Abstract
DNA methylation is a highly conserved epigenetic modification involved in many biological processes, including growth and development, stress response, and secondary metabolism. In the plant kingdom, cytosine-5 DNA methyltransferase (C5-MTase) and DNA demethylase (dMTase) genes have been identified in some plant species. However, to the best of our knowledge, no investigator has focused on the identification and analysis of C5-MTase and dMTase genes in tea plants (Camellia sinensis) based on genome-wide levels. In this study, eight CsC5-MTases and four dMTases were identified in tea plants. These CsC5-MTase genes were divided into four subfamilies, including CsMET, CsCMT, CsDRM and CsDNMT2. The CsdMTase genes can be classified into CsROS, CsDME and CsDML. Based on conserved domain analysis of these genes, the gene loss and duplication events occurred during the evolution of CsC5-MTase and CsdMTase. Furthermore, multiple cis-acting elements were observed in the CsC5-MTase and CsdMTase, including light responsiveness, phytohormone responsiveness, stress responsiveness, and plant growth and development-related elements. Then, we investigated the transcript abundance of CsC5-MTase and CsdMTase under abiotic stress (cold and drought) and withering processing (white tea and oolong tea). Notably, most CsC5-MTases, except for CsCMT1 and CsCMT2, were significantly downregulated under abiotic stress, while the transcript abundance of all four CsdMTase genes was significantly induced. Similarly, the same transcript abundance of CsC5-MTase and CsdMTase was found during withering processing of white tea and oolong tea, respectively. In total, our findings will provide a basis for the roles of CsC5-MTase and CsdMTase in response to abiotic stress and the potential functions of these two gene families in affecting tea flavor during tea withering processing.
Keywords: Camellia sinensis, C5-MTase, dMTase, Stress response, Withering processing, Transcript abundance
Introduction
Epigenetics is the mechanism by which gene expression and phenotype are changed without alterations in DNA sequence, and these changes can be passed on continuously with cell mitosis and meiosis. DNA methylation is not only the most common epigenetic modification but also is an important link between phenotype and genotype. Increasingly abundant evidence demonstrates that DNA methylation can be divided into three types, namely, 5-methylcytosine (5mC), N6-methyladenine and N4-methylcytosine (Chen, Zhao & He, 2016; Ratel et al., 2006; Zhang et al., 2015). In the plant kingdom, 5mC is the predominant form of DNA methylation, which transfers the methyl group from S-adenosyl methionine to the carbon-5 (C-5) atom in the pyrimidine ring of cytosine residues. Unlike in animals, 5mC methylation in plants usually occurs in the CG, CHG and CHH contexts (H represents A, C or T), where the methylation status of the CG context is most stable (Cokus et al., 2008). It has been reported that DNA methylation also plays a crucial role in genomic imprinting, X chromosome inactivation, transposon suppression, and gene silencing (Chan, Henderson & Jacobsen, 2005; Law & Jacobsen, 2010).
In plants, DNA methylation can be divided into two types: DNA maintenance methylation and de novo DNA methylation. Among these types, DNA methylation is mainly regulated by cytosine-5 DNA methyltransferase (C5-MTase), including methyltransferase (MET), chromomethylase (CMT), domains rearranged methylase (DRM), and de novo DNA methyltransferase 2 (DNMT2). The MET gene family was first originally isolated from Arabidopsis thaliana, including AtMET1, AtMET2a, AtMET2b and AtMET3 (Genger et al., 1999). In the plant kingdom, MET is a major regulatory gene affecting the DNA methylation status of plants and plays a major role in maintaining methylation in the symmetric CG context. CMT is a plant-specific DNA methyltransferase whose main function is to maintain methylation in CHG contexts through a reinforcing loop between CHG methylation and histone H3 lysine 9 (H3K9) methylation (Du et al., 2012, 2014). In addition, previous research has shown that CMT2 can participate in the maintenance of CHH methylation in plants (Zemach et al., 2013). A total of three CMT genes from A. thaliana were identified by genome sequencing, namely, AtCMT1, AtCMT2 and AtCMT3. In CMT mutants of A. thaliana, both CHG and CHH methylation decreased sharply (Stroud et al., 2013; Zemach et al., 2013). Similarly, it has been reported that the absence of CMT2 in Zea mays also results in the depletion of CHG methylation (Papa et al., 2001). Moreover, previous research has shown that DNMT2 also has C5-MTase activity (Tang et al., 2003). DNMT2 is the smallest MET gene family found in different eukaryotes. Due to the considerable variability in the specific target recognition domain, DNMT2 is also involved in RNA methylation modification (Jeltsch et al., 2017; Vieira et al., 2017). DRM is homologous to DNMT3 in mammals, and both differ only in the order of related catalytic domains (Cao et al., 2000). In plants, DRM is primarily responsible for catalyzing de novo DNA methylation in all known DNA sequence contexts (CG, CHG and CHH) through the RNA-directed DNA methylation pathway. DRM2 also plays a vital regulatory role in maintaining methylation in the asymmetric CHH context (Gent et al., 2013). After the targeted destruction of the DRM2 gene in rice, it was found that the genomic DNA methylation level was abnormal and adversely affected rice growth (Moritoh et al., 2012). In addition, double mutants (drm1–drm2) block de novo DNA methylation, resulting in a significant reduction in CHG methylation (Cao & Jacobsen, 2002).
The level of DNA methylation in plants is dynamic and depends not only on the establishment and maintenance of DNA methylation but also on DNA demethylation. DNA demethylation can be divided into passive demethylation and active demethylation. After DNA replication, the newly synthesized DNA strand cannot be methylated due to the maintenance of DNA methyltransferase inactivation, which is called passive methylation. Active demethylation is not dependent on DNA replication and is caused by a series of DNA demethylase (dMTase) catalysis. Therefore, the overall level of genomic DNA methylation is affected by both DNA methylase and demethylase. In contrast to DNA methylation catalyzed by a single C5-MTase gene, a series of genes are required for DNA demethylation, including repressor of silencing 1 (ROS1), demeter (DME), demeter-like 2 (DML2) and demeter-like 3 (DML3) (Gong et al., 2002; Ortega-Galisteo et al., 2008). On the basis of the base excision repair mechanism, these dMTases recognize and excise the 5mC base and replace it with an unmethylated cytosine. In the ros1 mutant of A. thaliana, both CHH and CHG methylation increased in the transposon region, and many genes were found to be highly methylated and silenced at the transcriptional level (Wang et al., 2016a; Yamamuro et al., 2014). Moreover, the expression level of some genes in the triple mutant (ros1–dml2–dml3) of A. thaliana was suppressed due to hypermethylation of the upstream promoter regions (Zhang, Lang & Zhu, 2018). Additionally, previous studies have shown that DME was more associated with genomic demethylation during pollen development (Schoft et al., 2011; Zhu, 2009). Deletion of these four dMTase genes leads to increased genomic methylation levels and hypermethylation of locus-specific DNA (Penterman et al., 2007).
Extensive studies have shown that dynamic changes in plant methylation levels are importantly associated with various abiotic stresses, including drought stress (Liu et al., 2018a; Sanchez & Paszkowski, 2014), cold stress (Steward et al., 2002), salt stress (Su et al., 2018; Sun et al., 2018), and ultraviolet (UV)/light radiation stresses (Eichten & Springer, 2015; Ganguly et al., 2018). In addition, DNA methylation also plays an important role in regulating growth and development (Liu et al., 2015; Xing et al., 2015) and secondary metabolism (Bharti et al., 2015; Zhang et al., 2016). Since DNA methylation is involved in the regulation of a wide range of biological processes, C5-MTase and dMTase genes have been extensively identified and analyzed in several plant species, including A. thaliana (Ogneva, Dubrovina & Kiselev, 2016), Solanum lycopersicum (Cao et al., 2014), Arachis hypogaea (Wang et al., 2016b), Cynara cardunculus (Gianoglio et al., 2017) and Ricinus communis (Victoria et al., 2018). However, to the best of our knowledge, no investigator focused on the identification and analysis of C5-MTase and dMTase genes in tea plants based on genome-wide levels.
Tea plant (Camellia sinensis) is an important economic crop that originates in China. With the extreme global climate, cold stress and drought stress may adversely affect the growth and development of tea plants and the quality of tea products. Previous studies have shown that plant methylation plays a crucial role in response to abiotic stress (Zhang, Lang & Zhu, 2018). Additionally, a recent report revealed that methylation levels are affected by C5-MTase and dMTase genes, and DNA methylation regulating transposon silencing may play a crucial role in genome size expansion (Wang et al., 2019a). However, no research has been published on exploring the roles of C5-MTase and dMTase genes in tea plants under abiotic stress. Furthermore, withering is the first indispensable process for improving flavors in the postharvest processing of oolong tea and white tea, which is closely associated with their unique aromas and flavors. Similar to tea plants are affected by stress, and fresh leaves (FL) are also affected by various stresses during different types of withering. During solar-withering, tea leaves may suffer from different types of stresses, including drought, heat, and UV/light radiation. However, the tea leaves are primarily affected by drought stress during indoor withering. The functions of C5-MTase and dMTase genes in the tea withering process have not been determined. With the release of the tea reference genome (Wei et al., 2018; Xia et al., 2017), it is possible to accurately identify C5-MTase and dMTase genes in tea plants. In the present study, all members of C5-MTase and dMTase were identified in tea plants, and their physical and chemical characteristics, phylogenetic relationships, gene structures, protein–protein interaction, and cis-acting elements were investigated. Moreover, the transcript abundance of C5-MTase and dMTase genes under various stresses and withering processing were analyzed. Our analysis provides valuable information for screening the C5-MTase and dMTase genes and their transcript abundance under abiotic stress and withering processing in tea plants. The results of this study may help to further elucidate the functional roles of the C5-MTase and dMTase genes in tea plants.
Materials and Methods
Genome-wide identification of C5-MTase and dMTase genes in tea plants
To perform genome-wide identification of C5-MTase and dMTase genes in tea plants, the known C5-MTase and dMTase sequences in A. thaliana (Table S1) were downloaded from The Arabidopsis Information Resource database (https://www.arabidopsis.org). These sequences were used to search against the tea reference genome (Xia et al., 2017) using the BLAST algorithm (p-value < 1.0E−5). Then, we obtained the putative C5-MTase and dMTase sequences in tea plants. To further verify the accuracy of these sequences in tea plants, we also downloaded the relevant sequences of C5-MTase and dMTase in S. lycopersicum (Table S1) from the Sol Genomics Network database (https://solgenomics.net). We also used these sequences in S. lycopersicum to search against the tea reference genome. The ProtParam tool (https://www.expasy.org/) and WoLF PSORT server (https://wolfpsort.hgc.jp/) were used to analyze the physical and chemical characteristics and the subcellular localizations of CsC5-MTase and CsdMTase, respectively. The percentage identity matrix of CsC5-MTase and CsdMTase was analyzed using DNAMAN 7.0 software. Moreover, the domain contained in CsC5-MTase and CsdMTase was identified and analyzed using the PfamScan tool (https://www.ebi.ac.uk/Tools/pfa/pfamscan/).
Analysis of phylogenetic trees, gene structures, protein–protein interaction, and cis-acting elements
To further understand the classification of C5-MTase and dMTase genes, related C5-MTase and dMTase sequences from eight plants (Oryza sativa, Glycine max, Z. mays, C. sinensis, A. thaliana, R. communis, C. cardunculus and S. lycopersicum) were used to construct the phylogenetic tree using MEGA X software (Kumar et al., 2018) by the neighbor-joining method (bootstrap value = 1,000).
Based on genetic feature format data from the tea genome dataset, the gene structures of C5-MTase and dMTase in C. sinensis were analyzed using the Tbtools software (Chen et al., 2018). The motifs of C5-MTase and dMTase in C. sinensis were analyzed with MEME suite (Bailey et al., 2009), the maximum number of motifs was set as 10, and the remaining parameters were the default. The STRING 11 tool (https://string-db.org) was used to construct the protein–protein interaction network.
To analyze the cis-acting elements of C5-MTase and dMTase, the upstream sequences (2,000 bp) of the start codon were retrieved from the tea genome dataset, and then the cis-acting elements were analyzed using the PlantCARE tool (Lescot et al., 2002).
Tea plant materials and treatments
The tea cultivar “Tieguanyin” was cultivated at Fujian Agriculture and Forestry University, Fuzhou, Fujian Province, China (E 119°14′, N 26°05′). To detect the transcript abundance of CsC5-MTase and CsdMTase in tea plants under cold stress, tea plants were subjected to cold temperature (4 °C). For drought treatments, tea plants were irrigated with 15% (w/v) PEG 4000 to simulate drought stress (Zhou et al., 2019). Then, the treated tea leaves were sampled at 0, 12, 24, 36 and 48 h.
For withering treatment of oolong tea, the fresh shoot and first to third leaves were uniformly picked from each tea plant. Then, the tea leaves were equally divided into three parts, each weighing 2 kg. The first part was collected without any processing. The second part was subjected to solar withering under sunlight for 45 min (temperature 25 ± 2 °C, relative humidity 60 ± 5%, illumination intensity 40,000 ± 1,000 Lx, and leaf layered thickness 1 cm). The third part of tea leaves were evenly layered and exposed to indoor light for 45 min (illumination intensity 100 ± 5 Lx). The other parameters in the indoor-withering processing were all consistent with those in the solar-withering processing. The FL, indoor-withered leaves (IW), and solar-withered leaves (SW) were collected for analyses in this study. The withering processing of white tea was performed according to the previous method (Yue et al., 2019) with minor modifications. For the withering treatment of white tea, one bud and two leaves were uniformly picked from each tea plant. The leaves were left in a withering room. The withered leaves of white tea were collected at 0, 12, 24, 36 and 48 h after withering.
All samples were frozen in liquid nitrogen immediately and stored at −80 °C for further experiments. Each sample was performed in three independent biological replicates.
Total RNA extraction and transcript abundance analyses of C5-MTase and dMTase genes in tea plants under abiotic stress and withering processing
Total RNA was extracted using TransZol Up Reagent (TransGen Biotech, Beijing, China). The RNA integrity was checked by gel electrophoresis and micro-ultraviolet spectrophotometry (Nanodrop). Then, total RNA was reverse transcribed into first-strand cDNA quantitative real-time polymerase chain reaction (qRT-PCR) using a Transcript First-Strand cDNA Synthesis SuperMix (TransGen Biotech, Beijing, China). The qRT-PCR was performed using the LightCycler 480 platform (Roche Applied Sciences, Basel, Switzerland) with TransStart Tip Green qPCR SuperMix (TransGen Biotech, Beijing, China). The qRT-PCR procedure and reaction system were both used in the previous method (Guo et al., 2019). The glyceraldehyde-3-phosphate dehydrogenase and β-actin genes were used as reference genes. The transcript abundance was calculated using the 2−ΔΔCt method (Livak & Schmittgen, 2001), and all primers used for qRT-PCR were designed using the Tea Plant Information Archive platform (Xia et al., 2019) (Table S2). All qRT-PCR analyses were performed in three biological replications, respectively. Statistical analyses were conducted using SPSS 25 software, and the data were analyzed by one-way analysis of variance followed by Tukey’s post-hoc test.
Results
Genome-wide identification and sequence feature analysis of CsC5-MTase and CsdMTase genes
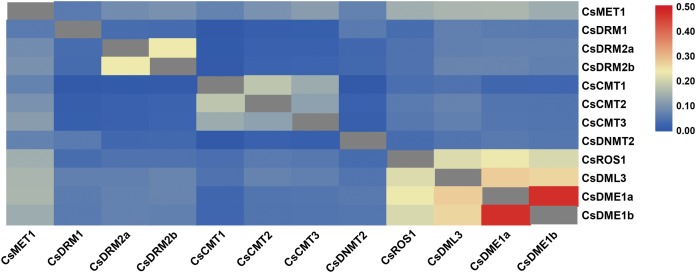
After searching the tea reference genome, a total of eight CsC5-MTase and four CsdMTase genes were identified in tea plants. Among these genes, CsC5-MTase included one CsMET gene, three CsDRM genes, three CsCMT genes, and one CsDNMT2 gene, while CsdMTase included one CsROS gene, one CsDML gene, and two CsDME genes. The sequence characteristic analysis showed that the open reading frames of eight CsC5-MTase genes ranged in size from 1,155 to 4,707 bp, while the ORF range of four CsdMTase genes was 3,015 to 5,856 bp (Table 1). The deduced proteins of CsC5-MTase varied between 384 and 1,568 amino acids. Among these proteins, the CsC5-MTase protein with the least number of amino acids is CsDNMT2, while CsMET1 contains the most amino acids. In CsdMTase proteins, protein length varied between 1,004 and 1,951 amino acids. The molecular weights of these proteins ranged from 42.99 to 217.83 kDa, and the theoretical pI values ranged from 5.03 to 8.19. Except for CsCMT1 and CsDME1a, the theoretical pI values of most CsC5-MTase and CsdMTase proteins were smaller than 7.0, indicating that these proteins were acidic. The instability index of CsDRM1, CsCMT1 and CsCMT3 were all smaller than 40, showing that these three proteins belong to stable protein, while the other nine proteins were unstable. According to grand average of hydropathicity (GRAVY) analysis, all CsC5-MTase and CsdMTase proteins were hydrophilic. In addition, the aliphatic index of these proteins ranged from 61.61 to 86.84. According to the result of putative subcellular localization, only CsDRM2b is located in the cytoplasm, whereas the other 11 proteins are localized in the nucleus. This result was consistent with previous findings that different RcDRM members are located in multiple organelles in R. communis (Victoria et al., 2018). The sequence similarity of these CsC5-MTase and CsdMTase proteins was also analyzed (Fig. 1). Compared with dMTase proteins, all C5-MTase proteins shared a low level of identity with each other, indicating that different members of C5-MTase proteins contain diverse functions. The sequences of CsDRM2a and CsDRM2b have significantly higher similarities than other CsC5-MTase proteins. In CsdMTase, CsDME1a and CsDME1b have the highest similarity. These results indicated that there are some duplicated genes in the CsC5-MTase and CsdMTase genes.
Table 1. Summary information of CsC5-MTase and CsdMTase genes in tea plant.
| Gene name | Genome ID | ORF (bp) | Amino acid (aa) | Molecular weight (kDa) | Theoretical pI | Instability index | Aliphatic index | GRAVY | Subcellular localization |
|---|---|---|---|---|---|---|---|---|---|
| CsMET1 | CSA018787.1 | 4,707 | 1,568 | 176.58 | 5.83 | 44.71 | 73.62 | −0.522 | Nucleus |
| CsDRM1 | CSA001898.1 | 1,410 | 469 | 52.98 | 7.17 | 39.64 | 86.84 | −0.325 | Nucleus |
| CsDRM2a | CSA001531.1 | 1,863 | 620 | 69.16 | 5.03 | 41.31 | 78.15 | −0.523 | Nucleus |
| CsDRM2b | CSA005657.1 | 1,875 | 624 | 70.69 | 5.12 | 43.38 | 80.96 | −0.382 | Cytoplasm |
| CsCMT1 | CSA032127.1 | 1,884 | 627 | 70.31 | 8.19 | 33.31 | 83.76 | −0.293 | Nucleus |
| CsCMT2 | CSA030966.1 | 2,511 | 836 | 93.66 | 5.77 | 45.56 | 80.45 | −0.384 | Nucleus |
| CsCMT3 | CSA021917.1 | 2,160 | 719 | 80.64 | 6.56 | 30.94 | 84.02 | −0.263 | Nucleus |
| CsDNMT2 | CSA000025.1 | 1,155 | 384 | 42.99 | 5.19 | 53.88 | 70.31 | −0.399 | Nucleus |
| CsROS1 | CSA023990.1 | 3,015 | 1,004 | 112.41 | 5.65 | 48.7 | 77.62 | −0.529 | Nucleus |
| CsDML3 | CSA015244.1 | 5,856 | 1,951 | 217.83 | 6.68 | 43.95 | 70.11 | −0.609 | Nucleus |
| CsDME1a | CSA015772.1 | 5,718 | 1,905 | 212.99 | 8.04 | 48.83 | 63.8 | −0.795 | Nucleus |
| CsDME1b | CSA017793.1 | 5,691 | 1,896 | 211.51 | 6.85 | 47.07 | 61.61 | −0.795 | Nucleus |
Figure 1. Sequence similarity of C5-MTases and dMTases in tea plant.
The colored bar indicates the correlation of two proteins. Blue represents a low correlation; red represents a high correlation; gray represents no correlation analysis on these proteins.
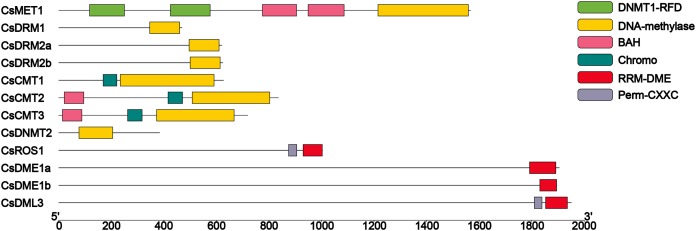
On the basis of structural domain analysis (Fig. 2), all of the C5-MTase included the DNA-methylase domain (PF00145). According to whether there are other domains and the location of the DNA-methylase domain in sequences, eight CsC5-MTases can be divided into four subfamilies, namely, MET, DRM, CMT and DNMT2. The CsMET harbors two DNMT1-RFD domains (PF12047), two BAH domains (PF01426) and one DNA-methylase domain (PF00145). Compared to CsCMTs, three CsDRMs and one CsDNMT2 each contained a single DNA-methylase domain (PF00145). All three CsMETs include one Chromo domain (PF00385) and one DNA-methylase domain (PF00145). Unlike CsCMT1, both CsCMT2 and CsCMT3 have a BAH domain (PF01426). Domain analysis of CsdMTase revealed that all four subfamilies of CsdMTase contain an RRM-DME domain (PF15628) at their C-terminus. Except for CsDME, CsDML3 and CsROS1 contain an extra Perm-CXXC domain. These results indicated that CsROS and CsDML are more structurally similar to each other than to CsDME.
Figure 2. Conserved domain analysis of CsC5-MTases and CsdMTases.
Phylogenetic classification, structures and motif analysis of CsC5-MTase and CsdMTase genes
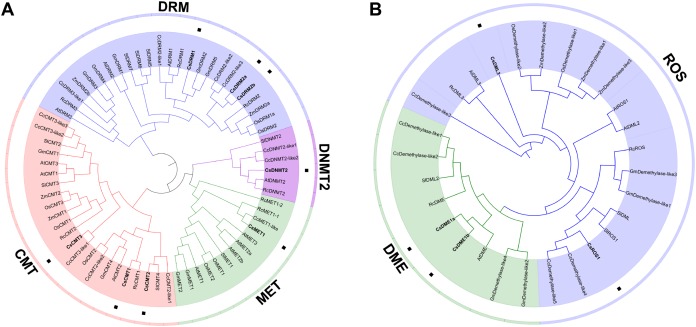
To elucidate the phylogenetic relationship of C5-MTase and dMTase in plants, 67 C5-MTase protein sequences and 28 dMTase protein sequences from two monocotyledons (O. sativa and Z. mays) and six dicotyledons (C. sinensis, A. thaliana, R. communis, C. cardunculus, S. lycopersicum, and G. max) were used to construct phylogenetic trees (Table S3). As a result, the C5-MTase can be naturally grouped into four categories, namely, DRM, CMT, MET and DNMT2 (Fig. 3A). Compared to the DRM category, the MET, CMT and DNMT2 categories were more similar and belonged to the same clade. DNMT2 was the smallest category in the phylogenetic tree and contained only six members from five plant species. In the CMT and MET categories, most of the CMTs and METs in O. sativa and Z. mays were clustered into a small branch, respectively. Compared to monocotyledons in these two categories, most CMTs and METs in dicotyledons also form a distinct branch. In the largest DRM category, there is also a divergence between monocotyledons and dicotyledons. This result is consistent with the findings of previous studies on S. lycopersicum (Cao et al., 2014). It is speculated that C5-MTase may have different functions in monocotyledons and dicotyledons.
Figure 3. Phylogenetic analysis of the CsC5-MTases (A) and CsdMTases (B).
Cs, Camellia sinensis; At, Arabidopsis thaliana; Rc, Ricinus communis; Cc, Cynara cardunculus; Sl, Solanum lycopersicum; Os, Oryza sativa; Zm, Zea mays; Gm, Glycine max.
In phylogenetic classification, 28 dMTase proteins can be divided into two categories (Fig. 3B). Compared to the DME category, ROSs and DMLs showed a closer relationship. Therefore, CsROS1 and CsDML3 were grouped into ROS category, while CsDME1a and CsDME1b were classified into another DME category. Compared to ROS category, the nine proteins in the DME category were more dense. Similar to the classification of monocotyledons in C5-MTases, all dMTases in two monocotyledons (O. sativa and Z. mays) also form a distinct branch.
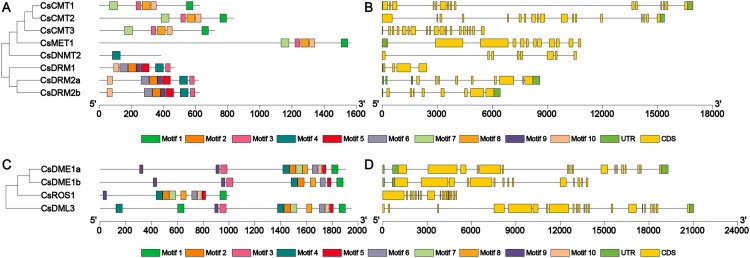
To further clarify the structural features of the CsC5-MTase and CsdMTase genes, the exon-intron structure of these genes was examined to elucidate the evolution of CsC5-MTase and CsdMTase in tea plants. The CsC5-MTase genes exhibited different exon-intron organizational patterns. In CsC5-MTase genes, CsCMT genes had the largest number of exons (Fig. 4). CsCMT1, CsCMT2 and CsCMT3 have 15, 18 and 19 exons, respectively. However, there are only five exons in CsDRM1. Moreover, the number of exons in the CsdMTase genes ranges from 15 to 22 (Fig. 4). Among them, the CsDML3 gene contains 22 exons, which is the largest number of exons in CsdMTase genes. However, there are only 15 exons in CsDME1b. Further analysis revealed that all exon-intron junction sites of the CsC5-MTase and CsdMTase genes are GT-AG sites, which is consistent with the GT-AG splice rules in eukaryotes (Modrek & Lee, 2002).
Figure 4. Phylogenetic relationships, conserved motifs, gene structures of CsC5-MTases and CsdMTases.
(A) Phylogenetic relationships and conserved motifs of CsC5-MTases. (B) Gene structures of CsC5-MTases. (C) Phylogenetic relationships and conserved motifs of CsdMTases. (D) Gene structures of CsdMTases.
Furthermore, the motifs in all members of CsC5-MTase and CsdMTase were analyzed. A total of 10 different motifs were identified in eight CsC5-MTase proteins (Table S4). Among the motifs, motifs 1, 3, 7, 8 and 10 were found in all CsCMTs and CsMET1. Hence, these two proteins were clustered together to be a small branch. The three CsDRMs were composed of 7 motifs, including motifs 3, 4, 5, 6, 8, 9 and 10. However, CsDNMT2 only contained motif 4, which has a large difference from the structure of CsDRMs. This finding may be why CsDNMT2 and CsDRMs belong to different branches. In the CsdMTase proteins, we also identified a total of 10 different motifs. Both CsDME1a and CsDME1b have all 10 motifs. In contrast, CsROS1 lacks motif 3, while CsDML3 has more than one motif 1 and 4. Therefore, both CsROS1 and CsDML3 differ from CsDMEs in their structure. To some extent, this finding is consistent with the results of the phylogenetic analysis. Consequently, CsC5-MTase and CsdMTase in the same cluster are similar in motif composition.
Protein–protein interaction of CsC5-MTase and CsdMTase
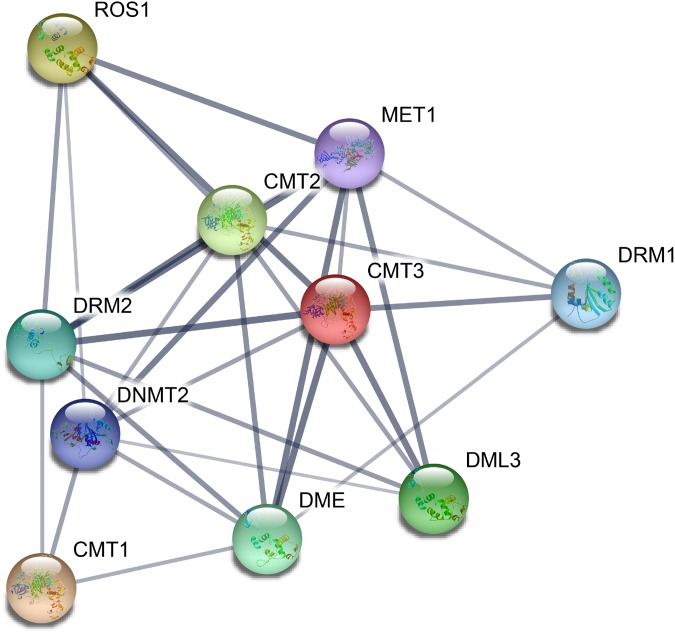
On the basis of the orthologs in A. thaliana, we constructed a protein–protein interaction network of CsC5-MTase and CsdMTase proteins using the STRING 11 tool. All eight CsC5-MTase and four CsdMTase are aligned to the corresponding AtC5-MTase and AtdMTase (Fig. 5). Among them, CsDRM2a and CsDRM2b were both homologous to AtDRM2. AtDME was the highest homologous protein of CsDME1a and CsDME1b. There were strong interactions among CMTs, METs and DRMs, and they may regulate the overall methylation level of plants by forming protein complexes. Moreover, we found that ROS1, DMEs and DMLs interact with three members of CMTs, indicating that plant methylation levels may be regulated by both C5-MTase and dMTase. Additionally, C5-MTase and dMTase may form a negative feedback loop that dynamically regulates the methylation level of plants.
Figure 5. Potential protein–protein interaction network of CsC5-MTases and CsdMTases.
Cis-acting element analysis of CsC5-MTase and CsdMTase genes
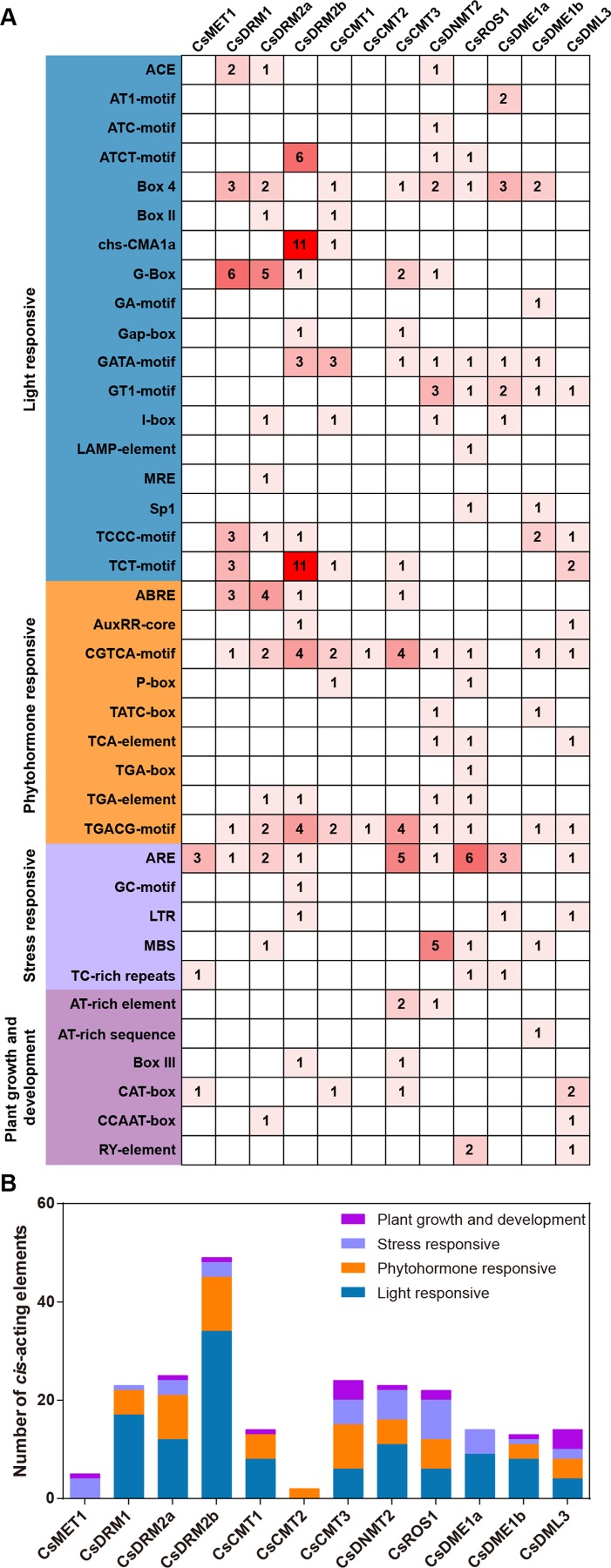
To investigate the potential biological functions of CsC5-MTase and CsdMTase in tea plants, the upstream genome sequences (2,000 bp) of the start codon were analyzed using the PlantCARE tool. The identified cis-acting elements of CsC5-MTase and CsdMTase were divided into four categories, including light responsiveness, phytohormone responsiveness, stress responsiveness, and plant growth and development-related elements (Fig. 6). Among these elements, ARE, GC-motif, LTR, MBS and TC-rich repeat elements belong to the stress responsiveness classification. Except for CsCMT1 and CsCMT2, the other 10 genes contain at least one cis-acting element involved in stress responsive classification, among which CsROS1 has the largest number of stress-responsive elements. This finding suggested that the transcript abundance of these CsC5-MTase and CsdMTase genes may be affected by diverse forms of stresses. The light responsive elements are present in all CsC5-MTase and CsdMTase genes, except CsMET1 and CsCMT2. In CsMET1 and CsDME1a, we did not find any phytohormone response-related elements, but other CsC5-MTase and CsdMTase genes contain several phytohormone response-related elements, such as ABRE, AuxRR-core, and CGTCA-motif elements.
Figure 6. Analysis of the cis-acting elements in the promoters of CsC5-MTases and CsdMTases.
(A) The numbers of different cis-acting regulatory elements in the promoters of CsC5-MTases and CsdMTases. (B) The cis-acting elements in four categories were represented by different colors.
Transcript abundance of CsC5-MTase and CsdMTase genes in tea plants under abiotic stress and withering processing
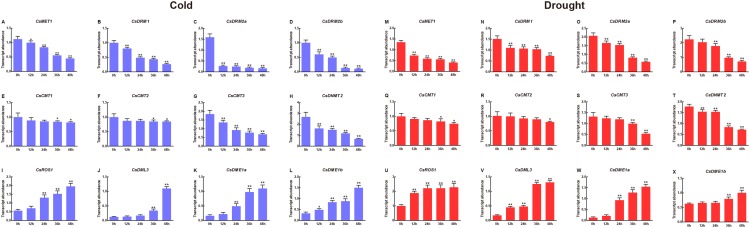
To clarify the potential roles of CsC5-MTase and CsdMTase genes involved in abiotic stress, we used qRT-PCR to determine the transcript abundance of CsC5-MTase and CsdMTase genes under cold and drought stresses. Under cold treatment, all members of the CsC5-MTase and CsdMTase genes showed significant changes in transcript abundance (Fig. 7). All eight CsC5-MTase genes were significantly reduced during cold treatment. At 12 h, the transcript abundance of these six genes (CsMET1, CsDRM1, CsDRM2a, CsDRM2b, CsCMT3 and CsDNMT2) was significantly downregulated, whereas CsCMT1 and CsCMT2 genes showed no significant change in transcript abundance. With the extension of cold stress time, the transcript abundance of all CsC5-MTase genes showed a trend of continuous decline and reached the lowest point of transcript abundance at 48 h. Except for CsCMT1 and CsMET2, the transcript abundance of six other CsC5-MTase genes was significantly suppressed at 48 h. In CsdMTase genes, the transcript abundance of all four genes was notably upregulated by cold treatment. The transcript abundance of CsROS1 and CsDME1a was significantly induced at 24 h, while the transcript abundance of CsDME1b was significantly increased at 12 h. Then, the transcript abundance of all CsdMTase genes peaked at 48 h, which was significantly higher than that at 0 h. Hence, CsC5-MTase and CsdMTase genes are cold stress-specific response genes.
Figure 7. Transcript abundance of CsC5-MTase and CsdMTase genes under cold stress and drought stress.
Transcript abundance of CsMET1 (A), CsDRM1 (B), CsDRM2a (C), CsDRM2b (D), CsCMT1 (E), CsCMT2 (F), CsCMT3 (G), CsDNMT2 (H), CsROS1 (I), CsDML3 (J), CsDME1a (K), and CsDME1b (L) under cold stress and CsMET1 (M), CsDRM1 (N), CsDRM2a (O), CsDRM2b (P), CsCMT1 (Q), CsCMT2 (R), CsCMT3 (S), CsDNMT2 (T), CsROS1 (U), CsDML3 (V), CsDME1a (W) and CsDME1b (X) under drought stress. Data are presented as mean ± standard deviation (SD). *indicates significant difference (p < 0.05) and **indicates highly significant difference (p < 0.01).
In response to drought stress, we observed that the transcript abundance of four CsC5-MTase genes (CsMET1, CsDRM1, CsDRM2a and CsDNMT2) changed immediately at 12 h, while the transcript abundance of the remaining CsC5-MTase genes was significantly inhibited at the late stage of drought stress. All the CsC5-MTase genes showed the lowest transcript abundance at 48 h. Compared with 0 h, the transcript abundance of the CsCMT2 gene did not show a notably significant decline at 36 h, while the other seven CsC5-MTase genes reached a notably significant decline.
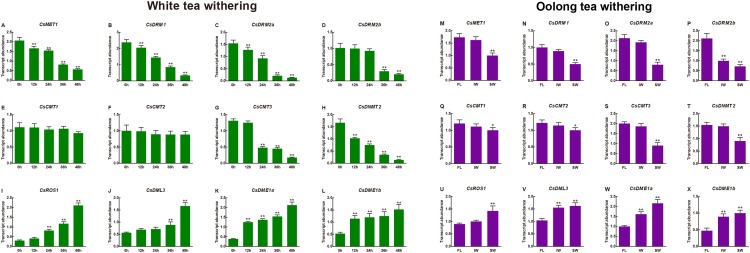
Moreover, the transcript abundance of the CsC5-MTase and CsdMTase genes during withering processing of white tea and oolong tea was also investigated. As shown in Fig. 8, the transcript abundance of CsC5-MTase was significantly upregulated during withering processing of white tea. Among them, the transcript abundance of CsMET1, CsDRM1, CsDRM2a, CsDRM2b, CsCMT3 and CsDNMT2 was strongly repressed, whereas the transcript abundance of CsCMT2 and CsCMT3 was not altered significantly. In CsdMTase genes, the transcript abundance of four genes was significantly enhanced. At 12 h, the genes CsDME1 and CsDME2 were highly significantly upregulated, while CsROS1 and CsDML3 showed no significant change in transcript abundance. When white tea withered to 24 h and 36 h, the transcript abundance of CsDML3 and CsROS1 was also significantly induced. In general, the transcript abundance of all CsC5-MTase genes reached their lowest point at 48 h. In contrast, the CsdMTase genes had the highest transcript abundance at 48 h.
Figure 8. Transcript abundance of CsC5-MTase and CsdMTase genes under white tea withering and oolong tea withering.
Transcript abundance of CsMET1 (A), CsDRM1 (B), CsDRM2a (C), CsDRM2b (D), CsCMT1 (E), CsCMT2 (F), CsCMT3 (G), CsDNMT2 (H), CsROS1 (I), CsDML3 (J), CsDME1a (K), and CsDME1b (L) under white tea withering and CsMET1 (M), CsDRM1 (N), CsDRM2a (O), CsDRM2b (P), CsCMT1 (Q), CsCMT2 (R), CsCMT3 (S), CsDNMT2 (T), CsROS1 (U), CsDML3 (V), CsDME1a (W) and CsDME1b (X) under oolong tea withering. FL, fresh leaves; IW, indoor-withered leaves; SW, solar-withered leaves. Data are presented as mean ± standard deviation (SD). *indicates significant difference (p < 0.05) and **indicates highly significant difference (p < 0.01).
In different withering processing of oolong tea, the transcript abundance of all eight CsC5-MTase was downregulated at IW and SW compared to FL. In FL vs. IW, only the CsDRM2b transcript abundance was significantly inhibited, while the other seven CsC5-MTase were slightly changed in transcript abundance. The transcript abundance of CsDML3, CsDME1a and CsDME1b was dramatically induced and maintained at high levels in IW and SW. Although the transcript abundance of CsROS1 was not affected in IW, the transcript abundance of this gene was significantly enhanced in SW. In SW, the transcript abundance of all CsC5-MTase genes was significantly downregulated, while the transcript abundance of four CsdMTase genes was significantly upregulated. In IW, only CsDRM2b, CsDML3, CsDME1a and CsDME1b showed significant change in transcript abundance.
Discussion
With the release of tea reference genome, gene family identification, classification, and function prediction have gradually become new research hotspots (Chen et al., 2019; Liu et al., 2019; Ma et al., 2019; Wang et al., 2018). With the extreme global climate, low temperature stress and drought stress may adversely affect the growth and development of tea plants and the quality of tea products. Meanwhile, it has been widely reported that CsC5-MTase and CsdMTase modulate methylation levels to participate in various stress responses (Zhang, Lang & Zhu, 2018). Fresh leaves are also affected by various stresses during the withering process, including drought, heat, and UV/light radiation (Gui et al., 2015). Therefore, the study of CsC5-MTase and CsdMTase is of considerable significance for understanding the anti-stress mechanism and tea processing. The C5-MTase and dMTase genes have been well investigated in several plants (Cao et al., 2014; Gianoglio et al., 2017; Qian et al., 2014; Wang et al., 2016b). However, there is no report on the identification of these genes in tea plants. The functional roles of these genes in stress response and tea withering processing have not been determined. To elucidate DNA methylation regulation in C. sinensis, we identified eight CsC5-MTase and four CsdMTase genes based on the tea reference genome. The physical and chemical characteristics, phylogenetic relationships, gene structures, protein–protein interactions, and cis-acting elements of the genes were also investigated.
Structural and evolutionary features of CsC5-MTase and CsdMTase in tea plant
Compared with other plant species, a total of eight C5-MTase and four dMTase genes have been identified in C. sinensis. However, the number of these two gene families discovered in A. thaliana (15), C. cardunculus (17) and G. max (13) is higher than that of tea plants (12). Due to the tea plant genome (3.02 GB) being approximately several-fold larger than that of A. thaliana (125 MB) (Cheng et al., 2017), R. communis (350 MB) (Chan et al., 2010) and G. max (978 MB) (Schmutz et al., 2010), indicating that CsC5-MTase and CsdMTase may have lost events in tea plants. Moreover, previous research has confirmed several gene contraction and expansion events occurred during the evolution of tea plants (Xia et al., 2017). Several studies have suggested that the contraction and expansion of these genes will directly affect the gene number in various plant species (Bennetzen, 2002; Choi, Jansen & Ruhlman, 2019; Lehtonen & Cárdenas, 2019). Hence, we inferred that gene duplication and loss events may be the key factors in the evolution of these two gene families. This finding is consistent with previous studies in maize (Qian et al., 2014) and globe artichoke (Gianoglio et al., 2017).
In phylogenetic classification, C5-MTase and dMTase genes in monocotyledons (O. sativa and Z. mays) are clustered in different branches from those in dicotyledons. It is speculated that the functions of C5-MTase and dMTase may be different between monocotyledons and dicotyledons. Consistent with previous research (Cao et al., 2014; Gianoglio et al., 2017), dicotyledons and monocotyledons may adopt different strategies in the evolution of C5-MTase and dMTase. Based on C5-MTase domain conservation, CsC5-MTase is classified into four distinct subfamilies, namely, MET, CMT, DRM and DNMT2. This classification is consistent with other dicotyledons, except soybean (lack of DNMT2). Similar to the MET gene in S. lycopersicum and C. cardunculus, only one MET gene was identified in tea plants, while in other plant species (A. thaliana, R. communis, O. sativa, G. max and Z. mays), each contains more than two MET genes. The number of CMT genes varies greatly among different plant species. In tea plants, a total of three CMT genes were identified. Similarly, three CMT genes were found in A. thaliana, S. lycopersicum and O. sativa, respectively. However, a total of four CMT genes were discovered in C. cardunculus. Compared to A. thaliana and R. communis, no DRM3 was found in the tea genome, suggesting that the DRM3 gene may be lost during the evolution of tea plants. At the same time, this effect may also be related to the presence of two CsDRM2 genes in the tea genome. Consistent with a previous study (Blanc & Wolfe, 2004), the function of DRM3 may be replaced by DRM2a and DRM2b. In contrast to the absence of DNMT2 in O. sativa and G. max, the tea plant genome harbors one CsDNMT2 gene. Previous studies have shown that DNMT2 has a wide substrate specificity, not only as a DNA methylase (Ashapkin, Kutueva & Vanyushin, 2016) but also as an RNA methylase to participate in various pathways to regulate the methylation level (Hermann, Schmitt & Jeltsch, 2003; Tuorto et al., 2012). Hence, the presence of CsDNMT2 may diversify the ways in which methylation levels are regulated in response to various stresses. In all plant species analyzed to date, the number of dMTase genes is considerably smaller than that in C5-MTase. In the present study, the number of dMTase genes in different plants ranged from 3 to 5. There is little difference in the number of these genes among different plant species. Based on the analysis of phylogenetic classification, dMTase genes can be divided into three distinct subfamilies, including ROS, DML and DME. In tea plants, all three subfamilies of dMTase genes were identified. Among the subfamilies, CsDME has two highly similar members, namely, CsDME1a and CsDME1b. These genes are closer in the tea genome, suggesting that they may result from localized replication events. Recent research also confirmed that methylation-related genes are affected by genome duplication and tandem replication (Pei et al., 2019). In total, the loss of CsDRM3 and duplication of CsDME suggested that CsC5-MTase and dMTase genes have functional redundancy and divergence.
Although the number of C5-MTase and dMTase genes varies widely among different plant species, the conserved domains contained in these proteins are similar. In this study, all members of CsC5-MTase contained one DNA-methylase domain, indicating that this is the core conserved domain of CsC5-MTase. The absence of this domain will make C5-MTase proteins unable to normally methylate the C-5 of cytosine in DNA (Wang, Lou & Li, 2019; Zhang, Lang & Zhu, 2018). In CsC5-MTase, the three members of CsDRM and CsDNMT2 only contain the DNA-methylase domain, while another domain (chromo domain) exists in CsCMT. The chromo domain is involved in chromatin interactions and is thought to mediate recognition and binding to target DNA. It was also reported that the chromo domain is responsible for gene regulation associated with chromatin remodeling, which can also bind to methylated histones, thereby affecting the methylation level of the genome (Jones, Cowell & Singh, 2000; Tajul-Arifin et al., 2003). Unlike CsCMT1, both CsCMT2 and CsCMT3 have a BAH domain. The BAH domain is involved in gene silencing and replication by mediating the association between chromatin and heterochromatin regions and protein–protein interaction (Saze et al., 2013). Combined with previous studies, the maintenance of CHG methylation in plants is catalyzed by CMT2 and CMT3 (Lindroth et al., 2001; Stroud et al., 2014). Similarly, it has been reported that the BAH domain in AtCMT1 is disrupted by frameshift mutation; therefore, AtCMT1 cannot function normally in methylation maintenance (Bewick et al., 2017). Hence, we inferred that the BAH domain is the key conserved structural domain for the methylation maintenance of CMT. However, CsCMT1 without this structural domain may have a limited contribution to affecting methylation level in tea plants, while CsCMT2 and CsCMT3 may be the key factors regulating the methylation level of tea plants. Additionally, we performed conserved domain identification of CsMET1. A total of five conserved domains were identified in CsMET1, including two DNMT1-RFD domains, two BAH domains, and one DNA-methylase domain. Compared to CsCMT2 and CsCMT3, CsMET1 has two extra DNMT1-RFD domains, which are responsible for the recognition of hemi-methylated CG dinucleotides and the methylation of unmodified cytosine (He, Chen & Zhu, 2011). We speculate that the presence of the DNMT1-RFD domain is an essential element for CsMET1 to participate in the maintenance of CG methylation.
Demethylase is an important factor involved in the dynamic regulation of methylation (Kapoor, Agius & Zhu, 2005). To further understand the specific functions of these genes in tea plants, the recognizable domains in all CsdMTases were also analyzed. All four CsdMTases contain an RRM-DME domain at their C-terminus. The RRM-DME domain is responsible for catalyzing the removal of 5mC base, which can effectively reduce the methylation level of plants (Iyer, Abhiman & Aravind, 2011). Moreover, CsROS1 and CsDML3 contain one Perm-CXXC domain, which is not found in two members of CsDMEs. This domain catalyzes the release of 5mC in DNA by a glycosylase/lyase mechanism, thereby effectively reducing the methylation level of plants (Mok et al., 2010; Morales-Ruiz et al., 2006). Genes with the same domain may have similar functions; therefore, CsROS1 and CsDML3 may play similar roles in plant demethylation. which is consistent with the result that these two genes belong to the same branch in the phylogenetic classification.
In total, we suggested that there is a difference in the number of CsC5-MTase and CsdMTase genes compared to monocotyledons, which may be related to gene replication and gene loss events in tea plant. Combined with loss of CsDRM3 and duplication of CsDME in tea plants, gene replication and loss events enrich the functional redundancy and divergence of C5-MTase and dMTase. Meanwhile, the number of CsC5-MTase and CsdMTase genes is not highly different from those of other dicotyledonous plants. Similar to other dicotyledons, the gain and loss of CsC5-MTase and CsdMTase genes experienced relaxed natural selection. In the evolutionary process of C5-MTase and dMTase, gene duplication and loss events promoted the abundant functions of CsC5-MTase and CsdMTase such that these genes have different roles in the regulation of methylation level of tea plant. This finding is consistent with the results of previous research (Gu et al., 2016; Wang et al., 2016b).
CsC5-MTase and CsdMTase genes play crucial roles in response to abiotic stress in tea plants and withering processing
In the production of tea plants, cold and drought are the major abiotic stresses that are not conducive to the growth and development of tea plants. Recent studies have shown that DNA methylation plays an important role in the plant stress response (Sanchez & Paszkowski, 2014; Xu et al., 2015). However, the transcript abundance of CsC5-MTase and CsdMTase genes in tea plants under these two abiotic stresses has rarely been studied. In this present study, we investigated the transcript abundance of the CsC5-MTase and CsdMTase genes under cold and drought stresses. These results showed that the transcript abundance of all CsC5-MTase and CsdMTase genes revealed dynamic trends at certain times from 0 to 48 h under cold and drought stresses, demonstrating a possible regulatory role of DNA methylation in the abiotic stress of tea plants. Except for CsCMT1 and CsCMT2, the transcript abundance of the other six CsC5-MTase genes showed a significant decrease after 12 h of cold treatment. With the extension of cold stress time, the transcript abundance of all CsC5-MTase genes showed a trend of continuous decline and reached the lowest point of transcript abundance at 48 h. In contrast, the transcript abundance of all CsdMTase genes showed a gradual increase under cold stress, reaching their highest transcript abundance at 48 h. In drought stress, the transcript abundance of CsC5-MTase and CsdMTase genes was similar under cold stress, and all CsC5-MTase genes also showed a descending trend under drought stress, while the transcript abundance of all CsdMTase genes increased significantly with increasing drought stress time. The results are consistent with the response of C5-MTase and dMTase to abiotic stress in R. communis (Victoria et al., 2018). These results indicated that these two gene families played different roles in the abiotic stress response.
Increasing evidences have shown that withering plays a predominant role in affecting the quality of white tea and oolong tea (Hu et al., 2018; Ma et al., 2018; Wang et al., 2019b). Similar to the effects of stress on tea plants, FL are also affected by various stresses during withering processing, including drought, high temperatures and UV/light radiation. To understand the role of the C5-MTase and dMTase genes in the withering process, the transcript abundance of these two gene families during the withering processing of white tea and oolong tea was analyzed. During withering of white tea, the transcript abundance of six C5-MTase genes (CsMET1, CsDRM1, CsDRM2a, CsDRM2b, CsCMT3 and CsDNMT2) was significantly suppressed with the extension of withering time. However, the transcript abundance of two CsCMT genes (CsCMT1 and CsCMT2) was only slightly (not significantly) downregulated. On the other hand, all four CsdMTase genes were significantly induced, and their transcript abundance was sharply increased. This indicated that the effects of withering on white tea might result from the transcriptional regulation of methylation-related genes.
Among oolong tea withering, solar-withering and indoor-withering are the most common processing methods (Lin et al., 2013). The flavor of tea products after different processing is quite different. In the withering of oolong tea, withered leaves will suffer from diverse types of stresses, such as drought, heat, and UV/light radiation. Combined with the above results, CsC5-MTase and CsdMTase may play different roles in solar-withering and indoor-withering. To understand the specific functions of CsC5-MTase and CsdMTase genes in solar-withering and indoor-withering, we investigated the transcript abundance of CsC5-MTase and CsdMTase genes in FL, IW and SW, respectively. In this study, we found that the transcript abundance of all CsC5-MTase showed a decrease in both IW and SW compared to FL. However, except for CsDRM2b, the transcript abundance of the other seven CsC5-MTase genes showed only a slight (not significant) decrease in IW compared to FL. In FL vs. SW, the transcript abundance of all CsC5-MTase was significantly inhibited. Compared with IW, the transcript abundance of these CsC5-MTase genes in SW was also significantly reduced. In these three samples, all eight CsC5-MTase showed lowest transcript abundance in SW. In the CsdMTase genes, the transcript abundance of these four genes is opposite to that of CsC5-MTase. All four CsC5-MTase were present at the highest transcript abundance in SW. Specifically, CsROS1 showed no significant change in transcript abundance between FL and IW, while the transcript abundance of CsDML3, CsDME1a and CsDME1b in IW was significantly higher than those in FL. Consistent with the detection of the transcript abundance of CsC5-MTase and CsdMTase genes during abiotic stress and white tea withering, the transcript abundance of six C5-MTase genes (CsMET1, CsDRM1, CsDRM2a, CsDRM2b, CsCMT3 and CsDNMT2) was significantly suppressed in SW, while the transcript abundance of all four CsdMTase genes was significantly induced. In the withering of oolong tea, the SW will suffer from different types of stress, including drought, heat, and UV/light radiation, while the indoor withered leaves are primarily affected by drought stress. Hence, solar-withering accelerates the dehydration process of tea leaves, and the stress degree in SW was deeper than that in IW. Due to the combination of multiple stresses, SW suffers a considerably higher stress degree than IW. Based on the above results, we concluded that the transcript abundance of CsC5-MTase and CsdMTase genes may be related to the stress degree of withered leaves. Similarly, previous work has demonstrated that C5-MTase and dMTase play important roles in response to salt stress (Liu et al., 2018b), cold stress (Gu et al., 2016), and drought stress (Victoria et al., 2018). Moreover, we observed a large number of light-responsive and stress-responsive cis-acting elements in the promoter regions of CsC5-MTase and CsdMTase. It has been reported that cis-acting elements are important molecular switches involved in dynamic network transcriptional regulation of gene activity and affect the transcript abundance of relevant genes (Shinozaki, Yamaguchi-Shinozaki & Seki, 2003; Yamaguchi-Shinozaki & Shinozaki, 2005). Consistent with a previous study (Cheng et al., 2013), these cis-acting elements have stress-related functions in CsC5-MTase and CsdMTase, which could respond to diverse stress signals and affect the transcript abundance of CsC5-MTase and CsdMTase. This effect may explain the significant changes in the transcript abundance of these two gene families under abiotic stress and withering processing. The presence of abundant light-responsive elements explained that the transcript abundance of CsC5-MTase and CsdMTase is affected by solar light. Compared with FL, the change of most CsC5-MTase and CsdMTase in SW are greater than that of IW. Of note is the observation that we did not identify a cis-acting element associated with stress-responsive in the promoter regions of the CsCMT1 and CsCMT2 genes. This may be the reason why the difference in transcript abundance between CsCMT1 and CsCMT2 genes is less than that in other CsC5-MTase during stress and withering.
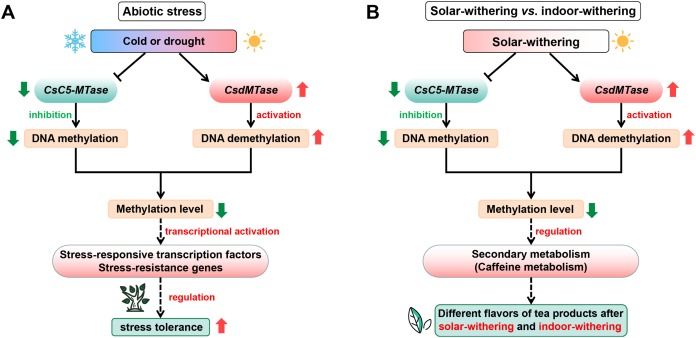
The transcript abundance of CsC5-MTase and CsdMTase directly affect the dynamic change of plant methylation levels (Zemach et al., 2010). Combined with the above results, this suggested that the DNA methylation patterns of tea plants might undergo a dramatic change during abiotic stress and withering. With the upregulation of CsdMTase, the DNA demethylation mechanism in tea plants is activated. Recent studies also revealed that the level of plant methylation is significantly reduced in response to abiotic stress (Dhar et al., 2014; Furner & Matzke, 2011). Moreover, hypomethylation level in DNA methylase mutants enhances resistance to biotrophic pathogens (López Sánchez et al., 2016). Under normal conditions, the promoter region of most genes in plants is maintained at a high methylation level. When plants are considered to be under stress, methylation markers from regulatory regions of stress-responsive transcription factors and other stress-resistance genes will be erased by demethylation mechanisms, which may enhance the ability of tea plants to cope with various stresses. In accordance with the studies in Hevea brasiliensis (Uthup et al., 2011) and R. communis (Victoria et al., 2018), the reduction of DNA methylation level may facilitate the activation of transposon transcription, and transposon transcription is often accompanied by stress conditions, thereby further activating the transcript abundance of related stress-resistant genes. Moreover, it has been reported that DNA methylation is involved in the regulation of secondary metabolism (Bharti et al., 2015; Conde et al., 2017). Caffeine is one of the most important secondary metabolites and plays an important role in tea flavor. In the caffeine metabolism, several METs participate in caffeine metabolism and convert xanthine to caffeine, and the methyl donor is also derived from S-adenosyl methionine (Deng et al., 2008; Kato et al., 1996). Meanwhile, S-adenosyl methionine is a methyl donor for cytosine methylation. Hence, methylation levels may be closely related to caffeine metabolism in tea plants. Increasing evidence has shown that drought stress and withering treatment can significantly affect the caffeine content in tea plants (Wang et al., 2016c, 2019b; Ye et al., 2018). Combined with the dynamic transcript abundance of CsC5-MTase and CsdMTase during abiotic stress and withering processing, it is suggested that CsC5-MTase and CsdMTase could affect caffeine metabolism in tea plants by regulating the methylation level. Due to the different transcript abundance of CsC5-MTase and CsdMTase in IW and SW, this may be one of the reasons for the different flavors of tea products after indoor-withering and solar-withering. On the basis of our data, we propose a hypothetical model to explain potential functions of CsC5-MTase and CsdMTase in abiotic stress and different withering processing (Fig. 9).
Figure 9. Schematic illustration showing the roles of CsC5-MTase and CsdMTase under abiotic stress (A) and withering treatment (B).
The solid line with the arrow represents direct regulation; the dashed line with the arrow represents indirect regulation; the T-shaped line represents inhibition.
Conclusions
Based on the tea reference genome, a total of eight CsC5-MTase and four CsdMTase genes were identified. Phylogenetic classification revealed that CsC5-MTase genes were divided into four subfamilies, including CsMET, CsCMT, CsDRM and CsDNMT2. These CsdMTase genes can be classified into CsROS, CsDME and CsDML subfamilies. The conserved domain analysis of these two gene families showed that gene loss and duplication events may occur in the evolution of CsC5-MTase and CsdMTase. Analysis of cis-acting elements revealed that these genes may be involved in a wide range of biological processes, including stress response, phytohormone response, and plant growth and development. In addition, the transcript abundance of CsC5-MTase and CsdMTase genes under abiotic stress (cold and drought) and withering processing (white tea and oolong tea) were detected, and the results showed that CsC5-MTase and CsdMTase genes may play vital roles in regulating responses to abiotic stress. Furthermore, these CsC5-MTase and CsdMTase may affect tea flavor in withering processing. We hope that the study helps to elucidate the possible roles of CsC5-MTase and CsdMTase in the abiotic stress response of tea plants and in the flavor of tea in withering processing and also provides a foundation for further understanding the epigenetic regulatory mechanism in tea plants.
Supplemental Information
Acknowledgments
We thank American Journal Experts (AJE) for English language editing.
Funding Statement
This work was supported by the Natural Science Foundation of Fujian Province (2018J01701), the Earmarked Fund for China Agriculture Research System (CARS-19), the Special Fund Project for Scientific and Technological Innovation of Fujian Agriculture and Forestry University (KF2015055, CXZX2017164 and CXZX2018069), the Rural Revitalization Tea Industry Technical Service Project of Fujian Agriculture and Forestry University (11899170102), the Construction of Plateau Discipline of Fujian Province (102/71201801101), and the Scientific Research Foundation of Horticulture College of Fujian Agriculture and Forestry University (2019B01). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Zhongxiong Lai, Email: laizx01@163.com.
Yuqiong Guo, Email: guoyq828@163.com.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Chen Zhu conceived and designed the experiments, performed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Shuting Zhang performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Chengzhe Zhou performed the experiments, prepared figures and/or tables, and approved the final draft.
Lan Chen performed the experiments, prepared figures and/or tables, and approved the final draft.
Haifeng Fu performed the experiments, prepared figures and/or tables, and approved the final draft.
Xiaozhen Li analyzed the data, prepared figures and/or tables, and approved the final draft.
Yuling Lin conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Zhongxiong Lai conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Yuqiong Guo conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
References
- Ashapkin, Kutueva & Vanyushin (2016).Ashapkin VV, Kutueva LI, Vanyushin BF. Dnmt2 is the most evolutionary conserved and enigmatic cytosine DNA methyltransferase in eukaryotes. Russian Journal of Genetics. 2016;52(3):237–248. doi: 10.1134/S1022795416030029. [DOI] [PubMed] [Google Scholar]
- Bailey et al. (2009).Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Research. 2009;37(Suppl. 2):W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetzen (2002).Bennetzen JL. Mechanisms and rates of genome expansion and contraction in flowering plants. Genetica. 2002;115(1):29–36. doi: 10.1023/A:1016015913350. [DOI] [PubMed] [Google Scholar]
- Bewick et al. (2017).Bewick AJ, Niederhuth CE, Ji L, Rohr NA, Griffin PT, Leebens-Mack J, Schmitz RJ. The evolution of CHROMOMETHYLASES and gene body DNA methylation in plants. Genome Biology. 2017;18(1):65. doi: 10.1186/s13059-017-1195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti et al. (2015).Bharti P, Mahajan M, Vishwakarma AK, Bhardwaj J, Yadav SK. AtROS1 overexpression provides evidence for epigenetic regulation of genes encoding enzymes of flavonoid biosynthesis and antioxidant pathways during salt stress in transgenic tobacco. Journal of Experimental Botany. 2015;66(19):5959–5969. doi: 10.1093/jxb/erv304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc & Wolfe (2004).Blanc G, Wolfe KH. Functional divergence of duplicated genes formed by polyploidy during Arabidopsis evolution. Plant Cell. 2004;16(7):1679–1691. doi: 10.1105/tpc.021410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao & Jacobsen (2002).Cao X, Jacobsen SE. Locus-specific control of asymmetric and CpNpG methylation by the DRM and CMT3 methyltransferase genes. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(Suppl. 4):16491–16498. doi: 10.1073/pnas.162371599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao et al. (2014).Cao D, Ju Z, Gao C, Mei X, Fu D, Zhu H, Luo Y, Zhu B. Genome-wide identification of cytosine-5 DNA methyltransferases and demethylases in Solanum lycopersicum. Gene. 2014;550(2):230–237. doi: 10.1016/j.gene.2014.08.034. [DOI] [PubMed] [Google Scholar]
- Cao et al. (2000).Cao X, Springer NM, Muszynski MG, Phillips RL, Kaeppler S, Jacobsen SE. Conserved plant genes with similarity to mammalian de novo DNA methyltransferases. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(9):4979–4984. doi: 10.1073/pnas.97.9.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan et al. (2010).Chan AP, Crabtree J, Zhao Q, Lorenzi H, Orvis J, Puiu D, Melake-Berhan A, Jones KM, Redman J, Chen G, Cahoon EB, Gedil M, Stanke M, Haas BJ, Wortman JR, Fraser-Liggett CM, Ravel J, Rabinowicz PD. Draft genome sequence of the oilseed species Ricinus communis. Nature Biotechnology. 2010;28(9):951–956. doi: 10.1038/nbt.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, Henderson & Jacobsen (2005).Chan SW-L, Henderson IR, Jacobsen SE. Gardening the genome: DNA methylation in Arabidopsis thaliana. Nature Reviews Genetics. 2005;6(5):351–360. doi: 10.1038/nrg1601. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2018).Chen CJ, Xia R, Chen H, He YH. TBtools, a Toolkit for Biologists integrating various biological data handling tools with a user-friendly interface. https://doi.org/10.1101/289660 BioRxiv. 2018 [Google Scholar]
- Chen et al. (2019).Chen W, Xu Y-X, Mao J, Hao W-J, Liu Y-F, Ni D-J, Chen L. Cloning and expression patterns of VQ-motif-containing proteins under abiotic stress in tea plant. Plant Growth Regulation. 2019;87(2):277–286. doi: 10.1007/s10725-018-0469-2. [DOI] [Google Scholar]
- Chen, Zhao & He (2016).Chen K, Zhao BS, He C. Nucleic acid modifications in regulation of gene expression. Cell Chemical Biology. 2016;23(1):74–85. doi: 10.1016/j.chembiol.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng et al. (2017).Cheng C-Y, Krishnakumar V, Chan AP, Thibaud-Nissen F, Schobel S, Town CD. Araport11: a complete reannotation of the Arabidopsis thaliana reference genome. Plant Journal. 2017;89(4):789–804. doi: 10.1111/tpj.13415. [DOI] [PubMed] [Google Scholar]
- Cheng et al. (2013).Cheng M-C, Liao P-M, Kuo W-W, Lin T-P. The Arabidopsis ETHYLENE RESPONSE FACTOR1 regulates abiotic stress-responsive gene expression by binding to different cis-acting elements in response to different stress signals. Plant Physiology. 2013;162(3):1566–1582. doi: 10.1104/pp.113.221911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, Jansen & Ruhlman (2019).Choi I-S, Jansen R, Ruhlman T. Lost and found: return of the inverted repeat in the legume clade defined by its absence. Genome Biology and Evolution. 2019;11(4):1321–1333. doi: 10.1093/gbe/evz076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cokus et al. (2008).Cokus SJ, Feng S, Zhang X, Chen Z, Merriman B, Haudenschild CD, Pradhan S, Nelson SF, Pellegrini M, Jacobsen SE. Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature. 2008;452(7184):215–219. doi: 10.1038/nature06745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde et al. (2017).Conde D, Moreno-Cortés A, Dervinis C, Ramos-Sánchez JM, Kirst M, Perales M, González-Melendi P, Allona I. Overexpression of DEMETER, a DNA demethylase, promotes early apical bud maturation in poplar. Plant, Cell & Environment. 2017;40(11):2806–2819. doi: 10.1111/pce.13056. [DOI] [PubMed] [Google Scholar]
- Deng et al. (2008).Deng W-W, Li YY, Ogita S, Ashihara H. Fine control of caffeine biosynthesis in tissue cultures of Camellia sinensis. Phytochemistry Letters. 2008;1(4):195–198. doi: 10.1016/j.phytol.2008.09.009. [DOI] [Google Scholar]
- Dhar et al. (2014).Dhar MK, Vishal P, Sharma R, Kaul S. Epigenetic dynamics: role of epimarks and underlying machinery in plants exposed to abiotic stress. International Journal of Genomics. 2014;2014(4):187146. doi: 10.1155/2014/187146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du et al. (2014).Du J, Johnson LM, Groth M, Feng S, Hale CJ, Li S, Vashisht AA, Gallego-Bartolome J, Wohlschlegel JA, Patel DJ, Jacobsen SE. Mechanism of DNA methylation-directed histone methylation by KRYPTONITE. Molecular Cell. 2014;55(3):495–504. doi: 10.1016/j.molcel.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du et al. (2012).Du J, Zhong X, Bernatavichute YV, Stroud H, Feng S, Caro E, Vashisht AA, Terragni J, Chin HG, Tu A, Hetzel J, Wohlschlegel JA, Pradhan S, Patel DJ, Jacobsen SE. Dual binding of chromomethylase domains to H3K9me2-containing nucleosomes directs DNA methylation in plants. Cell. 2012;151(1):167–180. doi: 10.1016/j.cell.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichten & Springer (2015).Eichten SR, Springer NM. Minimal evidence for consistent changes in maize DNA methylation patterns following environmental stress. Frontiers in Plant Science. 2015;6:308. doi: 10.3389/fpls.2015.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furner & Matzke (2011).Furner IJ, Matzke M. Methylation and demethylation of the Arabidopsis genome. Current Opinion in Plant Biology. 2011;14(2):137–141. doi: 10.1016/j.pbi.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Ganguly et al. (2018).Ganguly DR, Crisp PA, Eichten SR, Pogson BJ. Maintenance of pre-existing DNA methylation states through recurring excess-light stress. Plant, Cell & Environment. 2018;41(7):1657–1672. doi: 10.1111/pce.13324. [DOI] [PubMed] [Google Scholar]
- Genger et al. (1999).Genger RK, Kovac KA, Dennis ES, Peacock WJ, Finnegan EJ. Multiple DNA methyltransferase genes in Arabidopsis thaliana. Plant Molecular Biology. 1999;41(2):269–278. doi: 10.1023/A:1006347010369. [DOI] [PubMed] [Google Scholar]
- Gent et al. (2013).Gent JI, Ellis NA, Guo L, Harkess AE, Yao Y, Zhang X, Dawe RK. CHH islands: de novo DNA methylation in near-gene chromatin regulation in maize. Genome Research. 2013;23(4):628–637. doi: 10.1101/gr.146985.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianoglio et al. (2017).Gianoglio S, Moglia A, Acquadro A, Comino C, Portis E. The genome-wide identification and transcriptional levels of DNA methyltransferases and demethylases in globe artichoke. PLOS ONE. 2017;12(7):e181669. doi: 10.1371/journal.pone.0181669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong et al. (2002).Gong Z, Morales-Ruiz T, Ariza RR, Roldán-Arjona T, David L, Zhu J-K. ROS1, a repressor of transcriptional gene silencing in Arabidopsis, encodes a DNA glycosylase/lyase. Cell. 2002;111(6):803–814. doi: 10.1016/S0092-8674(02)01133-9. [DOI] [PubMed] [Google Scholar]
- Gu et al. (2016).Gu T, Ren S, Wang Y, Han Y, Li Y. Characterization of DNA methyltransferase and demethylase genes in Fragaria vesca. Molecular Genetics and Genomics. 2016;291(3):1333–1345. doi: 10.1007/s00438-016-1187-y. [DOI] [PubMed] [Google Scholar]
- Gui et al. (2015).Gui J, Fu X, Zhou Y, Katsuno T, Mei X, Deng R, Xu X, Zhang L, Dong F, Watanabe N, Yang Z. Does enzymatic hydrolysis of glycosidically bound volatile compounds really contribute to the formation of volatile compounds during the oolong tea manufacturing process? Journal of Agricultural and Food Chemistry. 2015;63(31):6905–6914. doi: 10.1021/acs.jafc.5b02741. [DOI] [PubMed] [Google Scholar]
- Guo et al. (2019).Guo Y, Zhu C, Zhao S, Zhang S, Wang W, Fu H, Li X, Zhou C, Chen L, Lin Y, Lai Z. De novo transcriptome and phytochemical analyses reveal differentially expressed genes and characteristic secondary metabolites in the original oolong tea (Camellia sinensis) cultivar ‘Tieguanyin’ compared with cultivar ‘Benshan’. BMC Genomics. 2019;20(1):265. doi: 10.1186/s12864-019-5643-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Chen & Zhu (2011).He X-J, Chen TP, Zhu J-K. Regulation and function of DNA methylation in plants and animals. Cell Research. 2011;21(3):442–465. doi: 10.1038/cr.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann, Schmitt & Jeltsch (2003).Hermann A, Schmitt S, Jeltsch A. The human Dnmt2 has residual DNA-(cytosine-C5) methyltransferase activity. Journal of Biological Chemistry. 2003;278(34):31717–31721. doi: 10.1074/jbc.M305448200. [DOI] [PubMed] [Google Scholar]
- Hu et al. (2018).Hu C-J, Li D, Ma Y-X, Zhang W, Lin C, Zheng X-Q, Liang Y-R, Lu J-L. Formation mechanism of the oolong tea characteristic aroma during bruising and withering treatment. Food Chemistry. 2018;269:202–211. doi: 10.1016/j.foodchem.2018.07.016. [DOI] [PubMed] [Google Scholar]
- Iyer, Abhiman & Aravind (2011).Iyer LM, Abhiman S, Aravind L. Natural history of eukaryotic DNA methylation systems. Progress in Molecular Biology and Translational Science. 2011;101:25–104. doi: 10.1016/b978-0-12-387685-0.00002-0. [DOI] [PubMed] [Google Scholar]
- Jeltsch et al. (2017).Jeltsch A, Ehrenhofer-Murray A, Jurkowski TP, Lyko F, Reuter G, Ankri S, Nellen W, Schaefer M, Helm M. Mechanism and biological role of Dnmt2 in nucleic acid methylation. RNA Biology. 2017;14(9):1108–1123. doi: 10.1080/15476286.2016.1191737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, Cowell & Singh (2000).Jones DO, Cowell IG, Singh PB. Mammalian chromodomain proteins: their role in genome organisation and expression. Bioessays. 2000;22(2):124–137. doi: 10.1002/(SICI)1521-1878(200002)22:2<124::AID-BIES4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Kapoor, Agius & Zhu (2005).Kapoor A, Agius F, Zhu J-K. Preventing transcriptional gene silencing by active DNA demethylation. FEBS Letters. 2005;579(26):5889–5898. doi: 10.1016/j.febslet.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Kato et al. (1996).Kato M, Kanehara T, Shimizu H, Suzuki T, Gillies FM, Crozier A, Ashihara H. Caffeine biosynthesis in young leaves of Camellia sinensis: in vitro studies on N-methyltransferase activity involved in the conversion of xanthosine to caffeine. Physiologia Plantarum. 1996;98(3):629–636. doi: 10.1111/j.1399-3054.1996.tb05720.x. [DOI] [Google Scholar]
- Kumar et al. (2018).Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution. 2018;35(6):1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law & Jacobsen (2010).Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nature Reviews Genetics. 2010;11(3):204–220. doi: 10.1038/nrg2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen & Cárdenas (2019).Lehtonen S, Cárdenas GG. Dynamism in plastome structure observed across the phylogenetic tree of ferns. Botanical Journal of the Linnean Society. 2019;190(3):229–241. doi: 10.1093/botlinnean/boz020. [DOI] [Google Scholar]
- Lescot et al. (2002).Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Research. 2002;30(1):325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin et al. (2013).Lin SY, Chen YL, Lee CL, Cheng CY, Roan SF, Chen IZ. Monitoring volatile compound profiles and chemical compositions during the process of manufacturing semi-fermented oolong tea. Journal of Horticultural Science and Biotechnology. 2013;88(2):159–164. doi: 10.1080/14620316.2013.11512951. [DOI] [Google Scholar]
- Lindroth et al. (2001).Lindroth AM, Cao X, Jackson JP, Zilberman D, McCallum CM, Henikoff S, Jacobsen SE. Requirement of CHROMOMETHYLASE3 for maintenance of CpXpG methylation. Science. 2001;292(5524):2077–2080. doi: 10.1126/science.1059745. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2015).Liu R, How-Kit A, Stammitti L, Teyssier E, Rolin D, Mortain-Bertrand A, Halle S, Liu M, Kong J, Wu C, Degraeve-Guibault C, Chapman NH, Maucourt M, Hodgman TC, Tost J, Bouzayen M, Hong Y, Seymour GB, Giovannoni JJ, Gallusci P. A DEMETER-like DNA demethylase governs tomato fruit ripening. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(34):10804–10809. doi: 10.1073/pnas.1503362112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2019).Liu Y, Jiang H, Zhao Y, Li X, Dai X, Zhuang J, Zhu M, Jiang X, Wang P, Gao L, Xia T. Three Camellia sinensis glutathione S-transferases are involved in the storage of anthocyanins, flavonols, and proanthocyanidins. Planta. 2019;250(4):1163–1175. doi: 10.1007/s00425-019-03206-2. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2018b).Liu CX, Li H, Lin J, Wang Y, Xu X, Cheng ZM, Chang Y. Genome-wide characterization of DNA demethylase genes and their association with salt response in Pyrus. Genes. 2018b;9(8):398. doi: 10.3390/genes9080398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2018a).Liu G, Xia Y, Liu T, Dai S, Hou X. The DNA methylome and association of differentially methylated regions with differential gene expression during heat stress in Brassica rapa. International Journal of Molecular Sciences. 2018a;19(5):1414. doi: 10.3390/ijms19051414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak & Schmittgen (2001).Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- López Sánchez et al. (2016).López Sánchez A, Stassen JHM, Furci L, Smith LM, Ton J. The role of DNA (de)methylation in immune responsiveness of Arabidopsis. Plant Journal. 2016;88(3):361–374. doi: 10.1111/tpj.13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma et al. (2018).Ma C, Li J, Chen W, Wang W, Qi D, Pang S, Miao A. Study of the aroma formation and transformation during the manufacturing process of oolong tea by solid-phase micro-extraction and gas chromatography–mass spectrometry combined with chemometrics. Food Research International. 2018;108:413–422. doi: 10.1016/j.foodres.2018.03.052. [DOI] [PubMed] [Google Scholar]
- Ma et al. (2019).Ma Q, Zhou Q, Chen C, Cui Q, Zhao Y, Wang K, Arkorful E, Chen X, Sun K, Li X. Isolation and expression analysis of CsCML genes in response to abiotic stresses in the tea plant (Camellia sinensis) Scientific Reports. 2019;9(1):8211. doi: 10.1038/s41598-019-44681-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrek & Lee (2002).Modrek B, Lee C. A genomic view of alternative splicing. Nature Genetics. 2002;30(1):13–19. doi: 10.1038/ng0102-13. [DOI] [PubMed] [Google Scholar]
- Mok et al. (2010).Mok YG, Uzawa R, Lee J, Weiner GM, Eichman BF, Fischer RL, Huh JH. Domain structure of the DEMETER 5-methylcytosine DNA glycosylase. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(45):19225–19230. doi: 10.1073/pnas.1014348107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Ruiz et al. (2006).Morales-Ruiz T, Ortega-Galisteo AP, Ponferrada-Marín MI, Martínez-Macías MI, Ariza RR, Roldán-Arjona T. DEMETER and REPRESSOR OF SILENCING 1 encode 5-methylcytosine DNA glycosylases. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(18):6853–6858. doi: 10.1073/pnas.0601109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritoh et al. (2012).Moritoh S, Eun C-H, Ono A, Asao H, Okano Y, Yamaguchi K, Shimatani Z, Koizumi A, Terada R. Targeted disruption of an orthologue of DOMAINS REARRANGED METHYLASE 2, OsDRM2, impairs the growth of rice plants by abnormal DNA methylation. Plant Journal. 2012;71(1):85–98. doi: 10.1111/j.1365-313X.2012.04974.x. [DOI] [PubMed] [Google Scholar]
- Ogneva, Dubrovina & Kiselev (2016).Ogneva ZV, Dubrovina AS, Kiselev KV. Age-associated alterations in DNA methylation and expression of methyltransferase and demethylase genes in Arabidopsis thaliana. Biologia Plantarum. 2016;60(4):628–634. doi: 10.1007/s10535-016-0638-y. [DOI] [Google Scholar]
- Ortega-Galisteo et al. (2008).Ortega-Galisteo AP, Morales-Ruiz T, Ariza RR, Roldán-Arjona T. Arabidopsis DEMETER-LIKE proteins DML2 and DML3 are required for appropriate distribution of DNA methylation marks. Plant Molecular Biology. 2008;67(6):671–681. doi: 10.1007/s11103-008-9346-0. [DOI] [PubMed] [Google Scholar]
- Papa et al. (2001).Papa CM, Springer NM, Muszynski MG, Meeley R, Kaeppler SM. Maize chromomethylase Zea methyltransferase2 is required for CpNpG methylation. Plant Cell. 2001;13(8):1919–1928. doi: 10.1105/TPC.010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei et al. (2019).Pei L, Zhang L, Li J, Shen C, Qiu P, Tu L, Zhang X, Wang M. Tracing the origin and evolution history of methylation-related genes in plants. BMC Plant Biology. 2019;19(1):307. doi: 10.1186/s12870-019-1923-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penterman et al. (2007).Penterman J, Zilberman D, Huh JH, Ballinger T, Henikoff S, Fischer RL. DNA demethylation in the Arabidopsis genome. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(16):6752–6757. doi: 10.1073/pnas.0701861104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian et al. (2014).Qian Y, Xi Y, Cheng B, Zhu S. Genome-wide identification and expression profiling of DNA methyltransferase gene family in maize. Plant Cell Reports. 2014;33(10):1661–1672. doi: 10.1007/s00299-014-1645-0. [DOI] [PubMed] [Google Scholar]
- Ratel et al. (2006).Ratel D, Ravanat J-L, Berger F, Wion D. N6-methyladenine: the other methylated base of DNA. BioEssays. 2006;28(3):309–315. doi: 10.1002/bies.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez & Paszkowski (2014).Sanchez DH, Paszkowski J. Heat-induced release of epigenetic silencing reveals the concealed role of an imprinted plant gene. PLOS Genetics. 2014;10(11):e1004806. doi: 10.1371/journal.pgen.1004806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saze et al. (2013).Saze H, Kitayama J, Takashima K, Miura S, Harukawa Y, Ito T, Kakutani T. Mechanism for full-length RNA processing of Arabidopsis genes containing intragenic heterochromatin. Nature Communications. 2013;4(1):2301. doi: 10.1038/ncomms3301. [DOI] [PubMed] [Google Scholar]
- Schmutz et al. (2010).Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, Hyten DL, Song Q, Thelen JJ, Cheng J, Xu D, Hellsten U, May GD, Yu Y, Sakurai T, Umezawa T, Bhattacharyya MK, Sandhu D, Valliyodan B, Lindquist E, Peto M, Grant D, Shu S, Goodstein D, Barry K, Futrell-Griggs M, Abernathy B, Du J, Tian Z, Zhu L, Gill N, Joshi T, Libault M, Sethuraman A, Zhang X-C, Shinozaki K, Nguyen HT, Wing RA, Cregan P, Specht J, Grimwood J, Rokhsar D, Stacey G, Shoemaker RC, Jackson SA. Genome sequence of the palaeopolyploid soybean. Nature. 2010;463(7278):178–183. doi: 10.1038/nature08670. [DOI] [PubMed] [Google Scholar]
- Schoft et al. (2011).Schoft VK, Chumak N, Choi Y, Hannon M, Garcia-Aguilar M, Machlicova A, Slusarz L, Mosiolek M, Park J-S, Park GT, Fischer RL, Tamaru H. Function of the DEMETER DNA glycosylase in the Arabidopsis thaliana male gametophyte. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(19):8042–8047. doi: 10.1073/pnas.1105117108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki, Yamaguchi-Shinozaki & Seki (2003).Shinozaki K, Yamaguchi-Shinozaki K, Seki M. Regulatory network of gene expression in the drought and cold stress responses. Current Opinion in Plant Biology. 2003;6(5):410–417. doi: 10.1016/S1369-5266(03)00092-X. [DOI] [PubMed] [Google Scholar]
- Steward et al. (2002).Steward N, Ito M, Yamaguchi Y, Koizumi N, Sano H. Periodic DNA methylation in maize nucleosomes and demethylation by environmental stress. Journal of Biological Chemistry. 2002;277(40):37741–37746. doi: 10.1074/jbc.M204050200. [DOI] [PubMed] [Google Scholar]
- Stroud et al. (2014).Stroud H, Do T, Du J, Zhong X, Feng S, Johnson L, Patel DJ, Jacobsen SE. Non-CG methylation patterns shape the epigenetic landscape in Arabidopsis. Nature Structural & Molecular Biology. 2014;21(1):64–72. doi: 10.1038/nsmb.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud et al. (2013).Stroud H, Greenberg MVC, Feng S, Bernatavichute YV, Jacobsen SE. Comprehensive analysis of silencing mutants reveals complex regulation of the Arabidopsis methylome. Cell. 2013;152(1–2):352–364. doi: 10.1016/j.cell.2012.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su et al. (2018).Su Y, Bai X, Yang W, Wang W, Chen Z, Ma J, Ma T. Single-base-resolution methylomes of Populus euphratica reveal the association between DNA methylation and salt stress. Tree Genetics & Genomes. 2018;14(6):86. doi: 10.1007/s11295-018-1298-1. [DOI] [Google Scholar]
- Sun et al. (2018).Sun L, Miao X, Cui J, Deng J, Wang X, Wang Y, Zhang Y, Gao S, Yang K. Genome-wide high-resolution mapping of DNA methylation identifies epigenetic variation across different salt stress in Maize (Zea mays L.) Euphytica. 2018;214(2):25. doi: 10.1007/s10681-017-2076-0. [DOI] [Google Scholar]
- Tajul-Arifin et al. (2003).Tajul-Arifin K, Teasdale R, Ravasi T, Hume DA, Mattick JS, RIKEN GER Group, GSL Members Identification and analysis of chromodomain-containing proteins encoded in the mouse transcriptome. Genome Research. 2003;13(6b):1416–1429. doi: 10.1101/gr.1015703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang et al. (2003).Tang L-Y, Reddy MN, Rasheva V, Lee T-L, Lin M-J, Hung M-S, Shen C-KJ. The eukaryotic DNMT2 genes encode a new class of cytosine-5 DNA methyltransferases. Journal of Biological Chemistry. 2003;278(36):33613–33616. doi: 10.1074/jbc.C300255200. [DOI] [PubMed] [Google Scholar]
- Tuorto et al. (2012).Tuorto F, Liebers R, Musch T, Schaefer M, Hofmann S, Kellner S, Frye M, Helm M, Stoecklin G, Lyko F. RNA cytosine methylation by Dnmt2 and NSun2 promotes tRNA stability and protein synthesis. Nature Structural & Molecular Biology. 2012;19(9):900–905. doi: 10.1038/nsmb.2357. [DOI] [PubMed] [Google Scholar]
- Uthup et al. (2011).Uthup TK, Ravindran M, Bini K, Thakurdas S. Divergent DNA methylation patterns associated with abiotic stress in Hevea brasiliensis. Molecular Plant. 2011;4(6):996–1013. doi: 10.1093/mp/ssr039. [DOI] [PubMed] [Google Scholar]
- Victoria et al. (2018).Victoria D, Aliki K, Venetia K, Georgios M, Zoe H. Spatial and temporal expression of cytosine-5 DNA methyltransferase and DNA demethylase gene families of the Ricinus communis during seed development and drought stress. Plant Growth Regulation. 2018;84(1):81–94. doi: 10.1007/s10725-017-0323-y. [DOI] [Google Scholar]
- Vieira et al. (2017).Vieira GC, Vieira GF, Sinigaglia M, Da Silva Valente VL. Linking epigenetic function to electrostatics: the DNMT2 structural model example. PLOS ONE. 2017;12(6):e178643. doi: 10.1371/journal.pone.0178643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2016b).Wang PF, Gao C, Bian XT, Zhao SZ, Zhao CZ, Xia H, Song H, Hou L, Wan SB, Wang XJ. Genome-wide identification and comparative analysis of cytosine-5 DNA methyltransferase and demethylase families in wild and cultivated peanut. Frontiers in Plant Science. 2016b;7:7. doi: 10.3389/fpls.2016.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2018).Wang M, Li Q, Sun K, Chen X, Zhou Q, Li H, Zhang X, Li X. Involvement of CsCDPK20 and CsCDPK26 in regulation of thermotolerance in tea plant (Camellia sinensis) Plant Molecular Biology Reporter. 2018;36(2):176–187. doi: 10.1007/s11105-018-1068-0. [DOI] [Google Scholar]
- Wang, Lou & Li (2019).Wang RB, Lou J, Li J. A mobile restriction modification system consisting of methylases on the IncA/C plasmid. Mobile DNA. 2019;10(1):26. doi: 10.1186/s13100-019-0168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2019a).Wang L, Shi Y, Chang X, Jing S, Zhang Q, You C, Yuan H, Wang H. DNA methylome analysis provides evidence that the expansion of the tea genome is linked to TE bursts. Plant Biotechnology Journal. 2019a;17(4):826–835. doi: 10.1111/pbi.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2016c).Wang W, Xin H, Wang M, Ma Q, Wang L, Kaleri NA, Wang Y, Li X. Transcriptomic analysis reveals the molecular mechanisms of drought-stress-induced decreases in Camellia sinensis leaf quality. Frontiers in Plant Science. 2016c;7:385. doi: 10.3389/fpls.2016.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2016a).Wang Y, Xue X, Zhu J-K, Dong J. Demethylation of ERECTA receptor genes by IBM1 histone demethylase affects stomatal development. Development. 2016a;143(23):4452–4461. doi: 10.1242/dev.129932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2019b).Wang Y, Zheng P-C, Liu P-P, Song X-W, Guo F, Li Y-Y, Ni D-J, Jiang C-J. Novel insight into the role of withering process in characteristic flavor formation of teas using transcriptome analysis and metabolite profiling. Food Chemistry. 2019b;272:313–322. doi: 10.1016/j.foodchem.2018.08.013. [DOI] [PubMed] [Google Scholar]
- Wei et al. (2018).Wei C, Yang H, Wang S, Zhao J, Liu C, Gao L, Xia E, Lu Y, Tai Y, She G, Sun J, Cao H, Tong W, Gao Q, Li Y, Deng W, Jiang X, Wang W, Chen Q, Zhang S, Li H, Wu J, Wang P, Li P, Shi C, Zheng F, Jian J, Huang B, Shan D, Shi M, Fang C, Yue Y, Li F, Li D, Wei S, Han B, Jiang C, Yin Y, Xia T, Zhang Z, Bennetzen JL, Zhao S, Wan X. Draft genome sequence of Camellia sinensis var. sinensis provides insights into the evolution of the tea genome and tea quality. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(18):E4151–E4158. doi: 10.1073/pnas.1719622115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia et al. (2019).Xia E-H, Li F-D, Tong W, Li P-H, Wu Q, Zhao H-J, Ge R-H, Li R-P, Li Y-Y, Zhang Z-Z, Wei C-L, Wan X-C. Tea Plant Information Archive: a comprehensive genomics and bioinformatics platform for tea plant. Plant Biotechnology Journal. 2019;17(10):1938–1953. doi: 10.1111/pbi.13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia et al. (2017).Xia E-H, Zhang H-B, Sheng J, Li K, Zhang Q-J, Kim C, Zhang Y, Liu Y, Zhu T, Li W, Huang H, Tong Y, Nan H, Shi C, Shi C, Jiang J-J, Mao S-Y, Jiao J-Y, Zhang D, Zhao Y, Zhao Y-J, Zhang L-P, Liu Y-L, Liu B-Y, Yu Y, Shao S-F, Ni D-J, Eichler EE, Gao L-Z. The tea tree genome provides insights into tea flavor and independent evolution of caffeine biosynthesis. Molecular Plant. 2017;10(6):866–877. doi: 10.1016/j.molp.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Xing et al. (2015).Xing M-Q, Zhang Y-J, Zhou S-R, Hu W-Y, Wu X-T, Ye Y-J, Wu X-X, Xiao Y-P, Li X, Xue H-W. Global analysis reveals the crucial roles of DNA methylation during rice seed development. Plant Physiology. 2015;168(4):1417–1432. doi: 10.1104/pp.15.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2015).Xu R, Wang Y, Zheng H, Lu W, Wu C, Huang J, Yan K, Yang G, Zheng C. Salt-induced transcription factor MYB74 is regulated by the RNA-directed DNA methylation pathway in Arabidopsis. Journal of Experimental Botany. 2015;66(19):5997–6008. doi: 10.1093/jxb/erv312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki & Shinozaki (2005).Yamaguchi-Shinozaki K, Shinozaki K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends in Plant Science. 2005;10(2):88–94. doi: 10.1016/j.tplants.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Yamamuro et al. (2014).Yamamuro C, Miki D, Zheng Z, Ma J, Wang J, Yang Z, Dong J, Zhu J-K. Overproduction of stomatal lineage cells in Arabidopsis mutants defective in active DNA demethylation. Nature Communications. 2014;5(1):4062. doi: 10.1038/ncomms5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye et al. (2018).Ye Y, Yan J, Cui J, Mao S, Li M, Liao X, Tong H. Dynamic changes in amino acids, catechins, caffeine and gallic acid in green tea during withering. Journal of Food Composition and Analysis. 2018;66:98–108. doi: 10.1016/j.jfca.2017.12.008. [DOI] [Google Scholar]
- Yue et al. (2019).Yue C, Cao H, Lin H, Hu J, Ye Y, Li J, Hao Z, Hao X, Sun Y, Yang Y, Wang X. Expression patterns of alpha-amylase and beta-amylase genes provide insights into the molecular mechanisms underlying the responses of tea plants (Camellia sinensis) to stress and postharvest processing treatments. Planta. 2019;250(1):281–298. doi: 10.1007/s00425-019-03171-w. [DOI] [PubMed] [Google Scholar]
- Zemach et al. (2013).Zemach A, Kim MY, Hsieh P-H, Coleman-Derr D, Eshed-Williams L, Thao K, Harmer SL, Zilberman D. The Arabidopsis nucleosome remodeler DDM1 allows DNA methyltransferases to access H1-containing heterochromatin. Cell. 2013;153(1):193–205. doi: 10.1016/j.cell.2013.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemach et al. (2010).Zemach A, McDaniel IE, Silva P, Zilberman D. Genome-wide evolutionary analysis of eukaryotic DNA methylation. Science. 2010;328(5980):916–919. doi: 10.1126/science.1186366. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2015).Zhang G, Huang H, Liu D, Cheng Y, Liu X, Zhang W, Yin R, Zhang D, Zhang P, Liu J, Li C, Liu B, Luo Y, Zhu Y, Zhang N, He S, He C, Wang H, Chen D. N6-methyladenine DNA modification in Drosophila. Cell. 2015;161(4):893–906. doi: 10.1016/j.cell.2015.04.018. [DOI] [PubMed] [Google Scholar]
- Zhang, Lang & Zhu (2018).Zhang H, Lang Z, Zhu J-K. Dynamics and function of DNA methylation in plants. Nature Reviews Molecular Cell Biology. 2018;19(8):489–506. doi: 10.1038/s41580-018-0016-z. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2016).Zhang B, Tieman DM, Jiao C, Xu Y, Chen K, Fei Z, Giovannoni JJ, Klee HJ. Chilling-induced tomato flavor loss is associated with altered volatile synthesis and transient changes in DNA methylation. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(44):12580–12585. doi: 10.1073/pnas.1613910113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou et al. (2019).Zhou C, Zhu C, Fu H, Li X, Chen L, Lin Y, Lai Z, Guo Y. Genome-wide investigation of superoxide dismutase (SOD) gene family and their regulatory miRNAs reveal the involvement in abiotic stress and hormone response in tea plant (Camellia sinensis) PLOS ONE. 2019;14(10):e223609. doi: 10.1371/journal.pone.0223609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu (2009).Zhu J-K. Active DNA demethylation mediated by DNA glycosylases. Annual Review of Genetics. 2009;43(1):143–166. doi: 10.1146/annurev-genet-102108-134205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability: