Abstract
Context
Experiencing early-life adversity plays a key role in the development of mood disorders in adulthood. Experiencing adversities during early life period negatively affects brain development. Sex steroids such as progesterone affect the brain structure and functions and subsequently affects behaviour.
Objective
We assess the antidepressant-like effect of progesterone in a mouse model of maternal separation (MS) stress, focussing on its anti-neuroinflammatory and antioxidative effects.
Materials and methods
NMRI mice were treated with progesterone (10, 50, and 100 mg/kg, i.p., respectively) for 14 days. Valid behavioural tests including forced swimming test (FST), splash test and open field test (OFT) were used. Quantitative reverse transcription-PCR (qRT-PCR) was used for evaluation of genetic expression in the hippocampus. Antioxidant capacity was assessed by the FRAP method and the level of malondialdehide by TBA.
Results
MS provoked depressive-like behaviour in mice. Treatment of MS mice with progesterone increased the grooming activity time in the splash test and decreased the immobility time in the FST. In addition, progesterone decreased the expression of inflammatory genes related to neuroinflammation (IL-1β, TNF-α, TLR4 and NLRP3) as well as increased the antioxidant capacity and decreased the lipid peroxidation (MDA) in the hippocampus.
Discussion and Conclusion
Administration of progesterone significantly mitigated the negative effects of MS on behaviours relevant to depressive-like behaviour as well as attenuated neuro-immune response and oxidative stress in the hippocampus of MS mice. In this context, we conclude that progesterone, at least partially, via attenuation of oxidative stress and neuroinflammation, exerts antidepressant-like effects.
Keywords: Early life stress, splash test, FST
Introduction
Depression is one of the most prevalent psychiatric disorders and the main cause of disability in the twenty-first century (Üstün 2001; Murray et al. 2012). It is characterised by sadness, loss of interest or pleasure, decreased energy or feelings of tiredness, feelings of low self-worth, disturbed sleep or appetite, and poor concentration (Chen et al. 2015). Experiencing early-life adversity plays a key role in the development of the mood disorders in humans and rodents (Lupien et al. 2009; Rao et al. 2010).
The early postnatal period is accompanied by significant maturation of the nervous system. Experiencing stressful conditions during this period results in the majority of neurobehavioral, neurochemical and immune-inflammatory abnormalities in later life (Rice and Barone Jr. 2000). In this regard, maternal separation (MS) is well-known as a valid animal model which leads to behavioural and developmental disturbances in adulthood (Solas et al. 2010; Martisova et al. 2012). Although various studies for introduction of new agents have been performed, there are no fully satisfactory treatments yet available for treatment of depression (Kupfer et al. 2012).
Clinical studies have shown that children who suffer adverse experiences have a greater risk for developing future psychiatric illnesses, including depression, anxiety disorders, attention deficit/hyperactivity disorders, and cognitive deficits (Heim and Nemeroff 2001). Although the neural circuits and biochemical signalling that account for the behavioural changes subsequent to this phenomenon are largely unknown, studies have shown that the development of the prefrontal cortex, the hippocampus and the amygdala can be modulated by emotional experiences during early postnatal life (Carlyle et al. 2012).
It has been determined that sex steroids such as progesterone affects the brains structure and functioning and subsequently affects behaviour (Hines 1982). Gonadotropins and sex steroids have been shown to have direct influence on regulating of the brain structure and function. In this regard, it has been suggested that progesterone exerts neuroprotective effects in various experimental neurological disorders (Roof et al. 1994, 1997; Shear et al. 2002; He et al. 2004; Stein and Wright 2010). Furthermore, progesterone and progesterone-derived metabolites play important roles in the pathogenesis of psychological diseases (Wirth 2011).
It has been shown that progesterone is metabolised to the allopregnanolone, which can affect neuron functions and behaviour. In this context, there is evidence that progesterone and allopregnanolone are not only produced by peripheral glands (e.g., ovary, adrenal gland) but are also produced in the brain (Wirth 2011; Frye et al. 2014). Progesterone has potent anti-inflammatory and antioxidant properties (Roof et al. 1997; He et al. 2004; Stein and Wright 2010). Previous studies have demonstrated that experiencing early life stress reduces the effectiveness of the progesterone and its metabolites in the brain (Lai and Huang 2011; Brunton 2015).
Ample evidence has indicated that neuro-inflammation is a contributing factor in the pathophysiology of psychiatric disorders such as depression (Maes et al. 2009; Jones and Thomsen 2013). Inflammatory cytokines are involved in neuronal dysfunctions as well as neuro-immune response in psychiatric disorders (Maes 1995; Dowlati et al. 2010). Toll like receptors (TLRs), tumour necrosis factor alpha (TNF-α), interleukin 1-beta (IL-1β) and inducible nitric oxide synthase (iNOS) are parts of components of the innate immunity in mood disorders such as depression (Andreasson et al. 2016).
It is well-known that activation of the NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome leads to production of inflammatory cytokines in the CNS. In this context, it has been suggested that NLRP3 could be considered a potential therapeutic target for affective disorders (Haneklaus et al. 2013; Xu et al. 2016).
In the current study we assess the antidepressant-like property of progesterone in a mouse model of MS stress, considering its anti-neuroinflammatory and antioxidative effects.
Materials and methods
Animals
The protocol for this project was approved by the ethics committee of the Shahrekord University of Medical Sciences and all experiments were performed according to the institutional guidelines for animal care and use. Forty-eight NMRI mice were housed in a facility with 12 h light/dark cycle, temperature 22 ± 1 °C and were given food and water ad libitum.
Maternal separation paradigm
Pregnant NMRI mice (Pasteur Institute, Tehran, Iran) were used in this study. The birth date was considered as postnatal day 0 (PND 0). After birth, at PND 2 the offspring were subjected to the MS paradigm. For this purpose, pups were separated from their mothers for 3 h daily during PND 2–14, beginning at 10:00 a.m., and returned to their mothers after the 3 h separation period (Amini-Khoei et al. 2017, 2019).
At the end of PND 14, pups were returned to their mother’s cages and remained undisturbed until PND 25. On PND 25, male mice were weaned and divided into six experimental groups (four mice per cage) with treatment beginning from PND 44 until experiment day (PND 58–60). We did not touch the control animals and they were left undisturbed and were weaned on PND 25 and grouped (four mice per cage) until PND 58–60 for experiments. All tests were performed between 9:00 a.m. and 1:00 p.m. All experimental groups involved eight mice, and we tried to minimise the use of animals and to improve their well-being.
Study design
Maternally separated and control mice were randomly divided into six experimental groups as follows (n = 8/group). Group 1: control group treated with normal saline for 14 days. Group 2: MS mice received normal saline for 14 days. Groups 3, 4 and 5: MS mice treated with progesterone (10, 50, and 100 mg/kg, i.p., respectively) for 14 days. Group 6: MS mice treated with fluoxetine (30 mg/kg, i.p.) for 14 days. All behavioural experiments were performed in adult mice (PND 58–60). At the end of our study, the animals were sacrificed (decapitated) and the hippocampi were separated on an ice-cold surface and directly snapped frozen in liquid nitrogen and kept in the freezer at −70 °C until the start of molecular assays. The doses and times of drug administrations were selected according to our pilot study as well as previous studies (Martínez-Mota et al. 1999; Molina-Hernández and Téllez-Alcántara 2001; Taniguti et al. 2019). Duration of treatments was from PND 44 (early adulthood) until PND 58–60 (adulthood), for 14 constant days.
Forced swimming test (FST)
FST is a valid test for evaluation of new antidepressants in which elevated immobility time is related to disappointment behaviour demonstrating the depressive-like behaviour in animals (Porsolt et al. 1977). Mice were individually forced to swim in a cylindrical glass tank (diameter: 10 cm, height: 25 cm) containing 19 cm of water (23 ± 1 °C) and passive behaviour (immobility time) was measured for 6 min and animal behaviour was recorded at last 4 min of test. Immobility was defined as a period during which the animal floated in the water, making only those movements necessary to keep its head above the water (Amiri et al. 2016).
Open-field test (OFT)
We used OFT to evaluate the effects of MS, FLX treatment and progesterone treatment and to validate the results of FSD test (Amiri et al. 2015; Sonei et al. 2017). The open-field apparatus was made of white opaque Plexiglas (50 cm × 50 cm × 30 cm), which was dimly illuminated. Each mouse was placed into the central zone of the open-field and its behaviours were recorded by camera for 5 min and evaluated by Ethovision software version 8 (Noldus, Netherlands). The surface of the apparatus was cleaned with 70% ethanol after testing each animal. The distance moved (horizontal activity) and the number of rearings (vertical activity) was measured in OFT.
Splash test
In order to evaluate motivational deficits and self-care difficulties in rodents we used the splash test (Haj-Mirzaian et al. 2016; Haj-Mirzaian, Amini-Khoei, et al. 2017). To do this, grooming behaviour, which can be considered an indirect measure of palatable solution intake, was measured. A 10% sucrose solution was squirted on the dorsal coat of animals in their home cage. First grooming latency and time spent grooming were recorded for a period of 5 min.
Quantitative reverse transcription-PCR (qRT-PCR) analysis for hippocampus inflammatory genes
Trizol (Invitrogen; Life Technologies, Grand Island, NY, USA) was used to isolate hippocampi RNA as described by the manufacturer’s protocol. The samples were verified to be free of contaminating DNA, as no signal originating from genomic DNA was observed among the no reverse transcription controls. Isolated RNA was checked for purity by ensuring that the OD 260/280 ratio was greater than 1.8. For qRT-PCR, SYBR green detection was used according to the manufacturer’s protocol (Bio-Rad QPCR kit; Hercules, CA, USA). A Rotor-Gene Q real time thermocycler was used to collect the data. Thermal cycling conditions included an initial activation step for 30 s at 95 °C after 45 cycles as well as a denaturation step for 5 s at 95 °C and a combined annealing/extension step for 20 s at 60 °C. Melting curve analysis was performed to certify whether all primers yielded a single PCR product. All PCR primer pairs used generated amplicons between 90 and 110 base pairs (Table 1). Histone H2A variant, H2afz, was used as a house-keeping gene (normalizer) and alterations in expression of each target mRNA in comparison with H2afz was measured based on the 2−ΔΔCt relative expression formula. Studies in mice have shown that this particular histone is required for development.
Table 1.
Primer Sequences.
| Primer | Forward sequence | Reverse sequence |
|---|---|---|
| H2AFZ | TCATCGACACCTGAAATCTAGGA | AGGGGTGATACGCTTTACCTTTA |
| TNF-α | CTGAACTTCGGGGTGATCGG | GGCTTGTCACTCGAATTTTGAGA |
| IL-1β | GAAATGCCACCTTTTGACAGTG | TGGATGCTCTCATCAGGACAG |
| TLR4 | ATGGCATGGCTTACACCACC | GAGGCCAATTTTGTCTCCACA |
| NLRP3 | ATCAACAGGCGAGACCTCTG | GTCCTCCTGGCATACCATAGA |
| iNOS | TTTGACCAGAGGACCCAGAG | AAGACCAGAGGCAGCACATC |
Ferric reducing/antioxidant power (FRAP) assay
Ferric reducing/antioxidant power was determined based on method described in previous studies (Heidarian and Soofiniya 2011). Briefly, plasma was mixed with FRAP reagent (composed of: 25 mL acetate buffer, 2.5 mL TPTZ solution and 2.5 mL FeCl3) then incubated at 37 °C for 10 min and optical density of blue colour was measured at 593 nm. FeSO4 was used as a standard of FRAP assay at a concentration range between 100 and 1000 μM. The results are reported as μmol/mg.
Determination of lipid peroxidation (LPO)
MDA content in the rat hippocampus was determined by the TBA reactive substance test as described previously (Heidarian and Soofiniya 2011). Briefly, plasma or supernatant was mixed with sodium dodecyl sulphate (8.1%) and TBA/buffer (composed of 0.53% thiobarbituric acid in 20% acetic acid as adjusted to pH 3.5 with NaOH), then incubated at 95 °C for 60 min. The reaction was stopped by placing tubes in ice followed by centrifugation at 4000 rpm for 10 min. The optical density of the pink colour was measured at 532 nm.1, 1, 3, 3-tetraethoxypropane was used as a standard of MDA assay. The measurements were done in duplicate and the results were expressed in μmol/mg.
Results
Progesterone attenuates the depressive-like behaviours of MS
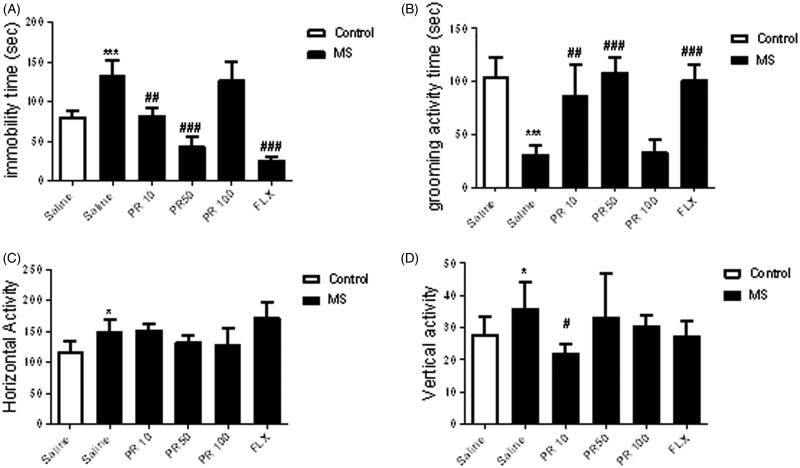
We assessed the effect of MS on depressive-like behaviours, including behavioural despair, motivational difficulty using the FST and splash test, respectively (Figure 1). In the FST, MS mice have an elevation in the immobility time when compared to control animals (p < 0.001). Results indicate that MS led to a remarkable decrease in grooming activity time in the splash test in comparison with control group (p < 0.001). In the OFT, MS remarkably elevated the total distance moved (horizontal activity) (p < 0.05) and number of rearings (vertical activity) (p < 0.05) compared to the control group.
Figure 1.
Effects of MS and progesterone on depressive-like behaviours in male mice: FST (A), splash test (B) and OFT (horizontal activity: C and vertical activity: D). Values are presented as mean ± S.E.M from eight animals and were analysed using one-way ANOVA followed by Tukey post hoc test. *p < 0.05 and ***p < 0.001 compared to the control group, #p < 0.05, ##p < 0.01 and ###p < 0.001 compared with the salinereceived MS mice.
The effects of various doses of progesterone (10, 50 and 100 mg/kg) was evaluated on depressive-like behaviours (Figure 1). One-way ANOVA analysis demonstrated that treatment with progesterone (10, 50 mg/kg) created significant changes in depressive-like behaviours in MS mice in compared to the saline-treated MS mice in the FST (p < 0.01) and splash test (p < 0.01). However, dose of 100 mg/kg of progesterone had no effect in the aforesaid behavioural tests in MS mice. In the OFT, Tukey’s post hoc analysis showed that administration of progesterone (10 mg/kg) to MS mice significantly decreased the vertical activity in comparison with the saline-treated MS mice (p < 0.05). However, MS mice did not significantly respond to progesterone (10, 50 and 100 mg/kg) in the horizontal activity in OFT.
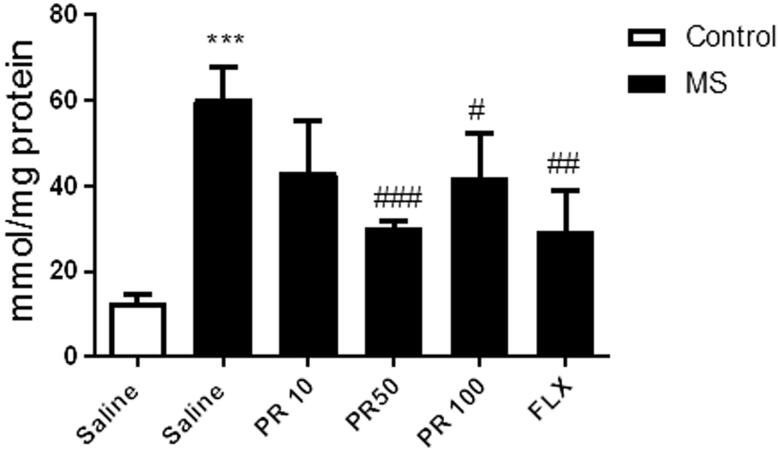
Progesterone decreases the MDA content of the hippocampus
Lipid peroxidation measurement was made in hippocampus because this tissue was directly involved on depression, so any damage in hippocampus should be related to depressive signs. Figure 2 indicates that MS led to a remarkable elevation in MDA level (p < 0.001) in compared to the control group. Treatment with progesterone at doses of 50 and 100 mg/kg in MS mice leads to significant reduction (p < 0.001 and p < 0.05, respectively) in MDA content in compared to the saline-treated MS mice. However, treatment with progesterone at dose of 10 mg/kg in MS mice could not exert significant change in MDA content in compared to the saline-treated MS mice. Fluoxetine also significantly decreased (p < 0.01) the hippocampal MDA level in compared to the saline-treated MS mice.
Figure 2.
Effects of MS and progestrone on MDA content in the hippocampus. Values are presented as mean ± S.E.M from six samples and were analysed using one-way ANOVA followed by Tukey post hoc test. ***p < 0.001 compared to the control group, #p < 0.05, ##p < 0.01 and ###p < 0.001 compared with the salinetreated MS mice.
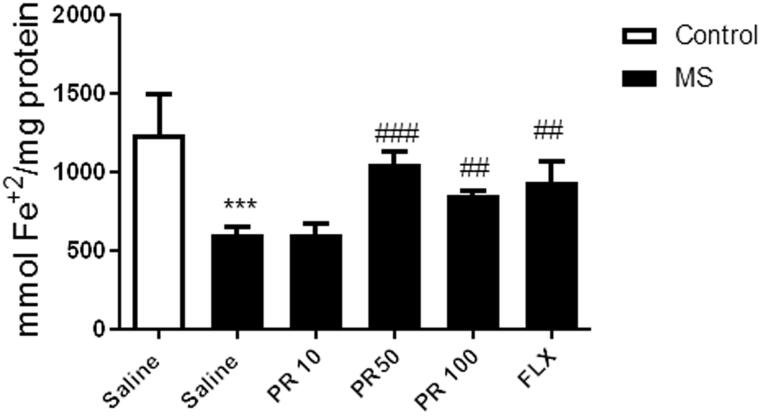
Progesterone reduses the hippocampal total antioxidant capacity
Figure 3 shows that MS caused a significant reduction in the hippocampus antioxidant capacity in comparison with the control group (p < 0.001). However, treatment with progesterone at doses of 50 and 100 mg/kg in MS mice significantly elevated the hippocampus antioxidant capacity in compared to the saline-treated MS mice (p < 0.001 and p < 0.01, respectively). However, MS mice did not significantly respond to treatment with progesterone at dose of 10 mg/kg. We showed that fluoxetine significantly increased the hippocampus antioxidant capacity in compared to the saline-treated MS mice (p < 0.01).
Figure 3.
Effects of MS and progestrone on total antioxidant capacity in the hippocampus. Values are presented as mean ± S.E.M from six samples and were analysed using one-way ANOVA followed by Tukey post hoc test. ***p < 0.001 compared to the control group, ##p < 0.01 and ###p < 0.001 compared with the saline-received MS mice.
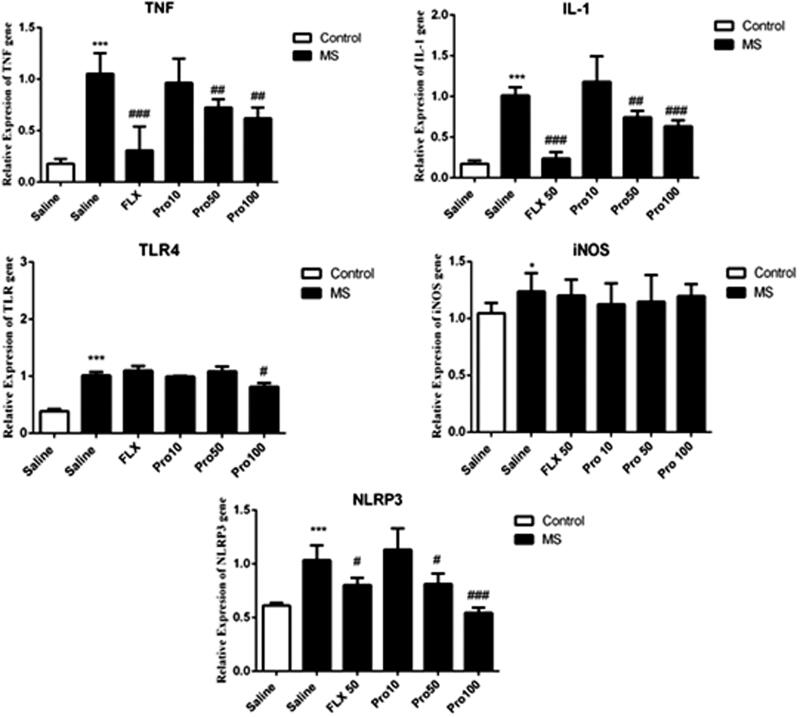
Progesterone decreased the expression of inflammatory genes in the hippocampus
Figure 4 shows the expression of genes related to neuro-immune responses in the hippocampus. One-way ANOVA analysis showed that there were significant differences between experimental groups. Tukey’s post hoc analysis demonstrated that expression of the IL-1β (p < 0.001), TNF-α (p < 0.001), iNOS (p < 0.05), TLR4 (p < 0.001) and NLRP3 (p < 0.001) significantly increased in the hippocampus of MS mice in comparison with the control mice. Results showed that treatment with progesterone at dose of 50 mg/kg significantly reduced expression of IL-1β (p < 0.01), TNF-α (p < 0.01), NLRP3 (p < 0.05) in the hippocampus in comparison with the saline-received counterpart. Also, treatment with progesterone at dose of 100 mg/kg significantly decreased the expression of IL-1β (p < 0.001), TNF-α (p < 0.01), TLR4 (p < 0.05) and NLRP3 (p < 0.001) in the hippocampus when compared with the saline-treated counterparts. However, treatment with progesterone at dose of 10 mg/kg did not lead to significant changes in the expression of inflammatory genes in MS mice.
Figure 4.
Effects of MS and progestrone on the gene expression of immune-inflammatory genes (Il-1β, TNFα, iNOS, TLR4 and NLRP-3) in the hippocampus. Data are expressed as the mean ± S.E.M from six samples and were analysed by one-way ANOVA. *p < 0.05 and ***p < 0.001 compared to the control group, #p < 0.05, ##p < 0.01 and ###p < 0.001 compared with the saline-received MS mice.
Discussion
Our results demonstrated that MS stress provokes depressive-like behaviours in adult male mice in behavioural tests including FST, OFT and the splash test. We observed that progesterone provokes antidepressant-like effects in MS mice. The expression of inflammatory genes related to neuro-immune response increased in the hippocampus of MS mice. Also, our results indicated that MS increased the MDA level and decreased the antioxidant capacity in the hippocampus of MS mice. Moreover, we found that progesterone attenuates the neuro-immune response, decreases the MDA level and increases the antioxidant capacity of the hippocampus of MS mice.
Ample evidence indicates that experiencing stressful events during early stages of life have profound and long-lasting effects on brain development and behaviour (Lupien et al. 2009). Our results are in line with previous studies that reported MS is able to provoke depressive-like behaviours in mice (Vetulani 2013; Marco et al. 2015). We showed that MS mice have increased immobility time in the FST compared with the control animals. An increase in immobility time in the FST reflects behavioural despair in mice (Cryan and Holmes 2005). Our findings showed that treatment with progesterone significantly reduced the immobility time in the FST, indicating that progesterone exerted antidepressant-like effects.
The splash test is an accepted method evaluating the self-care difficulties and motivation in mice (Marrocco et al. 2014). Our findings revealed that MS mice have decreased grooming activity time compared to control mice. Treatment with progesterone is able to increase the grooming activity time in the splash test. These results suggest that progesterone exerts antidepressant-like effects in MS mice.
In this study, OFT was applied immediately before the FST to evaluate the ambulatory behaviour of the mice and also confirm that variations which occur in motor activity did not affect the duration of immobility in the FST (Kaster et al. 2005). In the OFT, MS mice showed an increase in locomotion in both vertical and horizontal activities. In the case of OFT, it should be noted that there is an inconsistency in the literature regarding OFT results following the MS paradigm. In this regard, previous studies reported that MS decreases locomotion (Millstein and Holmes 2007), increases locomotion (Cannizzaro et al. 2006) or does not change ambulation in the OFT (Shalev and Kafkafi 2002).
To investigate the possible mechanism of progesterone, we measured lipid peroxidation (LPO) and the anti-oxidant capacity of the hippocampus. We examined the total antioxidant activity using FRAP as an indicator of the strength of non-enzymatic antioxidants and LPO using MDA content as a marker of LPO (Thirunavukkarasu et al. 2012; Nouri et al. 2017). In our study, the levels of MDA were significantly increased and the content of antioxidant capacity markedly decreased in MS mice, which indicates lipid proxidation and oxidative stress in the hippocampus which are in agreement with prior studies (Arabameri et al. 2017; Menezes et al. 2017). Treatment with progesterone not only increased antioxidant capacity, but also decreased the MDA level in the hippocampus of MS mice. These protective effects of progesterone might be due to its antioxidant and free radical scavenging properties. Therefore, it seems that progesterone can reduce the oxidative stress caused by MS.
It has been reported that stress induces immunomodulatory effects through modifications in the HPA axis and the sympathetic nervous system (Miller and Raison 2016). Recent studies have emphasised the role of inflammatory pathways in the pathophysiology of depression. Involvement of TLRs and subsequent activation of an inflammatory response in behavioural abnormalities has recently been reported in animal models of chronic stress (Pascual et al. 2015; Sadeghi et al. 2016).
Previous studies demonstrated the central role for NLRP3 inflammasome in stress-induced depressive-like behaviours. Stress had long been considered as an important contributing factor in the pathology of depression. Activation of TLR4 and NLRP3 leads to overproduction of inflammatory cytokines (Kupfer et al. 2012; Pan et al. 2014). Our data showed that MS caused an increase in the genes expression of TLR4, TNF-α, IL-1β, iNOS and NLRP3 in the hippocampus tissue. This result is consistent with recent studies that have demonstrated MS-induced depressive- like behaviours are associated with the activation of inflammatory responses in the hippocampus (Roque et al. 2016; Sadeghi et al. 2016; Haj-Mirzaian, Amiri, et al. 2017). In this research, we found that treatment with progesterone succeeded to attenuate the increased expression of TLR4, TNF-α, and IL-1β in the hippocampus of mice exposed to MS paradigm. Our results suggested that progesterone has anti-inflammatory effects through the downregulation of TLR4, TNF-α, IL-1β, and NLRP3.
Clinical and preclinical studies have demonstrated that progesterone exerted antidepressant (-like) effect (Gordon et al. 2018; Hu et al. 2019). As evidence for their importance to the brain, progesterone and allopregnanolone are produced not only by peripheral glands (e.g., ovary; adrenal gland), but also by the brain itself (Wirth 2011). There are studies demonstrating that stress decreases the level of steroids such as progesterone in the CNS (Weisz et al. 1982; Barbaccia et al. 2001). The impact of early‐life stress on the brain's capability to produce neurosteroids such as progesterone has been studied previously. In this regard, evidence demonstrated that experiencing early life stress could reduce the level of neurostroids in the brain (Brunton 2015; Santos et al. 2018).
Several studies have demonstrated that progesterone could stimulate neurogenesis in the hippocampus (Novais et al. 2018; Montes et al. 2019). So, considering involvement of the hippocampus in the pathophysiology of depression, understanding of the impact of early‐life stress on brain neurosteroid systems could help to identify new targets for developing treatments for stress‐related psychopathies like depression. In this study we found that facing early life stress is linked with depressive-like behaviour in adult male mice. Interestingly, we observed that administration of the progesterone attenuated the negative effects of maternal separation stress on behaviours of adult male mice. Our findings suggested that MS can, at least, disturb the neuronic circuits in the brain and disturb the function and/or production of steroids such as progesterone in the CNS. This indicates that further studies on this subject should be conducted to clarify the effect of MS on the steroidogenesis.
Conclusion
We concluded that progesterone through attenuation of neuro-inflammatory response and mitigation of oxidative stress, partially at least, exerts antidepressant-like effects in maternally separated mice.
Acknowledgment
The authors would like to thank Dr. Mahmoud Rafieian Kopaei, Dr. Gholam Reza Mobini, and Mrs. Elham Bijad for their collaboration on this study.
Funding Statement
This work was supported by a grant from Shahrekord University of Medical Sciences (SKUMS) with grant number 1395-01-75-3254.
References
- Amini-Khoei H, Amiri S, Mohammadi-Asl A, Alijanpour S, Poursaman S, Haj-Mirzaian A, Rastegar M, Mesdaghinia A, Banafshe H R, Sadeghi E, et al. 2017. Experiencing neonatal maternal separation increased pain sensitivity in adult male mice: involvement of oxytocinergic system. Neuropeptides. 61:77–85. [DOI] [PubMed] [Google Scholar]
- Amini-Khoei H, Haghani-Samani E, Beigi M, Soltani A, Mobini G R, Balali-Dehkordi S, Haj-Mirzaian A, Rafieian-Kopaei M, Alizadeh A, Hojjati MR, et al. 2019. On the role of corticosterone in behavioral disorders, microbiota composition alteration and neuroimmune response in adult male mice subjected to maternal separation stress. Int Immunopharmacol. 66:242–250. [DOI] [PubMed] [Google Scholar]
- Amiri S, Alijanpour S, Tirgar F, Haj-Mirzaian A, Amini-Khoei H, Rahimi-Balaei M, Rastegar M, Ghaderi M, Ghazi-Khansari M, Zarrindast MR.. 2016. NMDA receptors are involved in the antidepressant-like effects of capsaicin following amphetamine withdrawal in male mice. Neuroscience. 329:122–133. [DOI] [PubMed] [Google Scholar]
- Amiri S, Haj-Mirzaian A, Rahimi-Balaei M, Razmi A, Kordjazy N, Shirzadian A, Mehr SE, Sianati H, Dehpour AR.. 2015. Co-occurrence of anxiety and depressive-like behaviors following adolescent social isolation in male mice; possible role of nitrergic system. Physiol Behav. 145:38–44. [DOI] [PubMed] [Google Scholar]
- Andreasson KI, Bachstetter AD, Colonna M, Ginhoux F, Holmes C, Lamb B, Landreth G, Lee DC, Low D, Lynch MA, et al. 2016. Targeting innate immunity for neurodegenerative disorders of the central nervous system. J Neurochem. 138(5):653–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabameri A, Sameni H, Bandegi A.. 2017. The effects of propolis extract on ovarian tissue and oxidative stress in rats with maternal separation stress. IJRM. 15:509–520. [PMC free article] [PubMed] [Google Scholar]
- Barbaccia ML, Serra M, Purdy RH, Biggio G.. 2001. Stress and neuroactive steroids. Int Rev Neurobiol. 46:243–272. [DOI] [PubMed] [Google Scholar]
- Brunton P. 2015. Programming the brain and behaviour by early‐life stress: a focus on neuroactive steroids. J Neuroendocrinol. 27(6):468–480. [DOI] [PubMed] [Google Scholar]
- Cannizzaro C, Plescia F, Martire M, Gagliano M, Cannizzaro G, Mantia G, Cannizzaro E.. 2006. Single, intense prenatal stress decreases emotionality and enhances learning performance in the adolescent rat offspring: interaction with a brief, daily maternal separation. Behav Brain Res. 169(1):128–136. [DOI] [PubMed] [Google Scholar]
- Carlyle BC, Duque A, Kitchen RR, Bordner KA, Coman D, Doolittle E, Papademetris X, Hyder F, Taylor JR, Simen AA.. 2012. Maternal separation with early weaning: a rodent model providing novel insights into neglect associated developmental deficits. Dev Psychopathol. 24(4):1401–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lin D, Zhang C, Li G, Zhang N, Ruan L, Yan Q, Li J, Yu X, Xie X, et al. 2015. Antidepressant-like effects of ferulic acid: involvement of serotonergic and norepinergic systems. Metab Brain Dis. 30(1):129–136. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Holmes A.. 2005. Model organisms: the ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 4(9):775–790. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL.. 2010. A meta-analysis of cytokines in major depression. Biol Psychiatry. 67(5):446–457. [DOI] [PubMed] [Google Scholar]
- Frye CA, Koonce CJ, Walf AA.. 2014. Novel receptor targets for production and action of allopregnanolone in the central nervous system: a focus on pregnane xenobiotic receptor. Front Cell Neurosci. 8:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JL, Rubinow DR, Eisenlohr-Moul TA, Xia K, Schmidt PJ, Girdler SS.. 2018. Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: a randomized clinical trial. JAMA Psychiatry. 75(2):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Mirzaian A, Amini-Khoei H, Haj-Mirzaian A, Amiri S, Ghesmati M, Zahir M, Shafaroodi H, Dehpour AR.. 2017. Activation of cannabinoid receptors elicits antidepressant-like effects in a mouse model of social isolation stress. Brain Res Bull. 130:200–210. [DOI] [PubMed] [Google Scholar]
- Haj-Mirzaian A, Amiri S, Amini-Khoei H, Hosseini M-J, Haj-Mirzaian A, Momeny M, Rahimi-Balaei M, Dehpour A.. 2017. Anxiety-and depressive-like behaviors are associated with altered hippocampal energy and inflammatory status in a mouse model of Crohn’s disease. Neuroscience. 366:124–137. [DOI] [PubMed] [Google Scholar]
- Haj-Mirzaian A, Amiri S, Kordjazy N, Momeny M, Razmi A, Rahimi-Balaei M, Amini-Khoei H, Haj-Mirzaian A, Marzban H, Mehr SE, et al. 2016. Lithium attenuated the depressant and anxiogenic effect of juvenile social stress through mitigating the negative impact of interlukin-1β and nitric oxide on hypothalamic-pituitary-adrenal axis function. Neuroscience. 315:271–285. [DOI] [PubMed] [Google Scholar]
- Haneklaus M, O’Neill LA, Coll RC.. 2013. Modulatory mechanisms controlling the NLRP3 inflammasome in inflammation: recent developments. Curr Opin Immunol. 25(1):40–45. [DOI] [PubMed] [Google Scholar]
- He J, Evans C-O, Hoffman SW, Oyesiku NM, Stein DG.. 2004. Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Exp Neurol. 189(2):404–412. [DOI] [PubMed] [Google Scholar]
- Heidarian E, Soofiniya Y.. 2011. Hypolipidemic and hypoglycemic effects of aerial part of Cynara scolymus in streptozotocin-induced diabetic rats. J Med Plant Res. 5:2717–2723. [Google Scholar]
- Heim C, Nemeroff CB.. 2001. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 49(12):1023–1039. [DOI] [PubMed] [Google Scholar]
- Hines M. 1982. Prenatal gonadal hormones and sex differences in human behavior. Psychol Bull. 92(1):56–80. [PubMed] [Google Scholar]
- Hu Z, Du X, Yang Y, Botchway BO, Fang M.. 2019. Progesterone and fluoxetine treatments of postpartum depressive‐like behavior in rat model. Cell Biol Int. 5:539–552. [DOI] [PubMed] [Google Scholar]
- Jones KA, Thomsen C.. 2013. The role of the innate immune system in psychiatric disorders. Mol Cell Neurosci. 53:52–62. [DOI] [PubMed] [Google Scholar]
- Kaster MP, Ferreira PK, Santos AR, Rodrigues AL.. 2005. Effects of potassium channel inhibitors in the forced swimming test: possible involvement of l-arginine-nitric oxide-soluble guanylate cyclase pathway. Behav Brain Res. 165(2):204–209. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Frank E, Phillips ML.. 2012. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet. 379(9820):1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M-C, Huang L-T.. 2011. Effects of early life stress on neuroendocrine and neurobehavior: mechanisms and implications. Pediatr Neonatol. 52(3):122–129. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C.. 2009. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 10(6):434–445. [DOI] [PubMed] [Google Scholar]
- Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, Kubera M, Bob P, Lerer B, Maj M.. 2009. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis. 24:27–53. [DOI] [PubMed] [Google Scholar]
- Maes M. 1995. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuro-Psychopharmacol Biol Psychiatry. 19(1):11–38. [DOI] [PubMed] [Google Scholar]
- Marco EM, Llorente R, López-Gallardo M, Mela V, Llorente-Berzal Á, Prada C, Viveros M-P.. 2015. The maternal deprivation animal model revisited. Neurosci Biobehav Rev. 51:151–163. [DOI] [PubMed] [Google Scholar]
- Marrocco J, Reynaert M.-L, Gatta E, Gabriel C, Mocaer E, Di Prisco S, Merega E, Pittaluga A, Nicoletti F, Maccari S, et al. 2014. The effects of antidepressant treatment in prenatally stressed rats support the glutamatergic hypothesis of stress-related disorders. J Neurosci. 34(6):2015–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Mota L, Contreras CM, Saavedra M.. 1999. Progesterone reduces immobility in rats forced to swim. Arch Med Res. 30(4):286–289. [DOI] [PubMed] [Google Scholar]
- Martisova E, Solas M, Horrillo I, Ortega JE, Meana JJ, Tordera RM, Ramírez MJ.. 2012. Long lasting effects of early-life stress on glutamatergic/GABAergic circuitry in the rat hippocampus. Neuropharmacology. 62(5–6):1944–1953. [DOI] [PubMed] [Google Scholar]
- Menezes J, Neves B-H, Souza M, Mello-Carpes PB.. 2017. Green tea protects against memory deficits related to maternal deprivation. Physiol Behav. 182:121–127. [DOI] [PubMed] [Google Scholar]
- Miller AH, Raison CL.. 2016. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 16(1):22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millstein RA, Holmes A.. 2007. Effects of repeated maternal separation on anxiety-and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev. 31(1):3–17. [DOI] [PubMed] [Google Scholar]
- Molina-Hernández M, Téllez-Alcántara NP.. 2001. Antidepressant-like actions of pregnancy, and progesterone in Wistar rats forced to swim. Psychoneuroendocrinology. 26(5):479–491. [DOI] [PubMed] [Google Scholar]
- Montes P, Vigueras-Villaseñor RM, Rojas-Castañeda JC, Monfil T, Cervantes M, Moralí G.. 2019. Progesterone treatment in rats after severe global cerebral ischemia promotes hippocampal dentate gyrus neurogenesis and functional recovery. Neurol Res. 41(5):429–436. [DOI] [PubMed] [Google Scholar]
- Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, et al. 2012. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 380(9859):2197–2223. [DOI] [PubMed] [Google Scholar]
- Nouri A, Heidarian E, Nikoukar M.. 2017. Effects of N-acetyl cysteine on oxidative stress and TNF-α gene expression in diclofenac-induced hepatotoxicity in rats. Toxicol Mech Methods. 27(8):561–567. [DOI] [PubMed] [Google Scholar]
- Novais A, Silva A, Ferreira AC, Falcão AM, Sousa N, Palha JA, Marques F, Sousa JC.. 2018. Adult hippocampal neurogenesis modulation by the membrane-associated progesterone receptor family member neudesin. Front Cell Neurosci. 12:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Chen X-Y, Zhang Q-Y, Kong L-D.. 2014. Microglial NLRP3 inflammasome activation mediates IL-1β-related inflammation in prefrontal cortex of depressive rats. Brain Behav Immun. 41:90–100. [DOI] [PubMed] [Google Scholar]
- Pascual M, Baliño P, Aragón CM, Guerri C.. 2015. Cytokines and chemokines as biomarkers of ethanol-induced neuroinflammation and anxiety-related behavior: role of TLR4 and TLR2. Neuropharmacology. 89:352–359. [DOI] [PubMed] [Google Scholar]
- Porsolt R, Bertin A, Jalfre M.. 1977. Behavioral despair in mice: a primary screening test for antidepressants. Arc Int Pharmacody Therap. 229:327–336. [PubMed] [Google Scholar]
- Rao U, Chen L-A, Bidesi AS, Shad MU, Thomas MA, Hammen CL.. 2010. Hippocampal changes associated with early-life adversity and vulnerability to depression. Biol Psychiatry. 67(4):357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone S. Jr.. 2000. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 108:511–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, Braswell L, Stein DG.. 1994. Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp Neurol. 129(1):64–69. [DOI] [PubMed] [Google Scholar]
- Roof RL, Hoffman SW, Stein DG.. 1997. Progesterone protects against lipid peroxidation following traumatic brain injury in rats. Mol Chem Neuropathol. 31(1):1–11. [DOI] [PubMed] [Google Scholar]
- Roque A, Ochoa-Zarzosa A, Torner L.. 2016. Maternal separation activates microglial cells and induces an inflammatory response in the hippocampus of male rat pups, independently of hypothalamic and peripheral cytokine levels. Brain Behav Immun. 55:39–48. [DOI] [PubMed] [Google Scholar]
- Sadeghi M, Peeri M, Hosseini M-J.. 2016. Adolescent voluntary exercise attenuated hippocampal innate immunity responses and depressive-like behaviors following maternal separation stress in male rats. Physiol Behav. 163:177–183. [DOI] [PubMed] [Google Scholar]
- Santos A, Giráldez FJ, Valdés C, Trevisi E, Lucini L, Frutos J, Andrés S.. 2018. Milk replacer restriction during early life impairs the live body weight and progesterone patterns of ewe lambs during the replacement period. J Dairy Sci. 101(9):8021–8031. [DOI] [PubMed] [Google Scholar]
- Shalev U, Kafkafi N.. 2002. Repeated maternal separation does not alter sucrose-reinforced and open-field behaviors. Pharmacol Biochem Behav. 73(1):115–122. [DOI] [PubMed] [Google Scholar]
- Shear DA, Galani R, Hoffman SW, Stein DG.. 2002. Progesterone protects against necrotic damage and behavioral abnormalities caused by traumatic brain injury. Exp Neurol. 178(1):59–67. [DOI] [PubMed] [Google Scholar]
- Solas M, Aisa B, Mugueta MC, Del Río J, Tordera RM, Ramírez MJ.. 2010. Interactions between age, stress and insulin on cognition: implications for Alzheimer’s disease. Neuropsychopharmacology. 35(8):1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonei N, Amiri S, Jafarian I, Anoush M, Rahimi-Balaei M, Bergen H, Haj-Mirzaian A, Hosseini M-J.. 2017. Mitochondrial dysfunction bridges negative affective disorders and cardiomyopathy in socially isolated rats: pros and cons of fluoxetine. World J Biol Psychiatry. 18(1):39–53. [DOI] [PubMed] [Google Scholar]
- Stein DG, Wright DW.. 2010. Progesterone in the clinical treatment of acute traumatic brain injury. Expert Opin Investig Drugs. 19(7):847–857. [DOI] [PubMed] [Google Scholar]
- Taniguti EH, Ferreira YS, Stupp IJV, Fraga-Junior EB, Doneda DL, Lopes L, Rios-Santos F, Lima E, Buss ZS, Viola GG, et al. 2019. Atorvastatin prevents lipopolysaccharide-induced depressive-like behaviour in mice. Brain Res Bull. 146:279–286. [DOI] [PubMed] [Google Scholar]
- Thirunavukkarasu SV, Venkataraman S, Raja S, Upadhyay L.. 2012. Neuroprotective effect of Manasamitra vatakam against aluminium induced cognitive impairment and oxidative damage in the cortex and hippocampus of rat brain. Drug Chem Toxicol. 35(1):104–115. [DOI] [PubMed] [Google Scholar]
- Üstün TB. 2001. The worldwide burden of depression in the 21st century. In: Weissman MM, editor. Treatment of depression: bridging the 21st century. Washington (DC): American Psychiatric Publishing, Inc; p. 35–45. [Google Scholar]
- Vetulani J. 2013. Early maternal separation: a rodent model of depression and a prevailing human condition. Pharmacol Rep. 65(6):1451–1461. [DOI] [PubMed] [Google Scholar]
- Weisz J, Brown BL, Ward IL.. 1982. Maternal stress decreases steroid aromatase activity in brains of male and female rat fetuses. Neuroendocrinology. 35(5):374–379. [DOI] [PubMed] [Google Scholar]
- Wirth M. 2011. Beyond the HPA axis: progesterone-derived neuroactive steroids in human stress and emotion. Front Endocrinol (Lausanne). 2:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Sheng H, Bao Q, Wang Y, Lu J, Ni X.. 2016. NLRP3 inflammasome activation mediates estrogen deficiency-induced depression-and anxiety-like behavior and hippocampal inflammation in mice. Brain Behav Immun. 56:175–186. [DOI] [PubMed] [Google Scholar]