Abstract
Context
Zuogui Wan is a classic traditional Chinese prescription. Preliminary studies have confirmed that it could improve sperm quality significantly.
Objective
To investigate the effect of Zuogui Wan on testis structure and c-kitproto-oncogeneprotein (c-Kit) and octamer-binding transcription factor-4 (Oct4) expression in a rat model of impaired spermatogenesis.
Material and methods
Thirty-six Sprague-Dawley (SD) rats were divided into Blank control, Tripterygium glycosides (GTW) and Zuogui Wan groups (n = 12). GTW was used to generate models of impaired spermatogenesis. Then Zuogui Wan group was administered 6 g/kg/d of Zuogui Wan granules for 4 weeks. Changes in the pathological structure and ultrastructure were observed with optical microscope and transmission electron microscope. Expression of c-Kit and Oct4 were quantified by RT qPCR and Western blots.
Results
Both the pathological damage and the damages in the ultrastructure of spermatogenic epithelium had improved in Zuogui Wan group. Compared with the GTW model group (0.47 ± 0.19; 0.38 ± 0.14), c-Kit and Oct4 protein expression increased in the Zuogui Wan group (0.75 ± 0.27; 0.65 ± 0.23). C-Kit and Oct4 mRNA expression increased in Zuogui Wan group (1.06 ± 0.16; 1.85 ± 1.04) compared to the GTW model group (0.66 ± 0.23; 0.46 ± 0.29).
Conclusions
Zuogui Wan is capable of restoring the damage to the testis structure and ultrastructure and regulates the expression of c-Kit and Oct4 at protein and mRNA levels, inhibiting apoptosis and promoting proliferation of spermatogenic cells.
Keywords: Traditional Chinese medicine, spermatogenesis impairment, apoptosis, proliferation
Introduction
Abnormal semen quality is the main reason for male infertility and mainly presents as abnormal sperm quantity, sperm concentration, sperm number and sperm activity. Previous studies (Chen et al. 2012) have shown that oligospermia and asthenospermia account for about 46% of male infertility cases. In addition, with global changes in socioeconomic circumstances and cultures, many older couples may wish to have another child, but the quality of semen declines with age, causing a decline in fertility. Therefore, it is important to understand the role of semen quality in male sterility.
In terms of treatment of male infertility, surgical treatment has little application, assisted reproduction has a high cost, limited success rate, and unclear genetic risk, and most drug treatments have no clear effect. Traditional Chinese medicine (TCM) has a unique theoretical system and related diagnosis and treatment strategy, and has long been used for the prevention and treatment of male infertility. TCM treatment is favoured by many infertile patients, and thus it is necessary to evaluate the efficacy of TCM for this purpose in depth, and to explore better treatment methods for male infertility.
TCM holds that an ‘essence hidden in the kidney is crucial to reproduction’. In other words, physiologically speaking, male fertility is based on the kidney essence, and a deficiency in kidney essence and kidney Yin can result in fewer and weaker sperm cells. Preliminary clinical investigations (Li et al. 2013) have revealed that a deficiency in kidney Yin accounts for a large proportion of male infertility cases, but there have been few relevant clinical trials and experimental studies. Thus, it is necessary to elucidate the mechanism by which nourishing Yin and kidney essence could treat male infertility.
Zuogui Wan is a classic TCM prescription for nourishing Yin and tonifying the kidney. Preliminary clinical studies (Wang et al. 2017, 2018; Zhu et al. 2017) have confirmed that it has a significant effect on improving sperm quality in patients with less severe affected sperm. Zuogui Wan can relieve damage to the testis and inhibit spermatogenic cell apoptosis, and promote proliferation of these cells by regulating the expression of Bax, Bcl-2, SCF and Ki67 at protein and mRNA levels in male infertility rat models.
However, at present, few studies have investigated the regulating effects of Zuogui Wan on the structure and ultrastructure of the testis and SCF/c-Kit signalling system and Oct3/4 expression in animal models of spermatogenesis impairment. Therefore, in this study, we explored the effect of Zuogui Wan treatment on the pathology and ultrastructure of the testis, and the expression of c-Kit and Oct4 at protein and mRNA levels in testicular tissues of experimental rats. We aimed to elucidate the consequences of Zuogui Wan treatment in terms of inhibiting apoptosis and improving proliferation and differentiation in a rat model of spermatogenesis impairment induced by GTW, to shed light on the mechanism underlying its effects on spermatogenesis.
Materials and methods
Animals
Thirty-six 12-week-old male SPF-level SD rats, with an average body weight of 220 ± 15 g, were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. [Animal Licence No. SCXK (jing) 2011-0011]. They were allowed to acclimatize for 1 week prior to starting the experiments. The study was approved by the animal ethics committee of Beijing University of Chinese Medicine (No. 16-48).
Drugs and reagents
GTW tablets (batch No. 130801, 10 mg/tablet) were purchased from Anhui Xinlonghai Pharmaceutical Co., Ltd. (Suzhou, China). Zuogui Wan is a TCM formula granule (Beijing Tcmages Pharmaceutical Co., Ltd., Beijing, China), and includes the following, prepared radix rehmanniae 24 g, yam 12 g, dodder seed 12 g, radix achyranthis bidentatae 9 g, dogwood 12 g, deer-horn glue 12 g and tortoise-plastron glue 12 g. The GTW tablets were ground into a powder, and the GTW powder and Zuogui Wan granules were used to prepare a suspension with the required concentration, which was stored at 4 °C. Additionally, we purchased anti-c-Kit antibody (rabbit, bs-0672R), anti-Oct 3/4 antibody (rabbit, Bd-1111R), anti-actin antibody (rabbit, bs-10966R) from Bioss Antibodies (Boston, MA, USA).
Instruments
Instruments used in this study included a low-temperature centrifuge (Sigma-Aldrich 3K18, St Louis, MO, USA), a Sage Creation gel-imaging analysis system (Beijing Sage Creation Science Co., Ltd., Beijing, China), a centrifuge (Neofuge 15 R, Heal Force Bio-Meditech Ltd., Shanghai, China), a multi-sample grinding bead homogenizer (Omni Bead Ruptor 12, Omni Inc., Kennesaw, GA, USA), fluorescent quantitation PCR instrument (ABI 7300, ABI, Foster City, CA, USA), laminar flow cabinet (AIRTECH SW-CJ-1FD, Airtech, Huntington Beach, CA, USA), optical microscope (Olympus L340099, Olympus, Tokyo, Japan), and a transmission electron microscope (Hitachi H-7500, Hitachi, Tokyo, Japan).
Grouping and administration
Thirty-six SD rats were randomly divided into a Blank control group, GTW model group and Zuogui Wan group, each group consisting of 12 rats. The Blank control group was administered deionized water throughout the experiment. In the fourth week before the experiment, the GTW model group and the Zuogui Wan group were administered 40 mg/kg/d GTW to generate the model of impaired spermatogenesis (Ma et al. 2015), in the 5th to 8th weeks of the experiment, rats in the GTW model group were given deionized water by intragastric administration, and those in the Zuogui Wan group were given 6 g/kg/d of Zuogui Wan granules (equivalent to six times the human dose) by intragastric administration. After the end of the 8th week, all animals were sacrificed by cervical dislocation and the testicular tissue collected in sterile tubes for further analyses.
Observation of testicular pathology by optical microscopy
The left testis of each rat was dissected along its largest diameter, and half of the testis was fixed in 4% paraformaldehyde for 24 h, after which it was dissected along its horizontal axis, and then fixed in the same solution for another 12 h. Thereafter, it was rinsed under running water for 24 h and under ionized water for 2 h, following which it was dried with absorbent paper. The fixed tissue was embedded in wax and 4 μm thick slices obtained. Sections were stained with haematoxylin and eosin and observed with an optical microscope to analyse pathological changes in the testes of rats.
Observation of ultrastructure by transmission electron microscopy
A 1 mm × 1 mm × 1 mm sample of each tissue was fixed in 3% glutaraldehyde and 1% osmic acid, dehydrated in a graded series of acetone and ethyl alcohol, and then embedded in paraffin. This was used to prepare 100 nm thick ultrathin slices, which were stained with saturated uranyl acetate. A transmission electron microscope was used to identify the convoluted seminiferous tubules and observe the morphology, quantity and ultrastructure of spermatogenic cells (including the nucleus, mitochondria, lysosomes, etc.).
Expression of testicular c-Kit and Oct4 protein and mRNA
Western blot
The expression of c-Kit and Oct4 protein in the testicular tissue of the rats in the various groups was analysed and compared. Proteins were extracted from the testicular tissue of rats in the various groups, separated by gel electrophoresis, and transferred to a methanol-activated polyvinylidene difluoride (PVDF) membrane. The PVDF membrane was incubated in a solution containing 5% skim milk for 1 h at room temperature, after which the membrane was incubated with anti-c-Kit antibody or anti-Oct3/4 antibody (diluted 1:1000) overnight at 4 °C, thereafter, the PVDF membrane was washed three times in TBST solution, for 15 min each time. Then, the membrane was incubated with the secondary antibody for 1 hour at room temperature. Enhanced chemiluminescence was used to visualize bands and the Alpha software processing system was used to analyse the optical density of the target band on the film.
RT qPCR
Total RNA from the testicular tissue samples was extracted by Trizol, followed by cDNA synthesis using the PrimerScript RT reagent Kit (TaKaRa, Japan). The primers are listed in Table 1. The PCR conditions were as follows, pre-denaturation at 95 °C for 10 min, 40 cycles at 95 °C for 15 s, 60 °C for 60 s and 72 °C for 60 s. The quantity of mRNA was calculated through the cycle threshold (CT) values, and the relative mRNA expression levels were determined using the 2−ΔΔCT method.
Table 1.
List of primer sequences for real-time quantitative PCR.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| c-kit | 5 ′ - GTGCCCGAAACAAGTCATCTC -3 ′ | 5 ′ - GCTGAGGGTTCAACTTTATCCA -3 ′ NM_022264.1 |
| OCT3/4 | 5 ′ - TGGAGGAAGCTGACAACAACGA -3 ′ | 5 ′ - GCTGCTTGGCAATGCTAGTGAT -3 ′ NM_001009178.2 |
| GAPDH | 5 ′ - TTCCTACCCCCAATGTATCCG -3 ′ | 5 ′ - CATGAGGTCCACCACCCTGTT -3 ′ NM_017008.4 |
Statistical analyses
We used IBM SPSS v. 21.0 statistical software (SPSS Inc., Chicago, IL, USA) to analyse and process the obtained data. All measurement data are expressed as mean ± standard deviation. If the data had a normal distribution, one-way analysis of variance was used to compare data of multiple groups. If the variance was equal, multiple comparisons were performed with the Student-Newman-Keuls test and least significant difference test, if the variance was unequal, approximate variance analysis (Welch test) was used, and the Games-Howell test was used for multiple comparisons. If the data did not satisfy a normal distribution, nonparametric tests were used. p values <0.05 were considered to indicate statistically significant differences, while p < 0.01 was considered to indicate strongly significant differences.
Results
Pathological changes in the testis
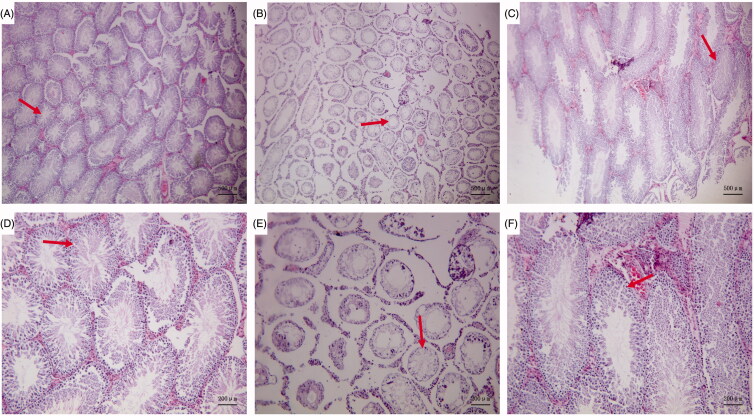
Light microscopy revealed that, in the Blank control group, the convoluted tubules in the testis appeared to have a normal structure and ordered arrangement, there were abundant sperm cells, and the spermatogenic cells had a normal morphology, with no evident anomalies (Figure 1(A,D)). In the GTW model group, the seminiferous tubules demonstrated a disordered arrangement, narrowed lumen, some metamorphic epithelium, and a large number of vacuoles. There were few mature sperms in the lumen and significantly increased numbers of sperm cells with an abnormal morphology. Spermatogenic cells were largely absent from the seminiferous tubules and had a disorderly arrangement (Figure 1(B,E)). In the Zuogui Wan group, the degree and extent of pathological changes were significantly alleviated as compared with those in the GTW model group. The lumen of the seminiferous tubules was obviously enlarged and there were significantly more sperm cells in the lumen, however, although some seminiferous tubules appeared to have recovered as compared to the GTW model group, they had not recovered to the levels of the Blank control group (Figure 1(C,F)).
Figure 1.
Pathological changes in the testis. Blank control group (A ×40, D ×100): the convoluted tubules in the testis appeared to have a normal structure and ordered arrangement, there were abundant sperm cells. GTW model group (B ×40, E ×100): there were few mature sperms in the lumen and significantly increased numbers of sperm cells with an abnormal morphology. Zuogui Wan group (C ×40, F ×100): The lumen of the seminiferous tubules was obviously enlarged and there were significantly more sperm cells in the lumen.
Ultrastructural changes in the testis and spermatogenic cells
Transmission electron microscopy revealed that the intrinsic structure of the convoluted tubules was intact, showing a clear boundary. Spermatogenic cells in various growth cycles were arranged in an orderly fashion on the spermatogenic epithelium, and had normal morphology. The nuclei were round or oval and the nucleoli were visible. The number of organelles in the cytoplasm was normal and there were abundant mitochondria. The intercellular bridge between the spermatogenic cells and the Sertoli cell junction complex was complete (Figure 2(A,D)). In the GTW model group, the cells of the spermatogenic epithelium of the convoluted tubules were disordered, non-compact, and showed vacuolation and degeneration. In the spermatogenic cells, an increase in cell organelles was noted, some nuclei were condensed, and some cells had no nucleus. Cytoplasmic vacuolar changes were present in the spermatogenic cells, and significantly fewer mitochondria were present than in those in the Blank control group (Figure 2(B,E)). In the Zuogui Wan group, transmission electron microscopy revealed that the ultrastructure of the spermatogenic epithelium of the testis was similar to that in the Blank control group. Spermatogenic cells showed a relatively intact morphology and orderly arrangement. Mitochondria and lysosomes were significantly increased as compared to the GTW model group. A few degenerated organelles and sperms were visible (Figure 2(C,F)).
Figure 2.
Ultrastructural changes in the testis and spermatogenic cells. Blank control group (A ×6000, D ×12,000): the intrinsic structure of the convoluted tubules was intact, showing a clear boundary. Spermatogenic cells in various growth cycles were arranged in an orderly fashion on the spermatogenic epithelium, and had normal morphology. GTW model group (B ×6000, E ×12,000): the cells of the spermatogenic epithelium of the convoluted tubules were disordered, non-compact, and showed vacuolation and degeneration. In the spermatogenic cells and some cells had no nucleus. Zuogui Wan group (C ×6000, F ×12,000): the spermatogenic cells showed a relatively intact morphology and orderly arrangement. Mitochondria and lysosomes were significantly increased.
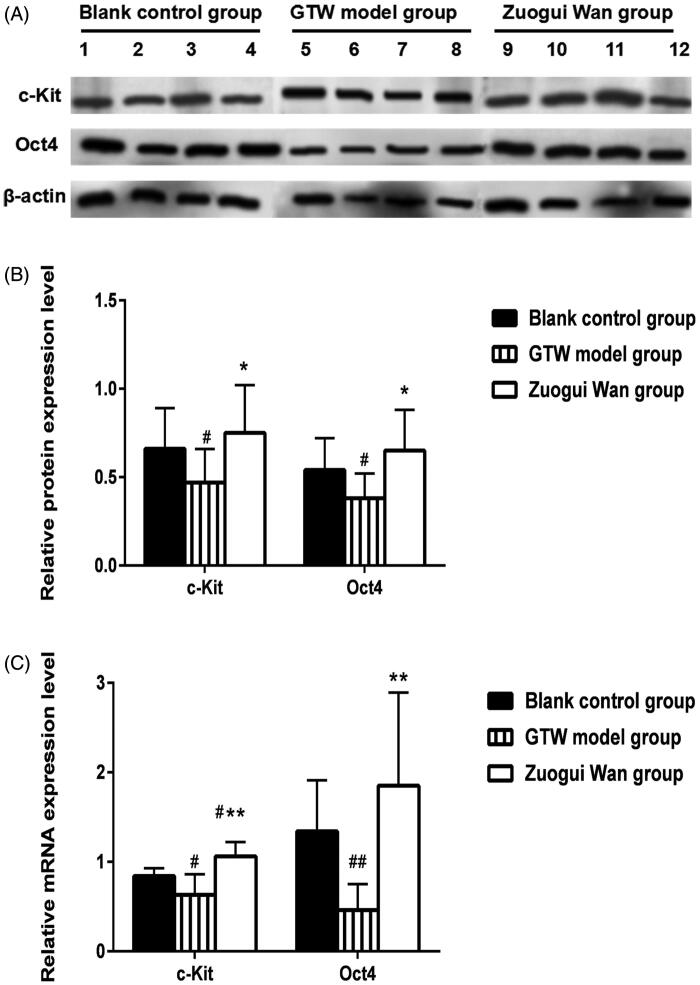
c-Kit protein expression in rat testicular tissue
Using western blotting, the relative c-Kit protein expression was determined in the testicular tissue of the rats in the various groups (representative results are shown in Figure 3(A)). Through analysis of relative expressions (Figure 3(B)), we found that the c-Kit protein expression in the GTW model group (0.47 ± 0.19) was significantly lower than that in the Blank control group (0.66 ± 0.23) (p < 0.05). Compared to the GTW model group, the c-Kit protein expression in the Zuogui Wan group (0.75 ± 0.27) was significantly higher (p < 0.05). While the difference between Blank control group and Zuogui Wan group was not statistically significant. See Figure 3(A,B) for specific results.
Figure 3.
(A) Western blotting analysis of c-Kit and Oct4 protein expression in rat testicular tissue in various groups; (B) proportions of c-Kit and Oct4 protein expression grey values in the testicular tissue of rats in various groups; (C) c-Kit and Oct4 mRNA expressions in rat testicular tissue. ##p < 0.01, #p < 0.05 compared to Blank control group, *p < 0.05, **p < 0.01 compared to GTW model group.
Oct4 protein expression in rat testicular tissue
The western blot method was used to test the Oct4 protein expression in rat’s testicular tissue in various groups (representative results are shown in Figure 3(A)). Through analysis of relative expressions (Figure 3(B)), we found that the Oct4 protein expression in the GTW model group (0.38 ± 0.14) was significantly lower than that in the Blank control group (0.54 ± 0.18) (p < 0.05). Compared to the GTW model group, the Oct4 protein expression in the Zuogui Wan group (0.65 ± 0.23) was significantly higher (p < 0.05). However, the Oct4 mRNA protein in the Zuogui Wan group was similar to that in the Blank control group (p > 0.05). See Figure 3(A,B) for specific results.
c-Kit mRNA expression in rat testicular tissue
Using RT qPCR, we found that the c-Kit mRNA expression in the GTW model group (0.63 ± 0.23) was significantly lower than that in the Blank control group (0.84 ± 0.09) (p < 0.05). Compared to the GTW model group, the c-Kit mRNA expression in the Zuogui Wan group (1.06 ± 0.16) was significantly higher (p < 0.01). This expression in the Zuogui Wan group was also statistically significantly lower than that in the Blank control group (p < 0.05). See Figure 3(C) for specific results.
Oct4 mRNA expression in rat testicular tissue
RT qPCR also revealed that Oct4 mRNA expression in the GTW model group (0.46 ± 0.29) was significantly lower than that in the Blank control group (1.34 ± 0.57) (p < 0.01). Compared with the GTW model group, the Oct4 mRNA expression in the Zuogui Wan group (1.85 ± 1.04) was significantly higher (p < 0.01), however, the Oct4 mRNA expression in the Zuogui Wan group was similar to that in the Blank control group (p > 0.05). See Figure 3(C) for specific results.
Discussion
The testis is a crucial part of the reproductive system of male mammals, and its major function is to produce androgen and sperms. Androgen is mainly synthetized and secreted by interstitial testis tissue. As sperms are produced in the seminiferous tubules of the testis, a normal testis structure is the foundation for sperm production and normal quality sperm. Typically, the intercellular bridges between spermatogenic cells and the Sertoli cell junction complexes should be intact, and mitochondria should be abundant. As GTW is a testicular toxin, it can damage the seminiferous tubules, degrade spermatogenic cells, and cause degeneration and necrosis in Leydig cells, decreasing the T-composition, and thus is widely used in studies of impaired spermatogenesis (Jing et al. 2016). Therefore, we used GTW to generate a rat model of spermatogenesis disorder.
The Zuogui Wan was created by Zhang Jingyue, a physician of the Ming Dynasty. In the Complete Works of Zhang Jingyue, Eight New Prescriptions, the following is recorded, ‘If you are good at nourishing yin, you must seek for yin in yang, then you will have yin from yang. The source is inexhaustible’, ‘treat Yin for Yang problems, and nourish kidney Yin in the left kidney’, and he named the prescription ‘Zuogui’. Zuogui Wan consists of prepared radix rehmanniae, dogwood, yam, deer-horn glue, tortoise-plastron glue, fructus lycii, dodder seed and radix achyranthis bidentatae. In the prescription, the prepared radix rehmanniae is used as the main drug to nourish kidney Yin and boost Qi. The tortoise-plastron glue and deer-horn glue are used as supporting drugs, the tortoise-plastron glue is sweet and cold, which can neutralize yang in addition to nourishing liver and kidney yin, the deer-horn glue is sweet and salty with warm property, which can boost essence, enrich blood, warm and invigorate kidney Yang, and when used with medicine for nourishing kidney Yin, it can boost both Yin and Yang, thereby nourishing Yin through Yang. Dogwood, yam and fructus lycii can all nourish Yin, dogwood nourishes the liver and kidneys, and prevents seminal emission, yam tonifies the spleen, replenishes Yin, and secures essence, fructus lycii nourishes the kidney and replenishes essence. The medicinal Cyathula root tonifies the liver and kidneys, and strengthens waist and bones, the dodder seed warms Yang and nourishes Yin, and when used with deer-horn glue, it can ‘seek Yin from Yang’, and balance nourishing of Yin and Yang. By combining these various medicines, it can generate the effects of nourishing kidney yin, replenishing essence and supplementing marrow. Based on own present study treatment with Zuogui Wan granules relieved the damage to the testes of GTW rats. Compared with the GTW model group, the structure and ordered arrangement of the seminiferous tubules repaired after treatment, and spermatogonia, spermatocytes, and spermatids increased. Thus, the cytopathic effects of GTW were rescued. Transmission electron microscopy further confirmed that the ultrastructure of spermatogenic epithelium of the testis in the Zuogui Wan group was similar to that of the Blank control group, spermatogenic cells in the former group had a relatively intact morphology, were arranged in an orderly fashion, and had increased numbers of Golgi and lysosomes. Although these features were not quite restored to the level of those in the Blank control group, the pathological damage had been markedly recovered as compared to the GTW model group. Therefore, Zuogui Wan improved the damage exerted by GTW on the testis and spermatogenic cells in rats, and the mechanism by which it reduces the reproductive toxicity of GTW may be related to repairing the morphology of spermatogenic cells and promoting the production of mitochondria in these cells.
As a receptor tyrosine kinase, c-Kit protein regulates the development of germ cells and can inhibit apoptosis by regulating mitochondrial function and the redox state of cells. After specific binding between this protein and stem cell factor (SCF) on the spermatogonial stem cell membrane, autophosphorylation occurs, which helps to maintain spermatogonial stem cells and regulate self-proliferation capacity. The SCF/c-Kit signal system thus regulates the proliferation, differentiation and apoptosis of spermatogonial stem cells during spermiogenesis. It can not only promote division of stem cells, but also inhibit apoptosis of spermatocytes and sperm cells, thereby regulating the development, growth and maturation of sperms (Unni et al. 2009; Shupe et al. 2011; Bhattacharya et al. 2012). A previous study (Rossi et al. 2000) showed that the SCF/c-Kit system plays a key regulatory role in the differentiation and proliferation of type A spermatogonia. Additionally, it has been shown (Bai et al. 2007) that expression of c-Kit is directly related to the differentiation of spermatogonia and plays a crucial role in their differentiation. In addition, c-Kit is closely related to Bcl-2/Bax, and the SCF/c-Kit signal is upstream to Bcl-2/Bax, which can indirectly regulate and activate the proliferation, and regulate the expression of Bcl-2/Bax by activating the PI3K/AKT path (Rossi et al. 2000). This is consistent with the results of a preliminary study conducted by our research group (Zhu et al. 2017).
Bax regulates apoptosis by affecting mitochondrial structure and function via regulation of permeability transition pores. In the ultrastructure analysis of testes in the present study, the number of mitochondria in the Zuogui Wan group was more than that in the GTW model group, providing morphological evidence for the relevance of c-Kit and Bcl-2/Bax to the effects of this treatment.
Oct3/4 is a POU-domain family transcription factor, which is related to totipotency during embryonic development, and is considered a marker of totipotent embryonic stem cells in mammals. Recent studies have shown that germ cell development is regulated by signalling pathways involving BMP, Kit/KL and FGFs. Oct3/4 participates in the regulation of BMP, FGFs and other signalling pathways. It is mainly expressed in embryonic stem cells and primordial germ cells, and regulates the growth and differentiation of embryonic stem cells and primordial germ cells, absence of Oct3/4 leads to failure of normal differentiation due to abnormal apoptosis. Therefore, over expression of Oct3/4 is associated with abnormal differentiation of germ cells, and it can be used as a marker to evaluate cell proliferative and differentiation ability (Looijenga et al. 2003; Kehler et al. 2004; Morrison and Brickman 2006).
Most kidney-tonifying TCMs exert their effects by inhibiting apoptosis of spermatogenic cells or promoting their proliferation. In previous preliminary studies (Zhu et al. 2017; Wang et al. 2018), we found that Zuogui Wan can inhibit apoptosis of spermatogenic cells and promote proliferation of spermatogenic cells by regulating the expression of Bax, Bcl-2, SCF and Ki67 proteins and their mRNA in model rats. We here showed that the expression of c-Kit protein and mRNA in rats with impaired spermatogenesis was significantly increased after Zuogui Wan treatment. Taken together with the results of our preliminary studies, this indicates that Zuogui Wan can promote the proliferation of spermatogenic cells by regulating the expression of SCF/c-Kit protein. As SCF/c-Kit is an upstream protein of Bcl-2/Bax, increased expression of c-Kit can increase Bcl-2 levels, and thus indirectly inhibit cell apoptosis.
Moreover, we showed that Zuogui Wan treatment also led to a significant increase in Oct4 protein in rats with impaired spermatogenesis. This suggests that one of the effects of Zuogui Wan is to improve and maintain the pluripotency of spermatogenic stem cells. Furthermore, Oct4 also reflects the differentiation ability of cells. We conclude that Zuogui Wan can also improve the ability of spermatogenic cells to differentiate from spermatogonial stem cells to mature spermatozoids.
At the same time, a relevant network pharmacology study (Gao et al. 2018) has shown that the active ingredient of Zuogui Wan such as isorhamnetin, sitosterol, stigmasterol, diosgenin, sesamin, piperlonguminine, ethyl oleate, iso-fucosterol, etc., target genes AKT1, BAX, BCL2, etc., which are closely related to the spermatogenesis process. It provides an important basis for Zuogui Wan’s improving the spermatogenic disorder and improving the pluripotency of spermatogenic stem cells, and is consistent with our previous research results. However, due to the multi-target and multi-channel effects of traditional Chinese medicine formula, its specific active ingredients and mechanism of action still need further study.
Conclusions
In conclusion, Zuogui Wan facilitates recovery of the damage in the testis structure and ultrastructure of rats with impaired spermatogenesis and regulates the expression of c-Kit and Oct4 at protein and mRNA levels, inhibiting spermatogenic cell apoptosis and promoting proliferation of these cells. Thus, the efficacy of Zuogui Wan is at least partly due to improvement of the pluripotency of spermatogenic stem cells. Furthermore, given the role of Oct4 in the differentiation ability of cells, Zuogui Wan may also enhance the gradual differentiation of spermatogenic cells from spermatogonial stem cells to mature spermatozoids.
Funding Statement
This work was supported by the Fundamental Research Funds for the Central Universities [No. 2016-JYB-QNJSZX009], Young Scientist Development Programme 2016, Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine, National Natural Science Foundation of China [No. 81273756 and No. 81774320].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bai Y, Ye ZW, Zeng PQ.. 2007. Characteristics of c-kit expression and differentiation of spermatogonia stem cell in rat testicles. Nat J Androl. 13:69–70. [Google Scholar]
- Bhattacharya I, Pradhan BS, Sarda K, Gautam M, Basu S, Majumdar SS.. 2012. A switch in Sertoli cell responsiveness to FSH may be responsible for robust onset of germ cell differentiation during prepubartal testicular maturation in rats. Am J Physiol Endocrinol Metab. 303:886–898. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Liang M, Yu Y, Sun W.. 2012. Research progress and preliminary mechanism of acupuncture treatment for improving semen quality in male infertility patients. Chinese J Androl. 26:69–72. [Google Scholar]
- Gao X, Ye ZH, Dai L, Li HM.. 2018. Molecular mechanism research of Zuogui Pill about “tonify-ing kidney to promote liver regeneration and repair by effecting stem cells and their micro-environment” by using the network pharmacology method. Chin J Integr Med. 28:96–100. [Google Scholar]
- Jing XP, Cheng WW, Zou Y, Pan HM, He L.. 2016. Reversibility of Tripterygium wilfordii Hook. F on the reproductive damage in male adolescence rats. Acad J Shanghai Univ Trad Chinese Med. 30:65–68. [Google Scholar]
- Kehler J, Tolkunova E, Koschorz B, Pesce M, Gentile L, Boiani M, Lomelí H, Nagy A, McLaughlin KJ, Schöler HR, et al. 2004. Oct4 is required for primordial germ cell survival. EMBO Rep. 5:1078–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HS, Jia YS, Han ZC, Wang B, Han L, Yang JZ, Dang J.. 2013. Analysis of different Chinese medicine syndrome in 800 infertile males. Chinese J Androl. 27:38–40, 41, 48. [Google Scholar]
- Looijenga LH, Stoop H, de Leeuw HP, de Gouveia Brazao CA, Gillis AJ, van Roozendaal KE, van Zoelen EJ, Weber RF, Wolffenbuttel KP, van Dekken H, et al. 2003. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. 63:2244–2250. [PubMed] [Google Scholar]
- Ma HF, Li HS, Zhao ZJ, Wang B, Zhao B, Mo XW, Liu Y, Cheng MX, Yang MJ.. 2015. Build the model of rat with spermatogenesis impairment induced by Tripterygium glycosides. Nat J Androl. 21:179–184. [Google Scholar]
- Morrison GM, Brickman JM.. 2006. Conserved roles for Oct4 homologues in maintaining multipotency during early vertebrate. Development. 133:2011–2022. [DOI] [PubMed] [Google Scholar]
- Rossi P, Sette C, Dolci S, Geremia R.. 2000. Role of c-kit in mammalian spermatogenesis. J Endocrinol Invest. 23:609–615. [DOI] [PubMed] [Google Scholar]
- Shupe J, Cheng J, Puri P, Kostereva N, Walker WH.. 2011. Regulation of Sertoli-germcell adhesion and sperm release by FSH and nonclassical testosterone signalling. Mol Endocrinol. 25:238–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unni SK, Modi DN, Pathak SG, Dhabalia JV, Bhartiya D.. 2009. Stage-specific localization and expression of c-kit in the adult human testis. J Histochem Cytochem. 57:861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Li X, Ma HF, Zhu YT, Ma JX, Wang JS, Dang J, Li HS.. 2018. Research on the influence of Zuogui Wan on the stem cell factor and its m RNA expression in the testicular tissues of model rats with oligospermia and asthenospermia. J Chin Gen Pract. 21:334–336. [Google Scholar]
- Wang B, Ma JX, Ma HF, Zhu YT, Wang JS, Dang J, Dong L, Li HS.. 2017. Preventive effect of Zuogui pills on sperm quality and reproductive organs of rats with Tripterygium glycosides-induced oligospermia and asthenospermia. China J Trad Chin Med Pharm. 32:5574–5577. [Google Scholar]
- Zhu YT, Wang B, Ma HF, Ma JX, Wang JS, Li HS.. 2017. Influence of Zuogui Wan on the expression of testicular Bcl-2 & Bax proteins and m RNA of rats with spermatogenesis impairment. China J Trad Chin Med Pharm. 32:5327–5330. [Google Scholar]