ABSTRACT
Background: A mural thrombus in the descending thoracic aorta frequently leads to distal organ and acute limb ischemia, increasing overall morbidity and mortality. Early diagnosis is imperative as thrombi are usually discovered after end organ damage has taken place. The formation of a mural thrombus in descending aorta has not been fully explained; however, the principle of Virchow’s triad for thrombogenesis (hypercoagulability, stasis of blood flow and endothelial injury) remains the likely pathophysiologic mechanism.
Case Presentation: We present a case of a descending aortic thrombus incidentally detected on computed tomography scan in a 65-year-old female and successfully treated with anticoagulation, preventing subsequent complications.
Conclusions: Suspicion for an aortic thrombus should arise when the origin is not known for acute onset distal limb or organ ischemia.
KEYWORDS: Complete blood profile, emergency department, transesophageal echo, transthoracic echo, interventional radiology, computed tomography, congestive heart failure
1. Introduction
A mural aortic thrombus is not encountered frequently in clinical practice and is in fact rare to diagnose prior to distal embolic complications, making the true incidence not well known.[1] Hematological disorders and states leading to hypercoagulability have been considered the main causes of aortic thrombi formation [2,3]. Though the complete mechanism of a direct mural aortic thrombus has not been fully explained, the state of hypercoagulability, stasis of blood flow, and endothelial injury (as expressed in Virchow’s triad) appear to play a significant role. Despite this, very few cases have been described in relatively healthy individual without these inciting factors. [4,5] The surgical and medical treatments for aortic thrombosis are still controversial regarding treatment modalities, and duration of treatment. [6,7] We present a case of an incidental thrombus in the descending aorta that was treated via anticoagulation.
2. Case description
The patient is a 65-year-old female who initially presented with severe abdominal pain and was eventually found to have bowel obstruction. At that time, she had undergone sigmoidectomy and primary anastomosis to manage the bowel obstruction. However, it was complicated by an anastomotic leak which required exploratory lap and end colostomy. During the same hospital course after surgery, the patient developed acute onset dyspnea and hypoxic respiratory failure. Based on clinical features of developing pitting edema and orthopnea, a diagnosis of CHF was made. Two doses of IV Lasix 20mg were given with significant clinical improvement then patient got discharged. However, patient presented to ED with similar complaints of dyspnea, orthopnea and pitting edema after 4 days of discharge. On lab investigation, CBC showed leukocytosis and hemoglobin of 11 with normal peripheral smear. Electrolytes and renal function were within normal limits. Urinalysis was unremarkable. A CT angiogram was performed which excluded pulmonary embolism, but instead discovered an incidental filling defect that was present along the right side of descending thoracic aorta at 9 o’clock position opposite the carina and near the esophagus measuring 15.5 mm x 12.8 mm in size (Figure 1)(Figure 3)(Figure 4). Differential diagnosis for the filling defect at the time included a mural thrombus, primary arterial/aortic tumor, primary myxoma, or xanthogranulomatous sarcomas. Infection of the aorta was less likely as there was no irregular wall thickening elsewhere or any suggestion of plaque with ulceration. The difficulty in determining the etiology prompted an MRI of the chest which better visualized and aided in diagnosing a thrombus in the descending aorta. She had also undergone a transthoracic echocardiogram which showed ejection fraction of 3540%, no valvular vegetations, and no septal defects. As the patient was hemodynamically stable, pursuing conservative management rather than invasive surgery was decided. Intravenous heparin infusion was commenced in view of a more favorable diagnosis of descending aorta thrombus and was later bridged to oral anticoagulation with warfarin. After discharge, the patient had close follow-up with her primary care doctor and cardiologist. Follow-up CTA chest with contrast after 3 months showed the filling defect was no longer present in descending thoracic aorta. Instead, there was some calcification measuring 3 mm x 4 mm at the level of intima with no other filling defects seen to suggest any residual blood clot (Figure 2). The second area of calcification was present posteriorly at the 10 o’clock position along the aortic margin in the intima at that level so there now is some calcific atherosclerosis where previously, there was a noncalcified blood clot and thrombus present. No dissection was seen in the descending thoracic aorta. No new areas of thrombus formation were seen along the walls of the vessel. The patient’s anticoagulation was discontinued after completing 3 months of therapy. Subsequent follow ups did not show any signs of recurrence of thrombus or signs of distal embolization. The patient was instructed to follow up for hypercoagulability work-up following completion of warfarin therapy.
Figure 1.
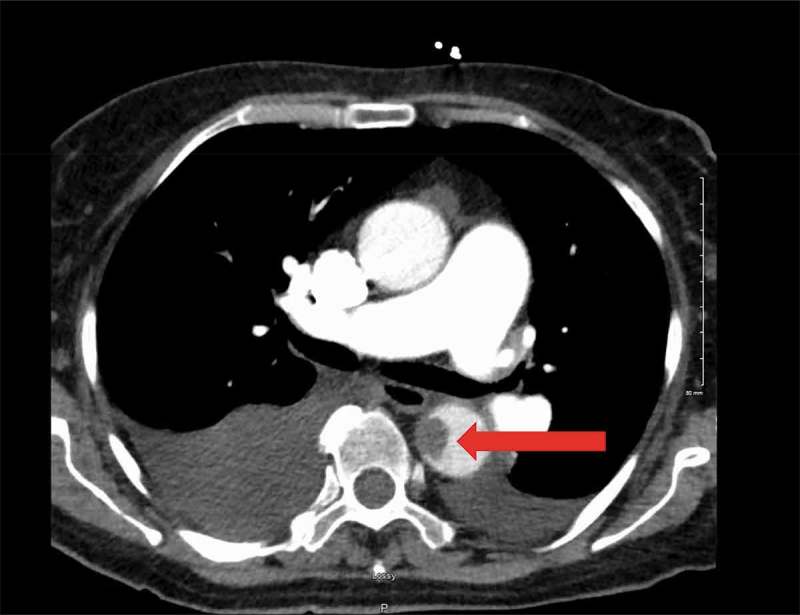
Filling defect in the descending thoracic aorta at 9 o’clock position opposite the carina and near the esophagus measuring 15.5 mm x 12.8 mm in size (Red Arrow).
Figure 3.
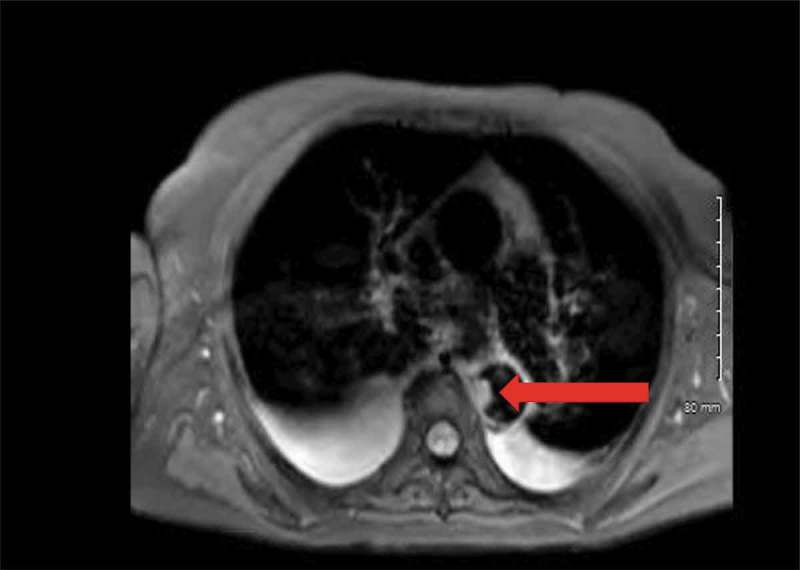
Irregular filling defect in the descending thoracic aorta 1.2 × 1.8 cm transverse by 3 cm craniocaudal. It is homogeneous signal on the T1 and T2 weighted sequences (Red Arrow).
Figure 4.
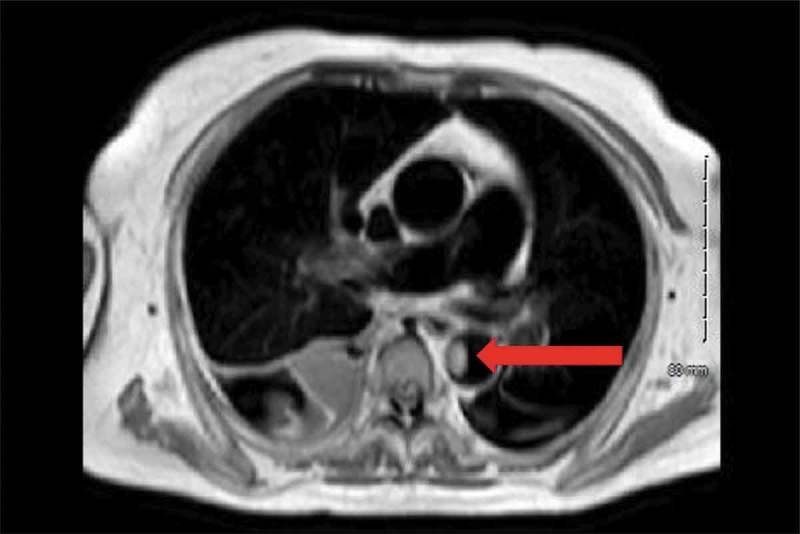
Irregular filling defect in the descending thoracic aorta 1.2 × 1.8 cm transverse by 3 cm craniocaudal. It does not enhance, unchanged in signal on the pre and postcontrast images. (Red Arrow).
Figure 2.
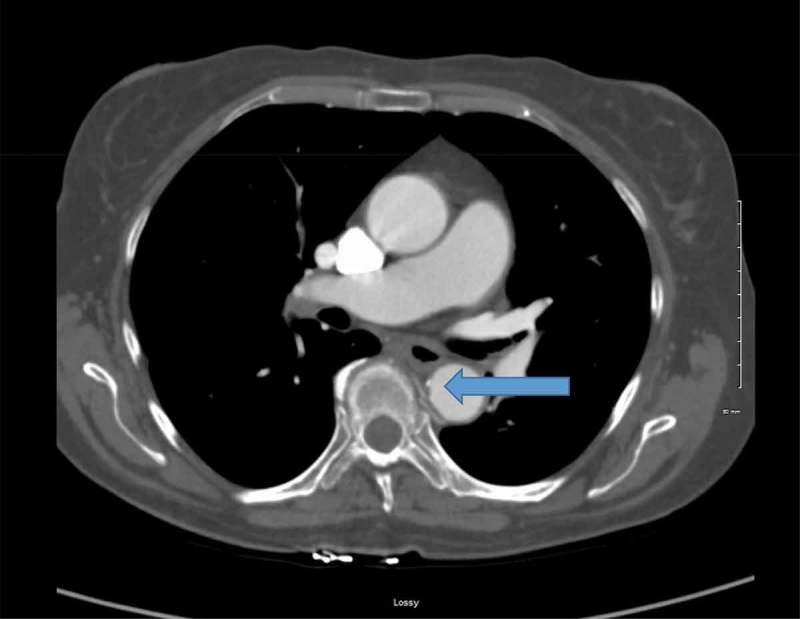
Small area of calcification (Blue Arrow) noted on repeat CT scan 3 months after treatment with anticoagulation. There is no sign of residual clotting.
3. Discussion
Thrombophilic states such as antiphospholipid syndrome, protein C or S deficiency, and APC resistance can be triggering cause for both venous and arterial thrombosis. [8] Generally, venous thrombosis is more frequent but less severe than arterial thrombosis. One of the clinical disorders which has exclusive association with venous thrombosis is antithrombin deficiency. In an aortic thrombus is not a common occurrence as the probability of forming an active thrombus originating from the aorta is low due to constant flow. A mural aortic thrombus may form with aortic dissection, aortic aneurysm, malignancy, and blood disorders and is rarely seen in a healthy subject. Other overlooked risk factors include patients on chemotherapy, smokers, patients with atherosclerosis, and iron deficiency. In the absence of these risk factors, formation of mural aortic thrombi are unlikely. It is hypothesized that mild atherosclerosis not detected on imaging may be a triggering the site for thrombus formation. The feared complication of an aortic thrombus is propagation and distal embolization. Depending on the original location of the thrombus, distal embolization can lead to stroke, mesenteric ischemia, organ dysfunction, and acute limb ischemia. In some studies, the incidence of distal embolization from an aortic thrombus can be as high as 73%. The most common encountered site of thrombus formation has been noted to be either in the distal aortic arch or in the region of the aortic isthmus.
Since the incidence of embolization is so high, most cases cannot be diagnosed until embolization has occurred. Numerous case reports have shown that the diagnosis of an aortic thrombi to be in part as a routine imaging work-up of the distal limb or end organs arterial systems. Notably in our patient’s case, the diagnosis was made prior to any consequential distal embolic phenomenon. Once diagnosed, screening for hypercoagulable disease in the absence of other risk factors can be pursued. [9] The discovery or absence of hypercoagulable diseases may then aid in guiding duration of treatment.
Our patient’s most significant risk factors included atherosclerosis and calcification of the aortic wall that was visualized on CT, and complicated abdominal surgery which we theorized to have triggered inflammatory cytokines, leading to a hypercoagulable state. The other possible risk factors may be advanced age, history of hypertension, and hyperlipidemia which caused microinjury and microinflammation causing increasing hypercoagulability, though previous studies mentioned there is no evidence that hypertension promotes thrombus formation. [10] Our patient may also have had an underlying hypercoagulability disorder, the evaluation of which was planned to be done after discontinuing anticoagulation so as not to be cofounded by acute inflammatory markers and anticoagulation treatment. Moreover, surgery-induced postoperative thrombocytosis and rise in fibrinogen and prothrombin levels leads to increasing the hypercoagulability. [11,12]
In accordance with the knowledge of the pathophysiologic mechanisms, appropriate therapy for an aortic thrombus is still controversial, varying between long-term anticoagulation and surgical options. The conventional method is conservative therapy. In our case report, the thrombus was successfully treated with warfarin anticoagulation therapy for about 3 months in duration. In acute patients who are hemodynamically unstable, aortic surgery was found to be beneficial. Combined therapy such as pharmacotherapy and surgery can sometimes be used for treatment. One emerging technology is an aortic stent graft which is used in emergency situations. However, in some previous studies, it is said that long-term anticoagulant is more reliant than surgery. [13] As our patient was asymptomatic and hemodynamically stable, conservative treatment with warfarin was prescribed along with follow-up. There was no incidence of recurrent thrombosis or distal embolization after termination of therapy.
4. Conclusion
We reported a case of an incidental finding of a mural thrombus in descending thoracic aorta without distal embolic complications. Suspicion for an aortic thrombus should arise when the origin is not known for acute onset distal limb or organ ischemia. Once discovered, it is more plausible to pursue conservative therapy with anticoagulation rather than utilizing a surgical approach.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Sugiura, T., Dohi, Y., Yamashita, S., Murai, S., & Ohte, N. A case report of asymptomatic aortic thrombosis incidentally detected by computed tomography in an apparently healthy subject with a history of cancer surgery. Thromb J. 2016;14(1):16. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4971728/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chong BK, Mun D, Kang CH, et al. Essential thrombocytosis-associated thromboembolism in the abdominal aorta. Korean J Thorac Cardiovasc Surg. 2016;49(5):397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fayad ZY, Semaan E, Fahoum B, et al. Aortic mural thrombus in the normal or minimally atherosclerotic aorta. Ann Vasc Surg. 2013;27(3):282–290. [DOI] [PubMed] [Google Scholar]
- [4].Haji K, Heron V, Davis R, et al. A case of massive aortic mural thrombus in the absence of atherosclerotic or aneurysmal disease. Int J Cardiol Heart Vasc. 2016;12:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Josephson, GD., Tiefenbrun, J., & Harvey, J.. Thrombosis of the descending thoracic aorta: a case report. Surgery. 1993;114(3):598–600. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8367817. [PubMed] [Google Scholar]
- [6].Wolberg AS, Aleman MM, Leiderman K, et al. Procoagulant activity in hemostasis and thrombosis: Virchow’s triad revisited. Anesth Analg. 2012;114(2):275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hassan I, Zehr KJ, Freeman WK, et al. A case of asymptomatic thoracic aorta mural thrombi. Ann Thorac Surg. 2001;72(5):1735–1737. [DOI] [PubMed] [Google Scholar]
- [8].Nakashima MO, Rogers HJ.. Hypercoagulable states: an algorithmic approach to laboratory testing and update on monitoring of direct oral anticoagulants. Blood Res. 2014;49(2):85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Habib H, Hsu J, et al. Mural thrombus in the normal-appearing descending thoracic aorta of a chronic smoker. Tex Heart Inst J. 2013;40(5):619. [PMC free article] [PubMed] [Google Scholar]
- [10].Horvei L, Grimnes G, Hindberg K, et al. C‐reactive protein, obesity, and the risk of arterial and venous thrombosis. J Thromb Haemost. 2016;14(8):1561–1571. [DOI] [PubMed] [Google Scholar]
- [11].Lison S, Weiss G, Spannagl M, et al. Postoperative changes in procoagulant factors after major surgery. Blood Coagul Fibrinolysis. 2011;22(3):190–196. [DOI] [PubMed] [Google Scholar]
- [12].Tsilimparis N, Hanack U, Pisimisis G, et al. Thrombus in the non-aneurysmal, non-atherosclerotic descending thoracic aorta–an unusual source of arterial embolism. Eur J Vasc Endovascular Surg. 2011;41(4):450–457. [DOI] [PubMed] [Google Scholar]
- [13].Wang, L., Djousse, L., Song, Y., Akinkuolie, A. O., Matsumoto, C., Manson, J. A. E., … Sesso, H. D Associations of diabetes and obesity with risk of an abdominal aortic aneurysm in men. J Obesity. 2017. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5343258/. [DOI] [PMC free article] [PubMed] [Google Scholar]


