Virus-virus interactions in plants can modify host symptoms. As a result, disease management strategies may be unsuccessful if they are based solely on visual assessment and diagnostic assays for known individual viruses. Papaya ringspot virus is an important limiting factor for papaya production and likely has interactions with other viruses that are not yet known. Using high-throughput sequencing, we recovered known and novel RNA and DNA viruses from papaya orchards in Chiapas, Mexico, and categorized them by host and, in the case of papaya, symptom type: asymptomatic papaya, papaya with ringspot virus symptoms, papaya with nonringspot symptoms, weeds, and insects. Using network analysis, we demonstrated virus associations within and among host types and described the ecological community patterns. Recovery of viruses from weeds and asymptomatic papaya suggests the need for additional management attention. These analyses contribute to the understanding of the community structure of viruses in the agroecological landscape.
KEYWORDS: bipartite networks, network analysis, phytobiome, plant viruses, viral ecology, viral metagenomics
ABSTRACT
The study of complex ecological interactions, such as those among host, pathogen, and vector communities, can help to explain host ranges and the emergence of novel pathogens. We evaluated the viromes of papaya orchards, including weed and insect viromes, to identify common viruses in intensive production of papaya in the Pacific Coastal Plain and the Central Depression of Chiapas, Mexico. Samples of papaya cultivar Maradol, susceptible to papaya ringspot virus (PRSV), were categorized by symptoms by local farmers (papaya ringspot symptoms, non-PRSV symptoms, or asymptomatic). These analyses revealed the presence of 61 viruses, where only 4 species were shared among both regions, 16 showed homology to known viruses, and 36 were homologous with genera including Potyvirus, Comovirus, and Tombusvirus (RNA viruses) and Begomovirus and Mastrevirus (DNA viruses). We analyzed the network of associations between viruses and host-location combinations, revealing ecological properties of the network, such as an asymmetric nested pattern, and compared the observed network to null models of network association. Understanding the network structure informs management strategies, for example, revealing the potential role of PRSV in asymptomatic papaya and that weeds may be an important pathogen reservoir. We identify three key management implications: (i) each region may need a customized management strategy; (ii) visual assessment of papaya may be insufficient for PRSV, requiring diagnostic assays; and (iii) weed control within orchards may reduce the risk of virus spread to papaya. Network analysis advances understanding of host-pathogen interactions in the agroecological landscape.
IMPORTANCE Virus-virus interactions in plants can modify host symptoms. As a result, disease management strategies may be unsuccessful if they are based solely on visual assessment and diagnostic assays for known individual viruses. Papaya ringspot virus is an important limiting factor for papaya production and likely has interactions with other viruses that are not yet known. Using high-throughput sequencing, we recovered known and novel RNA and DNA viruses from papaya orchards in Chiapas, Mexico, and categorized them by host and, in the case of papaya, symptom type: asymptomatic papaya, papaya with ringspot virus symptoms, papaya with nonringspot symptoms, weeds, and insects. Using network analysis, we demonstrated virus associations within and among host types and described the ecological community patterns. Recovery of viruses from weeds and asymptomatic papaya suggests the need for additional management attention. These analyses contribute to the understanding of the community structure of viruses in the agroecological landscape.
INTRODUCTION
Pathogen emergence results from interactions between susceptible hosts and pathogenic viruses in conducive environments, causing disease outbreaks in new geographic regions or hosts (1, 2). Emerging diseases caused by pathogen expansion to new hosts, or pathogen host jumping, are regularly reported in new hosts, vectors, and regions, causing yield losses in many parts of the world (3). An example is the emergence of diseases such as maize lethal necrosis in sub-Saharan Africa, caused by a synergistic interaction between two single-stranded (ss) RNA viruses, a potyvirus and a tombusvirus (4, 5). Another important example is the viral complex of several species of ssDNA begomoviruses that causes cassava mosaic disease, which caused a pandemic that spread throughout Africa via whiteflies (6, 7).
Several emerging viral diseases of papaya (Carica papaya L.) have been observed in recent years. A disease outbreak of a mixed infection of RNA and DNA viruses, the interaction of potyvirus, crinivirus, and begomovirus, caused severe symptoms in papaya orchards in Texas (8). Papaya ringspot virus (PRSV), a plant ssRNA potyvirus transmitted by aphids in a nonpersistent manner or mechanically transmitted through farm tools, causes major yield losses due to foliar deformation, resulting in reduced photosynthetic area, and lowers fruit quality by producing ringspots (9). Previous studies of papaya production areas across Mexico found six different strains of PRSV and mixed infections with papaya mosaic virus (PapMV) (10, 11). In the 1990s, a rhabdovirus was identified in southeast Mexico with similar etiology to that of papaya apical necrosis disease or papaya droopy fruit (12–15). A disease reported since the 1980s in Brazil, named meleira disease, or “sticky disease,” of papaya, caused by the papaya meleira virus (PMeV) (16), was reported in Yucatan, Mexico (17, 18). Recently, papaya meleira virus 2 (PMeV-2), with etiology similar to that of meleira disease (19), was discovered but is not yet known to cause problems for papaya production in Mexico. Plant-virus interactions can have devastating outcomes; however, interactions between viruses can also be antagonistic, as we recently reported in a time series study of coinfection of PapMV and PRSV (20).
Interactions among viruses, including synergistic and antagonistic interactions, may be a common feature in nature (21). Coinfections modify symptoms in different ways resulting in mild to severe symptoms or asymptomatic infected plants. Thus, coinfections can produce misleading diagnoses when planting material from tissue culture is tested. Plant-virus interactions can pass unnoticed and may be difficult to interpret by standard methods such as visual evaluation of symptoms, enzyme-linked immunosorbent assay (ELISA), PCR and/or reverse transcriptase PCR (RT-PCR) (22). Due to improvement of protocols for nucleic acid isolation of virus-like particles and availability of high-throughput sequencing technologies, it is now possible to explore all or almost all viruses associated within a given host. Viral metagenomics approaches support the discovery of a large number of virus species in wild plants, crops, and vectors (22–25). In addition to analyses of diversity indices, methods for evaluating associations among pathogens are needed to understand the potentially complex network of interactions among microbes, epidemiological dynamics, and bipartite networks specific to host-vector and plant-virus interactions (26, 27). Network analysis provides insights into biological relationships in viral communities and can generate hypotheses about mechanisms that promote and prevent the cooccurrence of viruses in communities (28). Systems that include multiple hosts and potential unknown interactions between RNA and DNA viruses add another layer of complexity. Bipartite networks have been evaluated extensively for plant-pollinator interactions, which are interesting analogs for host-pathogen systems (29, 30).
Bipartite network analysis can be used to evaluate community nestedness and modularity. In more nested communities, the viruses associated with one host will tend to be a subset of the viruses associated with another host. In modular communities, nodes tend to be divided into subsets, forming modules, such that one group of viruses tends to infect one group of hosts. Theoretical studies have shown that a nested network structure can minimize competition, increasing the number of coexisting species and potentially making nested communities more robust against random extinctions (31). The network of specialization has characteristics, such as modularity or compartmentalization patterns, that support the network stability between generalist and specialist species (31).
Our analysis synthesizes two conceptual frameworks: viral metagenomics to reveal plant viruses using high-throughput sequencing and analysis of plant viruses in bipartite networks. Using concepts from island biogeography (32), we defined an environment as a combination of host type, in the broad sense, and region. This analysis shows how hosts connect virus species through the network and how plant-vector-virus associations can be interpreted to inform management strategies for papaya orchards in Chiapas.
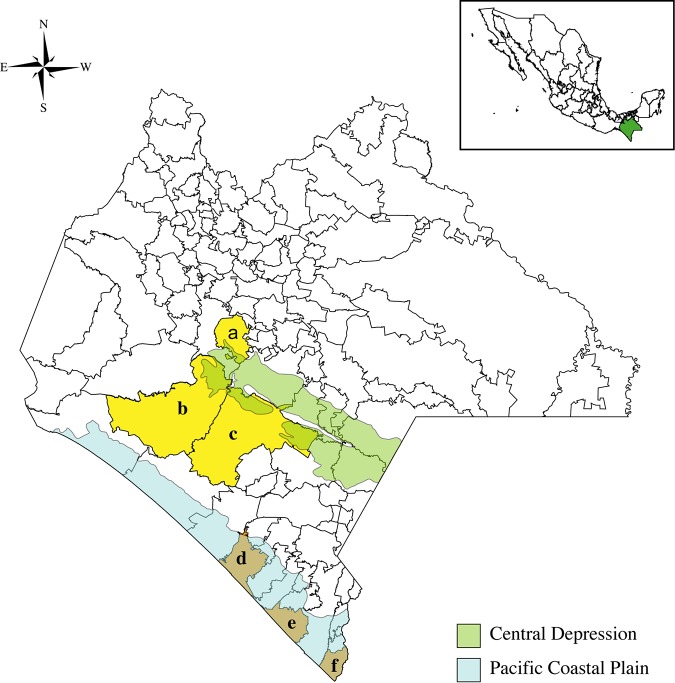
In the papaya orchard virome network, the link between PRSV and papaya plants displaying typical symptoms of ringspot is expected in both regions examined. Also, it is expected that some viruses will be nested within papayas and weeds or insects, indicating potential management strategies. What other types of interactions are present in the papaya orchard virome? We used metagenomics to characterize the papaya orchard virome and network analysis to characterize viral associations across the host types in two physiographic regions in southeast Mexico (Fig. 1) separated by the Sierra Madre de Chiapas mountain range at an elevation of 4,093 m (33). These two physiographic regions represent different ecosystems, sharing some characteristics but differing in the degree of land fragmentation, orchard size, and plant species, among other characteristics (34). The first, the Central Depression, is a tropical deciduous forest at 700 masl. The second, the Pacific Coastal Plain, is a highly fragmented semideciduous tropical forest at sea level, with forests being replaced to a great extent by grasslands for cattle grazing. Both physiographic regions are in the Federal State of Chiapas, one of the main producers and exporters of papaya in southern Mexico. Management strategies for papaya orchards in these regions emphasize reducing PRSV effects, and roguing of infected papaya is the primary strategy to control ringspot disease.
FIG 1.
Two physiographic regions in the Federal State of Chiapas in Mexico. Maps showing the Central Depression (at 700 masl; light green), the Pacific Coastal Plain (at sea level; light blue), and the municipalities from which samples were collected: Acalá (a), Villa Corzo (b), La Concordia (c), Acapetahua (d), Mazatán (e), and Suchiate (f).
Here, we (i) report 52 near-full-length genome sequences identified as plant viruses, 29 being novel viruses, associated with papaya, weeds, and insects, (ii) report the diversity and distribution of viruses in two contrasting physiographic regions, the Central Depression and the Pacific Coastal Plain of Chiapas, and (iii) evaluate the bipartite network structure of viruses and host-location combinations in the papaya orchard agroecosystem. The papaya orchard virome network has structures beyond stochastic associations. Bipartite network analysis of these complex viromes, generated through viral metagenomics, revealed virus associations and identified the roles of particular hosts in connecting the network.
RESULTS
To evaluate the papaya orchard virome, papaya samples were collected based on symptoms evaluated by local growers. Growers are generally familiar with PRSV symptoms, because management in orchards commonly includes roguing papaya plants with typical PRSV symptoms or other virus symptoms. To evaluate where PRSV and other viruses were present in papaya orchards, we used a viral metagenomics approach to assess the diversity of viruses in the region. We identified 82 sequences corresponding to at least 57 viruses, of which 52 sequences were nearly full viral genomes within 10 genera, and 10 were unassigned genera in 10 viral families. The bipartite network of viruses, based on metagenomic results and host-location combinations for the papaya orchards, was evaluated in terms of modularity, nestedness, and centrality measures (Table 1).
TABLE 1.
Metric definitions
| Network metric | Measurement definition | Reference(s) |
|---|---|---|
| Node degree | For hosts and regions, this is the number of viruses; for viruses, this is the number of hosts and locations from which the virus was recovered | 35, 36 |
| Species strength | Number of interactions per species in qualitative networks | 36 |
| Betweenness centrality | Describes the connectivity by host and location | 30 |
| Weighted betweenness | Describes the connectivity by sample type incorporating the relative abundance of viruses | 30 |
| Closeness centrality | Describes the proximity of a node to all other nodes in the network | 30 |
| Modularity | Describes the module connectivity within each node type, where higher numbers indicate more connected networks | 37 |
| Nestedness | Describes the degree to which the species associated with a host and region tend to be subsets of the species associated with another host and location | 38 |
Bipartite networks have frequently been described for cases such as pollinator networks, where the two types of nodes are pollinators and plants (29). We conceptualize bipartite networks for the papaya orchard virome as having one level representing hosts (in the broad sense, the primary host [papaya, divided into three categories based on symptoms], secondary hosts [weeds], and vectors [insects]) for each of two geographic regions and a second level representing viruses. A link between a virus and a host-location combination indicates that the virus was present in that host in that location. These analyses clarify the interactions among RNA and DNA viruses in papaya production areas and the prevalence and distribution of viruses in secondary hosts and insect vectors. Understanding these relationships can inform strategies for management of viruses that pose a risk to papaya production and can contribute to assessments of the risk of virus spillovers.
Virus-like particles.
The diversity of viruses was first evaluated by observing the purified virus-like particles (VLPs) with an electron microscope. Several viral particle morphotypes were confirmed, including filamentous, icosahedral, and pleomorphic. We estimated the diameters for icosahedral particles as ranging from 15 to 65 nm and lengths of up to 750 nm for filamentous particles. Viral morphologies resembling flexuous filamentous or rigid helical rod particles were observed in papaya plants from both the Pacific Coastal Plain and Central Depression. Large numbers of icosahedral particles were observed in all samples. The presence of pleomorphic particles suggested the presence of rhabdoviruses in papaya plants from the Pacific Coastal Plain (see Fig. S1 in the supplemental material).
Electron microscopy of virus-like particles (VLPs). Negative staining transmission electron microscopy (TEM) micrographs of VLPs obtained from the papaya orchard samples of the Central Depression (A) and the Pacific Coastal Plain (B). The numbers on images refer to VLPs extracted from papaya plants (1 to 6), weeds (7 and 8), and insects (9 and 10). Papaya with PRSV symptoms (PS) contained icosahedral, filamentous, and pleiomorphic particles (A1, A2, B1, and B2). Papaya with non-PRSV symptoms (OS) showed rigid helical rods, flexuous filamentous, and pleomorphic particles (A3, A4, B3, and B4). Papayas that were visually asymptomatic (VA) contained icosahedral, filamentous, and pleomorphic particles (A5, A6, B5, and B6). Weeds (W) contained icosahedral and filamentous particles (A7, A8, B7, and B8). Insects (I) contained icosahedral and filamentous particles (A9, A10, B9, and B10). Download FIG S1, PDF file, 1.4 MB (1.4MB, pdf) .
Copyright © 2020 Alcalá-Briseño et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Overall composition and relative abundance of plant viruses present in papaya orchards.
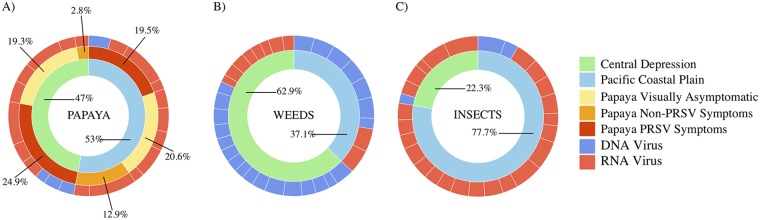
There were 82 sequences with homology to viruses and 61 unique viral sequences of 56 viruses. Only three virus species (four viral sequences) were recovered from both physiographic regions: PRSV, PapMV, and Euphorbia mosaic virus (EuMV). Thirty-three viral sequences (53% of the total number of virus-location-host type combinations) were obtained from samples from the Pacific Coastal Plain, and 32 sequences (47%) were obtained from the Central Depression (Fig. 2). The percentage of DNA and RNA viruses was calculated using the presence and sequence coverage as virus relative abundance (Fig. 2). The percentages of virus-location-host type combinations for asymptomatic papaya were similar in the Pacific Coastal Plain (20.6%) and the Central Depression (19.3%). However, samples from papaya with other viral symptoms showed differences in virus-location-host type combination percentages, 12.9% and 2.8%, respectively. Papaya identified by growers as having symptoms of PRSV had similar percentages, 19.5% in the Pacific Coastal Plain and 24.9% in the Central Depression. Interestingly, only samples from papaya with symptoms of PRSV were associated with both RNA and DNA viruses in both regions; samples from asymptomatic papaya and papaya with other type of symptoms were associated only with RNA viruses (Fig. 2A). The plant virus-location-host type combinations associated with weed samples (including Euphorbia sp., Ipomea sp., Sida sp., Portulaca sp., wild grasses, and maize) (for a list of weeds, see Table S1) were 9.4% RNA viruses and 27.7% DNA viruses for the Pacific Coastal Plain and 17.9% RNA viruses and 44.9% DNA viruses for the Central Depression (Fig. 2B). The plant virus-location-host type combinations associated with the insect samples (see Table S2) were 69% RNA and 8% DNA for the Pacific Coastal Plain and 20% RNA and 2% DNA for the Central Depression (Fig. 2C).
FIG 2.
Distribution of DNA and RNA viruses in papaya, weeds, and insects. The frequency of log-transformed relative sequence abundances of viruses from the papaya orchard virome by host and location: papaya plants (A), weeds (B), and insects (C). The inner rings indicate the proportions of viruses from the samples collected from the Pacific Coastal Plain (light green) and the Central Depression (light blue). The middle ring (papaya only) indicates the proportion of viruses for each of three different types of papaya symptoms: visually asymptomatic (yellow), non-PRSV symptoms (orange), and PRSV symptoms (red). The outer rings indicate the proportions of putative RNA (light red) and DNA (blue) virus species.
Weed accessions. Download Table S1, XLSX file, 0.01 MB (9.8KB, xlsx) .
Copyright © 2020 Alcalá-Briseño et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Insect accessions. Download Table S2, XLSX file, 0.01 MB (9.4KB, xlsx) .
Copyright © 2020 Alcalá-Briseño et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Virus classification.
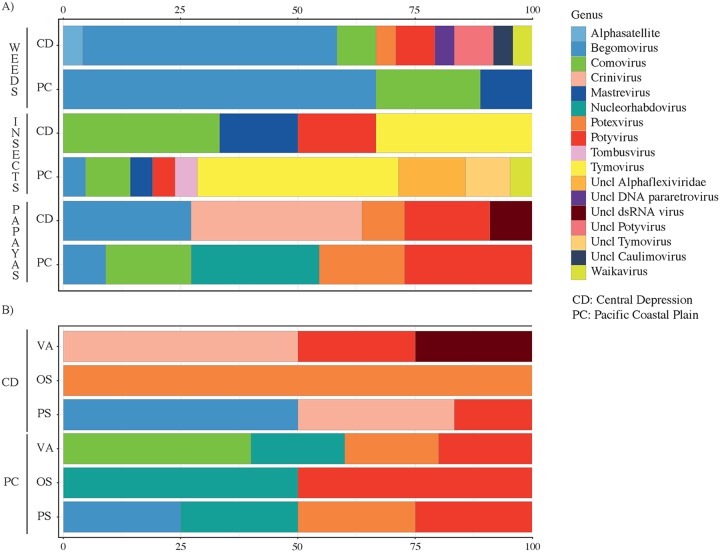
All viral sequences were grouped taxonomically within ten families, with ten approved genera and ten viral sequences that could not be classified at the genus level. Nine viral sequences in six genera (Comovirus, Crinivirus, Potexvirus, Potyvirus, Nucleorhabdovirus, and Begomovirus) and an unclassified double-stranded RNA (dsRNA) virus, PMeV-2, a toti-like virus, were identified from papaya. In the Pacific Coastal Plain, potexviruses, comoviruses, potyviruses, nucleorhabdoviruses, and begomoviruses were present, and potexviruses, criniviruses, potyviruses, unclassified dsRNA viruses, and begomoviruses were present in the Central Depression (Fig. 3A, bottom bars). In the Pacific Coastal Plain, asymptomatic papaya samples included potexviruses, comoviruses, potyviruses, and nucleorhabdoviruses; samples from papaya with non-PRSV symptoms yielded sequences identified as potyviruses and nucleorhabdoviruses. Samples from papaya with PRSV symptoms yielded sequences identified as potexviruses, potyviruses, nucleorhabdoviruses, and begomoviruses (Fig. 3B, top bars). In the Central Depression, asymptomatic papaya yielded sequences identified as criniviruses, potyviruses, and PMeV-2; papaya with non-PRSV symptoms yielded one virus identified as PapMV, a potexvirus. Papaya with symptoms of PRSV yielded sequences identified as criniviruses, potyviruses, and begomoviruses (Fig. 3B, bottom bars). Thirty-one viral sequences were recovered from weeds in both regions and classified in four RNA genera (Comovirus, Potyvirus, Potexvirus, and Waikavirus) and two DNA genera (Begomovirus and Mastrevirus) as two novel unclassified potyviruses, one unclassified caulimovirus, a DNA pararetrovirus, and a satellite virus (Fig. 3A, top bars). The alphasatellite sequence (accession number MN203219) showed 78% similarity with the dragonfly-associated alphasatellite characterized previously in Puerto Rico (39). Twenty-seven sequences were recovered from insects, four of them near-full-length genomes, and classified in five RNA genera: Comovirus, Potyvirus, Tombusvirus, Tymovirus, and Waikavirus. Two DNA genera were recovered from insects, Begomovirus and Mastrevirus, along with unclassified alphaflexiviruses and tymoviruses (Fig. 3A, middle bars).
FIG 3.
The log-transformed relative abundances of DNA and RNA viruses by genus and by host and location in the papaya orchard virome of Chiapas. (A) The diversity and relative abundances of viruses by host and location for weeds, insects, and papaya plants by physiographic region for the Central Depression (CD) and Pacific Coastal Plain (PC). (B) The diversity and relative abundances of viruses in papaya divided by symptoms: visually asymptomatic (VA), non-PRSV symptoms (OS), and PRSV symptoms (PS). Cold colors represent DNA viruses, and warm colors indicate RNA viruses.
Analysis of virome networks in papaya orchards.
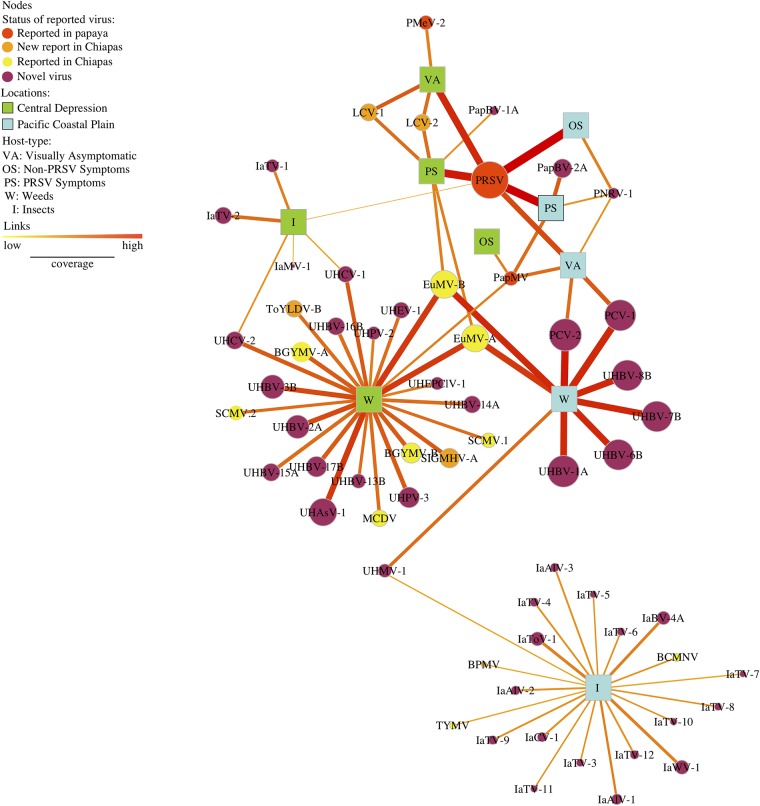
The papaya orchard virome network included 61 viral sequences and 82 links connecting virus nodes and host-location combination nodes (Fig. 4). The number of links for a region, equal to the number of viral sequences recovered from samples from that region, was 33 for the Pacific Coastal Plain and 32 for the Central Depression. Only four viral sequences (nodes) were shared by both regions: PRSV, PapMV, EuMV-A, and EuMV-B.
FIG 4.
Papaya orchard virome, represented in a bipartite network with two types of nodes: viruses (circles) and host-location combinations (squares) for two physiographic regions, the Pacific Coastal Plain (light blue) and Central Depression (light green). The size of circular nodes varies with the sum of the relative abundance by virus species. Links indicate that a virus is present in a host and location, and their width and color (yellow to red) represent relative abundance by host and location. Alternate hosts are weeds (W), insects (I), papaya with PRSV symptoms (PS), papaya with non-PRSV symptoms (OS), and visually asymptomatic papaya (VA). Viruses are indicated by their acronyms (Table S3). Node color represents the status of the virus: previously reported in papaya (red), new report for Chiapas in nonpapaya hosts (orange), previously reported in Chiapas in nonpapaya hosts (yellow), and novel viruses not yet reported anywhere else on any hosts (purple).
The node degree for each host-location combination indicates the number of viral sequences recovered from each group (Table 2; Fig. 4). The node species strength is a weighted version of the node degree, the weighted average of the number of interactions of virus by host (36). The highest node strength in the Central Depression was observed for weeds, and the highest in the Pacific Coastal Plain was for insects. The lowest node strength observed for the Pacific Coastal Plain was for asymptomatic papaya, and the lowest in the Central Depression was for papayas with non-PRSV symptoms (Table 2).
TABLE 2.
Diversity and network indices
| Host typea | Network metric values |
|||||
|---|---|---|---|---|---|---|
| Degree | Species strength | Betweenness | Weighted betweenness | Closeness | Closeness weighted | |
| Pacific Coastal Plain | ||||||
| VA | 5 | 0.586 | 0.270 | 0.181 | 0.122 | 0.001 |
| OS | 2 | 1.193 | 0.000 | 0.000 | 0.098 | 0.001 |
| PS | 4 | 1.840 | 0.090 | 0.168 | 0.112 | 0.001 |
| W | 9 | 8.259 | 0.333 | 0.272 | 0.093 | 0.001 |
| I | 21 | 20.058 | 0.000 | 0.000 | 0.060 | 0.000 |
| Central Depression | ||||||
| VA | 4 | 2.212 | 0.000 | 0.000 | 0.098 | 0.001 |
| OS | 1 | 0.114 | 0.000 | 0.000 | 0.084 | 0.000 |
| PS | 6 | 2.002 | 0.159 | 0.246 | 0.115 | 0.001 |
| W | 24 | 21.674 | 0.125 | 0.129 | 0.108 | 0.001 |
| I | 6 | 3.057 | 0.020 | 0.000 | 0.105 | 0.000 |
VA, visually asymptomatic papaya; OS, non-PRSV symptoms; PS, PRSV symptoms; W, weeds; I, insects.
Betweenness centrality indicates the importance of a species as a connector forming bridges between hosts. We calculated unweighted and weighted versions of betweenness centrality. The highest betweenness centrality (unweighted and weighted) was observed for papaya with PRSV symptoms (0.090 and 0.168, respectively), visually asymptomatic papaya (0.270 and 0.181, respectively), and weeds (0.333 and 0.272, respectively) in the Pacific Coastal Plain and for papaya with PRSV symptoms (0.159 and 0.246, respectively) and weeds (0.125 and 0.108, respectively) in the Central Depression. The rest of the nodes had no role in connecting the network (Table 2). To compare the weighted and unweighted betweenness values, we computed Kendall’s tau (τ) correlation coefficient (τ = 5.8, correlation = 0.9, P = 0.0004), indicating limited differences between the ranks for unweighted and weighted versions of betweenness centrality.
Closeness centrality is a measure of the proximity of one node (host-location) to all other nodes (host-locations) in the network. Surprisingly, asymptomatic papaya in the Pacific Coastal Plain had the highest closeness centrality (0.122) in the network, followed by papaya with PRSV symptoms (0.112 and 0.115) for the Pacific Coastal Plain and Central Depression, respectively; however, the weighted closeness was significantly lower (0.001) (Table 2). For the Kendall’s tau (τ) correlation coefficient, there was some evidence (τ = 2, correlation = 0.58, P = 0.07) for differences between the unweighted and weighted versions of closeness centrality (Table 1).
We represented the host-location interactions by generating a one-mode projection of the network (Fig. 5) showing the betweenness centrality measures. Kendall’s tau (τ) did not show differences between unweighted and weighted versions of the network. We are particularly interested in the associations between host and locations, information that can be translated for the development of management strategies. The one-mode host-location network represents as links the number of viruses shared between and among regions and indicates the weighted betweenness centrality.
FIG 5.

A one-mode network extracted from the bipartite network of associations. Nodes represent host types from the Central Depression and the Pacific Coastal Plain. Node size is proportional to the betweenness centrality of the node. Links represent associations of viruses, and link thickness is proportional to the number of viruses shared within (blue) and among (red) regions.
Network analysis and viral metagenomics reveal hidden associations in papaya orchards.
Viral metagenomics revealed previously unknown viruses in the papaya orchard and their associations with other known viruses such as PRSV. Network metrics indicate host associations with viruses. We can show more clearly how host and locations are linked to each other by removing virus nodes that are linked to only a single host and location (Fig. 6). PRSV was present in all papaya sample types in both regions, with the exception of non-PRSV symptoms in the Central Depression, where only PapMV was present. PRSV was also associated with insect samples from the Central Depression. On the other hand, PapMV was present in papaya with symptoms of PRSV and asymptomatic papaya in the Pacific Coastal Plain as well as in weeds in the Central Depression. A putative papaya nucleorhabdovirus 1 (PNRV-1) was present in all samples of papaya in the Pacific Coastal Plain and associated with PapMV and PRSV. Novel putative bipartite comoviruses, papaya comovirus (PCV) 1 and 2, were identified in weeds and were also present in asymptomatic papaya in the Pacific Coastal Plain. A novel mastrevirus provisionally named unknown host mastrevirus 1 (UHMV-1) was isolated from weeds and insects in the Pacific Coastal Plain. EuMV (Begomovirus) was identified in weeds from both regions, but only in the Central Depression was it associated with PRSV and lettuce chlorosis virus (LCV; Crinivirus) in papaya showing symptoms of PRSV. Interestingly, PRSV and LCV were present in asymptomatic papaya in the Central Depression. A novel bipartite comovirus was recovered from both weeds and insects in the Central Depression and was different from PCV from the Pacific Coastal Plain and two comoviruses tentatively named unknown host comovirus 1 (UHCV-1) and 2 (UHCV-2).
FIG 6.
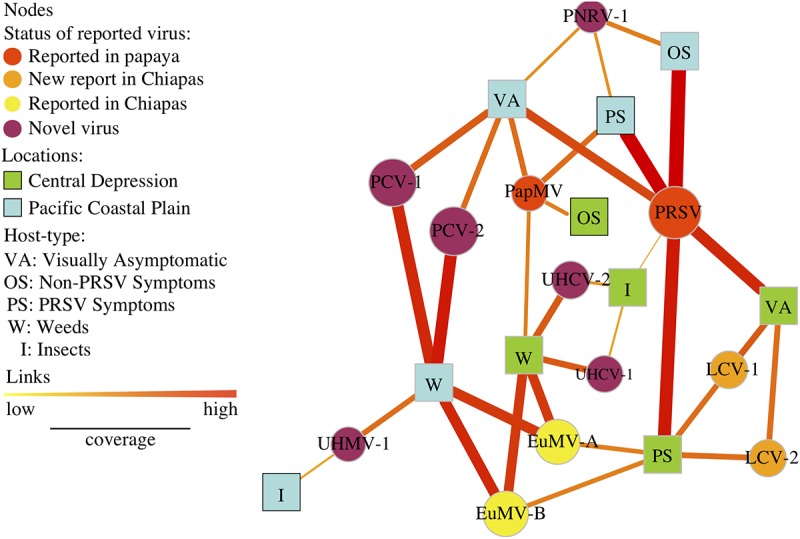
Bipartite network displaying only the nodes that have two or more links. Viruses (circles) and host-location combinations (squares) from two physiographic regions, the Pacific Coastal Plain (light blue) and Central Depression (light green) are represented. Node sizes are proportional to the relative abundance of virus species. Links indicate associations between nodes, and their width and color (yellow to red) represent low and high relative abundances, respectively, by host and location. Hosts are weeds (W), insects (I), papaya with PRSV symptoms (PS), non-PRSV symptomatic papaya (OS), and visually asymptomatic papaya (VA). Viruses are indicated by their acronyms. Node color represents the status of the virus: reported in papaya (red), new report for Chiapas in nonpapaya hosts (orange), previously reported in Chiapas in nonpapaya hosts (yellow), and novel viruses not yet reported anywhere (purple).
Analysis of the virome community structure in papaya orchards.
We used two network metrics to summarize the viral community structure: modularity and nestedness (Table 1). These metrics were calculated using the package bipartite (29) in the R programming environment (40). We tested whether there was evidence that the observed virus community patterns, in terms of these two metrics, differed from what would be expected under three null models (41). These three null models were used to generate new adjacency matrices based on specific properties of the observed data. Under null model 1, species richness is maintained for each host-location combination. Reshuffling occurs within host-location combinations, such that the specific viruses associated with each host and location can change while the number of viruses associated with the host and location remains the same. Under null model 2, the number of hosts and locations in which a species is found is maintained. Reshuffling occurs within each virus species, such that the specific hosts and locations associated with each virus can change while the number of hosts and locations for each virus remains the same. Under null model 3, neither species richness nor the number of hosts and locations associated with a virus is maintained, so reshuffling occurs across both. A comparison of the results for the three null models can be used to interpret whether deviations from random network structures may be due simply to “first-order” properties (such as the total number of species) (29). For example, if null model 1 is rejected but null model 3 is not rejected, this indicates that first-order properties alone do not explain the patterns (29). For each null model, the observed adjacency matrices were reshuffled 1,000 times, and the observed values were compared to 10,000 random null matrices using a Z-score test.
The modularity of the network describes the connectivity within each node type, where the more interactions there are between host-location nodes, the more connected the network is. The observed modularity for the papaya orchard virome was 0.37, where by comparison, modularity values observed in other real networks ranged between 0.3 and 0.7 (42). There was strong evidence to reject null model 1 (P < 0.001) but little evidence to reject null model 2 (P = 0.216) and null model 3 (P = 0.213) (Table 3). The nestedness of the network indicates the degree to which, for example, the viruses associated with one host and location tend to be a subset of the viruses associated with another host and location. The observed nestedness was 0.576, suggesting a moderate level of nestedness. There was strong evidence for rejecting all three null models for nestedness (Table 3). In general, the low level of modularity and the nestedness of the papaya orchard virome reflect the module formed by papaya plants across symptomatologies and the nestedness patterns for weeds and papaya plants, and for weeds and insects, in both locations.
TABLE 3.
Network metrics
| Metric | Observed value |
P value |
||
|---|---|---|---|---|
| Null 1 | Null 2 | Null 3 | ||
| Modularity | 0.373 | 4.32e−34a | 0.216 | 0.213 |
| Nestedness | 0.576 | 3.356e−07 | 4.533e−14 | 0.0047 |
Not normally distributed.
DISCUSSION
Expanding virome databases offer new opportunities for analysis and understanding of virome complexity. Typical approaches to virome analysis include the use of descriptive statistics, sequence analysis for the identification and discovery of novel viruses, and studies of evolution and viral diversity (43–49). More recently, some studies have captured the association of viruses and hosts using network analysis by mining databases or ELISA (50, 51). Here, we present a new application of bipartite network analysis coupled with viral metagenomics in a framework that reveals interactions of the entire community, including both known and new virus associations across hosts. Node centrality measures, such as node degree, betweenness, and closeness, provide information to motivate follow-up analyses of management strategies and more generally provide new insights into ecological interactions between viruses. There was strong evidence that the papaya orchard virome had a nested structure but only weak evidence for modularity. The patterns of nestedness in this virome indicate subsets of viruses and hosts and locations that may need to be managed together, where one host-location combination may act as a risk factor for another. To represent this, the bipartite network was converted to a one-mode network for host levels, where modularity and nestedness patterns connect the different host-location combinations emphasizing interactions. The one-mode network emphasized how viruses were recovered in asymptomatic papaya in both regions, suggesting that roguing papayas with viral symptoms may not be sufficient to manage virus incidence. We also found PapMV in weeds in the Central Depression and a novel comovirus (PCV) in papaya found in weeds in the Pacific Coast, suggesting that management strategies may need to expand to weed control, removing potential hosts for known or emerging pathogens of papaya.
Papaya and its virus interactions.
Carica papaya is the only species in the family Caricaceae, a cultivated species originating and domesticated in southern Mexico (52). Papaya viruses tend to be specialists, but some also may infect relatives of C. papaya. Horovitzia, Jarilla, and Jacaratia are the closest genera to Carica and are native to southern Mexico and Central America. Vasconcellea is the most closely related genus in South America (52). Plant viruses, known or emerging, are often reported in papaya plants, and 22 viral species are known to cause disease in papaya. Papaya was the host from which 11 of these were first reported, frequently in mixed infections with PRSV (8, 16, 17, 53, 54). For example, PMeV and papaya virus Q both have only been reported to infect papaya (16, 17, 54). Recently, PMeV-2 isolate PMeV-MX, identified in papaya in southeastern Mexico, was reported to also infect watermelon (18). PapMV, in addition to infecting papaya, has been reported naturally infecting pumpkin (Cucurbita pepo), Cnidoscolus chayamansa, and Jacaratia mexicana (10, 11). Mixed infections of PRSV and PapMV have been reported in Cucurbita moschata, Cucurbita pepo var. cylindrica, and Citrullus lanatus (11). Two strains of PRSV (P and W) have been reported, distinguished by host range, where PRSV strain P can infect papaya and cucurbits. In this analysis, we recovered PRSV only from papaya plants (in both regions), except for the recovery from insects in the Central Depression.
We recovered sequences similar to those of LCV (RNA 1, MN203147 and MN203150; and RNA 2 MN203148 and MN203149) and EuMV (DNA A and B, MN203156-61) that are new reports for viruses infecting papaya in Chiapas. The crinivirus, LCV, and a begomovirus, Tomato yellow leaf curl virus (TYLCV), were recently reported in papaya in Southern Texas, causing severe symptoms (8). Two novel sequences of begomoviruses provisionally named PapBV 1 (MN203166) and 2 (MN203167) were recovered from papaya plants in both regions. Both sequences shared homology to segment A of the genus Begomovirus, with lengths of 2.7 and 2.8 kb. Additionally, we identified a novel nucleorhabdovirus by transmission electron microscopy (TEM) images with pleomorphic virions (see Fig. S1) and sequences generated by high-throughput sequencing, up to 5.4 kb, isolated from papaya in the Pacific Coastal Plain. Only one rhabdovirus has been reported in papaya, causing apical necrosis disease in Florida and Venezuela in the 1980s (12, 55). In the late 1990s, similar symptoms were reported in southeast Mexico (15); however, no sequence accessions have been submitted for rhabdovirus from papaya. We suggest that PNRV-1 (MN203193-95) identified in Chiapas could be the causal agent of papaya apical necrosis, although further information is required to confirm this hypothesis. Additionally, in this study, we report novel viruses sharing characteristics with bipartite comoviruses, provisionally named papaya comovirus 1 and 2 (PCV 1 RNA1, MN203151 and MN203154; and RNA 2, MN203152 and MN203153), in papaya and weeds in the Pacific Coastal Plain. Interestingly, LCV, EuMV, PNRV-1, and PCV 1 and 2, together with PapMV and PMeV, were associated with PRSV.
Viruses may be protective agents, where interactions between viruses within the host are important determinants of the severity of infection, and a wide range of interaction types are possible (21, 56–59). Notice the similar compositions of RNA and DNA viruses in the papayas with symptoms of PRSV in both the Pacific Coastal Plain and the Central Depression. Interestingly, PapMV was only reported in papaya plants with non-PRSV symptoms in the Central Depression; however, the role of PapMV as a protective agent was not evaluated in situ. We have previously reported changes in the symptomology with either a coinoculation or a stepwise inoculation, where PRSV followed by PapMV caused synergism and the reciprocal stepwise inoculation of PapMV followed by PRSV led to antagonism (20). This observation suggested that PapMV could interact as a protective agent against PRSV and probably against other viruses as well. PapMV triggers systemic acquired resistance (SAR) in papaya, increasing the expression of a marker protein related to pathogenesis (PR1), which inhibits subsequent infections by PRSV (20). PapMV-triggered SAR may be a mechanism of plant defense against PRSV that could help in managing the disease, because papayas lack a set of genes conferring resistance to ringspot disease (20, 52). Future studies of the virome at the individual host level, compared to those from bulked samples, will help to clarify virus interactions. It would also be interesting to study these interactions at the molecular level to identify antagonistic or synergistic effects in individual papaya plants, as was recently reported for PapMV interactions (20).
The papaya virome network: metrics for disease management.
Bipartite network analysis has been used to characterize complex systems of trophic networks, such as plants and pollinators (including bees, bats, and birds), hosts and parasitoids (including fish and mammals, and ecto- and endoparasites), and bacteria and bacteriophages (29, 30, 60–62). We developed applications of bipartite network analysis for plant obligate intracellular parasites, for example, of viruses in interactions with plants and vectors. This bipartite network analysis includes both illustration of the network structure in images and quantitative analysis of the network structure. Plant viral metagenomics techniques are sensitive enough to reveal most viral sequences within a plant (23, 63). Also, interpretation of these metrics needs to take into account sampling effort, plant abundance, and insect behavior, such as the actions of vector species (64).
Bipartite network analysis of the papaya orchard virome also focused on hosts and locations, and a one-mode projection of the network was generated. Information about the role of hosts and viruses in the network can be translated into potential risk management strategies. For example, weeds and visually asymptomatic papayas in the Pacific Coastal Plain had the highest betweenness centrality (Table 3); however, the highest number of shared viruses within the Pacific Coastal Plain was for visually asymptomatic papaya and papaya with PRSV symptoms. This suggests that asymptomatic papaya plants and plants with PRSV symptoms both contribute as a source of viruses (Fig. 5, blue nodes). A scenario consistent with our expectations was observed in the Central Depression, where papaya with PRSV symptoms had the highest betweenness centrality, followed by weeds and insects. Asymptomatic papaya and non-PRSV-symptomatic papaya had betweenness centrality equal to 0 (Fig. 5, green nodes). These results suggest that different management strategies may be needed for each region. The Pacific Coastal Plain is a more fragmented ecosystem and is heavily managed compared to the Central Depression. Other agroecological differences between the two regions, such as farm size, diversity of plants, and management strategies, may also play a role in the virome dynamics in the papaya orchards. Translation of these results could include future studies to evaluate the cost-effectiveness of weed management and to consider diagnostic assays to evaluate viral thresholds in visually asymptomatic papayas.
Virome perspective.
The phytobiome is conceptualized as the interactions among microorganisms, the environment, and plants (65). Virome interactions in marine ecosystems have been described, but there is limited information about plant-pathogenic virus interactions beyond studies of virus pairs. Plant-virus interactions can produce a number of outcomes, and the possibilities increase when there are multiple viruses. Little is known about the virus community in agroecological landscapes and how the total number of interactions impact the susceptibility of infected plants to other viruses, where initial infection by one species may facilitate the establishment of another virus species (57). Bipartite network metrics help in identifying the properties of the community, including specialization patterns.
Plant-pollinator networks are asymmetric, relying on generalist species to maintain the nested structure of the network that supports specialized species (30, 66). Host-parasitoid networks are often asymmetric as well, with the nestedness of the network having the opposite effect—specialist species tend to parasitize hosts with more parasites, and generalist parasites tend to parasitize hosts with fewer parasites (31, 61). The papaya orchard virome was asymmetric, slightly modular, and strongly nested. Papaya viruses tended to be in a module with papayas. Viruses in weeds were identified in papayas, suggesting the potential for emerging pathogens.
In our study, there was strong evidence for the observed modularity of the papaya orchard virome for null model 1 but not for null models 2 and 3. There was strong evidence for nestedness of the papaya orchard virome for all three null models, indicating that the observed nestedness may be due to first-order properties (29). It is important to keep in mind that networks of specialization may differ by region in temperate or tropical areas, affecting the viral community structure (64). It will be interesting to compare the ecological properties, such as nestedness, of the papaya orchard virome network in Chiapas to networks in other papaya orchards and other global cropping systems. The identification of these ecological patterns in agroecosystems will support an understanding of the contrasting dynamics of tropical and temperate systems over time. Future work emphasizing the ecological properties of plant virome networks will support risk assessment for disease emergence and the discovery and management of new viruses and new virus interactions.
MATERIALS AND METHODS
Sample collection.
We collected samples in two physiographic regions in southern Mexico with significant papaya production, separated by a mountain range: the Pacific Coastal Plain, at sea level, and the Central Depression, at 700 m above sea level (masl). The regions have different levels of ecosystem fragmentation, with patches of deciduous forest, secondary vegetation, and farmland in the Central Depression, contrasting with former deciduous tropical forest replaced with grasslands and farms in the Pacific Coastal Plain (67, 68). The samples consisted of leaf pieces of papaya and weeds and insects, all collected in September 2014 from three papaya orchards in each physiographic region (Fig. 1). The farms were El Rocio, Ejido Aquiles Serdan, and Santa Lucia in the counties of Acapetahua, Mazatán, and Suchiate in the Pacific Coastal Plain and San Juan de Acala, La Unión, Monte Achiote, and La Fortuna in the counties of Acalá, Villa Corzo, and La Concordia in the Central Depression. At each farm, a zig zag sampling method was used, and farmers provided approximately seven young papaya leaves from the top of trees, categorized by the farmers into each of three symptom types: visually asymptomatic, symptoms that are PRSV like, and symptoms that are non-PRSV. Two samples from each leaf, approximately 6 cm2, were kept in a plastic bag (see below). Weeds were sampled by collecting asymptomatic and symptomatic leaves in the orchards or their surroundings and insects were actively sampled using insect sweep collecting nets by walking along the perimeter of the orchards and in a cross section inside the orchard and passively collecting insects from sticky traps placed in the orchard by the owner. Samples from these orchards were weed species in the Euphorbiaceae, Poaceae, Solanaceae, Convolvulaceae, Asteraceae, Lamiaceae, Anacardiaceae, Malvaceae, Salicaceae, Moraceae, Plantaginaceae, Cucurbitaceae, Portulacaceae, Caryophyllaceae, and Amaranthaceae families (see Table S1 in the supplemental material), and insects were in the orders Coleoptera, Dermaptera, Hemiptera, Homoptera, Hymenoptera, Odonata, and Orthoptera (see Table S2). Within each region, papaya samples were pooled by each of the three symptom types, weeds were pooled, and insects were pooled. Plant samples were excised with a knife treated with quaternary ammonium salts before each collection. All individual sampling bags were transported on ice. Samples were rinsed with nuclease-free water and stored at −80°C until processing.
Purification of virus-like particles.
To obtain enriched viral nucleic acids, VLPs and double-stranded RNA from plants and insects were extracted. To obtain the VLPs, a mixture of 100 g of frozen plant tissue from approximately 14.5 g of each of the seven leaf pieces collected per sample were pulverized and then homogenized with 40 ml of phosphate-buffered saline (PBS), to which 60 μl of 0.25 mM iodoacetamide and 125 μl of Triton X-100 (33%) were added. The homogenate was stirred for 10 min and centrifuged at 13,000 × g for 30 min. The supernatant was filtered through a 0.22-μm-pore-size sterile filter (Millipore, Billerica, MA) to eliminate particles of higher density and mass, including bacteria, eukaryotic cells, or their fragments. Afterwards, VLPs were precipitated with 10% (wt/vol) polyethylene glycol 8000 (PEG), incubated overnight at 4°C, and further centrifuged at 13,000 × g for 1 h. The pellet was resuspended in PBS and washed with an equal volume of chloroform 2 or 3 times; each time, the mixture was incubated for 15 min at room temperature and centrifuged at 13,000 × g for 10 min at 4°C, and the supernatant was recovered. Insect VLPs were partially purified only with SM buffer (50 mM Tris-HCl, 10 mM MgSO4, 0.1 M NaCl, pH 7.5) (69). Samples of the VLPs were deposited on Formvar-coated 200-mesh copper grids and negatively stained in 1% phosphotungstic acid for 10 min. Finally, they were examined by transmission electron microscopy (TEM). Pleomorphic, filamentous, and icosahedral particles were visualized (Fig. S1). Once the VLPs were confirmed, we proceeded with the nucleic acid extraction.
Nucleic acid extraction.
Nucleic acids were isolated from the VLP pellets using a procedure described for the extraction of PMeV-RNA in latex (70). VLPs were incubated for 2 h in the DNase and RNase cocktails (Invitrogen, Carlsbad, CA) with the addition of 14 μl of 20 mg/ml proteinase K (Thermo Scientific, Waltham, MA), and the mixtures were incubated at 37°C for 30 min. Nucleic acid suspensions were extracted with a volume of Tris-HCl (pH 7.5)-saturated phenol. After centrifugation at 8,000 × g for 4 min at 4°C, the aqueous phases were transferred to clean tubes, and a second step of extraction with chloroform/isoamyl alcohol (24:1) was repeated. One volume of 3 M sodium acetate (pH 5.2) and 2.5 volumes of ethanol were added to the samples. After centrifugation at 12,000 × g for 20 min at 4°C, the pellet was resuspended in 35 μl of RNase-free water.
Enrichment of dsRNA.
The dsRNA enrichment procedure is a microscale adaptation of a published method (71). Five grams of plant tissue per sample mix was flash frozen in liquid nitrogen, pulverized with a mortar and pestle, and deposited in a 1.5-ml tube for the immediate addition of 4% (vol/wt) extraction buffer STE (0.1 M NaCl, 50 mM Tris [pH 8], 1 mM EDTA, 1% SDS). Then, 0.1% 2-mercaptoethanol, 1% bentonite, and 2 volumes of Tris-EDTA (TE)-saturated phenol/chloroform (1:1) were added, and the mixture was shaken vigorously for 10 min. The resulting slurry was centrifuged at 8,000 × g for 15 min at 4°C, and the aqueous phase was recovered and deposited into a new 1.5-ml tube containing 0.02 g of CF-11 cellulose (Cole-Parmer Scientific, Vernon Hills, IL). Ethanol was added to a final concentration of 16%, and the mixture was shaken and then centrifuged at 8,000 × g for 5 min at 4°C. The cellulose was resuspended thoroughly, and the centrifugation process was repeated several times until there were no traces of color. Thereafter, 200 μl of STE was added, and the mixtures were centrifuged at 8,000 × g for 5 min at 4°C. This step was repeated three times, followed by 95% ethanol precipitation of dsRNA at −20°C overnight. After a centrifugation of 10,000 × g at 4°C for 30 min, the dsRNA pellet was dissolved in a volume of 30 μl of RNase-free water and stored at −20°C for further downstream applications.
First- and second-strand synthesis from RNA or dsRNA.
For the RNA viruses, the first DNA strand was synthesized with murine reverse transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer’s recommendations for cDNA synthesis, with 2 μl of 10 μM dT18 primer or 20 μM of each of three random primers based on the reported 5′-CCTTCGGATCCTCCN6-12-3′ (63): 5′-CCTTCGGATCCTCCGTACTA-3′, 5′-CCTTCGGATCCTCCGTCTCCATGTAC-3′, and 5′-CCTTCGGATCCTCCTCTAGT-3′. For the dsRNA viruses, 1 μl of dsRNA and 4 μl of viral nucleic acids were combined with 2 μl of those random primers and 5 μl of deoxynucleoside triphosphates (dNTPs; 10 mM each) in a microtube, denatured at 65°C for 2 min, and subsequently quenched on ice. Then, 4 μl of 5× first-strand buffer (250 mM Tris-HCl [pH 8.3], 75 mM KCl, 15 mM MgCl2), 2 μl dithiothreitol, and 1 μl of Superscript II (Invitrogen, Carlsbad, CA) were added to each microtube, and the mixture was then incubated first at 42°C for 60 min and then at 70°C for 15 min. This was followed by alcoholic precipitation using 3 M ammonium acetate and absolute ethanol. The second-strand cDNA was synthesized according to the manufacturer’s recommendations; 20 μl of purified single-stranded cDNA was mixed with 20 μl NEB 10× buffer, 6 μl dNTPs (10 mM), 2 μl RNase H (2 U/μl), 3 μl DNA polymerase I (50,000 U/ml; NEB), and 80 μl deionized H2O. The sample was incubated for 2.5 h at 16°C and purified with phenol/chloroform/isoamyl alcohol (24:25:1).
Library preparation, sequencing, and sequence analysis.
There were ten samples in total, with five sample types from each region (Pacific Coastal Plain and Central Depression). Three types of papaya symptom types were sampled (visually asymptomatic papaya, papaya with PRSV symptoms, and papaya with non-PRSV symptoms) along with bulked samples of weeds and insects. The duplicated cDNA libraries for each of the ten host-location combinations were obtained using the Nextera XT library preparation kit and sequenced by Illumina HiSeq 2500 fast mode with paired-end reads (2 × 100) (Cinvestav Sequencing Facility, Irapuato, Mexico). The total number of raw reads we obtained was 66,406,113, and an average of 2 million reads per library were obtained after removing low-quality reads. The libraries from papaya were filtered using the draft genome sequence of the C. papaya reference genome (accession number ABIM01) with BOWTIE2 (72, 73). All libraries were de novo assembled using Spades v.3.7 (74) enabling the metagenomics option, yielding a total of 220,792 contigs, where all sequences smaller than 500 nucleotides (nt) were discarded. The total number of contigs was analyzed with a first iteration of BLASTx (75) using a local database of viruses, VirDB (76), where sequences with >95% identity and sequences with homology to plant viruses and E values of >e−5 were retained. The recovered contigs were searched with a second iteration of BLASTn against the nonredundant database from NCBI with E values of >e−5. Sequences belonging to taxa other than plant viruses were discarded. The contigs were linked to scaffolds with Geneious v.R11. Contigs and scaffolds recovered were used for realigning the paired-end reads using a custom pipeline implementing trinity to estimate the abundance of reads and calculating the stats with bbmap (https://sourceforge.net/projects/bbmap/), using BOWTIE2, and SAMtools (77). All sequences generated for downstream analysis were nearly full length (with variations of ±0.2 kb), considering complete open reading frames (ORFs) for RNA viruses and, for DNA viruses, two ORFs for geminiviruses and one ORF for the alphasatellite. The only partial sequences included in the analysis from plants were for rhabdoviruses in papaya; it was visualized by TEM, and validated by RT-PCR.
Classification analysis.
The results of the search for homology by BLAST to known viruses against databases of nucleotide or amino acid sequences allowed the identification of sequences to distantly related viruses with low similarity to higher taxonomic levels (e.g., family or genus) or for sequences with high similarity to known virus species. The sequences assembled were aligned among them using Needleman and Wunsch in Geneious (78). Sequences grouped by similarity were classified taxonomically according to their species demarcation criteria according to ICTV rules (https://talk.ictvonline.org/), either by identities using BLAST or by pairwise similarities. Sequences were submitted to NCBI (see Table S3).
Papaya orchard virome accession numbers and classification. Download Table S3, XLSX file, 0.01 MB (15.2KB, xlsx) .
Copyright © 2020 Alcalá-Briseño et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Bipartite network analysis.
We constructed a bipartite incidence matrix, where one group comprised viral taxa and the other group comprised the combinations of host type and region. The entries in the incidence matrix indicated the presence or absence of each viral taxon in each host-location combination. We also considered weighted matrices with weights representing the read coverage across the contig length as an indicator of relative sequence abundance. Network metrics such as node degree, nestedness, and others were calculated using the bipartite package in R (79). Hypothesis tests for modularity and nestedness of networks were evaluated using a standardized effect size (SES). A Z-score test was calculated for these two metrics as follows: Z = (observed metric − mean under null)/standard deviation under null. Calculations used a two-tailed normal distribution and comparison to three null models. Null model 1 maintained sample richness, null model 2 maintained species frequency (37, 80, 81), and null model 3 maintained neither species frequency nor sample richness (41). Ten thousand matrices were generated with 1,000 iterations each using the picante package in R (82). Bipartite network figures were generated using the igraph package in R using the Davidson-Harel (DH) algorithm layout (83, 84). Pie plots were generated with sunburstR, and other figures were generated with ggplot; these analyses were conducted in the R programming environment version 3.4.2 (40).
Data availability.
The read sequence accessions of the papaya orchard virome from the Central Depression and the Pacific Coastal Plain were deposited in the NCBI database as BioProject PRJNA592837. Ten BioSamples were submitted corresponding to visually asymptomatic papaya (SAMN13440963 and SAMN13440956), papaya with non-PRSV symptoms (SAMN13440964 and SAMN13440957), papaya with PRSV symptoms (SAMN13440965 and SAMN13440960), weeds (SAMN13440966 and SAMN13440961) and insects (SAMN13440967 and SAMN13440962). Virus accession numbers (MN203139 to MN204623) are also listed in Table S3.
ACKNOWLEDGMENTS
This work was carried out with support from Agromod through a sponsorship of the PEI-PROINNOVA program of CONACYT, Mexico. R. I. Alcalá-Briseño’s Ph.D. scholarship (number 410151) was sponsored by CONACYT. Publication of this article was funded in part by the University of Florida Open Access Publishing Fund.
We also appreciate support from the University of Florida. We thank Javier Luévano-Borroel from Cinvestav for the identification of the insect families. We also thank José C. Huguet-Tapía for helpful discussions about the bioinformatic analysis and Masatoshi Katabuchi for helpful discussions about the ecological models.
REFERENCES
- 1.Anderson PK, Cunningham AA, Patel NG, Morales FJ, Epstein PR, Daszak P. 2004. Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnology drivers. Trends Ecol Evol 19:535–544. doi: 10.1016/j.tree.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 2.Elena SF, Bedhomme S, Carrasco P, Cuevas JM, de la Iglesia F, Lafforgue G, Lalić J, Pròsper A, Tromas N, Zwart MP. 2011. The evolutionary genetics of emerging plant RNA viruses. Mol Plant Microbe Interact 24:287–293. doi: 10.1094/MPMI-09-10-0214. [DOI] [PubMed] [Google Scholar]
- 3.Garrett KA, Thomas-Sharma S, Forbes GA, Hernandez Nopsa J, Ziska LH, Dukes JS. 2014. Climate change and plant pathogen invasions, p 22–44. In Ziska LH, Dukes JS (eds), Invasive species and global climate change. CABI Publishing, Oxfordshire, United Kingdom. [Google Scholar]
- 4.Adams IP, Harju VA, Hodges T, Hany U, Skelton A, Rai S, Deka MK, Smith J, Fox A, Uzayisenga B, Ngaboyisonga C, Uwumukiza B, Rutikanga A, Rutherford M, Ricthis B, Phiri N, Boonham N. 2014. First report of maize lethal necrosis disease in Rwanda. New Dis Rep 29:22–22. doi: 10.5197/j.2044-0588.2014.029.022. [DOI] [Google Scholar]
- 5.Mahuku G, Wangai A, Sadessa K, Teklewold A, Wegary D, Ayalneh D, Adams I, Smith J, Bottomley E, Bryce S, Braidwood L, Feyissa B, Regassa B, Wanjala B, Kimunye JN, Mugambi C, Monjero K, Prasanna BM, Others. 2015. First report of maize chlorotic mottle virus and maize lethal necrosis on maize in Ethiopia. Plant Dis 99:1870–1870. doi: 10.1094/PDIS-04-15-0373-PDN. [DOI] [Google Scholar]
- 6.Legg JP, Jeremiah SC, Obiero HM, Maruthi MN, Ndyetabula I, Okao-Okuja G, Bouwmeester H, Bigirimana S, Tata-Hangy W, Gashaka G, Mkamilo G, Alicai T, Lava Kumar P. 2011. Comparing the regional epidemiology of the cassava mosaic and cassava brown streak virus pandemics in Africa. Virus Res 159:161–170. doi: 10.1016/j.virusres.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Legg JP, Lava Kumar P, Makeshkumar T, Tripathi L, Ferguson M, Kanju E, Ntawuruhunga P, Cuellar W. 2015. Cassava virus diseases: biology, epidemiology, and management. Adv Virus Res 91:85–142. doi: 10.1016/bs.aivir.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Alabi OJ, Al Rwahnih M, Jifon JL, Sétamou M, Brown JK, Gregg L, Park J-W. 2017. A mixed infection of lettuce chlorosis virus, papaya ringspot virus, and tomato yellow leaf curl virus-IL detected in a Texas papaya orchard affected by a virus-like disease outbreak. Plant Dis 101:1094–1102. doi: 10.1094/PDIS-01-17-0118-RE. [DOI] [PubMed] [Google Scholar]
- 9.Adams MJ, Antoniw JF. 2006. DPVweb: a comprehensive database of plant and fungal virus genes and genomes. Nucleic Acids Res 34:D382–D385. doi: 10.1093/nar/gkj023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noa Carrazana JC, Silva-Rosales L. 2001. First report of a Mexican isolate of papaya mosaic virus in papaya (Carica papaya) and pumpkin (Cucurbita pepo). Plant Dis 85:558. doi: 10.1094/PDIS.2001.85.5.558A. [DOI] [PubMed] [Google Scholar]
- 11.Noa Carrazana JC, González de León D, Ruiz Castro BS, Piñero D, Silva-Rosales L. 2006. Distribution of papaya ringspot virus and papaya mosaic virus in papaya plants (Carica papaya) in Mexico. Plant Dis 90:1004–1011. doi: 10.1094/PD-90-1004. [DOI] [PubMed] [Google Scholar]
- 12.Lastra R. 1981. Papaya apical necrosis, a new disease associated with a rhabdovirus. Plant Dis 65:439. doi: 10.1094/PD-65-439. [DOI] [Google Scholar]
- 13.Wan S-H. 1983. Incidence and distribution of papaya viruses in southern Florida. Plant Dis 67:353. doi: 10.1094/PD-67-353. [DOI] [Google Scholar]
- 14.Mejías Y, Rodríguez D, González G. 1987. Rhabdovirus asociado a síntomas de necrosis apical en fruta bomba Carica papaya en Cuba. Cie Tec Agric Prot Plant 3:57–62. [Google Scholar]
- 15.Becerra EN, Cárdenas E, Lozoya H, Mosqueda R. 2006. Rhabdovirus en papayo (Carica papaya L.) en el sureste de México. Agron Mesoam 10:85–90. doi: 10.15517/am.v10i2.17947. [DOI] [Google Scholar]
- 16.Rodrigues CH, Ventura JA, Maffia LA. 1989. Distribuição e transmissão da meleira em pomares de mamão no Espírito Santo. Fitopatol Bras 14:118. [Google Scholar]
- 17.Pérez-Brito D, Tapia-Tussell R, Cortés-Velázquez A, Quijano-Ramayo A, Nexticapan-Garcés A, Martín-Mex R. 2012. First report of papaya meleira virus (PMeV) in Mexico. Afr J Biotechnol 11:13564–13570. [Google Scholar]
- 18.García-Cámara I, Pérez-Brito D, Moreno-Valenzuela O, Magaña-Álvarez A, Fernandes PMB, Tapia-Tussell R. 2018. Molecular and experimental evidence of watermelon (Citrullus lanatus) as host of the Mexican variant of papaya meleira virus. Eur J Plant Pathol 151:117–123. [Google Scholar]
- 19.Zamudio-Moreno E, Ramirez-Prado JH, Moreno-Valenzuela OA, Lopez-Ochoa LA. 2015. Early diagnosis of a Mexican variant of papaya meleira virus (PMeV-Mx) by RT-PCR. Genet Mol Res 14:1145–1154. doi: 10.4238/2015.February.6.18. [DOI] [PubMed] [Google Scholar]
- 20.Chávez-Calvillo G, Contreras-Paredes CA, Mora-Macias J, Noa-Carrazana JC, Serrano-Rubio AA, Dinkova TD, Carrillo-Tripp M, Silva-Rosales L. 2016. Antagonism or synergism between papaya ringspot virus and papaya mosaic virus in Carica papaya is determined by their order of infection. Virology 489:179–191. doi: 10.1016/j.virol.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Roossinck MJ. 2011. The good viruses: viral mutualistic symbioses. Nat Rev Microbiol 9:99–108. doi: 10.1038/nrmicro2491. [DOI] [PubMed] [Google Scholar]
- 22.Hadidi A, Flores R, Candresse T, Barba M. 2016. Next-generation sequencing and genome editing in plant virology. Front Microbiol 7:1325. doi: 10.3389/fmicb.2016.01325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stobbe AH, Roossinck MJ. 2014. Plant virus metagenomics: what we know and why we need to know more. Front Plant Sci 5:150. doi: 10.3389/fpls.2014.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.François S, Filloux D, Fernandez E, Ogliastro M, Roumagnac P. 2018. Viral metagenomics approaches for high-resolution screening of multiplexed arthropod and plant viral communities. Methods Mol Biol 1746:77–95. doi: 10.1007/978-1-4939-7683-6_7. [DOI] [PubMed] [Google Scholar]
- 25.Roossinck MJ. 2012. Plant virus metagenomics: biodiversity and ecology. Annu Rev Genet 46:359–369. doi: 10.1146/annurev-genet-110711-155600. [DOI] [PubMed] [Google Scholar]
- 26.Poudel R, Jumpponen A, Schlatter DC, Paulitz TC, Gardener BBM, Kinkel LL, Garrett KA. 2016. Microbiome networks: a systems framework for identifying candidate microbial assemblages for disease management. Phytopathology 106:1083–1096. doi: 10.1094/PHYTO-02-16-0058-FI. [DOI] [PubMed] [Google Scholar]
- 27.Garrett KA, Alcalá-Briseño RI, Andersen KF, Buddenhagen CE, Choudhury RA, Fulton JC, Hernandez Nopsa JF, Poudel R, Xing Y. 2018. Network analysis: a systems framework to address grand challenges in plant pathology. Annu Rev Phytopathol 56:559–580. doi: 10.1146/annurev-phyto-080516-035326. [DOI] [PubMed] [Google Scholar]
- 28.Anthony SJ, Islam A, Johnson C, Navarrete-Macias I, Liang E, Jain K, Hitchens PL, Che X, Soloyvov A, Hicks AL, Ojeda-Flores R, Zambrana-Torrelio C, Ulrich W, Rostal MK, Petrosov A, Garcia J, Haider N, Wolfe N, Goldstein T, Morse SS, Rahman M, Epstein JH, Mazet JK, Daszak P, Lipkin WI. 2015. Non-random patterns in viral diversity. Nat Commun 6:8147. doi: 10.1038/ncomms9147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dormann CF, Fründ J, Blüthgen N. 2009. Indices, graphs and null models: analyzing bipartite ecological networks. Open Ecol J 2:7–24. doi: 10.2174/1874213000902010007. [DOI] [Google Scholar]
- 30.Martín González AM, Dalsgaard B, Olesen JM. 2010. Centrality measures and the importance of generalist species in pollination networks. Ecol Complex 7:36–43. doi: 10.1016/j.ecocom.2009.03.008. [DOI] [Google Scholar]
- 31.Fortuna MA, Stouffer DB, Olesen JM, Jordano P, Mouillot D, Krasnov BR, Poulin R, Bascompte J. 2010. Nestedness versus modularity in ecological networks: two sides of the same coin? J Anim Ecol 79:811–817. [DOI] [PubMed] [Google Scholar]
- 32.Gotelli NJ. 2008. A primer of ecology, 4th ed Sinauer Associates, Sunderland, MA. [Google Scholar]
- 33.Cervantes-Zamora Y, Cornejo-Olgín SL, Lucero-Márquez R, Espinoza-Rodríguez JM, Miranda-Viquez E, Pineda-Velázquez A. 1990. Clasificación de regiones naturales de México II IV.10.2. Atlas Nacional de México, vol. II. Instituto de Geografía, Universidad Nacional Autónoma de México, Mexico City, Mexico. [Google Scholar]
- 34.Sosa V, De-Nova JA, Vásquez-Cruz M. 2018. Evolutionary history of the flora of Mexico: dry forests cradles and museums of endemism. J Syst Evol 56:523–536. doi: 10.1111/jse.12416. [DOI] [Google Scholar]
- 35.Holland JN, Okuyama T, DeAngelis DL. 2006. Comment on “Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science 313:1887. doi: 10.1126/science.1129547. [DOI] [PubMed] [Google Scholar]
- 36.Bascompte J, Jordano P, Olesen JM. 2006. Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science 312:431–433. doi: 10.1126/science.1123412. [DOI] [PubMed] [Google Scholar]
- 37.Beckett SJ. 2016. Improved community detection in weighted bipartite networks. R Soc Open Sci 3:140536. doi: 10.1098/rsos.140536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galeano J, Pastor JM, Iriondo JM. 2009. Weighted-Interaction Nestedness Estimator (WINE): a new estimator to calculate over frequency matrices. Environ Model Softw 24:1342–1346. doi: 10.1016/j.envsoft.2009.05.014. [DOI] [Google Scholar]
- 39.Rosario K, Marr C, Varsani A, Kraberger S, Stainton D, Moriones E, Polston JE, Breitbart M. 2016. Begomovirus-associated satellite DNA diversity captured through vector-enabled metagenomic (VEM) surveys using whiteflies (Aleyrodidae). Viruses 8:36. doi: 10.3390/v8020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.R Core Team. 2019. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 41.Gotelli NJ. 2000. Null model analysis of species co-occurrence patterns. Ecology 81:2606–2621. doi: 10.1890/0012-9658(2000)081[2606:NMAOSC]2.0.CO;2. [DOI] [Google Scholar]
- 42.Newman MEJ, Girvan M. 2004. Finding and evaluating community structure in networks. Phys Rev E Stat Nonlin Soft Matter Phys 69:026113. doi: 10.1103/PhysRevE.69.026113. [DOI] [PubMed] [Google Scholar]
- 43.Muthukumar V, Melcher U, Pierce M, Wiley GB, Roe B. a, Palmer MW, Thapa V, Ali A, Ding T. 2009. Non-cultivated plants of the tallgrass prairie preserve of northeastern Oklahoma frequently contain virus-like sequences in particulate fractions. Virus Res 141:169–173. doi: 10.1016/j.virusres.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 44.Roux S, Enault F, Robin A, Ravet V, Personnic S, Theil S, Colombet J, Sime-Ngando T, Debroas D. 2012. Assessing the diversity and specificity of two freshwater viral communities through metagenomics. PLoS One 7:e33641. doi: 10.1371/journal.pone.0033641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aguirre de Cárcer D, López-Bueno A, Alonso-Lobo JM, Quesada A, Alcamí A. 2016. Metagenomic analysis of lacustrine viral diversity along a latitudinal transect of the Antarctic Peninsula. FEMS Microbiol Ecol 92:fiw074. doi: 10.1093/femsec/fiw074. [DOI] [PubMed] [Google Scholar]
- 46.Fraile A, McLeish MJ, Pagán I, González-Jara P, Piñero D, García-Arenal F. 2017. Environmental heterogeneity and the evolution of plant-virus interactions: viruses in wild pepper populations. Virus Res 241:68–76. doi: 10.1016/j.virusres.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 47.Jo Y, Choi H, Kim S-M, Kim S-L, Lee BC, Cho WK. 2017. The pepper virome: natural co-infection of diverse viruses and their quasispecies. BMC Genomics 18:453. doi: 10.1186/s12864-017-3838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boukari W, Alcalá-Briseño RI, Kraberger S, Fernandez E, Filloux D, Daugrois J-H, Comstock JC, Lett J-M, Martin DP, Varsani A, Roumagnac P, Polston JE, Rott PC. 2017. Occurrence of a novel mastrevirus in sugarcane germplasm collections in Florida, Guadeloupe and Réunion. Virol J 14:146. doi: 10.1186/s12985-017-0810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corinaldesi C, Tangherlini M, Dell'Anno A. 2017. From virus isolation to metagenome generation for investigating viral diversity in deep-sea sediments. Sci Rep 7:8355. doi: 10.1038/s41598-017-08783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moury B, Fabre F, Hébrard E, Froissart R. 2017. Determinants of host species range in plant viruses. J Gen Virol 98:862–873. doi: 10.1099/jgv.0.000742. [DOI] [PubMed] [Google Scholar]
- 51.McLeish M, Sacristán S, Fraile A, Garcia-Arenal F. 2018. Co-infection organises epidemiological networks of viruses and hosts and reveals hubs of transmission. Phytopathology 109:1003–1010. doi: 10.1094/PHYTO-08-18-0293-R. [DOI] [PubMed] [Google Scholar]
- 52.Chávez-Pesqueira M, Núñez-Farfán J. 2017. Domestication and genetics of papaya: a review. Front Ecol Evol 5:1095. [Google Scholar]
- 53.Mishra R, Gaur RK, Patil BL. 2016. Current knowledge of viruses infecting papaya and their transgenic management, p 189–203. In Gaur RK, Petrov NM, Patil BL, Stoyanova MI (ed), Plant viruses: evolution and management. Springer, Singapore. [Google Scholar]
- 54.Cornejo-Franco JF, Alvarez-Quinto RA, Quito-Avila DF. 2018. Transmission of the umbra-like papaya virus Q in Ecuador and its association with meleira-related viruses from Brazil. Crop Prot 110:99–102. doi: 10.1016/j.cropro.2018.04.006. [DOI] [Google Scholar]
- 55.Wan SH, Conover RA. 1981. A rhabdovirus associated with a new disease of Florida papayas. Proc Fla State Hortic Soc 94:318–321. [Google Scholar]
- 56.Roossinck MJ. 2005. Symbiosis versus competition in plant virus evolution. Nat Rev Microbiol 3:917–924. doi: 10.1038/nrmicro1285. [DOI] [PubMed] [Google Scholar]
- 57.Syller J. 2012. Facilitative and antagonistic interactions between plant viruses in. Mol Plant Pathol 13:204–216. doi: 10.1111/j.1364-3703.2011.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roossinck MJ. 2013. Plant virus ecology. PLoS Pathog 9:e1003304. doi: 10.1371/journal.ppat.1003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bell TH, Hockett KL, Alcalá-Briseño RI, Barbercheck M, Beattie GA, Bruns MA, Carlson JE, Chung T, Collins A, Emmett B, Esker P, Garrett KA, Glenna L, Gugino BK, Jiménez-Gasco M del M, Kinkel L, Kovac J, Kowalski KP, Kuldau G, Leveau JHJ, Michalska-Smith MJ, Myrick J, Peter K, Salazar MFV, Shade A, Stopnisek N, Tan X, Welty AT, Wickings K, Yergeau E. 2019. Manipulating wild and tamed phytobiomes: challenges and opportunities. Phytobiomes 3:3–21. doi: 10.1094/PBIOMES-01-19-0006-W. [DOI] [Google Scholar]
- 60.Saavedra S, Stouffer DB, Uzzi B, Bascompte J. 2011. Strong contributors to network persistence are the most vulnerable to extinction. Nature 478:233–235. doi: 10.1038/nature10433. [DOI] [PubMed] [Google Scholar]
- 61.Vázquez DP, Poulin R, Krasnov BR, Shenbrot GI. 2005. Species abundance and the distribution of specialization in host–parasite interaction networks. J Anim Ecol 74:946–955. doi: 10.1111/j.1365-2656.2005.00992.x. [DOI] [Google Scholar]
- 62.Gurney J, Aldakak L, Betts A, Gougat-Barbera C, Poisot T, Kaltz O, Hochberg ME. 2017. Network structure and local adaptation in co-evolving bacteria-phage interactions. Mol Ecol 26:1764–1777. doi: 10.1111/mec.14008. [DOI] [PubMed] [Google Scholar]
- 63.Roossinck MJ, Saha P, Wiley GB, Quan J, White JD, Lai H, Chavarría F, Shen G, Roe BA. 2010. Ecogenomics: using massively parallel pyrosequencing to understand virus ecology. Mol Ecol 19 Suppl 1:81–88. doi: 10.1111/j.1365-294X.2009.04470.x. [DOI] [PubMed] [Google Scholar]
- 64.Dormann CF. 2011. How to be a specialist? Quantifying specialisation in pollination networks. Netw Biol 1:1–20. [Google Scholar]
- 65.Young CA, Kinkel LL. 2018. A year of phytobiomes. Phytobiomes 2:53–54. doi: 10.1094/PBIOMES-05-18-0023-E. [DOI] [Google Scholar]
- 66.Bascompte J, Jordano P, Melián CJ, Olesen JM. 2003. The nested assembly of plant-animal mutualistic networks. Proc Natl Acad Sci U S A 100:9383–9387. doi: 10.1073/pnas.1633576100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rocha-Loredo AG, Ramírez-Marcial N, González-Espinosa M. 2010. Riqueza y diversidad de árboles del bosque estacional caducifolio en la Depresión Central de Chiapas. Bot Sci 87:89. doi: 10.17129/botsci.313. [DOI] [Google Scholar]
- 68.González-Espinosa M, Ramírez-Marcial N, Ruiz-Montoya L. 2005. Diversidad biológica en Chiapas. Plaza y Valdés, San Rafael, Mexico. [Google Scholar]
- 69.Rosario K, Capobianco H, Ng TFF, Breitbart M, Polston JE. 2014. RNA viral metagenome of whiteflies leads to the discovery and characterization of a whitefly-transmitted carlavirus in North America. PLoS One 9:e86748. doi: 10.1371/journal.pone.0086748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tavares ET, Tatagiba JS, Ventura JA, Souza MT Jr. 2004. Dois novos sistemas de diagnose precoce da meleira do mamoeiro. Fitopatol Bras 29:563–566. doi: 10.1590/S0100-41582004000500018. [DOI] [Google Scholar]
- 71.Morris TJ, Dodds JA, Hillman B, Jordan RL, Lommel SA, Tamaki SJ. 1983. Viral specific dsRNA: diagnostic value for plant virus disease identification. Plant Mol Biol Rep 1:27–30. doi: 10.1007/BF02680259. [DOI] [Google Scholar]
- 72.Ming R, Hou S, Feng Y, Yu Q, Dionne-Laporte A, Saw JH, Senin P, Wang W, Ly BV, Lewis KLT, Salzberg SL, Feng L, Jones MR, Skelton RL, Murray JE, Chen C, Qian W, Shen J, Du P, Eustice M, Tong E, Tang H, Lyons E, Paull RE, Michael TP, Wall K, Rice DW, Albert H, Wang M-L, Zhu YJ, Schatz M, Nagarajan N, Acob RA, Guan P, Blas A, Wai CM, Ackerman CM, Ren Y, Liu C, Wang J, Wang J, Na J-K, Shakirov EV, Haas B, Thimmapuram J, Nelson D, Wang X, Bowers JE, Gschwend AR, Delcher AL, et al. 2008. The draft genome of the transgenic tropical fruit tree papaya (Carica papaya Linnaeus). Nature 452:991–996. doi: 10.1038/nature06856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 76.Goodacre N, Aljanahi A, Nandakumar S, Mikailov M, Khan AS. 2018. A reference viral database (RVDB) to enhance bioinformatics analysis of high-throughput sequencing for novel virus detection. mSphere 3:e00069-18. doi: 10.1128/mSphereDirect.00069-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup. 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Needleman SB, Wunsch CD. 1970. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol 48:443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- 79.Dormann CF, Strauss R. 2014. A method for detecting modules in quantitative bipartite networks. Methods Ecol Evol 5:90–98. doi: 10.1111/2041-210X.12139. [DOI] [Google Scholar]
- 80.Borgatti SP, Everett MG. 1997. Network analysis of 2-mode data. Soc Netw 19:243–269. doi: 10.1016/S0378-8733(96)00301-2. [DOI] [Google Scholar]
- 81.Staniczenko PPA, Kopp JC, Allesina S. 2013. The ghost of nestedness in ecological networks. Nat Commun 4:1391. doi: 10.1038/ncomms2422. [DOI] [PubMed] [Google Scholar]
- 82.Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO. 2010. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26:1463–1464. doi: 10.1093/bioinformatics/btq166. [DOI] [PubMed] [Google Scholar]
- 83.Csardi G, Nepusz T. 2006. The igraph software package for complex network research. InterJ Complex Syst 1695:1–9. [Google Scholar]
- 84.Davidson R, Harel D. 1996. Drawing graphs nicely using simulated annealing. ACM Trans Graph 15:301–331. doi: 10.1145/234535.234538. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electron microscopy of virus-like particles (VLPs). Negative staining transmission electron microscopy (TEM) micrographs of VLPs obtained from the papaya orchard samples of the Central Depression (A) and the Pacific Coastal Plain (B). The numbers on images refer to VLPs extracted from papaya plants (1 to 6), weeds (7 and 8), and insects (9 and 10). Papaya with PRSV symptoms (PS) contained icosahedral, filamentous, and pleiomorphic particles (A1, A2, B1, and B2). Papaya with non-PRSV symptoms (OS) showed rigid helical rods, flexuous filamentous, and pleomorphic particles (A3, A4, B3, and B4). Papayas that were visually asymptomatic (VA) contained icosahedral, filamentous, and pleomorphic particles (A5, A6, B5, and B6). Weeds (W) contained icosahedral and filamentous particles (A7, A8, B7, and B8). Insects (I) contained icosahedral and filamentous particles (A9, A10, B9, and B10). Download FIG S1, PDF file, 1.4 MB (1.4MB, pdf) .
Copyright © 2020 Alcalá-Briseño et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Weed accessions. Download Table S1, XLSX file, 0.01 MB (9.8KB, xlsx) .
Copyright © 2020 Alcalá-Briseño et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Insect accessions. Download Table S2, XLSX file, 0.01 MB (9.4KB, xlsx) .
Copyright © 2020 Alcalá-Briseño et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Papaya orchard virome accession numbers and classification. Download Table S3, XLSX file, 0.01 MB (15.2KB, xlsx) .
Copyright © 2020 Alcalá-Briseño et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
The read sequence accessions of the papaya orchard virome from the Central Depression and the Pacific Coastal Plain were deposited in the NCBI database as BioProject PRJNA592837. Ten BioSamples were submitted corresponding to visually asymptomatic papaya (SAMN13440963 and SAMN13440956), papaya with non-PRSV symptoms (SAMN13440964 and SAMN13440957), papaya with PRSV symptoms (SAMN13440965 and SAMN13440960), weeds (SAMN13440966 and SAMN13440961) and insects (SAMN13440967 and SAMN13440962). Virus accession numbers (MN203139 to MN204623) are also listed in Table S3.