Abstract
L-asparaginase is an important anticancer enzyme that is used in the first line treatment of acute lymphoblastic leukemia. This study was conducted to isolate L-asparaginase-producing endophytic fungi from medicinal plants of family Asteraceae. Seven healthy medicinal plants from family Asteraceae were selected for the isolation of endophytic fungi using standard surface sterilization techniques. A total of 837 isolates belonging to 84 species were comprised of the stem (55.6%), leaf (31.1%), root (10.6%) and flower (2.7%). Initial screening of L-asparaginase-producing endophytes was performed by qualitative plate assay on modified Czapex dox’s agar medium. L-asparaginase activity of fungal endophytes was quantified by the nesslerization method. Identification of endophytic fungi was performed using both morphological characteristics and phylogenetic analyses of DNA sequence data including ribosomal DNA regions of ITS (Internal transcribed spacer) and LSU (partial large subunit rDNA), TEF1 (Translation Elongation Factor) and TUB (β-tubulin). Of the 84 isolates, 38 were able to produce L-asparaginase and their L-asparaginase activities were between 0.019 and 0.492 unit/mL with Fusarium proliferatum being the most potent. L-asparaginase-producing endophytes were identified as species of Plectosphaerella, Fusarium, Stemphylium, Septoria, Alternaria, Didymella, Phoma, Chaetosphaeronema, Sarocladium, Nemania, Epicoccum, Ulocladium and Cladosporium. This study showed that endophytic fungi from Asteraceae members have a high L-asparaginase-producing potential and they can be used as an alternative source for production of anticancer enzymes.
Keywords: Asparaginase activity, Colonization frequency, Endophytic fungi, Fusariumproliferatum
Introduction
Endophytes are microorganisms that reside inside plant tissues without causing any symptoms or obvious negative effects to the host plants (Gond et al., 2012). Endophytic fungi from medicinal plants have been considered to be a rich source of novel natural products for medical and commercial exploitation (Gutierrez, Gonzalez & Ramirez, 2012). The close symbiotic relationship between endophytic fungi and host plants gives endophytes a potent ability to produce novel bioactive compounds whose production is fueled by host plant carbohydrates (Aly, Debbab & Proksch, 2011). These bioactive compounds increase plant resistance to pathogens and herbivores, enhanced competitive abilities and enhanced growth (Zhang, Song & Tan, 2006). Endophytic fungal bioactive metabolites may be useful as novel drugs due to their wide variety of biological activities (Guo et al., 2008). In recent years, endophytic fungi have been viewed as a source of secondary metabolites, including anticancer, anti-inflammatory, antibiotic and antioxidant agents (Guo et al., 2008; Bungihan et al., 2011; Debbab et al., 2009; Gutierrez, Gonzalez & Ramirez, 2012; Strobel et al., 2004).
Enzymes produced by microorganisms are used for medical and industrial purposes. L-asparaginase is one such enzyme that hydrolyzes asparagine to aspartic acid and ammonia (Patil, Patil & Mahjeshwari, 2012). L-asparaginase enzymes in the food industry are used as an admixture to reduce the acrylamide produced by the high temperature in starchy foods and reduce the risk of cancer (Xu, OrunaConcha & Elmore, 2016). This enzyme is one of the most important biochemical therapeutic enzymes used in the treatment of various types of leukemia, such as acute lymphoblastic leukemia in children (McCredie & Ho, 1973; Patil, Patil & Mahjeshwari, 2012). In cancer treatment, L-asparaginase removes L-asparagine in the serum, depriving tumor cells of the large amounts of asparagine required for growth (Asthana & Azmi, 2003). Currently, L-asparaginase derived from Escherichia coli is the main source of L- asparaginase (Batool et al., 2015). However, side effects of this enzyme derived from bacteria include chills, fever, abdominal cramps and fatal hyperthermia (Hosamani & Kaliwal, 2011). L-asparaginase derived from eukaryotes may induce relatively less toxicity and reduced immune response (Asthana & Azmi, 2003). Considering the importance of L-asparaginase in the treatment of leukemia, finding new sources of this enzyme that can produce high levels of enzyme with minimum side effects is a priority (Theantana, Hyde & Lumyong, 2009). Microorganisms such as fungi have the ability to produce extracellular enzymes in high quantities, which are easily extracted and purified, may provide ideal sources of L-asparaginase. In addition, fungal species due to their eukaryotic nature may have enzymes more comparable to human enzymes that may be used in treatment of cancer with better success than enzymes of other microorganisms (Serquis & Oliveira, 2004). L-asparaginase from endophytic fungi isolated from medicinal plants has been reported in recent years (Theantana, Hyde & Lumyong, 2007).
The appropriate selection of host plants is important to increase the chances of isolation of novel endophytes which may produce new bioactive metabolites (Ratklao, 2013). In this study, seven Iranian medicinal plants, including: Matricaria chamomilla, Matricaria parthenium, Athemis triumfetii, Anthemis altissima, Achillea millefolium, Achillea filipendulina and Cichorium intybus, were selected for the isolation of fungal endophytes and screening them for L-asparaginase activity based on use in traditional medicine, habitat, and diversity of these plant species in the Golestan province of Iran.
Materials & Methods
Isolation and identification of fungal endophytes
From May until September 2015, the plant specimen (stem, root, leaf and flower) were obtained from seven healthy medicinal plants: Matricaria chamomilla, Matricaria parthenium, Anthemis triumfetii,, Anthemis altissima, Achillea millefolium, Achillea filipendulina and Cichorium intybus growing in the natural ecosystem in Northeastern of Iran. The samples were stored in polyethylene bags at 4 °C (Waksman, 1916). Samples were washed thoroughly in distilled water. The Surface sterilization was performed by sequential immersion of samples in sodium hypochlorite (3 times in 5% NaOCl for 3–8 min each, depending on the type of samples) followed by 75% ethanol for 1 min and rinsed five times in sterile distilled water. The samples were dried on sterile blotters under the laminar air flow. The surface-sterilized samples were cut into about 0.5 × 1 cm2 using a sterile scalpel. 200 segments from stem, leaf, root and flower (four segments per Petri plate) for each plant species were placed equidistantly on Potato Dextrose Agar medium (PDA, Merck, Darmstadt, Germany) supplemented with tetracycline (50 mg/L) to inhibit bacterial growth. Three replicates of Petri dishes were used per plant sample. The petri plates were incubated at 28 ± 2 °C with 12 h light and dark cycles for up to 6 to 8 weeks. To verify sterility controls were prepared by spreading 100 µL aliquots of the water from final rinse solutions onto PDA medium plates and incubated for 2 weeks at 28 ± 2 °C with 12 h light and dark cycles. The absence of fungal growth in controls indicated effective sterilization, while mycelial growth from plant samples was indicative of endophyte isolation. Colonies that emerged from tissue segments were picked up and transferred to antibiotic-free PDA to enable identification. Colonization Frequency (CF) of endophytes was calculated as described by Khan et al. (2010).
Identification was achieved using morphological and molecular methods. Morphological identification of isolates was performed based on the fungal colony morphology, characteristics of the spores and reproductive structures using standard identification manuals (Barnett & Hunter, 1999; Bensch et al., 2012; Boerema et al., 2004; Simmons, 2007; Booth, 1971).
Molecular identification of endophytic fungi
Endophytic fungal isolates were grown in 200 mL of potato dextrose broth (PDB) for 7 days at 28 °C. The mycelia were washed with distilled water and ground with liquid nitrogen. The nucleic acid was extracted using the cetyl trimethyl ammonium bromide (CTAB) method (Dayle et al., 2001). Strains were sequenced with four: molecular markers, including ITS (Internal transcribed spacer), LSU (partial large subunit nrDNA), TEF1 (Translation elongation factor) and TUB (β-tubulin) using primer sets listed in Table 1. PCR amplifications were performed on a GeneAmp PCR System 9600 (Perkin Elmer, USA) in a total volume of 12.5 µL solution containing 10–20 ng of template DNA, 1 × PCR buffer, 0.7 µL DMSO (99.9%), 2 mM MgCl2, 0.5 µM of each primer, 25 µM of each dNTP and 1.0 U Taq DNA polymerase (NEB). The amplification process was initiated by pre-heating at 95 °C for1 min, followed by 40 cycles of denaturation at 95 °C for 30s, with primer annealing at the temperature stipulated in Table 2, extension at 72 °C for 10s, and a final extension at 72 °C for 5 min. The products of the PCR reaction were then examined by electrophoresis using 1% (w/v) agarose gel, stained with gel red (Biotium®) and visualized with a UV transilluminator (UVP MultiDoc-It™, Analytik Jena, Germany). BLAST analysis was carried out in the NCBI database. All sequences were deposited in NCBI’s GenBank Database.
Table 1. Primer combinations used for molecular identification.
| Locus | Primer | Primer sequence 5′ to 3′: | Orientation | Reference |
|---|---|---|---|---|
| TEF-1α | EF1-983F | GCCYGGHCAYCGTGAYTTYAT | Forward | Rehner & Buckley (2005) |
| Efgr | GCAATGTGGGCRGTRTGRCARTC | Reverse | Rehner & Buckley (2005) | |
| β-tubulin | T1 | AACATGCGTGAGATTGTAAGT | Forward | O’Donnell & Cigelnik (1997) |
| β-Sandy-R | GCRCGNGGVACRTACTTGTT | Reverse | O’Donnell & Cigelnik (1997) | |
| LSU | LROR | CC CGC TGA ACT TAA GC | Forward | Vilgalys & Hester (1990) |
| LR5 | TCCTGAGGGAA ACTTCG | Reverse | Vilgalys & Hester (1990) | |
| ITS | ITS5 | GGAAGTAAAAGTCGTAACAAGG | Forward | White et al. (1990) |
| ITS4 | TCCTCCGCTTATTGATATGC | Reverse | White et al. (1990) |
Table 2. Colonization frequency of endophytic fungi.
Two hundred segments of each sample were plated for frequency analysis. (n = 10) Not detected: –.
| Colonization frequency | ||||||
|---|---|---|---|---|---|---|
| Endophytic Fungi | Host Plant | Stem | Leaf | Root | Flower | Total |
| Fusarium redolens | Achillea millefolium | 5.5 | – | – | – | 11 |
| Septoria saposhnikoviae | A. millefolium | 1 | – | – | – | 2 |
| Paraophiobolus arundinis | A. millefolium | 4.5 | – | – | – | 9 |
| Stemphylium amaranthi | A. millefolium | 2.5 | – | – | – | 5 |
| Cladosporium ramotenellum | A. millefolium | – | 5 | – | – | 10 |
| Septoria tormentillae | A. millefolium | 1.5 | – | – | – | 3 |
| Septoria lycopersici var. lycopersici | A. millefolium | 1 | – | – | – | 2 |
| Septoria sp. | A. millefolium | 2.5 | – | – | – | 5 |
| Fusarium oxysporum | A. millefolium | 5 | – | – | – | 10 |
| Septoria malagutii | A. millefolium | 1.5 | – | – | – | 3 |
| Fusarium sp. | A. millefolium | – | 5.5 | – | – | 11 |
| S. tormentillae | A. millefolium | 3.5 | – | – | – | 7 |
| Alternaria infectoria | A. millefolium | 5.5 | – | – | – | 11 |
| Leptosphaerulina saccharicola | A. millefolium | 2.5 | – | – | – | 5 |
| Alternaria burnsii | A. millefolium | – | 4.5 | – | – | 9 |
| Alternaria sp. | A. millefolium | – | 5.5 | – | – | 11 |
| Nemania serpens | A. millefolium | 3.5 | – | – | – | 7 |
| Stemphylium vesicarium | A. millefolium | 4 | – | – | – | 8 |
| Fusarium avenaceum | A. millefolium | – | – | 6 | – | 12 |
| Fusarium sp. | A. millefolium | – | 6 | – | – | 12 |
| Paraphoma chrysanthemicola | A. millefolium | – | 5 | – | – | 10 |
| F. oxysporum | Achillea filipendulina | 8 | – | – | – | 16 |
| Fusarium sp. | A. filipendulina | 5.5 | – | – | – | 11 |
| Preussia africana | A. filipendulina | 1.5 | – | – | – | 3 |
| Plectosphaerella cucumerina | A. filipendulina | 6.5 | – | – | – | 13 |
| Antennariella placitae | A. filipendulina | 5 | – | – | – | 10 |
| Fusarium acuminatum | A. filipendulina | – | 6.5 | – | – | 13 |
| Acremonium sclerotigenum | A. filipendulina | – | 6 | – | – | 12 |
| Colletotrichum tanaceti | A. filipendulina | 4.5 | – | – | – | 9 |
| Trametes versicolor | A. filipendulina | 1.5 | – | – | – | 3 |
| A. burnsii | Anthemis altissima | 4 | – | – | – | 8 |
| Lewia infectoria | A. altissima | – | – | 8 | – | 16 |
| P. chrysanthemicola | A. altissima | 6 | – | – | – | 12 |
| Aspergillus calidoustus | A. altissima | – | 5.5 | – | – | 11 |
| Bjerkandera adusta | A. altissima | 2.5 | – | – | – | 5 |
| Schizophyllum commune | A. altissima | 3.5 | – | – | – | 7 |
| A. infectoria | A. altissima | 3 | – | 1.5 | 9 | |
| Paraphoma sp. | A. altissima | 4.5 | 1 | – | – | 11 |
| F. acuminatum | A. altissima | 8 | – | – | 16 | |
| Stemphylium botryosum | A. altissima | – | 6.5 | – | – | 13 |
| N. serpens | A. altissima | 4 | – | – | – | 8 |
| Fusarium proliferatum | A. altissima | 8.5 | – | – | – | 17 |
| Plenodomus tracheiphilus | A. altissima | 4.5 | – | – | – | 9 |
| Phoma tracheiphila | A. altissima | 6 | 1.5 | – | – | 15 |
| Ulocladium consortiale | A. altissima | – | 6.5 | – | – | 13 |
| P. cucumerina | A. altissima | – | – | 6 | – | 12 |
| Cladosporium limoniforme | A. altissima | 6 | – | 6 | – | 24 |
| Sarocladium strictum | A. altissima | – | – | 5.5 | – | 11 |
| Verticillium dahliae | A. altissima | 4.5 | – | – | – | 9 |
| F. avenaceum | A. altissima | – | – | – | 5.5 | 11 |
| Didymella tanaceti | A. altissima | 2 | – | – | – | 4 |
| Chaetosphaeronema sp. | Anthemis triumfetii | – | 6 | – | – | 12 |
| Chaetosphaeronema hispidulum | A. triumfetii | – | 7 | – | – | 14 |
| P. chrysanthemicola | A. triumfetii | 6.5 | – | – | – | 13 |
| Chaetosphaeronema achilleae | A. triumfetii | 5 | 1 | – | – | 12 |
| C. achilleae | A. triumfetii | – | 4 | – | – | 8 |
| S. amaranthi | A. triumfetii | – | 7 | – | – | 14 |
| Paraphoma sp. | A. triumfetii | 7 | – | – | – | 14 |
| Alternaria sp. | A. triumfetii | 6 | 2 | – | – | 16 |
| Alternaria sp. | A. triumfetii | 7 | 2 | – | – | 18 |
| S. vesicarium | Matricaria parthenium | – | 4.5 | – | – | 9 |
| Arthrinium phaeospermum | M. parthenium | – | – | – | 1 | 2 |
| Epicoccum nigrum | M. parthenium | 4 | – | – | – | 8 |
| Aspergillus chevalieri | M. parthenium | – | 4.5 | – | – | 9 |
| Trichaptum biforme | M. parthenium | 1.5 | – | – | – | 3 |
| Phoma haematocycla | Matricaria chamomilla | 5 | 1 | – | – | 12 |
| Paramyrothecium roridum | M. chamomilla | – | – | 6.5 | – | 13 |
| S. amaranthi | M. chamomilla | – | 7 | – | – | 14 |
| Xylariaceae sp. | M. chamomilla | 6 | – | – | – | 12 |
| E. nigrum | M. chamomilla | 4 | – | – | – | 8 |
| Cladosporium tenuissimum | Cichorium intybus | – | 5.5 | – | – | 11 |
| E. nigrum | C. intybus | 3.5 | – | – | – | 7 |
| Septoria cerastii | C. intybus | 3.5 | – | – | – | 7 |
| P. cucumerina | C. intybus | – | – | 5 | – | 10 |
| C. tanaceti | C. intybus | 7.5 | – | – | – | 15 |
| Stephanonectria keithii | C. intybus | – | 2 | – | – | 4 |
| Alternaria solani | C. intybus | 6 | – | – | – | 12 |
| B. adusta | C. intybus | 3.5 | – | – | – | 7 |
| Torula herbarum | C. intybus | – | 3.5 | – | – | 7 |
| Alternaria embellisia | C. intybus | – | 5.5 | – | – | 11 |
| Stemphylium globuliferum | C. intybus | 4 | – | – | – | 8 |
| A. sclerotigenum | C. intybus | – | – | – | 5 | 10 |
| Penicillium canescens | C. intybus | – | 2 | – | – | 4 |
| Diaporthe novem | C. intybus | 9.5 | – | – | – | 19 |
| Number of isolates | 466 | 259 | 89 | 23 | 837 | |
Screening of L-asparaginase-producing endophytes
The isolated endophytic fungi were screened for their ability to produce asparaginase. Mycelial plug was inoculated onto Modified Czapex Dox (McDox) agar [agar powder (20.0 g/ L−1), glucose (2.0 g/L−1), L-asparagine (10.0 g/L−1), KH2PO4 (1.52 g/L−1), KCl (0.52 g/L−1), MgSO4⋅7H2O (0.52 g/L−1), CuNO3⋅3H2O (0.001g/L−1), ZnSO4⋅7H2O (0.001 g/L−1), FeSO4⋅7H2O (0.001 g/L−1)], l-asparagine (10.0 g/L−1) and 0.3 mL of 2.5% phenol red dye (indicator). Controls were prepared by inoculating mycelial plugs on Czapex Dox agar without asparagine. Triplicates for each isolate were prepared. All Petri plates were incubated at 26 ± 2 °C. After 5 days of incubation, the diameter of the pink zone was evaluated (Gulati, Saxena & Gupta, 1997).
Estimation of L-asparaginase activity
The L-asparaginase positive fungal isolates were inoculated using 5 mm fungal mycelial plugs into 200 mL of McDox broth and incubated for 5 days at 36 ± 2 °C and 120 rpm. L-asparaginase was estimated by Nesslerization as described by Imada et al. (1973). After incubation, 100 µl of broth (crude enzyme) was pipetted into 2 ml tubes. After that, 100 µl of Tris HCl (pH 7), 200 µl of 0.04 M asparagine and 100 µl of sterile distilled water (SDW) were added. The mixture was incubated at 37 ± 2 °C for 1 h. After incubation, 100 µl of 1.5 M Trichloroacetic Acid (TCA) was then added to stop the enzymatic reaction. Finally, 100 µl of the mixture was pipetted into fresh tubes containing 750 µl SDW and 300 µl of Nessler’s reagent and incubated at 28 ± 2 °C for 20 min and the amount of enzyme activity was measured by determining the absorbance of samples at 450 nm using UV-Visible spectrophotometer (Jenway model 6315). One unit of asparaginase is expressed as the amount of enzyme that catalyzes the formation of 1 µmol of ammonia per minute at 37 ± 2 °C (Theantana, Hyde & Lumyong, 2007).
0.6 = Initial volume of enzyme mixture in mL
0.1 = Volume of enzyme mixture used in final reaction in mL
60 = Incubation time in minutes
0.2 = Volume of enzyme used in mL
Statistical analysis
The final experiment was conducted using a completely randomized design with triplicates for each parameter assessed. The data were statistically analyzed using the software Statistical Package for the Social Sciences (SPSS) version 17.0. One-way ANOVA with least significant difference (LSD(0.01)) were applied to analyze all the data collected.
Results
Identification of fungal endophytes
Endophytes were obtained from all seven medicinal plant species with a total of 837 isolates from 200 each of leaf, stem and root segments. Endophytes were mostly recovered from A. altissima (241 isolates), followed by A. millefolium (163 isolates), A. triumfetii (121 isolates), C. intybus (132 isolates), A. filipendulina (90 isolates), M. chamomilla (59 isolates) and M. parthenium (31 isolates) (Table 2). Due to the large number of fungal endophytes, the isolates were further classified into 84 morphotypes based on the different morphological and cultural characteristics. Eighty-four endophytic fungal species belonging to Ascomycota and Basidiomycota were identified using morphological and molecular methods. Few endophytic fungi such as Acremonium sclerotigenum, Alternaria burnsii, Bjerkandera adusta, Colletotrichum tanaceti, Epicoccum nigrum, Fusarium acuminatum, Paraphoma chrysanthemicola, Plectosphaerella cucumerina and Stemphylium amaranthi showed wide distributions in the host plants and were isolated from most plants studied. Also, a higher number of endophytes were recovered from stem tissues of all seven plant species (Table 2). The percent colonization frequency of endophytes varied in the plant parts with stem fragments harboring 55.6% of endophytic isolates followed by leaves with 31.1% and least for the isolates from flower samples. Many isolates belonged to the genera Alternaria, Fusarium, Phoma, Chaetosphaeronema and Plectosphaerella which colonized more than one plant part. The isolates of Fusarium were recovered from stem, leaf, flower and root while Phoma spp. was obtained from stem and leaf sample. Tissue specificity was also observed for some endophytes. This was most evident in the Septoria species that were found only in the stem tissues. Basidiomycetous endophytes such as Trametes versicolor, Bjerkandera adusta, Trichaptum biforme and Schizophyllum commune was isolated from stem tissues. Fusarium spp. was found as the dominant endophytes with 140 isolates, followed by Alternaria spp. (105 isolates). The results indicated that the species composition and frequency of endophyte species was found to be dependent on the tissue and host plant.
Screening of L-asparaginase-producing endophytes by qualitative plate assay
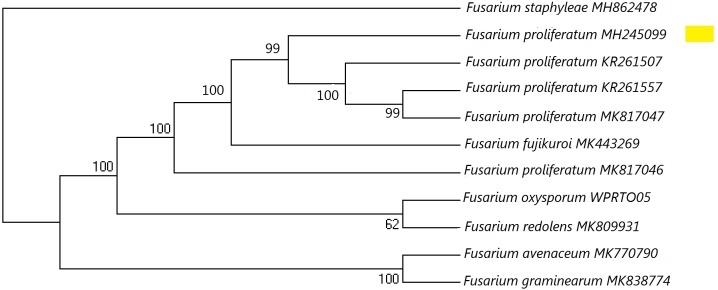
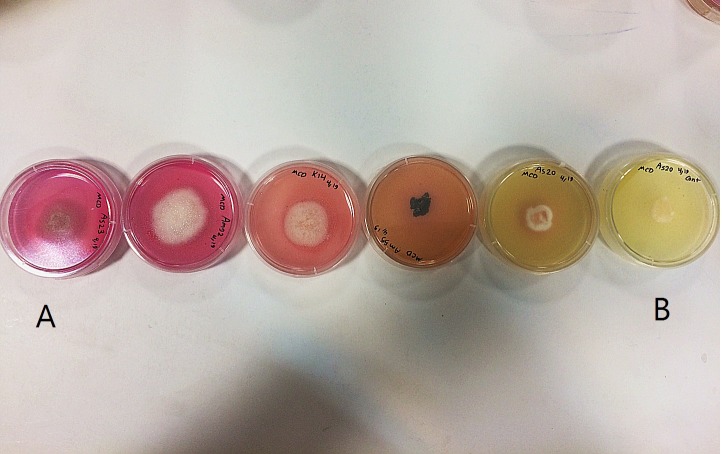
All endophytic fungal isolates were screened for their ability to produce L-asparaginase by qualitative rapid plate assay. Of the eighty-four fungal endophyte isolates tested for L-asparaginase activity (Table 3), thirty-eight isolates were positive for extracellular L-asparaginase and formation of pink zones was evident on Modified Czapex Dox (McDox) medium. The pink zone diameter varied from 15.3 to 58.2 mm (Table 3). Fusarium proliferatum showed maximum enzyme activity (Fig. 1), followed by Plenodomus tracheiphilus. All six Fusarium species in this study could produce L-asparaginase and the pink zones were measured above 27.3 mm. Forty-six isolates did not produce L-asparaginase, including all endophytic fungi isolated from M. parthenium. Also, our results demonstrated that the basidiomycetous endophytes did not produce L-asparaginase. Thirty-eight fungal strains exhibiting positive enzyme activities were selected for quantitative assay of L-asparaginase.
Table 3. Fungal endophytic strains from various medicinal plants and their L-asparaginase activity.
Least Significant Difference (LSD) test; The results with different superscripts were different significantly (p < 0.01) according to LSD test.
| Isolate code | Fungus | Host plant | GenBank accession number | Similarity (%) | Qualitative assay (mm) | L-asparaginase Enzyme in unit/mL | LSD test |
|---|---|---|---|---|---|---|---|
| Br08 | Fusarium proliferatum | Anthemis altissima | MH245099 | 100 | 58.2 | 0.492 | A |
| Br12 | Plenodomus tracheiphilus | A. altissima | MH245100 | 99 | 58.1 | 0.481 | b |
| k100 | Torula herbarum | Cichorium intybus | MH258980 | 100 | 57.1 | 0.442 | c |
| Br18 | Fusarium avenaceum | A. altissima | MH245076 | 99 | 57.2 | 0.424 | d |
| Am72 | Fusarium oxysporum | Achillea millefolium | MH259174 | 100 | 42.4 | 0.332 | e |
| Br15 | Cladosporium limoniforme | A. altissima | MH245072 | 100 | 42.1 | 0.309 | f |
| Am13 | Fusarium redolens | A. millefolium | MH259166 | 99 | 37.7 | 0.252 | g |
| Am91 | Alternaria infectoria | A. millefolium | MH259179 | 100 | 37.3 | 0.244 | h |
| AS26 | Fusarium sp. | Achillea filipendulina | MH250005 | 98 | 37.3 | 0.242 | h |
| AM55 | Cladosporium ramotenellum | A. millefolium | MH259170 | 100 | 37.2 | 0.232 | i |
| BB05 | Chaetosphaeronema hispidulum | Anthemis triumfetii | MH245081 | 100 | 36.4 | 0.224 | ij |
| Am03 | Septoria sp. | A. millefolium | MH259176 | 99 | 35.3 | 0.208 | k |
| k11 | Alternaria embellisia | C. intybus | MH258981 | 99 | 35.3 | 0.203 | l |
| BB28 | Alternaria sp. | A. altissima | MH245085 | 98 | 35.2 | 0.202 | l |
| K24 | Plectosphaerella cucumerina | C. intybus | MH258974 | 100 | 27.5 | 0.192 | m |
| Am87 | Fusarium sp. | A. millefolium | MH259177 | 98 | 27.3 | 0.187 | mn |
| BA18 | Epicoccum nigrum | Matricaria chamomilla | MH245107 | 100 | 27.1 | 0.166 | 0 |
| Br42 | Didymella tanaceti | A. altissima | MH245108 | 100 | 26.8 | 0.157 | p |
| Br09 | Verticillium dahliae | A. altissima | MH245075 | 100 | 26.4 | 0.155 | p |
| Am39 | Paraophiobolus arundinis | A. millefolium | MH259168 | 100 | 26.1 | 0.146 | q |
| Br41 | Ulocladium consortiale | A. altissima | MH245090 | 99 | 26.1 | 0.145 | q |
| Am04 | Septoria malagutii | A. millefolium | MH259172 | 100 | 26 | 0.144 | q |
| Ba24 | Didymella tanaceti | M. chamomilla | MH245097 | 99 | 26 | 0.143 | q |
| BB26 | Stemphylium amaranthi | A. triumfetii | MH245085 | 100 | 25.7 | 0.132 | r |
| Br38 | Aspergillus calidoustus | A. altissima | MH245078 | 100 | 25.7 | 0.131 | r |
| Am64 | Nemania serpens | A. millefolium | MH259183 | 100 | 25.5 | 0.125 | s |
| k29 | Alternaria solani | C. intybus | MH258977 | 99 | 25.4 | 0.123 | s |
| BA06 | Phoma haematocycla | M. chamomilla | MH245096 | 100 | 25.1 | 0.112 | t |
| As01 | Antennariella placitae | A. filipendulina | MH250008 | 100 | 24.8 | 0.108 | w |
| Am88 | Alternaria burnsii | A. millefolium | MH259181 | 99 | 24.8 | 0.107 | w |
| Am28 | Stemphylium amaranthi | A. millefolium | MH259169 | 100 | 24.7 | 0.107 | w |
| As16 | Acremonium sclerotigenum | A. filipendulina | MH250010 | 100 | 24.5 | 0.106 | w |
| Br92 | Lewia infectoria | A. altissima | MH245070 | 99 | 24.5 | 0.105 | w |
| BR25 | Paraphoma sp. | A. altissima | MH245091 | 98 | 21.4 | 0.083 | x |
| Br31 | Sarocladium strictum | A. altissima | MH245074 | 100 | 21.1 | 0.079 | x |
| K15 | Cladosporium tenuissimum | C. intybus | MH258971 | 100 | 18.2 | 0.029 | y |
| Br34 | Stemphylium botryosum | A. altissima | MH245094 | 100 | 17.9 | 0.027 | y |
| Am51 | Septoria tormentillae | A. millefolium | MH259171 | 99 | 15.3 | 0.019 | z |
Figure 1. A maximum parsimony phylogeny for Fusarium proliferatum from ITS (Internal transcribed spacer).
Phylogenetic position of isolate MH245099 was highlighted. Bootstrap tests were performed with 1,000 replications. Fusarium staphyleae (MH862478) was used as an outgroup.
Estimation of L-asparaginase production by Nesslerization
L-asparaginase activities of thirty-eight fungal endophytes were recorded to range of 0.019–0.492 unit/mL−1 (Table 3). The isolates of F. proliferatum obtained from A. altissima, exhibited a maximum enzyme activity with 0.492 unit/mL−1, followed by P. tracheiphilus isolated from A. altissima (0.481 unit/mL−1). F. oxysporum and Cladosporium limoniforme exhibited moderate enzyme activity, while Septoria tormentillae showed the least activity with 0.019 unit/mL−1 of enzyme (Fig. 2). Results showed that there were significant differences among the isolates at 1% (Table 3). The percentage of L-asparaginase-producing fungal endophytes was 45.2% of the total isolated endophytes (84 isolates) with 2.5%, 3.5%, 15%, 14.2%, 3.5% and 6.5% respectively for M. chamomilla, A. triumfetii, A. altissima, A. millefolium, A. filipendulina and C. intybus.
Figure 2. L-asparaginase activity detected by plate assay.
Colour change in the medium (yellow to pink) around colony indicates production of enzyme. (A) Isolates showing high production of L-asparaginase; (B) non-producer isolates.
Discussion
Eighty-four fungal endophytes belonging to Ascomycota (95%) and Basidiomycota (5%) were obtained from seven medicinal plants in Iran. All species obtained in the present study are reported for the first time as endophytes from M. chamomilla, M. parthenium, A. triumfetii, A. altissima, A. millefolium, A. filipendulina and C. intybus. In recent years, most records of fungal endophytes are Ascomycota (Carroll, 1988; Rodrigues, 1994; Gonthier, Gennaro & Nicolotti, 2006; Arnold, 2007) with a few species of Basidiomycota (Petrini, 1986; Chapela & Boddy, 1988; Oses et al., 2006; Sánchez Márquez, Bills & Zabalgogeazcoa, 2007). Endophytic Basidiomycota such as T. versicolor, B. adusta, T. biforme and S. commune were isolated from medicinal plants during this study are white- rot fungi. This finding is consistent with research that showed that most basidiomycetous endophytes, although not pathogenic in some hosts, incite white-rot in decaying wood (Oses et al., 2006; Thomas et al., 2008).
The frequency of colonization of endophytic fungi was higher in the stem compared to the other plant tissues. Previous studies have also reported high colonization frequency of endophytic fungi in stem tissues (Bezerra et al., 2015). Bezerra et al. (2015) extrapolated that highest frequency of colonization in the stem may be due to spore abundance of a few dominant endophytes in stem tissue. Similarly, Verma et al. (2013) demonstrated that the diversity of endophytic fungi was highest in the stem. The diversity and frequency of colonization of fungal endophytes are influenced by the host tissue (Rodrigues, 1994) and environmental factors (Clay, 1986). However, most studies reported that leaf tissues yield a higher diversity of endophytes (Verma et al., 2007; Gond et al., 2012).
In our study, most of the fungal species (e.g., T. versicolor) were isolated from a single host. In contrast, few fungal species, such as A. sclerotigenum, were common in multiple host plants. Endophytic assemblages tend to be distributed in specific hosts and specific tissues (Siqueira et al., 2011; Xing, Guo & Fu, 2010). Some dominant endophytes have been recovered from every part of plants such as F. avenaceum. This may be due to the ability of endophytes to penetrate from one part of plant to another (Manasa & Nalini, 2014).
L-asparaginase is one of the most effective antineoplastic agents for the treatment of acute leukemia (Nakamura, Wilkinson & Woodruff, 1999). It is produced by plants and a variety of microbial sources including fungi (Serquis & Oliveira, 2004). Endophytes from medicinal plants are rich sources of novel compounds (Hwang et al., 2011). In this study, L-asparaginase activities of the fungal endophytes from different medicinal plant species were evaluated. Eighty-four fungal endophytes were examined for the L-asparaginase activity. Thirty-eight of these demonstrated the ability to metabolize L-asparagine. The fungi that were good producers of this enzyme belonged to the genus Fusarium, followed by species of Alternaria and Cladosporium. Furthermore, these species has been reported to produce L-asparaginase (Serquis & Oliveira, 2004; Theantana, Hyde & Lumyong, 2009). L-asparaginase activity was not observed in the endophytes from M. parthenium. This may be due to the low diversity of endophytes that were obtained from this medicinal plant. Although, the S. tormentillae showed pink zones in the agar assay, enzymatic activity was low based on further quantitative analysis. The reason for the absence of enzyme activity in the quantitative estimation may be attributed to differences in the ability of the fungi to produce the enzyme in solid and liquid states (Holker, Hofer & Lenz, 2004). According to available literature, this is the first record of L-asparaginase production by endophytic fungi of the host plants examined in the present study.
Conclusions
Studies here revealed that the diversity of some endophytic fungal communities was influenced by host plants and tissues. We isolated numerous fungal endophytes from seven healthy medicinal plants of Iran. Endophytes that were able to produce L-asparaginase belonged to the genera Plectosphaerella, Fusarium, Stemphylium, Septoria, Alternaria, Didymella, Phoma, Chaetosphaeronema, Sarocladium, Nemania, Epicoccum, Ulocladium and Cladosporium. An isolate of Fusarium proliferatum was found to have the highest L-asparaginase enzyme activity. Our findings are consistent with the hypothesis that endophytes associated with medicinal plants have potential medicinal properties. We found that production of L-asparaginase by endophytic fungi may provide an alternative source for this enzyme. Further studies involving enzyme isolation are necessary to prove the utility of the L-asparaginases derived from fungal endophytes.
Supplemental Information
Funding Statement
This work was supported by grants to the first author Sareh Hatamzadeh from the postgraduate committee of the Research Vice President of Gorgan university of Agricultural Sciences and Natural Resources, Iran. This work was also supported by the New Jersey Agricultural Experiment Station and Multistate (Project number 4147), United States of America. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Sareh Hatamzadeh conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Kamran Rahnama conceived and designed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Saeed Nasrollahnejad analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Khalil Berdi Fotouhifar, Khodayar Hemmati and Fakhtak Taliei performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
James F. White conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data are available as a Supplemental File.
References
- Aly, Debbab & Proksch (2011).Aly AH, Debbab A, Proksch P. Fungal endophytes: unique plant inhabitants with great promises. Applied Microbiology and Biotechnology. 2011;90:1829–1845. doi: 10.1007/s00253-011-3270-y. [DOI] [PubMed] [Google Scholar]
- Arnold (2007).Arnold AE. Understanding the diversity of foliar endophytic fungi: progress, challenges, and frontiers. Fungal Biology Review. 2007;21:51–66. doi: 10.1016/j.fbr.2007.05.003. [DOI] [Google Scholar]
- Asthana & Azmi (2003).Asthana N, Azmi W. Microbial L-asparaginase: a potent antitumour enzyme. Indian Journal of Biotechnology. 2003;2:184–194. [Google Scholar]
- Barnett & Hunter (1999).Barnett HL, Hunter BB. Illustrated genera of imperfect fungi. American Phytopathological Society press; Minnesota: 1999. [Google Scholar]
- Batool et al. (2015).Batool T, Makky EA, Jalal M, Yusoff MM. A comprehensive review on L-asparaginase and its applications. Applied Biochemistry Biotechnology. 2015;178(5):900–923. doi: 10.1007/s12010-015-1917-3. [DOI] [PubMed] [Google Scholar]
- Bensch et al. (2012).Bensch K, Braun U, Groenewald JZ, Crous PW. The genus Cladosporium. Studies in Mycology. 2012;72:1–401. doi: 10.3114/sim0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra et al. (2015).Bezerra JDP, Santos MGS, Barbosa RN, Svedese VM, Lima DMM, Fernandes MJS, Gomes BS, Paiva LM, Almeida-Cortez JS, Souza-Motta CM. Endophytic fungi from medicinal plant Bauhinia forficata: diversity and biotechnological potential. Brazilian Journal of Microbiology. 2015;46(1):49–57. doi: 10.1590/S1517-838246120130657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerema et al. (2004).Boerema GH, Gruyter J, Noordeloos ME, Hamers MEC. Phoma identification manual differentiation of specific and infra-specific taxa in culture. CABI Publisher; Oxfordshire: 2004. [Google Scholar]
- Booth (1971).Booth C. The genus Fusarium. Commonwealth Mycological Institute Press; Kew: 1971. [Google Scholar]
- Bungihan et al. (2011).Bungihan ME, Tan MA, Kitajima M, Kogure N, Franzblau SG, Cruz TE, Takayama H, Nonat MG. Bioactive metabolites of Diaporthe sp. Journal of Natural Medicine. 2011;65:606–609. doi: 10.1007/s11418-011-0518. [DOI] [PubMed] [Google Scholar]
- Carroll (1988).Carroll GC. Fungal endophytes in stems and leaves: from latent pathogen to mutualistic symbiont. Journal of Ecology. 1988;69:2–9. doi: 10.2307/1943154. [DOI] [Google Scholar]
- Chapela & Boddy (1988).Chapela IH, Boddy L. Fungal colonization of attached beech branches. I. Early stages of development of fungal communities. The New Phytologist. 1988;110:39–45. doi: 10.1111/j.1469-8137.1988.tb00235.x. [DOI] [Google Scholar]
- Clay (1986).Clay K. Grass endophytes. In: Fokkema NJ, Van den Heuvel J, editors. Microbiology of the phyllosphere. Cambridge University Press; Cambridge: 1986. [Google Scholar]
- Dayle et al. (2001).Dayle ES, Poiiliis NO, Paul DS, Melvin RD. Angiosperm DNA contamination by endophytic fungi: detection and methods of avoidance. Plant Molecular Biology Reporter. 2001;19:249–260. doi: 10.1007/BF02772897. [DOI] [Google Scholar]
- Debbab et al. (2009).Debbab A, Aly AH, Edrada RA, Wray V, Werner EGM, Totzke F, Zirrgiebel U, Schachtele CH, Kubbutat MHG, Lin WH, Mosaddak M, Hakiki A, Proksch P, Ebel R. Bioactive metabolites from the endophytic fungus stemphylium globuliferum isolated from Mentha pulegium. Journal of Natural Product. 2009;72:626–631. doi: 10.1021/np8004997. [DOI] [PubMed] [Google Scholar]
- Gond et al. (2012).Gond SK, Mishra A, Sharma VK, Verma SK, Kumar J, Kharwar RN, Kumar A. Diversity and antimicrobial activity of endophytic fungi isolated from Nyctanthes arbor-tristis, a well-known medicinal plant of India. Mycoscience. 2012;53:113–121. doi: 10.1007/S10267-011-0146-Z. [DOI] [Google Scholar]
- Gonthier, Gennaro & Nicolotti (2006).Gonthier P, Gennaro M, Nicolotti G. Effects of water stress on the endophytic mycota of Quercus robur. Fungal Diversity. 2006;21:69–80. [Google Scholar]
- Gulati, Saxena & Gupta (1997).Gulati R, Saxena RK, Gupta R. A rapid plate assay for screening L-asparaginase producing micro-organisms. Letters in Applied Microbiology. 1997;24:23–26. doi: 10.1046/j.1472-765X.1997.00331. [DOI] [PubMed] [Google Scholar]
- Guo et al. (2008).Guo B, Wang Y, Sun X, Tang K. Bioactive natural products from endophytes: a review. Applied Biochemistry and Microbiology. 2008;44(2):136–142. doi: 10.1134/S0003683808020026. [DOI] [PubMed] [Google Scholar]
- Gutierrez, Gonzalez & Ramirez (2012).Gutierrez RMP, Gonzalez AMN, Ramirez AM. Compounds derived from endophytes: a review of phytochemistry and pharmacology. Current Medicinal Chemistry. 2012;19(18):2992–3030. doi: 10.2174/092986712800672111. [DOI] [PubMed] [Google Scholar]
- Holker, Hofer & Lenz (2004).Holker U, Hofer M, Lenz J. Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Applied Microbiology and Biotechnology. 2004;64:175–186. doi: 10.1007/s00253-003-1504-3. [DOI] [PubMed] [Google Scholar]
- Hosamani & Kaliwal (2011).Hosamani R, Kaliwal BB. Isolation, molecular identification and optimization of fermentation parameters for the production of L-asparaginase, an anticancer agent by Fusarium equisetii. International Journal of Microbiology Research. 2011;3:108–119. doi: 10.9735/0975-5276.3.2.108-119. [DOI] [Google Scholar]
- Hwang et al. (2011).Hwang JS, You YH, Bae JJ, Khan SA, Kim JG, Choo YS. Effects of endophytic fungal secondary metabolites on the growth and physiological response of Carex kobomugi. Coastal Journal of Coastal Research. 2011;27:544–548. doi: 10.2112/JCOASTRES-D-10-00090.1. [DOI] [Google Scholar]
- Imada et al. (1973).Imada A, Igarasi S, Nakahama K, Isono M. Asparaginase and glutaminase activities of micro-organisms. Journal of General and Applied Microbiology. 1973;73:85–99. doi: 10.1099/00221287-76-1-85. [DOI] [PubMed] [Google Scholar]
- Khan et al. (2010).Khan R, Shahzad S, Choudhary M, Shakeel I, Khan A, Aqeel A. Communities of endophytic fungi in medicinal plant Withania somnifera. Pakistan Journal of Botany. 2010;42:1281–1287. [Google Scholar]
- Manasa & Nalini (2014).Manasa C, Nalini MS. L-Asparaginase Activity of Fungal Endophytes from Tabernaemontana heyneana Wall. (Apocynaceae), Endemic to the Western Ghats (India) International Scholarly Research Notices. 2014;2:1–7. doi: 10.1155/2014/925131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCredie & Ho (1973).McCredie KB, Ho DHW. L-Asparaginase for treatment of cancer. Cancer Journal for Clinicians. 1973;23:220–227. doi: 10.3322/canjclin.23.4.220. [DOI] [PubMed] [Google Scholar]
- Nakamura, Wilkinson & Woodruff (1999).Nakamura CT, Wilkinson R, Woodruff K. Pancreatitis and perotitis following therapy with L-asparaginase. International Journal of Pediatrics. 1999;14:25–27. [Google Scholar]
- O’Donnell & Cigelnik (1997).O’Donnell K, Cigelnik E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus fusarium are nonorthologous. Molecular Phylogenetics and Evolution. 1997;7:103–107. doi: 10.1006/mpev.1996.0376. [DOI] [PubMed] [Google Scholar]
- Oses et al. (2006).Oses R, Valenzuela S, Freer J, Baesa J, Rodriguez J. Evaluation of fungal endophytes for lignocellulolytic enzyme production and wood biodegradation. International Biodeterioration and Biodegradation. 2006;57:129–135. doi: 10.1016/j.ibiod.2006.01.002. [DOI] [Google Scholar]
- Patil, Patil & Mahjeshwari (2012).Patil MP, Patil RH, Mahjeshwari VL. A novel and sensitive agar plug assay for screening of asparagine-producing endophytic fungi from Aegle marmelos. Acta Biologica Szegediensis. 2012;56:175–177. [Google Scholar]
- Petrini (1986).Petrini O. Taxonomy of endophytic fungi of aerial plant tissues. In: Fokkema NJ, Van den Heuvel J, editors. Microbiology of the phyllosphere. Cambridge University Press; Cambridge: 1986. pp. 175–187. [Google Scholar]
- Ratklao (2013).Ratklao S. Screening of novel secondary metabolites from endophytic fungi by chemical library analysis. Osaka University Knowledge Archives. 2013 doi: 10.4172/2469-9853.1000135. [DOI] [Google Scholar]
- Rehner & Buckley (2005).Rehner SA, Buckley EA. Beauveria phylogeny inferred from nuclear ITS and EF1- α sequences: evidence for cryptic diversification and links to cordyceps teleomorphs. Mycologia. 2005;97:84–98. doi: 10.3852/mycologia.97.1.84. [DOI] [PubMed] [Google Scholar]
- Rodrigues (1994).Rodrigues KF. The foliar fungal endophytes of the Amazonian palm Euterpe oleracea. Mycologia. 1994;83:376–385. [Google Scholar]
- Sánchez Márquez, Bills & Zabalgogeazcoa (2007).Sánchez Márquez S, Bills GF, Zabalgogeazcoa I. The endophytic mycobiota of the grass Dactylis glomerata. Fungal Diversity. 2007;27:171–195. [Google Scholar]
- Serquis & Oliveira (2004).Serquis M, Oliveira EMM. Production of L-asparaginase by filamentous fungi. Memórias do Instituto Oswaldo Cruz. 2004;99:489–492. doi: 10.1590/S0074-02762004000500005. [DOI] [PubMed] [Google Scholar]
- Simmons (2007).Simmons EG. Alternaria: an identification manual. CBS Fungal Biodiversity Centre; Utrecht: 2007. [Google Scholar]
- Siqueira et al. (2011).Siqueira VM, Conti R, Araújo JM, Souza-Motta CM. Endophytic fungi from the medicinal plant Lippia sidoides Cham and their antimicrobial activity. Symbiosis. 2011;53:89–95. doi: 10.1007/s13199-011-0113-7. [DOI] [Google Scholar]
- Strobel et al. (2004).Strobel GA, Daisy B, Castillo U, Harper J. Natural products from endophytic microorganisms. Journal of Natural Products. 2004;67:257–268. doi: 10.1021/np030397. [DOI] [PubMed] [Google Scholar]
- Theantana, Hyde & Lumyong (2007).Theantana T, Hyde KD, Lumyong S. Asparaginase production by endophytic fungi isolated some Thai medicinal plants. KMITL Science and Technology Journal. 2007;7:13–18. [Google Scholar]
- Theantana, Hyde & Lumyong (2009).Theantana T, Hyde KD, Lumyong S. Asparaginase production by endophytic fungi from Thai medicinal plants: cytotoxicity properties. International Journal of Integrative Biology. 2009;7:1–8. [Google Scholar]
- Thomas et al. (2008).Thomas SE, Crozier J, Aime MC, Evans HC, Holmes KA. Molecular characterization of fungal endophytic morphospecies associated with the indigeneous forest tree, Theobroma gileri, in Ecuador. Mycological Research. 2008;112:852–860. doi: 10.1016/j.mycres.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Verma et al. (2013).Verma SK, Gond SK, Mishra A, Sharma VK, Kumar J, Singh DK, Kumar A, Goutam J, Kharwar RN. Impact of environmental variables on the isolation, diversity and antibacterial activity of endophytic fungal communities from Madhuca indica Gmel. at different locations in India. Annals of Microbiology. 2013;51:469–476. doi: 10.1007/s13213-013-0707-9. [DOI] [Google Scholar]
- Verma et al. (2007).Verma VC, Gond SK, Kumar A, Kharwar RN, Strobel GA. The endophytic mycoflora of bark, leaf, and stem tissues of Azadirachta indica A. Juss (Neem) from Varanasi (India) Microbial Ecology. 2007;54:119–125. doi: 10.1007/s00248-006-9179-9. [DOI] [PubMed] [Google Scholar]
- Vilgalys & Hester (1990).Vilgalys R, Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology. 1990;172:4239–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waksman (1916).Waksman SA. Do fungi live and produce mycelium in the soil? Science. 1916;44:320–322. doi: 10.1126/science.44.1131.320. [DOI] [PubMed] [Google Scholar]
- White et al. (1990).White TJ, Bruns T, Lee S, Taylor JW. Amplification and direct sequencing of fungal ribosomal RNA Genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. Academic Press; New York: 1990. pp. 315–322. [Google Scholar]
- Xing, Guo & Fu (2010).Xing X, Guo S, Fu J. Biodiversity and distribution of endophytic fungi associated with Panax quinquefolium L. cultivated in a forest reserve. Symbiosis. 2010;51:161–166. doi: 10.1007/s13199-010-0062-6. [DOI] [Google Scholar]
- Xu, OrunaConcha & Elmore (2016).Xu F, OrunaConcha MJ, Elmore JS. The use of asparaginase to reduce acrylamide levels in cooked food. Food Chemistry. 2016;210(1):163–171. doi: 10.1016/j.foodchem.2016.04.105. [DOI] [PubMed] [Google Scholar]
- Zhang, Song & Tan (2006).Zhang HW, Song YC, Tan RX. Biology and chemistry of endophytes. Journal of Natural products. 2006;23:753–771. doi: 10.1039/b609472b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are available as a Supplemental File.