Abstract
Background
De novo Donor Specific Antibodies (DSA) are considered as a risk factor for the kidney allograft outcomes in recipients after simultaneous liver–kidney transplantation (SLKT). We hypothesized that length of hospital stay (LOS) might be associated with de novo DSA development of due to the increased likelihood of receiving blood transfusions with reduced immunosuppressive regimens.
Methods
This study is a single-center, retrospective cohort study consisting of 85 recipients who underwent SLKT from 2009 to 2018 in our hospital. We divided the patients into two groups according to LOS [long hospital stay (L) group (LOS >14 days) and short hospital stay (S) group (LOS ≤14 days)]. Propensity score (PS) has been created using logistic regression to predict LOS greater than median of 14 days. The association between the presence of de novo DSA and LOS was assessed by logistic regression models adjusted for PS.
Results
The mean age at transplantation of the entire cohort was 55.5 ± 10.1 years. Sixty percent of the recipients were male and Caucasian. Median LOS in (L) group was three-fold longer than (S) group [L: median 30 days (IQR: 21–52), S: median 8.5 days (IQR: 7–11)]. Eight patients developed de novo DSA after SLKT (9.4%), all of them were in (L) group. Longer LOS was significantly associated with higher risk of development of de novo DSA in unadjusted (OR+ each 5 days: 1.09, 95% CI:1.02–1.16) and PS adjusted (OR+ each 5 days: 1.11, 95% CI:1.02–1.21) analysis.
Conclusion
Longer hospitalization is significantly associated with the development of de novo DSA in SLKT.
Keywords: Donor specific antibody, DSA, de novo DSA, simultaneous liver–kidney transplantation, length of hospital stay, hospitalization
Introduction
Post-transplant donor-specific antibodies (DSA), either identified pre-transplant (persistent DSA) or newly developed (de novo DSA) beyond the absorptive capacity conferred by allograft liver [1–4], present a risk factor for patient- and allograft kidney outcome after simultaneous liver–kidney transplantation (SLKT) [5,6]. While the majority of pre-transplant DSA become undetectable after liver transplantation alone (LTA) [7] and after SLKT [8,9], about 10–20% of recipients develop de novo DSA after LTA and SLKT [5,6,10]. Currently, the risk factors associated with newly developed de novo DSA have not been well investigated in SLKT. The identification of potentially modifiable risk factors influencing de novo DSA development after SLKT might have positive effects on patient and graft survival.
Length of hospital stay (LOS) after surgery is one of the relevant clinical outcomes measured in many clinical settings [11–13]. Longer LOS has been shown to be associated with patient characteristics such as age, higher morbidity, worsened frailty, increased number and severity of comorbidities and unfavorable clinical outcomes and complications [11–16]. Previous studies also showed longer LOS was associated with more infectious complications; which could lead to decreased use of immunosuppressive medications or larger amount of blood product transfusions [14,15,17].
Infectious complications and blood transfusions have also been identified as risk factors for longer LOS in liver transplant recipients [18–20]. Infectious complications can cause cessation or reduction of immunosuppressive medications; while blood transfusions can cause allo-sensitization [21,22]. Furthermore, early allograft liver dysfunction (EAD) was also identified as a risk factor for longer LOS [23]. EAD grafts may lose the capacity to fully absorb existing pre-transplant DSA, which might lead to persistent DSA after SLKT. Longer hospital stay might serve as a surrogate marker for these sensitization events, in addition to demonstrating association with de novo DSA development after SLKT.
In this retrospective study, we hypothesized that LOS is associated with a higher probability of de novo DSA development after SLKT. We evaluated the association between LOS and de novo DSA development using a single-center cohort in the modern immunosuppressant era.
Materials and methods
Cohort definition and data source
This is a single-center, retrospective cohort study. We enrolled 85 consecutive recipients who underwent SLKT from 1 April 2009 to 28 February 2018 at Methodist University Hospital in Memphis, TN, USA. Exclusion criteria being those who were less than 18 years old or equal, but no patients were excluded from this study. Any information from recipients or deceased donors, as well as immunologic information were extracted from local electronic medical record (EMR), from the UNOS database, and from our HLA laboratory database until February 9th, 2019. We captured all data into a Research Electronic Data Capture (REDCap) system, which is an electronic data capturing tool hosted at the Center for Biomedical Informatics, the University of Tennessee Health Science Center [24]. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the ‘Declaration of Istanbul on Organ Trafficking and Transplant Tourism’. All deceased donated organs were procured based on SLKT allocation policy in Organ Procurement and Transplantation Network (OPTN)/United Network for Organ Sharing (UNOS) [25] and thus no organs were procured from prisoners [26].
This study was approved by the Institutional Review Committee of The University of Tennessee Health Science Center (18-06146-XP). This is retrospective observational data collection and waiver for the consent form from participated recipients was approved by our IRB. Furthermore, this study did not need the consent from deceased donor either since this was not an interventional study [27].
Immunosuppression protocol
The applied immunosuppression protocol was similar for all patients regardless of pre-transplant sensitization status [28]. As induction therapy, all patients received intravenous methylprednisolone (500 mg) on day 0, and rabbit anti-thymocyte globulin (1.5 mg/kg) on day 0 and again on post-operative day 2. Mycophenolate mofetil (MMF) or equivalent mycophenolic acid was started immediately post-operatively and continued until month three. Tacrolimus was started after improvement in kidney function, usually between post-operative day 3–7, and target trough range was 6–8 ng/mL until 3 months post-transplantation and 3–5 ng/mL thereafter. No patients received pre-transplant desensitization before SLKT. All patients were maintained on a steroid-free protocol.
Exposure
The LOS was defined as the exposure in this study. LOS was calculated as sum of the days from date of admission to date of discharge. LOS was divided into two groups according to the median of 14 days. Long hospital stay group was defined as LOS greater than 14 days [(L) group] and short hospital stay group was defined as LOS less than 14 days or equal [(S) group]. For the sensitivity analysis, the threshold of long LOS was replaced with 28 days, which was two-times longer compared to the main analysis and based on the previous literature [29,30].
Covariates
We extracted data about recipients’ baseline characteristics including age, gender, race, body mass index (BMI), marital status, insurance, cause of end-stage liver disease (ESLD), cause of chronic kidney disease (CKD)/end stage renal disease (ESRD), pre-SLKT dialysis information including length and type (maintenance dialysis was defined as dialysis for ≥3 months; acute dialysis initiation was defined as dialysis for <6 weeks; while sub-acute dialysis was defined as dialysis for ≥6 weeks-<3 months before transplantation), comorbid conditions (diabetes: DM and hypertension: HTN), the Model for End-stage Liver Disease (MELD) score at SLKT, the number of Human Leucocyte Antigen (HLA) mismatches, calculated panel reactive antibody (cPRA), and cold-ischemic time (CIT) of donated kidney from the above mentioned sources. As post-SLKT information, first discharge destination, delayed allograft kidney function (DGF), primary non-function (PNF) on allograft kidney, death following transplant hospitalization. DGF was defined as needs of dialysis within one-week post SLKT [31] and PNF was defined as the condition on dialysis dependence after SLKT. Deceased donors’ information included age, sex, race, cause of death, history of hypertension and diabetes, and expanded criteria donor (ECD) status were also collected. All donations occurred after brain death. There was only one recipient with missing value of CIT, thirteen recipients had missing value of cPRA, and one recipient had missing value of HLA mismatches. These missing values were not imputed.
Outcomes
Primary outcome was incidence of development of de novo DSA after SLKT. Furthermore, prevalence of post-transplant DSA (persistent or de novo DSA), was also assessed as secondary outcome. Persistent DSA was defined as DSA detected before and after SLKT, while de novo DSA was defined as newly developed DSA following SLKT. We assessed the incidence of persistent DSA or de novo DSA or in each patient after SLKT. HLA specificities were identified using a solid phase single antigen bead platform (SAB; One Lambda Inc, a division of Thermo-Fisher, Canoga Park, CA, USA) combined with Luminex xMAP technology (Luminex Corporation., Northbrook, IL, USA). Patients with any observed class DSA were categorized as de novo DSA (+), while those negative for identified de novo DSA were classified de novo DSA (−). We defined post-DSA that has a mean fluorescence intensity (MFI) value that is elevated compared to pre-transplant levels or has an MFI of greater than 1000. The median days of measurement of DSA from SLKT in both any post-transplant DSA and de novo DSA were also calculated as sum of days. The DSA measurements were performed as per clinical indication. The methodology of detecting DSA including the decision of measurements, technical issues, and thresholds of DSA was not changed during the study period.
Statistical analysis
Baseline characteristics were described for the entire cohort and for groups categorized based on the LOS and presented as mean ± standard deviation (SD) or median and interquartile range (IQR) for continuous variables and percent for categorical variables as appropriate. Differences between groups were assessed by student T-test or Mann-Whitney test for continuous variables and chi-square-test (or Fisher’s exact test) for categorical variables.
We calculated the propensity score (PS) for probability of long hospital stay in the main analysis (LOS >14 days versus ≤14 days) and in the sensitivity analysis (LOS >28 days versus ≤28 days) using logistic regression model (presented in Table S1, Supplementary material), including all available covariates without missing values for LOS, including age, gender, race, marital status, insurance, cause of ESLD, diabetes, hypertension, BMI, blood type, MELD score, first discharge destination, and DGF. The purpose of this step was to be able to adjust for co-variates which showed association with LOS. Because only 8 patients developed de novo DSA, we were able to adjust for only one variable in our adjusted logistic regression analysis used for assessing association between LOS (as exposure) and development of de novo DSA or any post-transplant DSA (as outcomes). We then assessed logistic regression analysis, unadjusted and PS score adjusted, to calculate the relative risk (odds ratio) for newly developing de novo DSA or any post-transplant DSA.
p values were two-sided and significance level was set at less than .05 for all analyses. All analyses were conducted using STATA Version 13 (STATA Corporation, College Station, TX).
Results
Baseline characteristics
Table 1 shows the baseline characteristics of the entire cohort for both (L) and (S) groups. The median age at SLKT was 55.5 ± 10.1 years old and approximately 60% of the patients were Caucasian males. The leading causes of ESLD were hepatitis C and alcoholic hepatitis. The patients in (L) group had significantly higher MELD scores, prevalence of acute dialysis initiation shorter length of dialysis before SLKT (maintenance and sub-acute dialysis) and higher prevalence of alcoholic hepatitis as a cause of ESLD compared with (S) group patients.
Table 1.
Baseline characteristics of the entire cohort and divided by long and short hospital stay groups.
| Baseline characteristics | Entire cohort, N = 85 | Long hospital stay group (L), N = 43 | Short hospital stay group (S), N = 42 | p Value* |
|---|---|---|---|---|
| Recipient information | ||||
| Age, years, mean ± SD | 55.5 ± 10.1 | 56.7 ± 9.4 | 54.4 ± 10.7 | .292 |
| Gender, male, n (%) | 53 (62.4) | 28 (65.1) | 25 (59.5) | .595 |
| BMI, kg/m2, mean ± SD | 27.0 ± 6.4 | 28.2 ± 7.2 | 28.6 ± 5.6 | .827 |
| Race, n (%) | .866 | |||
| African American | 22 (25.9) | 11 (25.6) | 11 (26.2) | |
| Caucasian | 51 (60.0) | 26 (60.5) | 25 (59.5) | |
| Other | 12 (14.1) | 6 (14.0) | 6 (14.3) | |
| Marital status, Married, n (%) | 52 (61.2) | 27 (62.8) | 25 (59.5) | .783 |
| Insurance, n (%) | .680 | |||
| Private | 31 (36.5) | 16 (37.2) | 15 (35.7) | |
| Medicaid | 6 (7.1) | 4 (9.3) | 2 (4.8) | |
| Medicare | 48 (56.5) | 23 (53.5) | 25 (59.5) | |
| Presence of preexisting CKD, n (%) | 74 (87.1) | 32 (74.4) | 42 (100) | <.001 |
| Cause of CKD, n (%) | .647 | |||
| Hypertension | 8/74 (10.8) | 4/32 (12.5) | 4/42 (9.5) | |
| Diabetes | 14/74 (18.9) | 6/32 (18.8) | 8/42 (19.0) | |
| Glomerulonephritis | 4/74 (5.4) | 2/32 (6.3) | 2/42 (4.8) | |
| Cystic disease | 3/74 (4.1) | 0 | 3/42 (7.1) | |
| Metabolic/inherited disease | 2/74 (2.7) | 0 | 2/42 (4.8) | |
| Other/unknown | 43/74 (58.1) | 20/32 (62.5) | 23/42 (54.8) | |
| Dialysis status before SLKT, n (%) | .003 | |||
| Maintenance dialysis, n (%) | 38 (44.7) | 19 (44.2) | 19 (45.2) | |
| Sub-acute dialysis, n (%) | 9 (10.6) | 6 (14.0) | 3 (7.1) | |
| Acute dialysis initiation before SLKT, n (%) | 16 (18.8) | 13 (30.2) | 3 (7.1) | |
| Length of dialysis before SLKT (maintenance and sub-acute dialysis group), months, median (IQR) | 8.9 (3.9, 36.8) | 7.1 (3.3, 20.4) | 13.0 (7.4, 44.6) | .077 |
| Length of acute dialysis before SLKT (acute dialysis group only), days, median (IQR) | 13.5 (6.5, 24.5) | 12.0 (8.0, 24.0) | 19.0 (5.0, 27.0) | .638 |
| Cause of ESKD (maintenance and sub-acute group), n (%) | .278 | |||
| Acute on CKD, | 12/47 (25.5) | 8/25 (32.0) | 4/22 (18.2) | |
| Same as CKD | 35/47 (74.5) | 17/25 (68.0) | 18/22 (81.8) | |
| Cause of ESLD, n (%) | .127 | |||
| HCV | 24 (28.2) | 7 (16.3) | 17 (40.5) | |
| Alcoholic hepatitis | 22 (25.9) | 14 (32.6) | 8 (19.0) | |
| HCV and Alcoholic hepatitis | 3 (3.5) | 1 (2.3) | 2 (4.8) | |
| NASH | 16 (18.8) | 9 (20.9) | 7 (16.7) | |
| Other | 20 (23.5) | 12 (27.9) | 8 (19.0) | |
| Comorbidity – diabetes, n (%) | 35 (41.2) | 18 (41.9) | 17 (40.5) | .897 |
| Comorbidity – hypertension, n (%) | 61 (71.8) | 29 (67.4) | 32 (76.2) | .370 |
| HLA mismatches locus A, n, mean ± SD | 1.6 ± 0.6 | 1.5 ± 0.7 | 1.5 ± 0.6 | .859 |
| HLA mismatches locus B, n, mean ± SD | 1.7 ± 0.5 | 1.7 ± 0.5 | 1.7 ± 0.5 | .820 |
| HLA mismatches locus DR, n, mean ± SD | 1.5 ± 0.5 | 1.5 ± 0.6 | 1.6 ± 0.5 | .535 |
| Total HLA mismatches, n, mean ± SD | 4.8 ± 1.0 | 4.8 ± 1.1 | 4.9 ± 0.9 | .750 |
| cPRA, %, median (IQR) | 0 (0, 8) | 0 (0, 3.0) | 0 (0, 13.0) | .866 |
| Cold ischemic time of donated kidney, minutes, mean ± SD | 496.5 ± 114.6 | 514.3 ± 114.1 | 478.6 ± 113.6 | .155 |
| MELD score, mean ± SD | 28.3 ± 6.5 | 31.7 ± 6.3 | 24.9 ± 4.6 | <.001 |
| Donor information | ||||
| Age, years, mean ± SD | 28.9 ± 11.1 | 29.2 ± 10.9 | 28.6 ± 11.4 | .793 |
| Gender, male, n (%) | 47 (55.3) | 27 (62.8) | 20 (47.6) | .160 |
| Donor Race, n (%) | .163 | |||
| Caucasian | 65 (76.5) | 36 (83.7) | 29 (69.0) | |
| African American | 18 (21.2) | 7 (16.3) | 11 (26.2) | |
| Hispanic | 2 (2.4) | 0 | 2 (4.8 | |
| Donation after brain death, n (%) | 85 (100) | 43 (100) | 42 (100) | – |
| Cause of death, n (%) | .785 | |||
| Anoxia | 27 (31.8) | 14 (32.6) | 13 (31.0) | |
| Cerebrovascular/stroke | 18 (21.2) | 8 (18.6) | 10 (23.8) | |
| Head trauma | 33 (38.8) | 18 (41.9) | 15 (35.7) | |
| Central nerve system tumor | 1 (1.2) | 0 | 1 (2.4) | |
| Other | 5 (5.9) | 2 (4.7) | 3 (7.1) | |
| Comorbidity-diabetes, n (%) | 1 (1.2) | 1 (2.3) | 0 | .320 |
| Comorbidity-hypertension, n (%) | 11 (12.9) | 7 (16.3) | 4 (9.5) | .354 |
| Expanded criteria donor, n (%) | 1 (1.2) | 1 (2.3) | 0 | .320 |
BMI: Body mass index; CKD: Chronic kidney disease; cPRA: calculated panel reactive antibody; ESKD: End-stage kidney disease; ESLD: End-stage liver disease; HCV: Hepatitis C; HLA: Human Leukocyte Antigen; IQR: Interquartile range; MELD: Model of end-stage liver disease; NASH: Nonalcoholic steatohepatitis; SLKT: Simultaneous liver–kidney transplantation.
Compared between Long hospital stay group (L) group and short hospital stay group (L) groups. p values for continuous variables with mean ± SD are result of t-test and with median (IQR) are result of Mann–Whitney test, and categorical variables are chi-square test.
Length of hospital stay and post-transplant events
Table 2 shows post-SLKT information of LOS, first discharge destination, and incidence of outcomes, DGF, PNF, and death following SLKT hospitalization. The median LOS of entire cohort was 15 days (IQR: 9–30 days) and duration from transplantation to discharge was median 11 days (IQR: 8–21 days). The median LOS in (L) group was significantly longer than that in (S) group [30 days (IQR: 21–52 days) and 8.5 days (IQR: 7–11 days), p < .001], respectively. In (L) group, incidence of returning to home as first discharge destination was significantly lower than in (S) group (27.9% and 69.0%, respectively, p = .001). No PNF was observed; however, DGF of the allograft kidney was significantly higher in (L) group. Incidence of death during SLKT hospitalization was higher in (L) group compared to (S) group.
Table 2.
Post-transplant characteristics of the entire cohort and divided by long and short hospital stay groups.
| Entire cohort, N = 85 | Long hospital stay group, (L) N = 43 | Short hospital stay group (S), N = 42 | p Value* | |
|---|---|---|---|---|
| Length of stay for SLKT admission, days, me\dian (IQR) | 15.0 (9.0, 30.0) | 30.0 (21.0, 52.0) | 8.5 (7.0, 11.0) | <.001 |
| Duration from admission to transplantation, days, median (IQR) | 1.0 (1.0, 6.0) | 6.0 (1.0, 16.0) | 0 (0, 1.0) | <.001 |
| Duration from transplantation to discharge, days, median (IQR) | 11.0 (8.0, 21.0) | 21.0 (12.0, 42.0) | 8.0 (6.0, 10.0) | <.001 |
| Delayed graft function (kidney), n (%) | 15 (17.6) | 12 (27.9) | 3 (7.1) | .012 |
| Primary non-function (kidney), n (%) | 0 | 0 | 0 | – |
| Death during hospitalization, n (%) | 9 (10.6) | 7 (16.3) | 2 (4.8) | .084 |
| First discharge destination, n (%) | .001 | |||
| Home | 41 (48.2) | 12 (27.9) | 29 (69.0) | |
| Home and taking health service | 22 (25.9) | 13 (30.2) | 9 (21.4) | |
| Rehabilitation hospital | 12 (14.1) | 10 (23.3) | 2 (4.8) | |
| Others | 10 (11.8) | 8 (18.6) | 2 (4.8) | |
| Incidence of developing of de novo DSA, n (%) | 8 (9.4) | 8 (18.6) | 0 | .003 |
| Duration from transplant to measurement of de novo DSA, days, median (IQR) | 53.0 (14.0, 280.5) | 53.0 (14.0, 280.5) | N/A | – |
| Prevalence of any post-transplant DSA, n (%) | 12 (14.1) | 11 (25.6) | 1 (2.4) | .002 |
DSA: Donor specific antibody; IQR: Interquartile range; SLKT: Simultaneous liver–kidney transplantation.
*Compared between long hospital stay and short hospital stay groups. p values for continuous variables with median (IQR) are result of Mann–Whitney test and categorical variables are chi-square test.
Developing of de novo DSA and prevalence of any post-transplant DSA
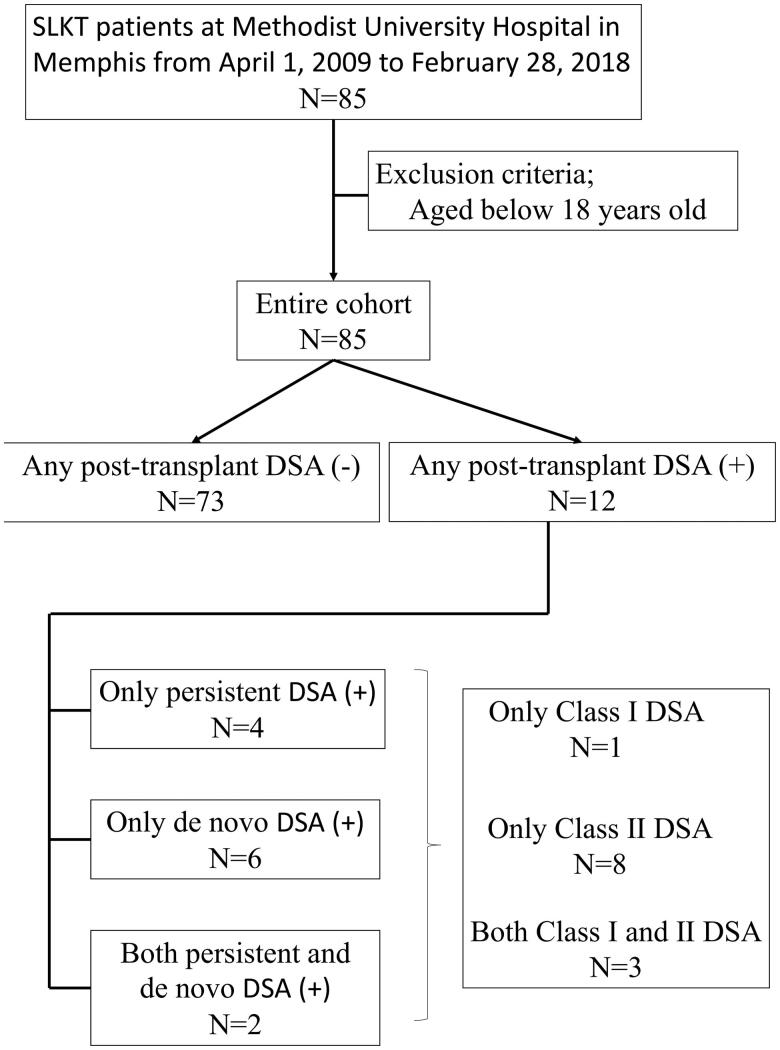
Eight patients developed de novo DSA (9.4%) and 12 patients were identified with any post-transplant DSA consisted of both persistent and de novo DSA (14.1%) after SLKT (Figure 1). All patients who developed de novo DSA and 11 out of 12 patients with any post-transplant DSA were in (L) group. The median days from SLKT was 22.0 days (IQR: 8.0–280.5 days) for measurement of any post-transplant DSA and 53.0 days was (IQR: 14.0–280.5 days) for measurement of de novo DSA (Table 2).
Figure 1.
Flow chart of patient selection and incidence and prevalence of de novo DSA and persistent DSA. Abbreviations: DSA: Donor-specific antibody; de novo DSA: newly developed DSA; N: Number; SLKT: Simultaneous liver–kidney transplantation.
Probability of developing de novo DSA and any post-transplant DSA
Longer LOS was significantly associated with higher risk of development of de novo DSA in unadjusted (OR+ each 5 days: 1.09, 95% CI: 1.02–1.16) and PS adjusted (OR+ each 5 days: 1.11, 95% CI: 1.02–1.21) analysis (Table 3). Longer LOS was also significantly associated with higher risk of prevalence of any post-transplant DSA consisted of both persistent and de novo DSA in unadjusted (OR+ each 5 days: 1.08, 95% CI: 1.02–1.15) and PS adjusted (OR+ each 5 days: 1.08, 95% CI: 1.01–1.16) analysis (Table 3).
Table 3.
Probability of development of de-novo DSA and any post-transplant DSA using unadjusted and propensity score adjusted logistic regression models.
| Risk of de novo DSA |
Risk of any post-transplant DSA |
|||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p value | |
| Un-adjusted | ||||||
| Length of hospital stay (each 5 days) | 1.09 | 1.02–1.16 | .010 | 1.08 | 1.02–1.15 | .011 |
| Adjusted | ||||||
| Length of hospital stay (each 5 days) | 1.11 | 1.02–1.21 | .014 | 1.08 | 1.01–1.16 | .028 |
| PS score | 0.27 | 0.02–4.13 | .343 | 1.08 | 0.13–8.68 | .944 |
DSA: Donor specific antibody; PS: Propensity score; 95% CI: 95% confidence interval.
Probability of developing de novo DSA and any post-transplant DSA in the sensitivity analysis
Longer LOS defined as >28 days was significantly associated with higher risk of development of de novo DSA and prevalence of any post-transplant DSA consisted of both persistent and de novo DSA in unadjusted and PS adjusted analysis (Table S2, Supplementary material).
Discussion
In this single center, retrospective study, we found significant associations between longer hospitalization and higher probability of both persistent post-transplant DSA and de novo DSA development after SLKT. In addition, our study indicates that longer LOS occurred more frequently in SLKT patients with higher MELD scores and higher incidence of DGF in allograft kidney. Furthermore, 30% of the patients in (L) group, compared to exact percentage in (S) group, were discharged to their home after SLKT. All of those who developed de novo DSA had longer hospitalizations. Our study implies that LOS might be a useful surrogate marker of a higher probability for persistent DSA and de novo DSA development. Patients, if not all, with longer hospitalization should be routinely screened for DSA after SLKT. We believe this is the first report to evaluate an association between the length of hospitalization and post-transplant DSA development in SLKT.
Sensitized status in SLKT has tended to be neglected due to the absorptive capacity by allograft liver [1–4]. In fact, sensitization before SLKT is not a contraindication of transplantation from several clinical practice guidelines [32–34]. However, post-transplant DSA, especially de novo ClassII DSA, has been thought to be a significant risk factor for patient-, allograft liver-, and allograft kidney outcome [5]. No previous study has assessed the risk factors of developing de novo DSA in SLKT, although the potential for exposure to sensitizing events might be expected to be higher in SLKT than liver or kidney transplantation alone (LTA and KTA). Although LTA patients typically do not undergo maintenance dialysis therapy before LTA, 30–50% of SLKT candidates undergo dialysis therapy immediately prior to SLKT [35,36]. We observed this finding in our cohort as well. Simultaneous liver–kidney transplant patients could be more fragile prior to transplantation and require more blood transfusions peri-operatively compared to KTA patients [37]. Higher prevalence of maintenance dialysis therapy and history of blood transfusions in SLKT patients has been associated with sensitization [38–40], which leads to higher probability of sensitization before and after transplantation compared to LTA and KTA patients.
We constructed a conceptual model of the assessed relationship between LOS and DSA. We identified an association between longer LOS and developing de novo DSA. The relationship between assumptive exposures, (blood transfusions, infectious events) and EAD and LOS has already been reported in other patient cohorts [14,15] as well as recipients with LTA [18–20,23]. We wanted to clarify a direct relationship between presumptive exposures and de novo DSA development. Our preliminary findings suggest LOS might be a consequence of DGF on allograft kidney secondary to persistent DSA or de novo DSA development or other potential intermediated mediators (indicator). Although we could not identify the exposure in each patient, a relationship between presumptive exposures and de novo DSA development could exist in accordance with this conceptual model.
Longer hospitalization also showed significant association with a higher probability of persistent or de novo post-transplant DSA. In our cohort, 4 patients had persistent DSA; 2 patients had both persistent and de novo DSA after SLKT (Figure 1). Five of the six patients with persistent DSA after SLKT, were in (L) group. Our results suggest patients with pre-transplant DSA and longer LOS should be routinely monitored for post-transplant DSA.
Several limitations should be noted with this study. Although this is one of the largest SLKT cohorts studied to date, our patient group was still small and had a relatively low number of events. Because this was a retrospective cohort study, we could not conclude causal relationship between LOS and developing de novo DSA despite the finding the median days of measurement of DSA after SLKT was almost double compared to length of hospital stay in (L) group. Furthermore, DSA measurement was indication based and not routine, which might have caused observational bias. In this study, we were not able to assess the pathophysiological role of Class I and II DSA separately, as almost all of the patients with post-transplant DSA had at least Class II DSA (N = 11/12) (Figure 1). Finally, the observed differences in clinical practices observed across different transplant programs may lead to different definitions of LOS. These differences could be the generalizability of our findings. In fact, albeit LOS was replaced with 28 days or longer in our sensitivity analysis, LOS was still significant risk factor for the developing of de novo DSA and prevalence of any post-transplant DSA. Additional prospective and larger studies that include protocol DSA measurement are highly warranted.
Despite some limitations, our study has confirmed several previous findings. LOS has been shown to be predicted by factors such as ethnicity, discharge destination, and type of insurance [13]. In our study, (L) group patients were more likely to be discharged to rehabilitation or discharged with home service than (S) group. Despite have low event numbers, we were able to adjust for these variables using a PS score. This is the first report to identify LOS as a risk factor for de novo DSA development in SLKT. Our results support the conclusion that DSA monitoring can be implemented into clinical practice for SLKT patients.
In conclusion, longer hospitalization was significantly associated with higher probability of persistent DSA and de novo DSA development after SLKT. Although longer hospitalization could be an indicator and not a direct cause for de novo DSA development, DSA monitoring after LOS might be able to help providers improve outcomes after SLKT. Additional prospective and larger studies are highly warranted to identify more modifiable and non-modifiable predictors for persistent and de novo DSA development following SLKT.
Supplementary Material
Acknowledgments
The results of this paper have not been published previously in whole or part.
Author contributions
All authors contributed to conception and design of the study and approved the final version of the manuscript. M.Y., O.C., P.S.B.P., and M.Z.M. collected the clinical data. S.F examined all donor specific antibodies. M.Y. and M.Z.M. performed the analysis and contributed to interpretation of results. M.Y., and M.Z.M. wrote the manuscript. M.Z.M. takes responsibility for the data and analysis accuracy and all other aspect the work.
Disclosure statement
The authors report no conflict of interest.
Data availability statement
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
- 1.Zavazava N. Soluble HLA class I molecules: biological significance and clinical implications. Mol Med Today. 1998;4:116–121. [DOI] [PubMed] [Google Scholar]
- 2.Gugenheim J, Amorosa L, Gigou M, et al. Specific absorption of lymphocytotoxic alloantibodies by the liver in inbred rats. Transplantation. 1990;50:309–313. [DOI] [PubMed] [Google Scholar]
- 3.Taner T, Park WD, Stegall MD.. Unique molecular changes in kidney allografts after simultaneous liver-kidney compared with solitary kidney transplantation. Kidney Int. 2017;91:1193–1202. [DOI] [PubMed] [Google Scholar]
- 4.Taner T, Gustafson MP, Hansen MJ, et al. Donor-specific hypo-responsiveness occurs in simultaneous liver-kidney transplant recipients after the first year. Kidney Int. 2018;93:1465–1474. [DOI] [PubMed] [Google Scholar]
- 5.O’Leary JG, Gebel HM, Ruiz R, et al. Class II alloantibody and mortality in simultaneous liver-kidney transplantation. Am J Transplant. 2013;13:954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yazawa M, Cseprekal O, Helmick AR, et al. Association between post-transplant donor specific antibodies and recipient outcomes in simultaneous liver-kidney transplant recipients: single center, cohort study. Transpl Int. 2019; doi: 10.1111/tri.13543. [Epub ahead of print] PubMed PMID: 31647143. [DOI] [PubMed] [Google Scholar]
- 7.Taner T, Gandhi MJ, Sanderson SO, et al. Prevalence, course and impact of HLA donor-specific antibodies in liver transplantation in the first year. Am J Transplant. 2012;12:1504–1510. [DOI] [PubMed] [Google Scholar]
- 8.Dar W, Agarwal A, Watkins C, et al. Donor-directed MHC class I antibody is preferentially cleared from sensitized recipients of combined liver/kidney transplants. Am J Transplant. 2011;11:841–847. [DOI] [PubMed] [Google Scholar]
- 9.Paterno F, Girnita A, Brailey P, et al. Successful simultaneous liver-kidney transplantation in the presence of multiple high-titered Class I and II antidonor HLA antibodies. Transplant Direct. 2016;2:e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jucaud V, Shaked A, DesMarais M, et al. Prevalence and impact of de novo donor-specific antibodies during a multicenter immunosuppression withdrawal trial in adult liver transplant recipients. Hepatology (Baltimore, MD). 2019;69:1273–1286. [DOI] [PubMed] [Google Scholar]
- 11.O’Sullivan K, Martensson J, Robbins R, et al. Epidemiology of long-stay patients in a university teaching hospital. Intern Med J. 2017;47:513–521. [DOI] [PubMed] [Google Scholar]
- 12.McAdams-DeMarco MA, King EA, Luo X, et al. Frailty, length of stay, and mortality in kidney transplant recipients: a national registry and prospective cohort study. Ann Surg. 2017;266:1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brasel KJ, Lim HJ, Nirula R, et al. Length of stay: an appropriate quality measure? Arch Surg. 2007;142:461–465. discussion 465–466. [DOI] [PubMed] [Google Scholar]
- 14.Vincent JL, Baron JF, Reinhart K, et al. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499–1507. [DOI] [PubMed] [Google Scholar]
- 15.Micek S, Johnson MT, Reichley R, et al. An institutional perspective on the impact of recent antibiotic exposure on length of stay and hospital costs for patients with gram-negative sepsis. BMC Infect Dis. 2012;12:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baek H, Cho M, Kim S, et al. Analysis of length of hospital stay using electronic health records: a statistical and data mining approach. PLoS One. 2018;13:e0195901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arefian H, Hagel S, Heublein S, et al. Extra length of stay and costs because of health care-associated infections at a German university hospital. Am J Infect Control. 2016;44:160–166. [DOI] [PubMed] [Google Scholar]
- 18.Singh N, Paterson DL, Gayowski T, et al. Predicting bacteremia and bacteremic mortality in liver transplant recipients. Liver Transpl. 2000;6:54–61. [DOI] [PubMed] [Google Scholar]
- 19.Ruiz J, Dugan A, Davenport DL, et al. Blood transfusion is a critical determinant of resource utilization and total hospital cost in liver transplantation. Clin Transplant. 2018;32:e13164. [DOI] [PubMed] [Google Scholar]
- 20.Nedelcu E, Wright MF, Karp S, et al. Quality improvement in transfusion practice of orthotopic liver transplantation reduces blood utilization, length of hospital stay, and cost. Am J Clin Pathol. 2019;151:395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang R. Donor-specific antibodies in kidney transplant recipients. Clin J Am Soc Nephrol. 2018;13:182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knoll GA, MacDonald I, Khan A, et al. Mycophenolate mofetil dose reduction and the risk of acute rejection after renal transplantation. J Am Soc Nephrol. 2003;14:2381–2386. [DOI] [PubMed] [Google Scholar]
- 23.Croome KP, Hernandez-Alejandro R, Chandok N.. Early allograft dysfunction is associated with excess resource utilization after liver transplantation. Transplant Proc. 2013;45:259–264. [DOI] [PubMed] [Google Scholar]
- 24.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.OPTN/UNOS Kidney Transplantation Committee . OPTN/UNOS public comment proposal. Simultaneous liver kidney (SLK) allocation policy. Available from: https://optn.transplant.hrsa.gov/media/1192/0815-1112_SLK_Allocation.pdf
- 26.Health Resources and Services Administration USDoHHS. The Ethics of Organ Donation from Condemned Prisoners . Available from: https://optn.transplant.hrsa.gov/resources/ethics/the-ethics-of-organ-donation-from-condemned-prisoners/
- 27.Mone T, Heldens J, Niemann CU.. Deceased organ donor research: the last research frontier? Liver Transpl. 2013;19:118–121. [DOI] [PubMed] [Google Scholar]
- 28.Yoo MC, Vanatta JM, Modanlou KA, et al. Steroid-free liver transplantation using rabbit antithymocyte globulin induction in 500 consecutive patients. Transplantation. 2015;99:1231–1235. [DOI] [PubMed] [Google Scholar]
- 29.Catalano G, Tandoi F, Mazza E, et al. Simultaneous liver-kidney transplantation in adults: a single-center experience comparing results with isolated liver transplantation. Transplant Proc. 2015;47:2156–2158. [DOI] [PubMed] [Google Scholar]
- 30.Baccaro ME, Pepin MN, Guevara M, et al. Combined liver-kidney transplantation in patients with cirrhosis and chronic kidney disease. Nephrol Dial Transplant. 2010;25:2356–2363. [DOI] [PubMed] [Google Scholar]
- 31.Siedlecki A, Irish W, Brennan DC.. Delayed graft function in the kidney transplant. Am J Transplant. 2011;11:2279–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis CL, Feng S, Sung R, et al. Simultaneous liver-kidney transplantation: evaluation to decision making. Am J Transplant. 2007;7:1702–1709. [DOI] [PubMed] [Google Scholar]
- 33.Eason JD, Gonwa TA, Davis CL, et al. Proceedings of consensus conference on simultaneous liver kidney transplantation (SLK). Am J Transplant. 2008;8:2243–2251. [DOI] [PubMed] [Google Scholar]
- 34.Hussain SM, Sureshkumar KK.. Refining the role of simultaneous liver kidney transplantation. J Clin Transl Hepatol. 2018;6:289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Locke JE, Warren DS, Singer AL, et al. Declining outcomes in simultaneous liver-kidney transplantation in the MELD era: ineffective usage of renal allografts. Transplantation. 2008;85:935–942. [DOI] [PubMed] [Google Scholar]
- 36.Tanriover B, MacConmara MP, Parekh J, et al. Simultaneous liver kidney transplantation in liver transplant candidates with renal dysfunction: importance of creatinine levels, dialysis, and organ quality in survival. Kidney Int Rep. 2016;1:221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Triulzi DJ. Specialized transfusion support for solid organ transplantation. Curr Opin Hematol. 2002;9:527–532. [DOI] [PubMed] [Google Scholar]
- 38.Witczak BJ, Leivestad T, Line PD, et al. Experience from an active preemptive kidney transplantation program–809 cases revisited. Transplantation. 2009;88:672–677. [DOI] [PubMed] [Google Scholar]
- 39.Haller MC, Kammer M, Oberbauer R.. Dialysis vintage and outcomes in renal transplantation. Nephrol Dial Transplant. 2019;34:555–560. [DOI] [PubMed] [Google Scholar]
- 40.Fidler S, Swaminathan R, Lim W, et al. Peri-operative third party red blood cell transfusion in renal transplantation and the risk of antibody-mediated rejection and graft loss. Transpl Immunol. 2013;29:22–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.