ABSTRACT
Rapid and significant range expansion of both Zika virus (ZIKV) and its Aedes vector species has resulted in ZIKV being declared a global health threat. Mean temperatures are projected to increase globally, likely resulting in alterations of the transmission potential of mosquito-borne pathogens. To understand the effect of diurnal temperature range on the vectorial capacity of Ae. aegypti and Ae. albopictus for ZIKV, longevity, blood-feeding and vector competence were assessed at two temperature regimes following feeding on infectious blood meals. Higher temperatures resulted in decreased longevity of Ae. aegypti [Log-rank test, χ2, df 35.66, 5, P < 0.001] and a decrease in blood-feeding rates of Ae. albopictus [Fisher's exact test, P < 0.001]. Temperature had a population and species-specific impact on ZIKV infection rates. Overall, Ae. albopictus reared at the lowest temperature regime demonstrated the highest vectorial capacity (0.53) and the highest transmission efficiency (57%). Increased temperature decreased vectorial capacity across groups yet more significant effects were measured with Ae. aegypti relative to Ae. albopictus. The results of this study suggest that future increases in temperature in the Americas could significantly impact vector competence, blood-feeding and longevity, and potentially decrease the overall vectorial capacity of Aedes mosquitoes in the Americas.
KEYWORDS: Climate change, Aedes mosquitoes, Zika virus, vectorial capacity, transmission potential
Background
Zika virus (ZIKV; Flavivirus, Flaviviridae), which prior to 2007 was geographically limited to Africa and Asia [1–3], has undergone an unprecedented range expansion in recent years and evolved into a global health threat. ZIKV was first identified in Brazil in May 2015 and subsequently spread throughout the Americas [3–7]. The explosive spread of ZIKV from Asia to the Americas and the association with disease outcomes such as microcephaly among infants and Guillain-Barre syndrome among adults necessitates the need to better understand the transmission dynamics of the virus and the potential impact of temperature increases on ZIKV transmission.
Increases in average and maximum temperatures have been recorded for over a century in the contiguous United States, with an annual temperature increase of 1.7°C [8]. These increases are expected to continue to accelerate, with an additional rise of 1.6–6.6°C in the late twenty-first century [9].
It is expected that the global increase in temperature [10,11] will lead to a geographical expansion of tropical disease, particularly vector-borne pathogens, throughout temperate regions [12]. Higher temperatures are known to accelerate biochemical reactions, yet heightened metabolism can come at a cost. While temperature increases might increase viral replication, decrease the length of extrinsic incubation and accelerate development rates, there could also be a negative impact on vector survival and alterations in host-seeking behaviour that could decrease their capacity to transmit pathogens [13–16]. As an ectothermic organism, the physiology [17,18], life history [19,20] and vectorial capacity [21–23] of mosquitoes will depend significantly on changes in temperature. Successful pathogen transmission depends on mosquito susceptibility, survivability during the extrinsic incubation period (EIP), and subsequent host feeding and transmission. Hence, the impact of varying temperature on survival, feeding behaviour, vector competence and EIP differentially affects the transmission of pathogens by mosquitoes [24]. In addition, the infection rates of mosquitoes with pathogens may be dependent on the interaction between temperature and mosquito genotype [25].
Multiple studies have also demonstrated that the geographic origin of both the mosquito and the viral strain affect the vector competence of Aedes mosquitoes for ZIKV [1,7,26,27].
A recent study by [2] showed that a mutation in the non-structural protein 1 (NS1) may have enhanced the infectivity of ZIKV and facilitated transmission by Aedes aegypti in the Americas.
Comparing mosquito strains, a study by [28] reared populations of Ae. aegypti and Aedes albopictus derived from different ecological and climatic conditions under a single temperature and demonstrated high infection rates but low transmission of ZIKV for all populations. While a study by [29] reared Ae. aegypti populations at a different temperature from the temperature they were held during the extrinsic incubation period. Their results indicated inconsistent differences across treatments for both infection and dissemination rates of ZIKV, yet in nature, it is unlikely that there would be a sudden abrupt change in temperature from immature development to adult blood feeding.
Despite progress made to understand the biology of ZIKV infection and its interactions with its vectors, questions remain regarding the intrinsic and extrinsic factors that govern the vectorial capacity for ZIKV.
Our study aimed to define differences in vectorial capacity of unique populations of Ae. aegypti and Ae. albopictus for ZIKV held under diurnal, fluctuating temperature regimes mimicking field conditions during peak transmission in regions from which the populations were derived. Given that the Intergovernmental Panel on Climate Change (IPCC) has predicted a 2–4°C mean global temperature rise over the next century due to global warming [30], we then modelled a 2°C increase over these baseline temperatures and assessed population and species-specific differences in the effect of rising temperatures on vectorial capacity. Insights into the impact of future increase in temperature in the Americas on ZIKV transmission dynamics of Ae. aegypti and Ae. albopictus will improve our understanding of potential influence of climate change on the epidemiology of this disease in the region.
Methods
Mosquitoes
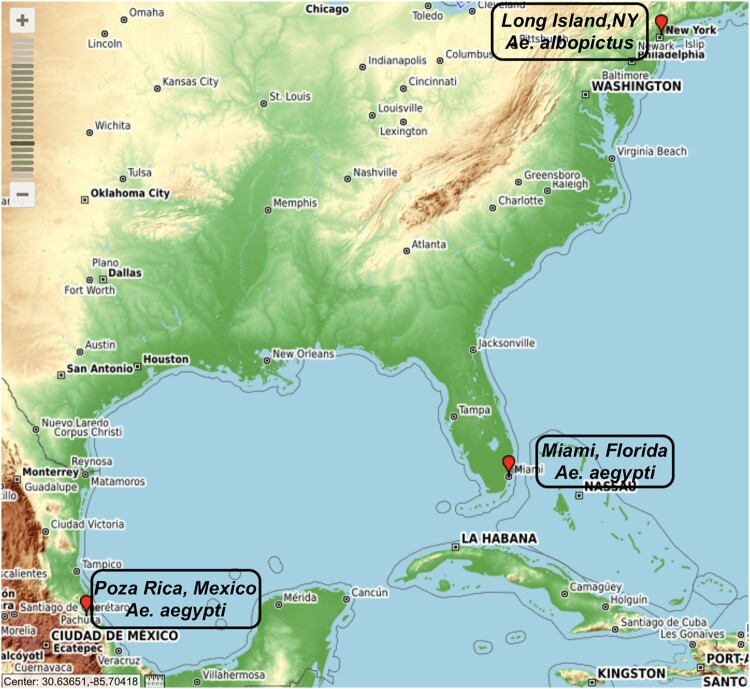
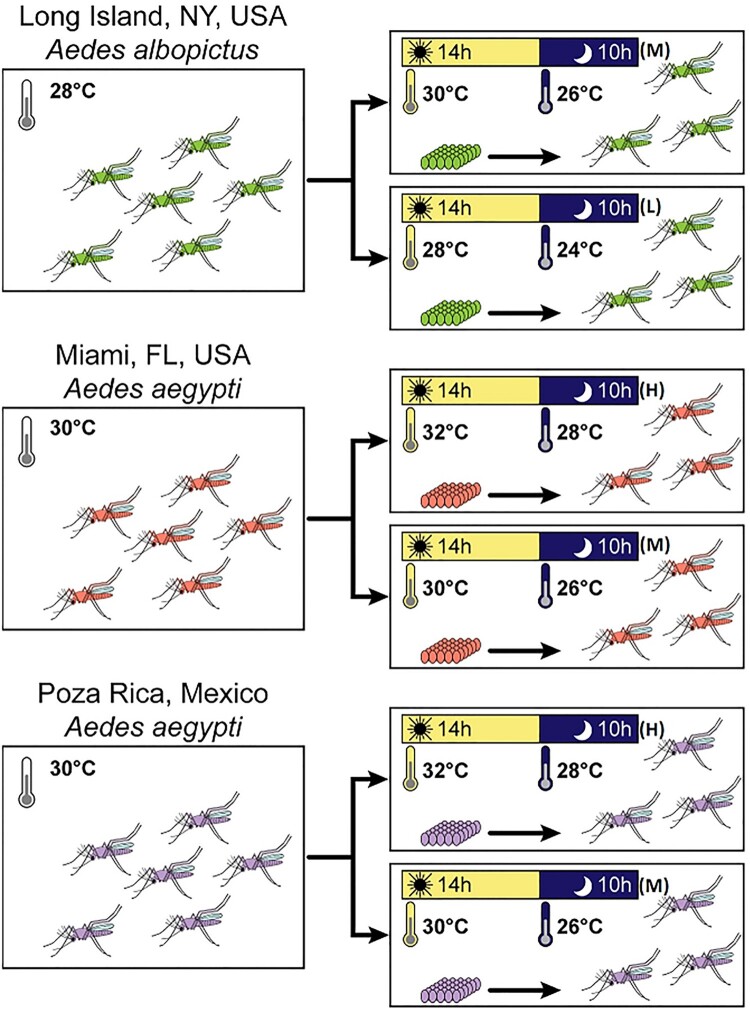
Three populations and two species of Aedes mosquitoes originally collected from three distinct geographical and environmental regions (Figure 1) were utilized in this study. Ae. albopictus (ALB LI; kindly provided by Illia Rochlin, Suffolk County Health Department) were originally collected in Suffolk County, NY in 2014 and subsequently colonized in the insectary of the New York State Department of Health (NYSDOH) Arbovirus Laboratory to F15. Mexican Ae. aegypti (AEG MX; kindly provided by GD Ebel, Colorado State University) were originally collected in Poza Rica, Mexico, in 2016 and maintained to F23. Ae. aegypti (Miami, AEG MI) were originally collected in Miami-Dade County, Florida in October 2017 (kindly provided by M DeGennaro, Florida International University) and reared to F3.
Figure 1.
Collection sites of the Aedes mosquitoes. Source: https://www.gpsvisualizer.com/mapsite.
Note: The AEG MI mosquitoes were collected from Miami, Florida, AEG MX population from Poza Rica, Mexico while the ALB LI from Long Island New York. The different sites of collection have different climatic conditions that include hot and humid summers, short warm winters in Miami, tropical wet and dry in Mexico and cold and temperate in Long Island, New York. The temperature used to determine the temperature regimes utilized in this study are the estimated temperatures during the peak transmission season. These include; day 30°C, night 26°C in Miami and Mexico and day 28°C, night 24°C for Long Island.
Colonies were maintained at 27°C under standard rearing conditions [14] before the eggs were hatched. F23 AEG MX, F3 AEG MI and F15 ALB LI eggs were hatched under vacuum pressure in 1 l dechlorinated water initially incubated for 3 h at the three distinct diurnal temperature regimes (Figure 2). The Ae. aegypti were reared and maintained at high (H) (day 32°C/ night 28°C [D32N28]) and moderate (M) (day 30°C/ night 26°C [D30N26]) temperatures; Ae. albopictus at M and low (L) (day 28°C/ night 24°C [D28N24]) temperature regimes. One population of Ae. albopictus (Ae. albopictus Long Island, ALB LI) and two populations of Ae. aegypti (Ae. aegypti Mexico, AEG MX and Ae. aegypti Miami, AEG MI) were used in this study. Baseline temperature regimes (L for Ae. albopictus and M for Ae. aegypti) were estimates of mean day and nighttime temperatures during peak transmission in regions from which populations were derived (https://www.noaa.gov). A subgroup of each population was reared and held at day and night temperatures 2°C higher than baseline (M for Ae. albopictus and H for Ae. aegypti; Figure 2).
Figure 2.
Schematic illustrating the experimental workflow.
Note: The temperature regime selected for each region was based on a 2°C increase in the day and the night temperature at peak transmission season of each geographical sample origin [D30N26 (M); D32N28 (H) and D28N24 (L)]. The eggs were vacuum hatched after warming the water to the day time temperature and the immature and adult stages were reared at the stated temperature regime.
Collected larvae were maintained in plastic rectangular flat containers [35.6 cm length × 27.9 cm width × 8.3 cm height (Sterilite, catalogue no. 1963)] at a density of 1 larva per 5 ml of dechlorinated water and reared at 40-60% relative humidity and a light–dark (LD) photoperiod 14:10 h. The larvae were fed 1.25 mg/larvae of Tetra pond Koi growth feed for first and second instar larvae and 2.5 mg/larvae for third and fourth instar larvae [27]. The larvae were counted, and all the food was pre-weighed before feeding the mosquitoes.
Both male and female adults were transferred to 3.8 l cardboard cartons upon emergence and housed together for 9 days under the different temperature regimes (Figure 2) while being provided with sugar and water ad libitum. To stimulate blood feeding, the 9-day old females were starved of water and sugar 18 h before an infectious blood meal. We based our duration of starvation of the mosquitoes on a standard operating procedure (SOP) for rearing mosquitoes in our insectary.
Zika virus vector competence
To test for the effect of temperature, population and mosquito species on vector competence for ZIKV, female Aedes mosquitoes were orally exposed to bloodmeal containing 8.3 log10 PFU/ml ZIKV HND (2016-19563, GenBank accession no. KX906952) [7].
The virus was diluted 1:1 with defibrinated sheep blood plus 2.5% sucrose; sodium bicarbonate was included to adjust pH to 8.0. The female mosquitoes were offered the infectious blood meal through a 37°C pre-heated Hemotek membrane feeding system (Discovery Workshops, Acrington, UK) with a porcine sausage casing membrane. After an hour, the mosquitoes were anaesthetized with CO2 and immobilized on a pre-chilled tray connected to 100% CO2. Engorged females were separated and placed in three separate 0.6 l cardboard cartons (30 individuals per carton). In addition, 1 ml of each blood meal was transferred to a 1.5 ml Safe Seal microtube (Eppendorf, Hamburg Germany) and stored at −80°C to allow for the determination of ZIKV titres. The engorged females were maintained on 10% sucrose solution provided ad libitum. The 0.6 l cardboard cartons were kept at the respective temperature regimes (Figure 2).
Infection and transmission analysis
On days 4, 7 and 14 post-infectious blood meal, mosquitoes were immobilized by exposure to triethylamine (Sigma Aldrich, St. Louis, MO, USA), the legs were removed from 30 mosquitoes of each population and species, and placed in 500 µl mosquito diluent (MD; 20% heat-inactivated foetal bovine serum in Dulbecco phosphate-buffered saline plus 50 µg/ml penicillin/streptomycin, 50 µg/ml gentamicin and 2 µg/ml Fungizone [Sigma Aldrich, St. Louis, MO, USA]) containing a 4 mm bead (Daisy Rogers, Arkansas). Saliva was collected by inserting the proboscis of the female mosquitoes into a capillary tube containing ∼20 µl foetal bovine serum plus 50% sucrose 1:1 for 30 min and subsequently ejecting the mixture into 125 µl MD. Mosquito bodies were then placed in individual tubes containing 500 µl MD and a bead. All samples were held at −80°C until assayed.
Infection, dissemination and transmission results were obtained by screening the whole bodies, legs and saliva, respectively, collected at different time points, as described by [7]. To obtain viral titre, ZIKV-specific quantitative PCR assay that targets the NS1 region was utilized [31].
Statistical analysis
Statistical analysis was performed with GraphPad Prism version 5.0. Infection (body); dissemination (legs) and transmission (saliva) rates were compared using Fischer’s exact test. Viral loads were compared using an ANOVA test.
Vectorial capacity
Vectorial capacity (VC), which is a calculation of the probability that an individual mosquito in a given population will transmit a pathogen, incorporates blood-feeding behaviour and vector longevity along with vector competence. In this study, VC was calculated using the following formula [32–34]:
| (1) |
where h = host feeding rate (proportions of mosquitoes acquiring at least two artificial blood meals in their lifetime) and p = the probability of daily survival. Death of each individual was plotted in days and a linear regression analysis with a runs test was used to determine if the relationship between time and mortality is linear. The slope of the best fit line is used to calculate p as follows, p = (100 − [ − slope]). N = the mean extrinsic incubation period (EIP), calculated here as the mean days to the transmission and b = vector competence (mean proportion of exposed mosquitoes transmitting days 7–14). The host feeding rate and the longevity were evaluated in a separate study using the same populations and temperature regimes. Briefly, six populations of Aedes mosquitoes were reared at similar temperature regimes and conditions as described above. Eggs were vacuum hatched as described before [1360 eggs AEG MI (hatched and reared at the H temperature regime); 1360 eggs AEG MI (hatched and reared at the M temperature regime); 1047 eggs AEG MX (hatched and reared at the H temperature regime); 952 eggs AEG MX (hatched and reared at the M temperature regime); 750 eggs ALB LI (hatched and reared at the M temperature regime) and 721 eggs ALB LI (hatched and reared at the L temperature regime)], and the larvae reared as described above.
The emerged female mosquitoes were allowed to mate and after three days, then placed in separate 3.8 l cartons and offered a non-infected blood meal. Mortality was monitored and recorded daily. Blood meals were subsequently offered every three days four times, until the last female mosquito died. The linear regression analysis of these data was completed using GraphPad Prism 5.0.
Results
Vector competence
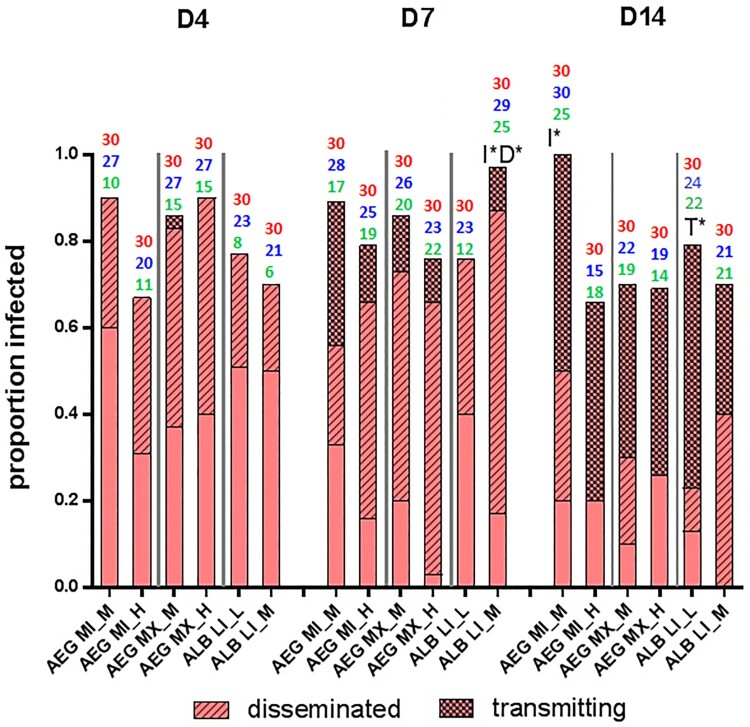
Ae. aegypti and Ae. albopictus were equally susceptible to ZIKV infection [Fisher’s exact test P = .44]. Overall, temperature influenced infection rates in a population and species-specific manner. Higher temperatures were associated with lower ZIKV infection rates of AEG MI population, i.e. the individuals reared and held at baseline temperature (M) had a significantly higher proportion of individuals infected (85/90) as compared to those reared at higher temperatures (H) (67/90) [Fisher’s exact test P < .001]. However, the temperature did not influence infection rates of the AEG MX population [H (72/90) M (76/90) Fisher’s exact test P = .56]. Although the temperature did not influence overall infection rates of the ALB LI population [M (71/90) L (73/90) Fisher’s exact test P = .85], we noted differences at 7 dpi. Specifically, an increase in temperature significantly increased infection of ALB LI individuals [M (29/30) L (23/30) Fisher’s exact test P < .05] (Figure 3).
Figure 3.
Vector competence of Aedes mosquitoes infected with Zika virus and reared at different temperature regimes.
Note: The height of the bar plot represents the proportion of blood-fed individuals successfully infected with Zika virus. Above each bar plot, the total number of females screened for infection is indicated in red, the total number screened for dissemination, blue and transmission, green. The proportion of these that disseminated and subsequently transmitted the virus are further indicated. The temperature affected infection and dissemination rates in a population and species-specific manner. Increased temperature reduced the infection rates of AEG MI at the last time point (14 dpi) [Fisher’s exact test P < .05]. The opposite effect was measured in ALB LI, with significantly higher infection [Fisher’s exact test P < .05] and dissemination rates [Fisher’s exact test P < .05] with an increase in temperature at 7 dpi. The ALB LI (L) population had the highest transmission efficiency [Fisher’s exact test P < .05]. I* represents significantly different infection rates P < .05; D* represents significantly different dissemination rates P < .05; T* represents significantly different transmission efficiency P < .05, when comparing among temperature regimes.
There were no significant differences in dissemination or transmission rates among temperature regimes, with the exception of the ALB LI population. At 7 dpi, increased temperature significantly increased ALB LI dissemination [M (25/29) L (12/23) Fisher’s exact test P < .05]. Conversely, at 14 dpi the ALB LI L population had a significantly higher transmission efficiency than the ALB LI M population [Fisher’s exact test P < .05] (Table 1; Figure 3).
Table 1. Vector competence of Aedes mosquitoes in this study following ZIKV exposure.
| Species | Geographical origin | Temp. regime | % Infection (whole bodies) | % Dissemination (infected bodies) | % Transmission (disseminated bodies) | % Transmission efficiency (no. of mosquitoes initially screened) |
|---|---|---|---|---|---|---|
| 4 days post-infection | ||||||
| Ae. aegypti | Miami | D32N28 (H) | 67 (30) | 55 (20) | 0 (11) | 0 (30) |
| Ae. aegypti | Miami | D30N26 (M) | 90 (30) | 37 (27) | 0 (10) | 0 (30) |
| Ae. aegypti | Mexico | D32N28 (H) | 90 (30) | 56 (27) | 0 (15) | 0 (30) |
| Ae. aegypti | Mexico | D30N26 (M) | 90 (30) | 56 (27) | 13 (15) | 7 (30) |
| Ae. albopictus | Long Island | D30N26 (M) | 70 (30) | 29 (21) | 0 (6) | 0 (30) |
| Ae. albopictus | Long Island | D28N24 (L) | 77 (30) | 35 (23) | 0 (8) | 0 (30) |
| 7 days post-infection | ||||||
| Ae. aegypti | Miami | D32N28 (H) | 83 (30) | 76 (25) | 26 (19) | 17 (30) |
| Ae. aegypti | Miami | D30N26 (M) | 93 (30) | 61 (28) | 6 (17) | 3 (30) |
| Ae. aegypti | Mexico | D32N28 (H) | 77 (30) | 96 (23) | 14 (22) | 10 (30) |
| Ae. aegypti | Mexico | D30N26 (M) | 87 (30) | 77 (26) | 21 (20) | 13 (30) |
| Ae. albopictus | Long Island | D30N26 (M) | 97 (30) | 86 (29) | 12 (25) | 10 (30) |
| Ae. albopictus | Long Island | D28N24 (L) | 77 (30) | 52 (23) | 0 (12) | 0 (30) |
| 14 days post-infection | ||||||
| Ae. aegypti | Miami | D32N28 (H) | 67 (30) | 80 (15) | 78 (18) | 47 (30) |
| Ae. aegypti | Miami | D30N26 (M) | 100 (30) | 83 (30) | 60 (25) | 50 (30) |
| Ae. aegypti | Mexico | D32N28 (H) | 70 (30) | 63 (19) | 93 (14) | 43 (30) |
| Ae. aegypti | Mexico | D30N26 (M) | 73 (30) | 86 (22) | 68 (19) | 43 (30) |
| Ae. albopictus | Long Island | D30N26 (M) | 70 (30) | 100 (21) | 43 (21) | 30 (30) |
| Ae. albopictus | Long Island | D28N24 (L) | 83 (30) | 91 (24) | 71 (22) | 57 (30) |
Significant differences in viral titre were measured in whole-body samples at 4 and 14 dpi (ANOVA, Tukey HSD post-hoc test, P < .001). At 14 dpi, a significantly higher viral load was measured in AEG populations relative to ALB LI populations (ANOVA, Tukey HSD post-hoc test, P < .001) (Figure 4). No significant effect of population or temperature on viral load was measured.
Figure 4.
Viral load of ZIKV in Aedes mosquitoes at 4 and 14 days post-infection (DPI).
Note: The plot represents the absolute viral load of ZIKV in individuals of different populations of Aedes mosquitoes utilized in this study. The distribution of the viral titre around the mean is represented by the error bar. The crossbars in the figure are indicative of a statistically significant heterogenous viral titres measured between populations. The viral load was determined by quantitative PCR for mosquitoes held at L (D28N24), M (D30N26) and H (D32N28) temperature regimes. Statistically significant differences were determined by 1-way ANOVA test indicated by *P < .05, **P < .01 and ***P < .001.
Vectorial capacity
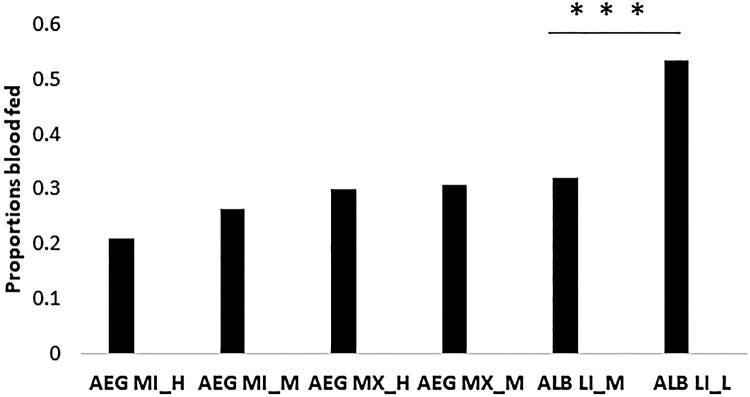
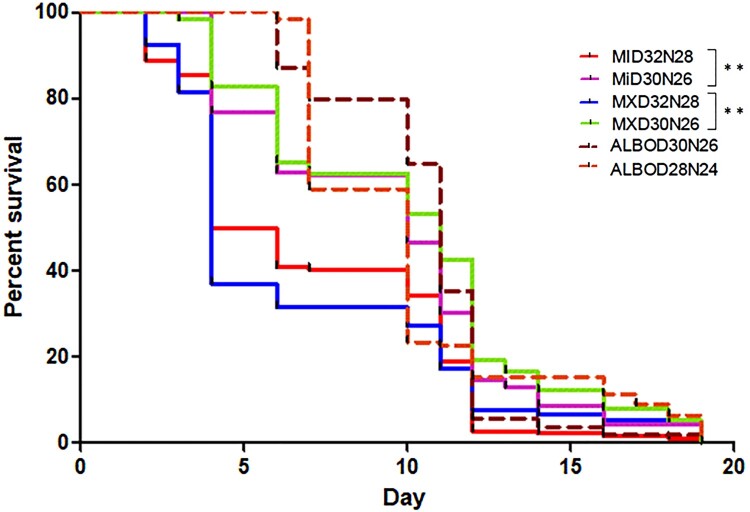
Increased temperature resulted in decreased blood feeding frequency by ALB LI population in [M (32%), L (53%) Fisher’s exact test P < .001] (Figure 5) while the AEG MI [M (36%), H (27%) Fisher’s exact test P = .09]and AEG MX [M (44%), H (43%) Fisher’s exact test P = .92] populations blood-feeding frequencies were not significantly affected. Ae. aegypti reared at the H temperature regimes had significantly reduced longevity relative to the M temperature regime population (Log-rank [Mantel–Cox] test, Chi-square, df 35.66, 5, P < .001; Figure 6). Ae. albopictus longevity was not affected by temperature (Log-rank [Mantel–Cox] test, P > .001).
Figure 5.
Blood feeding frequency of Aedes mosquitoes at different temperatures.
Note: The bar graphs represent the frequency of blood meal acquisition for individuals offered non-infectious blood meals twice a week post-mating. Mosquitoes were reared at similar temperature regimes as described for vector competence experiments: including L (D28N24), M (D30N26) and H (D32N28) regimes. Significantly higher feeding rates were measured for the ALB LI population at higher temperatures ***Fisher’s exact test (P < .001).
Figure 6.
The longevity of Aedes mosquitoes at varying temperature regimes.
Note: Mosquitoes were reared at similar temperature regimes as described for vector competence experiments: including L (D28N24), M (D30N26) and H (D32N28) regimes. Mortality was monitored and recorded daily. Graphs are Kaplan–Meier survival curves depicting proportions surviving at various days post-emergence. Significant differences were measured between populations and temperature regimes **Log-rank test (P < .01).
Overall, ALB LI (L) had the highest vectorial capacity (0.53), while AEG MI (H) had the lowest (0.09). Across all populations, increasing temperatures decreased vectorial capacity (Table 2). When comparing both the Ae. aegypti and Ae. albopictus populations at the same temperature regime (M), vectorial capacity was modestly higher for Ae. aegypti (ALB LI (M) = 0.17, AEG MI (M) = 0.26, AEG MX (M) = 0.33; Table 2). The decreased transmissibility at a higher temperature in Ae. albopictus can be wholly attributed to decreased feeding frequency while decreases in Ae. aegypti are influenced primarily by decreased longevity, but additionally by decreased feeding, decreased competence and increased EIPs (Table 2).
Table 2. Vectorial capacity of the Aedes mosquito populations for ZIKV.
| Source | Temp. regime | h | P | N | b | VC = h2PNb/−ln(P) | |
|---|---|---|---|---|---|---|---|
| AEG MI (H) | Miami | D32N28 | 0.21 | .92 | 11.12 | 0.30 | 0.09 |
| AEG MI (M) | Miami | D30N26 | 0.30 | .94 | 8.17 | 0.42 | 0.26 |
| AEG MX (H) | Mexico | D32N28 | 0.26 | .92 | 11.38 | 0.27 | 0.08 |
| AEG MX (M) | Mexico | D30N26 | 0.31 | .95 | 8.06 | 0.27 | 0.33 |
| ALB LI (M) | Long Island | D30N26 | 0.32 | .94 | 10.50 | 0.20 | 0.17 |
| ALB LI (L) | Long Island | D28N24 | 0.53 | .94 | 14.00 | 0.28 | 0.53 |
Discussion
This study demonstrates that increasing temperatures decreased the vectorial capacity of Aedes mosquitoes for ZIKV. Measured decreases in vectorial capacity resulted from alterations to vector competence, blood-feeding frequency and /or longevity. While the magnitude of these effects was dependent on species, population and temperature regime, an overall decrease in ZIKV transmissibility was measured with all three populations evaluated following a 2°C rise in diurnal temperature cycle. A larger decrease in ZIKV transmission was measured for Ae. aegypti mosquitoes when modelling warmer current and predicted future temperature regimes of the southern US and Mexico. These results support the idea that future temperature increase could result in a northern shift in the suitability of Aedes populations for transmission of ZIKV and other invasive arboviruses.
Higher temperatures decreased the blood-feeding rates of the Aedes mosquitoes in this study, although this difference was only significant for Ae. albopictus. Ae. aegypti has been shown to take several blood meals in a single gonotrophic cycle [35] and the frequency of their blood feeding has been noted to be positively correlated with the temperature of the environment [36]. However, this correlation has been attributed to indirect effects of environmental temperature on the mosquito development, energy storage and rate of blood meal digestion [36,37]. A caveat in our study is that Ae. aegypti populations were reared at a higher temperature of 32°C but the high temperature for Ae. albopictus was 30°C. While this was done to model current (and future) temperatures in locations where these populations were derived, it is not clear how further increases in temperature above 30°C would affect this population of Ae. albopictus. Previous studies demonstrate that population differences have a highly significant effect on the influence of temperature on feeding behaviour [27]. In spite of this, our results suggest future climate change resulting in increased temperature may lead to a reduction in vectorial capacity of Aedes mosquitoes.
Overall, increased temperature of 2°C negatively impacted the longevity of the Ae. aegypti populations while Ae. albopictus were not affected. The higher survival capacity of Ae. albopictus both in the field and laboratory settings could contribute to these differences. Since [38] showed a wider temperature tolerance of Ae. aegypti up to 40°C, the contribution of senescence may also play a role in the difference between our findings and [38].
At all rearing temperatures, we noted that there was a higher proportion of males compared to females on day one and two of emergence, as is generally seen. Three days after emergence, we blood fed the females and immediately separated the engorged females from the males. Studies have demonstrated that when female Aedes mosquitoes are associated with the males, they lived longer [39,40]. The male reproductive gland substances deposited by the male during mating increase female fitness [41]. The reason for a shorter survival time (19 days) may be due to the fact that a higher percentage of the females may not have mated before their first blood meal hence reducing their longevity.
In our study, the increased temperature did not consistently increase competence or accelerate EIP. In fact, for Ae. aegypti, overall competence was either higher or statistically similar at the lowest temperature regime and EIP was shorter. Our findings were contrary to the findings of [24] who demonstrated a unimodal effect of temperature on the EIP of ZIKV on Ae. aegypti across eight constant temperatures (16–38°C), with the shortest EIP at 36.4°C and the peak infectivity at 30.6°C. In addition to the use of unique populations and strains, a critical difference between our study and studies that have found a direct correlation between temperature and EIP [42–44] is that our mosquitoes were reared under fluctuating diurnal temperatures. Unlike adult holding temperature, some previous studies have found an indirect correlation between larval rearing temperatures and vector competence [45–47]. This effect could be related to altered RNA interference functionality at different temperatures [18]. In addition, fluctuation of temperatures has been shown to result in reduced longevity, lower midgut infection rates and increased EIP for virus compared to a constant temperature [48–51].
Viral loads in Ae. albopictus reared at the M temperature regime were significantly lower than Ae. aegypti reared at the M temperature regime across time points. Our results corroborate findings of [7] with experiments conducted at 28°C. Previous studies have demonstrated Ae. albopictus is a competent vector for ZIKV in the laboratory [1,7]. However, the vector competence has been shown to be influenced by the ZIKV strain and the geographic origin of the mosquito population [1]. The explosive spread of ZIKV in the Americas raised concerns that another vector in addition to Ae. aegypti might be involved in ZIKV transmission [52]. Our study confirms the potential for Ae. albopictus to play a role in ZIKV epidemics in the Americas, although they have not been demonstrated to be significantly involved to date.
However, since variation in vector competence among Ae. aegypti populations and virus strains has been reported previously [7,53–55], and our results further support this, a comprehensive assessment of the potential effect of increase in temperature would require additional studies with multiple populations and ZIKV strains.
There are two important caveats to this study. First, it is unclear how mosquitoes will adapt or evolve in response to incremental changes in temperature and this could significantly alter the vectorial capacity of future populations. Second, alterations to life-history traits including blood-feeding behaviour and longevity can result from arbovirus infection [56].
We measured lower infection rates at later timepoints, particularly at high temperatures. Since arboviral infections in mosquitoes are persistent, these data suggest that ZIKV infected individuals were less likely to survive the experimental period and that this increased mortality was facilitated by increased temperatures. Further studies monitoring life-history traits following infection at altered temperatures will help clarify this relationship.
Taken together, these data demonstrate that temperature is a significant component of ZIKV transmission; and defining optimum conditions for individual populations and species is important for an accurate prediction of how future increases in temperature will affect geographic distribution and intensity of transmission in the Americas.
Conclusion
The results of our study suggest that future global climate change resulting in increased global temperature will have negative fitness cost on Aedes aegypti mosquitoes for ZIKV and could significantly alter their vector competence. Further, the impact of future climate change will be driven by unique intrinsic interactions between specific vectors and pathogens and therefore is unlikely to be uniform across different species and populations [24].
Acknowledgements
This publication was supported by the Cooperative Agreement Number U01CK000509 funded by the Centers for Disease Control and Prevention. Its content is solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services. MDG was supported by the CDC Southeastern Center of Excellence in Vector-borne Disease. We would like to acknowledge the Arbovirus laboratory insectary crew for mosquito maintenance and insightful discussions and comments by Dr Constentin Dieme. MGO: Involved in the study design, conducted the experiments, analysed the results and drafted the paper. BSM: Involved in the mosquito rearing, assisted in mosquito manipulation exercises. PAF: Assisted in mosquito manipulation exercises. MN: Involved in the mosquito rearing, assisted in mosquito manipulation exercises. KL: Generated and propagated the ZIKV infectious clone utilized in this study. AV: Designed the linear regression model. MDG: Provided field-collected mosquitoes. CAT: Involved in the study design, participated in the writing of the paper. KLD: Involved in the study design, participated in the writing of the paper.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Funding Statement
This work was supported by Centers for Disease Control and Prevention [Cooperative Agreement Number U01CK000509].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Azar SR, Roundy CM, Rossi SL, et al. . Differential vector competency of Aedes albopictus populations from the Americas for Zika virus. Am J Trop Med Hyg. 2017;97(2):330–339. doi: 10.4269/ajtmh.16-0969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y, Liu J, Du S, et al. . Evolutionary enhancement of Zika virus infectivity in Aedes aegypti mosquitoes. Nature [Internet]. 2017;545(May):482–486. Available from: 10.1038/nature22365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyer S, Calvez E, Chouin-Carneiro T, et al. . An overview of mosquito vectors of Zika virus. Microbes Infect. 2018;20:1–15. doi: 10.1016/j.micinf.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 4.Bogoch II, Brady OJ, Kraemer MUG, et al. . Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;387(10016):335–336. doi: 10.1016/S0140-6736(16)00080-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hennessey M, Ficher M, Staples JE.. Zika virus spreads to new areas—region of the Americas, May 2015–January 2016. Morb Mortal Wkly Rep. 2016;65(3):55–58. doi: 10.15585/mmwr.mm6503e1 [DOI] [PubMed] [Google Scholar]
- 6.Petersen E, Wilson ME, Touch S, et al. . Rapid spread of Zika virus in the Americas – implications for public health preparedness for mass gatherings at the 2016 Brazil Olympic Games. Int J Infect Dis. 2016;44(May):11–15. doi: 10.1016/j.ijid.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 7.Ciota A, Bialosuknia S, Zink S, et al. . Effects of Zika virus strain and Aedes mosquito species on vector competence. Emerg Infect Dis. 2017;23(7):1110–1117. doi: 10.3201/eid2307.161633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vose RS, Easterling DR, Kunkel KE, et al. . Temperature changes in the United States. In: Wuebbles D, Fahey D, Hibbard K, et al., editors. Climate science special report: fourth national climate assessment. Washington (DC: ): U.S. Global Change Research Program; 2017. p. 185–206. [Google Scholar]
- 9.Romero-Lankao P, Smith J, Davidson D, et al. . Climate change 2014: impacts, adaptation and vulnerability. In: Barros V, Field D, Dokken M, et al., editors. Regional aspects contribution of working group ii to the fifth assessment report of the Intergovernmental Panel on Climate Change. New York: Cambridge University Press; 2014. p. 1439–1498. [Google Scholar]
- 10.Pachauri R. Avoiding dangerous climate change. In: Schellnhuber H, Cramer W, Nakicenovic N, et al., editors. Avoiding dangerous climate change. 1st ed. New York: Cambridge University Press; 2006. p. 3–6. [Google Scholar]
- 11.IPCC, et al. . Summary for policymakers. In: Solomon S, Manning Q, Chen Z, et al., editors. Climate change 2007: the physical science basis contribution of working group i to the fourth assessment report of the Intergovernmental Panel on Climate Change. 1st ed. New York: Cambridge University Press; 2007. p. 1–18. [Google Scholar]
- 12.Epstein PR. Is global warming harmful to health? Sci Am. 2000;283:50–57. doi: 10.1038/scientificamerican0800-50 [DOI] [PubMed] [Google Scholar]
- 13.Lafferty KD. The ecology of climate change and infectious diseases. Ecology. 2009;90(4):888–900. doi: 10.1890/08-0079.1 [DOI] [PubMed] [Google Scholar]
- 14.Ciota A, Matacchiero A, Kilpatrick AM, et al. . The effect of temperature on life history traits of Culex mosquitoes. J Med Entomol. 2014;51(1):55–62. doi: 10.1603/ME13003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huey B. Integrating thermal physiology and ecology of ectotherms: a discussion of approaches. Am Zool. 1979;19:357–366. doi: 10.1093/icb/19.1.357 [DOI] [Google Scholar]
- 16.McKechnie A, Wolf B.. Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biol Lett. 2010;6(September):253–256. doi: 10.1098/rsbl.2009.0702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murdock C, Krijn P, Bell A, et al. . Complex effects of temperature on mosquito immune function. Proc R Soc B Biol Sci. 2012;279(May):3357–3366. doi: 10.1098/rspb.2012.0638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adelman ZN, Anderson MAE, Wiley MR, et al. . Cooler temperatures destabilize RNA interference and increase susceptibility of disease vector mosquitoes to viral infection. PLoS Negl Trop Dis. 2013;7(5):1–8. doi: 10.1371/journal.pntd.0002239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mordecai EA, Cohen JM, Evans MV, et al. . Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLoS Negl Trop Dis. 2017;11(4):e0005568. doi: 10.1371/journal.pntd.0005568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delatte H, Gimonneau G, Triboire A, et al. . Influence of temperature on immature development, survival, longevity, fecundity, and gonotrophic cycles of Aedes albopictus, vector of chikungunya and dengue in the Indian Ocean. J Med Entomol. 2009;46(1):33–41. doi: 10.1603/033.046.0105 [DOI] [PubMed] [Google Scholar]
- 21.Liu-Helmersson J, Stenlund H, Wilder-Smith A, et al. . Vectorial capacity of Aedes aegypti: effects of temperature and Implications for global dengue epidemic potential. PLoS One. 2014;9(3):e89783. doi: 10.1371/journal.pone.0089783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mordecai EA, Paaijmans KP, Johnson LR, et al. . Optimal temperature for malaria transmission is dramatically lower than previously predicted. Ecol Lett. 2013;16:22–30. doi: 10.1111/ele.12015 [DOI] [PubMed] [Google Scholar]
- 23.Kilpatrick AM, Meola MA, Moudy RM, et al. . Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 2008;4(6):e1000092. doi: 10.1371/journal.ppat.1000092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tesla B, Demakovsky LR, Mordecai EA, et al. . Temperature drives Zika virus transmission: evidence from empirical and mathematical models. Proc R Soc B Biol Sci. 2018;285(1884):1–9. doi: 10.1098/rspb.2018.0795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gloria-Soria A, Armstrong P, Powell J, et al. . Infection rate of Aedes aegypti mosquitoes with dengue virus depends on the interaction between temperature and mosquito genotype. Proc R Soc B. 2017;284:20171506. doi: 10.1098/rspb.2017.1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weger-Lucarelli J, Rückert C, Chotiwan N, et al. . Vector competence of American mosquitoes for three strains of Zika virus. PLoS Negl Trop Dis. 2016;10(10):e0005101. doi: 10.1371/journal.pntd.0005101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muttis E, Balsalobre A, Chuchuy A, et al. . Factors related to Aedes aegypti (Diptera: Culicidae) populations and temperature determine differences on life-history traits with regional implications in disease transmission. J Med Entomol. 2018;20(April):1–8. [DOI] [PubMed] [Google Scholar]
- 28.Chouin-carneiro T, Vega-rua A, Vazeille M, et al. . Differential susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika virus. PLoS Negl Trop Dis. 2016;10(3):e0004543. doi: 10.1371/journal.pntd.0004543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tramonte AR, Christofferson RC.. Investigating the probability of establishment of Zika virus and detection through mosquito surveillance under different temperature conditions. PLoS Negl Trop Dis. 2019;14(3):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.IPCC . Third assessment report. Cambridge, UK; 2007.
- 31.Lanciotti RS, Calisher CH, Gubler DJ, et al. . Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30(3):545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Black WI, Moore CG.. Population biology as a tool for studying vector-borne diseases. Beaty BJ, Marquards WC, editors. Niwot: University Press of Colorado; 1996. p. 393–416. [Google Scholar]
- 33.Macdonald G. The epidemiology and control of malaria. London: Oxford University Press; 1957. 77 p. [Google Scholar]
- 34.Ciota A, Ehrbar D, Matacchiero A, et al. . The evolution of virulence of West Nile virus in a mosquito vector: implications for arbovirus adaptation and evolution. BMC Evol Biol. 2013;13(71):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott T, Clark G, Lorenz L, et al. . Detection of multiple blood feeding in Aedes aegypti (Diptera: Culicidae) during a single gonotrophic cycle using a histologic technique. J Med Entomol. 1993;30(1):94–99. doi: 10.1093/jmedent/30.1.94 [DOI] [PubMed] [Google Scholar]
- 36.Scott TW, Amerasinghe PH, Morrison AC, et al. . Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: blood feeding frequency. J Med Entomol. 2000;37(1):89–101. doi: 10.1603/0022-2585-37.1.89 [DOI] [PubMed] [Google Scholar]
- 37.Yasuno M, Tonn RJ.. A study of biting habits of Aedes aegypti in Bangkok, Thailand. Bull World Heal Organ. 1970;43(2):319–325. [PMC free article] [PubMed] [Google Scholar]
- 38.Brady OJ, Johansson MA, Guerra CA, et al. . Modelling adult Aedes aegypti and Aedes albopictus survival at different temperatures in laboratory and field settings. Parasit Vectors. 2013;6(351):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liles J. Effects of mating or association of the sexes on longevity in Aedes aegypti. Mosq News. 1965;25(4):434–439. [Google Scholar]
- 40.Liles J, Delong D.. The longevity and productivity of adult male and female Aedes aegypti when reared separately and together on three different diets. Ann Entomol Soc Am. 1960;53(2):277–280. doi: 10.1093/aesa/53.2.277 [DOI] [Google Scholar]
- 41.Villarreal SM, Pitcher S, Helinski MEH, et al. . Male contributions during mating increase female survival in the disease vector mosquito Aedes aegypti. J Insect Physiol [Internet]. 2018;108(August):1–9. Available from: 10.1016/j.jinsphys.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao F, Zhang Y, Yan-Qin D, et al. . The effect of temperature on the extrinsic incubation period and infection rate of dengue virus serotype 2 infection in Aedes albopictus. Arch Virol. 2014;159:3053–3057. doi: 10.1007/s00705-014-2051-1 [DOI] [PubMed] [Google Scholar]
- 43.Christofferson RC, Chisenhall DM, Wearing HJ, et al. . Chikungunya viral fitness measures within the vector and subsequent transmission potential. PLoS One. 2014;9(10):e110538. doi: 10.1371/journal.pone.0110538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johansson MA, Arana-vizcarrondo N, Biggerstaff BJ, et al. . Incubation periods of yellow fever virus. Am J Trop Med Hyg. 2010;83(1):183–188. doi: 10.4269/ajtmh.2010.09-0782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turell MJ. Effect of environmental temperature on the vector competence of Aedes taeniorhynchus for rift valley fever and equine encephalitis viruses. Am J Trop Med Hyg. 1993;49(6):672–676. doi: 10.4269/ajtmh.1993.49.672 [DOI] [PubMed] [Google Scholar]
- 46.Kay BH, Jennings C.. Enhancement or modulation of the vector competence of Ochlerotatus vigilax (Diptera: Culicidae) for Ross River virus by temperature. J Med Entomol. 2002;39(1):99–105. doi: 10.1603/0022-2585-39.1.99 [DOI] [PubMed] [Google Scholar]
- 47.Westbrook CJ, Reiskind MH, Pesko KN, et al. . Larval environmental temperature and the susceptibility of Aedes albopictus Skuse (Diptera: Culicidae) to chikungunya virus. Vector Borne Zoonotic Dis. 2010;10(3):241–247. doi: 10.1089/vbz.2009.0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lambrechts L, Paaijmans KP, Fansiri T, et al. . Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc Natl Acad Sci U S A. 2011;108(18):7460–7465. doi: 10.1073/pnas.1101377108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carrington LB, Armijos MV, Lambrechts L, et al. . Fluctuations at a low mean temperature accelerate dengue virus transmission by Aedes aegypti. PLoS Negl Trop Dis. 2013;7(4):e2190. doi: 10.1371/journal.pntd.0002190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watts DM, Burke DS, Harrison BA, et al. . Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. Am J Trop Med Hyg. 1987;36(1):143–152. doi: 10.4269/ajtmh.1987.36.143 [DOI] [PubMed] [Google Scholar]
- 51.Rohani A, Wong YC, Zamre I, et al. . The effect of extrinsic incubation temperature on development of dengue serotype 2 and 4 viruses in Aedes aegypti (L.). Southeast Asian J Trop Med Public Health. 2009;40(5):942–950. [PubMed] [Google Scholar]
- 52.Gardner LM, Chen N, Sarkar S.. Global risk of Zika virus depends critically on vector status of Aedes albopictus. Lancet Infect Dis. 2016;16(5):522–523. doi: 10.1016/S1473-3099(16)00176-6 [DOI] [PubMed] [Google Scholar]
- 53.Ciota AT, Chin PA, Ehrbar DJ, et al. . Differential effects of temperature and mosquito genetics determine transmissibility of arboviruses by Aedes aegypti in Argentina. Am J Trop Med Hyg. 2018;99(2):417–424. doi: 10.4269/ajtmh.18-0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Failloux AB, Vazeille M, Rodhain F.. Geographic genetic variation in populations of the dengue virus vector Aedes aegypti. J Mol Evol. 2002;55(6):653–663. doi: 10.1007/s00239-002-2360-y [DOI] [PubMed] [Google Scholar]
- 55.Lambrechts L, Chevillon C, Albright RG, et al. . Genetic specificity and potential for local adaptation between dengue viruses and mosquito vectors. BMC Evol Biol. 2009;9(1):160. doi: 10.1186/1471-2148-9-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ciota AT, Styer LM, Meola MA, et al. . The costs of infection and resistance as determinants of West Nile virus susceptibility in Culex mosquitoes. BMC Ecol. 2011;11(23):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]