in the patients with less bleeding (bleeding score 2 or less). Similarly Abdelmottalb et al. have found that there was a statistically significant decrease in bleeding score in group D as compared with group M (1.2 ± 0.7 v/s 12.0 ± 1.0) (P < 0.018).[15] Aboushanab OH et al. concluded that both MgSO4 and dexmedetomidine were equally successful in inducing deliberate hypotension in patients undergoing middle ear surgery and produced satisfactory surgical field.[16] Postextubation sedation for 5 to 30 min was more with dexmedetomidine which was similar to study by Bajwa et al. where significantly higher RSS was observed with dexmedetomidine when compared with NTG and esmolol.[17] The post-operative sedation is often desirable but sometimes prolongs the recovery and discharge of the patient from PACU. Longer duration of recovery time can be explained by sedative and analgesic effects of dexmedetomidine via central actions in the locus coeruleus and in the dorsal horn of spinal cord.
Preoperative and post-operative haemoglobin values along with intra-operative blood loss were not compared because the blood loss during FESS is not expected to cause any significant change in laboratory values, although even small amounts restrict surgical vision, especially in the narrow operative field. To get more authentic results further studies can be done in a larger population with a multicentric trial.
CONCLUSION
Dexmedetomidine and MgSO4 are equally effective in inducing controlled hypotension during FESS but Dexmedetomidine achieves target MAP earlier with low doses of infusion, provides good surgical field and lesser intra-operative bleeding. However, sedation and recovery during the post-operative period may be prolonged.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published, and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Jacobi K, Rickauer AJ. Prophylactic analgesia in functional endoscopic sinus surgery. Hemodynamics, surgical conditions, stress response. Anästhesiol Intensivmed Notfallmed Schmerzther. 1999;4:278–87. doi: 10.1055/s-1999-10822. [DOI] [PubMed] [Google Scholar]
- 2.Shams T, El Bahnasawe NS, Abu-Samra M, El-Masry R. Induced hypotension for functional endoscopic sinus surgery: A comparative study of dexmedetomidine versus esmolol. Saudi J Anesth. 2013;7:175–80. doi: 10.4103/1658-354X.114073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Degoute CS. Controlled hypotension. Drugs. 2007;67:1053–76. doi: 10.2165/00003495-200767070-00007. [DOI] [PubMed] [Google Scholar]
- 4.Degoute CS, Ray MJ, Manchon M, Dubreuil C, Banssillon V. Remifentanil and controlled hypotension; comparison with nitroprusside or esmolol during tympanoplasty. Can J Anaesth. 2001;48:20–7. doi: 10.1007/BF03019809. [DOI] [PubMed] [Google Scholar]
- 5.Coursin DB, Coursin DB, Maccioli GA. Dexmedetomidine. Curr Opin Crit Care. 2001;7:221–6. doi: 10.1097/00075198-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Ghodraty MR, Homaee MM, Farazmehr K, Nikzad-Jamnani AR, Soleymani-Dodaran M, Pournajafian AR, et al. Comparative induction of controlled circulation by magnesium and remifentanil in spine surgery. World J Orthop. 2014;18:51–6. doi: 10.5312/wjo.v5.i1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boezaart AP, van der Merwe J, Coetzee A. Comparison of sodium nitroprusside-and esmolol-induced controlled hypotension for functional endoscopic sinus surgery. Can J Anaesth. 1995;42:373–6. doi: 10.1007/BF03015479. [DOI] [PubMed] [Google Scholar]
- 8.Aldrete JA, Vazeery A. Is magnesium sulfate an anesthetic? Anesth Analg. 1989;168:186–7. doi: 10.1213/00000539-198902000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khalifa OS, Awad OG. A comparative study of dexmedetomidine, magnesium sulphate, or glyceryltrinitrate in deliberate hypotension during functional endoscopic sinus surgery. Ain-Shams J Anaesthesiol. 2015;8:320–6. [Google Scholar]
- 11.Khan ZP, Munday IT, Jones RM, Thornton C, Mant TG, Amin D. Effects of dexmedetomidine on isoflurane requirements in healthy volunteers.1: Pharmacodynamic and pharmacokinetic interactions. Br J Anaesth. 1999;83:372–80. doi: 10.1093/bja/83.3.372. [DOI] [PubMed] [Google Scholar]
- 12.Fragen RJ, Fitzgerald PC. Effect of dexmedetomidine on the minimum alveolar concentration (MAC) of sevoflurane in adults age 55 to 70 years. J Clin Anesth. 1999;11:466–70. doi: 10.1016/s0952-8180(99)00081-1. [DOI] [PubMed] [Google Scholar]
- 13.Bayram A, Ulgey A, Güneş I, Ketenci I, Capar A, Esmaoǧlu A, et al. Comparison between magnesium sulfate and dexmedetomidine in controlled hypotension during functional endoscopic sinus surgery. Rev Bras Anestesiol. 2015;65:61–7. doi: 10.1016/j.bjan.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Soliman R, Fouad E. The effects of dexmedetomidine and magnesium sulphate in adult patients undergoing endoscopic transnasaltranssphenoidal resection of pituitary adenoma: A double-blind randomised study. Indian J Anaesth. 2017;61:410–17. doi: 10.4103/ija.IJA_581_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdelmottalb NA. Magnesium sulphate versus dexmedetomedine in hypotensive technique in patients underwent functional endoscopic sinus surgery. AAMJ. 2014;12:119–36. [Google Scholar]
- 16.Aboushanab OH, El-Shaarawy AM, Omar AM, Abdelwahab HH. A comparative study between magnesium sulphate and dexmedetomidine for deliberate hypotension during middle ear surgery. Egypt J Anaesth. 2011;27:227–32. [Google Scholar]
- 17.Bajwa SJ, Kaur J, Kulshrestha A, Haldar R, Sethi R, Singh A. Nitroglycerine, esmolol and dexmedetomidine for induced hypotension during functional endoscopic sinus surgery: A comparative evaluation. J Anaesthesiol Clin Pharmacol. 2016;3:192–7. doi: 10.4103/0970-9185.173325. [DOI] [PMC free article] [PubMed] [Google Scholar]


