S. aureus remains a significant cause of disease within hospitals and communities. To reduce the burden of S. aureus infections, antiseptics are ubiquitously used in our daily lives. Furthermore, many antiseptic compounds are dual purpose and are found in household products. The increased abundance of antiseptic compounds has selected for S. aureus strains that carry efflux pumps that increase resistance to antiseptic compounds; however, the effect of carrying multiple pumps within S. aureus is unclear. We demonstrated that an isogenic strain carrying multiple efflux pumps had an additive resistance phenotype to cetrimide. Moreover, in a strain carrying qacA and norA, increased chlorhexidine tolerance was observed after the strain was preexposed to subinhibitory concentrations of a different common-use antiseptic. Taken together, our findings demonstrate cooperation between antiseptic resistance efflux pumps and suggest that their protective phenotype may be exacerbated by priming with subinhibitory concentrations of household antiseptics.
KEYWORDS: Staphylococcus aureus, antimicrobial agents, benzalkonium chloride, chlorhexidine, efflux pumps
ABSTRACT
Staphylococcus aureus-associated infections can be difficult to treat due to multidrug resistance. Thus, infection prevention is critical. Cationic antiseptics, such as chlorhexidine (CHX) and benzalkonium chloride (BKC), are liberally used in health care and community settings to prevent infection. However, increased administration of antiseptics has selected for S. aureus strains that show reduced susceptibilities to cationic antiseptics. This increased resistance has been associated with carriage of specific efflux pumps (QacA, QacC, and NorA). Since prior published studies focused on different strains and on strains carrying only a single efflux gene, the relative importance of these various systems to antiseptic resistance is difficult to ascertain. To overcome this, we engineered a collection of isogenic S. aureus strains that harbored norA, qacA, and qacC, individually or in combination. MIC assays showed that qacA was associated with increased resistance to CHX, cetrimide (CT), and BKC, qacC was associated with resistance to CT and BKC, and norA was necessary for basal-level resistance to the majority of tested antiseptics. When all three pumps were present in a single strain, an additive effect was observed in the MIC for CT. Transcriptional analysis revealed that expression of qacA and norA was significantly induced following exposure to BKC. Alarmingly, in a strain carrying qacA and norA, preexposure to BKC increased CHX tolerance. Overall, our results reveal increased antiseptic resistance in strains carrying multiple efflux pumps and indicate that preexposure to BKC, which is found in numerous daily-use products, can increase CHX tolerance.
IMPORTANCE S. aureus remains a significant cause of disease within hospitals and communities. To reduce the burden of S. aureus infections, antiseptics are ubiquitously used in our daily lives. Furthermore, many antiseptic compounds are dual purpose and are found in household products. The increased abundance of antiseptic compounds has selected for S. aureus strains that carry efflux pumps that increase resistance to antiseptic compounds; however, the effect of carrying multiple pumps within S. aureus is unclear. We demonstrated that an isogenic strain carrying multiple efflux pumps had an additive resistance phenotype to cetrimide. Moreover, in a strain carrying qacA and norA, increased chlorhexidine tolerance was observed after the strain was preexposed to subinhibitory concentrations of a different common-use antiseptic. Taken together, our findings demonstrate cooperation between antiseptic resistance efflux pumps and suggest that their protective phenotype may be exacerbated by priming with subinhibitory concentrations of household antiseptics.
INTRODUCTION
Hospital- and community-acquired infections by Staphylococcus aureus are a significant cause of disease and economic burden in the United States. In particular, infections by methicillin-resistant S. aureus (MRSA) are difficult to prevent and treat because of the ability of the bacterium to persist on nosocomial surfaces, including within inpatient populations, and because of antibiotic resistance, respectively (1, 2). In spite of the treatment difficulties associated with hospital-acquired MRSA (HA-MRSA) infections, improved hygiene and disinfection strategies are decreasing the number of invasive infections (3). Indeed, the increased application of antiseptics has been able to reduce the number of hospital-acquired infections (HAI) in certain settings (4, 5). However, our dependency on antiseptics is not without consequence, as bacteria are evolving an increased tolerance to some common biocides (6, 7).
Two common antiseptic compounds, chlorhexidine (CHX) and benzalkonium chloride (BKC), have become standard biocides in our daily lives. CHX was developed in the 1950s but was not put into common usage until after 2000 (8, 9). As a presurgical scrub and skin prep, CHX has been shown to be effective at infection prevention and is well tolerated on patients. However, CHX use is not limited to the hospitals; CHX is widely available in over-the-counter soaps and is frequently used as a disinfectant (10). Prior to CHX, BKC was developed for antiseptic usage in 1935 and was broadly used in surgical preparations and hand soaps (11, 12). Today, BKC is found in numerous common-use products, including cosmetics, antiseptics/hand sanitizers, and pharmaceutical products. This overwhelming prevalence of BKC has prompted an FDA request for additional scientific data to support the safety and effectiveness of BKC in over-the-counter antiseptic rubs; this analysis is currently ongoing (13). Throughout the past 70 years, both CHX and BKC have demonstrated efficacy as antiseptics (14); nonetheless, over time their excessive use is selecting for bacterial strains that show increased resistance to both compounds (9, 15).
Efflux pumps provide one mechanism for increased antiseptic tolerance of bacteria to CHX and BKC. In S. aureus, the antiporter efflux pumps QacA, NorA, and QacC are known to offer increased resistance to a wide range of common antiseptics (16). Besides norA, which has recently been shown to be part of the core genome of S. aureus (17), qacA and qacC reside on a variety of conjugative and nonconjugative plasmids (18, 19). The majority of qac prevalence studies demonstrate that while qacA and qacC do not commonly coexist within S. aureus, the presence of all three efflux pumps within S. aureus isolates does occur (20–22); however, the functional consequences of harboring all three pumps are unknown. For S. aureus strains that carry either qacA or qacC, the prevalence varies greatly and depends on the geographical location and sample population being studied. Typically, qacA and qacC prevalence is higher in isolates from Asia and Europe than in those from North America (23). Overall, there has been an increasing trend in qacA prevalence in Asian countries (24), and new data from the United States suggest a similar trend as well (22, 25). The identification of qacA and qacC carried on conjugative plasmids suggests the presence of selective pressures that drive the mobilization of these genes across S. aureus strains. Furthermore, this observation is further supported by the expansion of qacA to other pathogenic bacteria (26, 27).
As the prototypical antiseptic resistance pump in S. aureus, QacA has been well characterized and shown to impart increased tolerance to bisbiguanides, quaternary ammonium compounds (QACs), diamides, and aromatic dyes (28). QacA is composed of 14 transmembrane segments and is grouped in the major facilitator superfamily of transporters (29). Expression of qacA is controlled by the QacR repressor, which is coded for by the adjacently encoded qacR (30). To induce qacA expression, substrates of QacA directly bind to QacR and elicit a structural change that releases QacR from the qacA promoter (31, 32). Different compounds have been shown to stimulate qacA expression to various degrees (30, 33).
As another plasmid-encoded efflux pump, qacC, which is also known as smr, has been shown to impart increased resistance to QACs and to aromatic dyes (28). In addition to large low-copy-number multiresistance plasmids, qacC is found on small rolling-circle replicating (RCR) plasmids (34). qacC is largely conserved among different plasmids; however, the promoter of qacC is known to differ between low-copy-number and RCR plasmids (34). The small (107-amino-acid [aa]) QacC efflux pump is member of the small multidrug resistance (SMR) family, has 4 transmembrane segments, and likely functions as a homodimer (35, 36).
As mentioned above, norA was recently described as part of the core genome of S. aureus, but was shown to exist as multiple possible alleles (17). norA is known to impart resistance to a variety of structurally diverse compounds, and previous work suggests that NorA may be responsible for the majority of basal antiseptic resistance in S. aureus (37, 38). In terms of expression, many different norA promoter mutations are documented that cause increased expression of the gene. These mutations result in increased tolerance to hydrophilic fluoroquinolones and to antiseptics (37–39). The importance of norA in antiseptic tolerance is understated, and it is plausible that different norA alleles and/or expression changes account for the large portion of qacA-negative strains that show increased CHX tolerance (9).
Collectively, qacA, qacC, and norA have been characterized individually. Nonetheless, antiseptic resistance phenotypes are not well documented for strains that contain various combinations of these pumps. We engineered isogenic S. aureus strains that contain various combinations of all three pumps and then examined their MICs and minimal bactericidal concentrations (MBCs) to various substrates. Additionally, we assessed the expression of the different efflux pumps after exposure to various biocides. From these data we developed, tested, and confirmed the hypothesis that preexposure to one biocide could subsequently increase the resistance to a different antiseptic. Given the widespread prevalence of these antiseptics in common-use items, this finding presents a potential cause for concern.
RESULTS
Creation and characterization of isogenic strains.
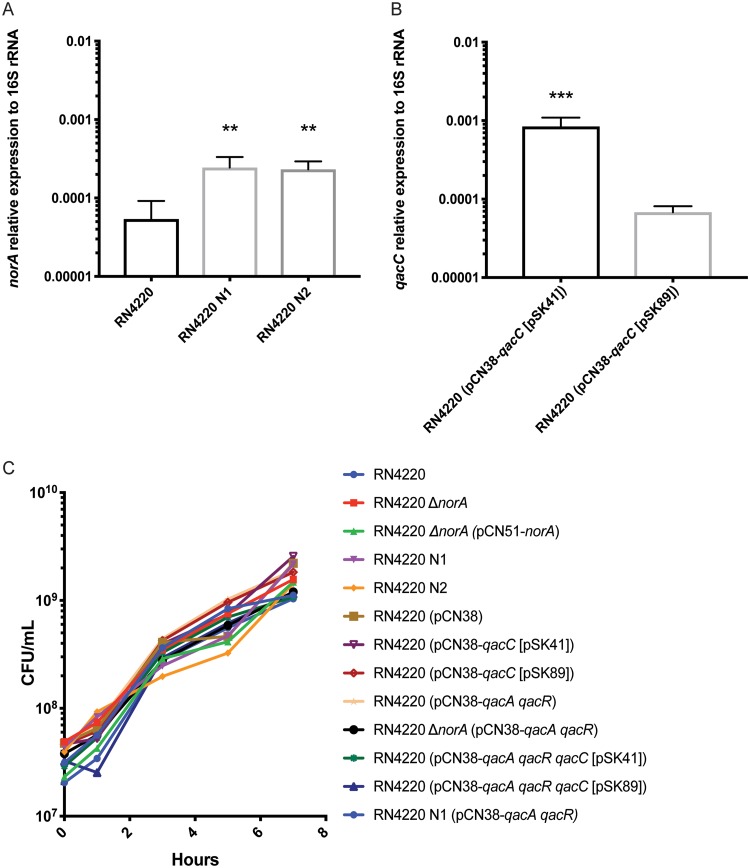
To examine the phenotypic effect of carrying norA, qacA, and qacC in different combinations, we constructed 13 isogenic strains in the S. aureus RN4220 strain background (Table 1). For the norA isogenic strains, mutations/changes were made in the chromosome via allelic exchange. Previous work indicates that norA is important for basal-level resistance to a wide variety of compounds (37, 38). To test this phenotype, we deleted norA in RN4220 to create RN4220 ΔnorA and then complemented the deletion using pCN51-norA. In addition to the phenotypic changes associated with the presence or absence of norA, a number of studies demonstrate that increased expression of norA is associated with higher levels of antiseptic and antibiotic resistance (38, 40). Therefore, we created two strains bearing naturally occurring distinct promoter mutations in norA that have been demonstrated to increase norA expression (38). The first mutation, a polymorphism in the −10 promoter region where 5′TACAAT3′ is mutated to 5′TATAAT3′, likely increases the binding affinity of RNA polymerase to the promoter. The second mutation is also known as the flq mutation and represents a polymorphism in the 5′ untranslated region (UTR) of norA whereby a polynucleotide tract is mutated from 5′TTTTT3′ to 5′TTCTT3′; this mutation is known to increase the half-life of the mRNA transcript (39, 41). The strains containing these promoter mutations were named RN4220 N1 and RN4220 N2, respectively. Both of these mutations increased the relative expression of norA (Fig. 1A).
TABLE 1.
Strain and plasmid descriptions
| Strain or plasmid | Lab strain designation | Descriptiona | Gene (norA, qacC, and/or qacA) present or other descriptionb | Reference |
|---|---|---|---|---|
| S. aureus strains | ||||
| RN4220 | DSM1935 | Wild type | norA | 62 |
| RN4220 ΔnorA | DSM1936 | S. aureus RN4220 with a clean deletion of norA | None | This study |
| RN4220 N1 | DSM1937 | RN4220 with a norA promoter mutation in the −10 element (TACAAT to TATAAT) | norA+ | This study |
| RN4220 N2 | DSM1938 | RN4220 with a norA promoter mutation in the 5′ untranslated region (TTTTT to TTCTT) | norA+ | This study |
| RN4220 (pCN38) | DSM1715 | RN4220 carrying cloning vector pCN38 | norA | 47 |
| RN4220 (pCN38-qacC [pSK41]) | DSM1939 | RN4220 carrying pCN38 containing qacC from pSK41 (accession number AF051917.1) | norA, qacC+ | This study |
| RN4220 (pCN38-qacC [pSK89]) | DSM1940 | RN4220 carrying pCN38 containing qacC from pSK89 (accession number M37889.1) | norA, qacC | This study |
| RN4220 (pCN38-qacA qacR) | DSM1941 | RN4220 carrying pCN38 containing qacA and qacR from pC02 (accession number CP012121.2) | norA, qacA | This study |
| RN4220 (pCN38-qacA qacR qacC [pSK41]) | DSM1942 | RN4220 carrying pCN38 containing qacA, qacR, and qacC from pSK41 | norA, qacA, qacC+ | This study |
| RN4220 (pCN38-qacA qacR qacC [pSK89]) | DSM1943 | RN4220 carrying pCN38 containing qacA, qacR, and qacC from pSK89 | norA, qacA, qacC | This study |
| RN4220 ΔnorA (pCN38-qacA qacR) | DSM1944 | RN4220 ΔnorA carrying pCN38 containing qacA and qacR | qacA | This study |
| RN4220 N1 (pCN38-qacA qacR) | DSM1945 | RN4220 N1 carrying pCN38 containing qacA and qacR | norA+, qacA | This study |
| RN4220 (pCN51) | DSM1946 | RN4220 carrying pCN51 | norA | This study |
| RN4220 ΔnorA (pCN51) | DSM1947 | RN4220 ΔnorA carrying pCN51 | None | This study |
| RN4220 ΔnorA (pCN51-norA) | DSM1948 | RN4220 ΔnorA carrying pCN51 containing norA from S. aureus RN4220 | norA | This study |
| RN4220 (pCN51-qacC) | DSM1950 | RN4220 carrying pCN51 containing qacC from pSK41 (accession number AF051917.1) | norA, qacC | This study |
| RN4220 (pCN51-qacA) | DSM1949 | RN4220 carrying pCN51 containing qacA from pC02 (accession number CP012121.2) | norA, qacA | This study |
| 2014.C02 (also known as C02) | DSM1418 | Clinical isolate carrying qacA-positive pC02 (accession number CP012121.2) | norA, qacA | 18 |
| 1969.N | DSM1504 | Clinical isolate carrying qacA-positive p1969.Nb (accession number CP016860.2) | norA, qacA | 18 |
| 2148.C01 | DSM1499 | Clinical isolate carrying qacA-positive p2148.C01b (accession number CP017095.2) | norA, qacA | 18 |
| 5118.N | DSM1491 | Clinical isolate carrying qacA-positive p5118.Nb (accession number CP016854.2) | norA, qacA | 18 |
| E. coli strains | ||||
| DH5 α (pCN38) | DSM1595 | DH5 α carrying pCN38 | 47 | |
| TOP10 (pCN38-qacC [pSK41]) | DSM1972 | TOP10 carrying pCN38 containing qacC from pSK41 (accession number AF051917.1) | This study | |
| TOP10 (pCN38-qacC [pSK89]) | DSM1973 | TOP10 carrying pCN38 containing qacC from pSK89 (accession number M37889.1) | This study | |
| TOP10 (pCN38-qacA qacR) | DSM1974 | TOP10 carrying pCN38 containing qacA and qacR from pC02 (accession number CP012121.2) | This study | |
| TOP10 (pCN38-qacA qacR qacC [pSK41]) | DSM1975 | TOP10 carrying pCN38 containing qacA, qacR, and qacC from pSK41 | This study | |
| TOP10 (pCN38-qacA qacR qacC [pSK89]) | DSM1976 | TOP10 carrying pCN38 containing qacA, qacR, and qacC from pSK89 | This study | |
| DH5 α (pCN51) | DSM1933 | DH5 α carrying pCN51 | This study | |
| TOP10 (pCN51-norA) | DSM1977 | TOP10 carrying pCN51 containing norA from S. aureus RN4220 | This study | |
| TOP10 (pCN51-qacC) | DSM1978 | TOP10 carrying pCN51 containing qacC from pSK41 (accession number AF051917.1) | This study | |
| TOP10 (pCN51-qacA) | DSM1979 | TOP10 carrying pCN51 containing qacA from pC02 (accession number CP012121.2) | This study | |
| TOP10 (pKOR1-ΔnorA) | DSM1930 | TOP10 carrying pKOR1 containing a fused upstream and downstream region of norA | This study | |
| TOP10 (pKOR1-N1) | DSM1931 | TOP10 carrying pKOR1 containing norA promoter with the N1 mutation | This study | |
| TOP10 (pKOR1-N2) | DSM1932 | TOP10 carrying pKOR1 containing norA promoter with the N2 mutation | This Study | |
| Plasmids | ||||
| pCN38 | Shuttle vector for E. coli and S. aureus | Encodes ampicillin resistance in E. coli and chloramphenicol resistance in S. aureus | 67 | |
| pCN38-qacC [pSK41] | pCN38 containing qacC from pSK41 | Encodes ampicillin resistance in E. coli and chloramphenicol resistance in S. aureus | This study | |
| pCN38-qacC [pSK89] | pCN38 containing qacC from pSK89 | Encodes ampicillin resistance in E. coli and chloramphenicol resistance in S. aureus | This study | |
| pCN38-qacA qacR | pCN38 containing qacA and qacR from S. aureus 2014.C02 | Encodes ampicillin resistance in E. coli and chloramphenicol resistance in S. aureus | This study | |
| pCN38-qacA qacR qacC [pSK41] | pCN38 containing qacA and qacR from S. aureus 2014.C02 and qacC from pSK41 | Encodes ampicillin resistance in E. coli and chloramphenicol resistance in S. aureus | This study | |
| pCN38-qacA qacR qacC [pSK89] | pCN38 containing qacA and qacR from S. aureus 2014.C02 and qacC from pSK89 | Encodes ampicillin resistance in E. coli and chloramphenicol resistance in S. aureus | This study | |
| pCN51 | Shuttle vector for E. coli and S. aureus. Plasmid multicloning site is located downstream of a cadmium-inducible promoter. | Encodes ampicillin resistance in E. coli and erythromycin resistance in S. aureus | 67 | |
| pCN51-norA | pCN51 containing norA from S. aureus RN4220 | Encodes ampicillin resistance in E. coli and erythromycin resistance in S. aureus | This study | |
| pCN51-qacA | pCN51 containing qacA from S. aureus 2014.C02 | Encodes ampicillin resistance in E. coli and erythromycin resistance in S. aureus | This study | |
| pCN51-qacC | pCN51 containing qacC from pSK41 | Encodes ampicillin resistance in E. coli and erythromycin resistance in S. aureus | This study | |
| pKOR1-ΔnorA | pKOR1 containing the ΔnorA construct | Encodes ampicillin resistance in E. coli and chloramphenicol resistance in S. aureus. Plasmid used to create S. aureus RN4220 ΔnorA. | This study | |
| pKOR1-N1 | pKOR1 containing the N1 norA promoter construct | Encodes ampicillin resistance in E. coli and chloramphenicol resistance in S. aureus. Plasmid used to create S. aureus RN4220 N1. | This study | |
| pKOR1-N2 | pKOR1 containing the N2 norA promoter construct | Encodes ampicillin resistance in E. coli and chloramphenicol resistance in S. aureus. Plasmid used to create S. aureus RN4220 N2. | This study |
Accession numbers are from GenBank.
“+” indicates increased expression.
FIG 1.
Isogenic strain characterization. (A) The expression of norA relative to that of the 16S rRNA gene was examined in the isogenic strains RN4220, RN4220 N1, and RN4220 N2. Bar graphs indicate the geometric means from 3 biologically independent replicates, and error bars represent the geometric SD. A statistically significant difference from RN4220 (P < 0.01) is indicated by double asterisks. Statistical analysis was conducted by one-way analysis of variance (ANOVA) with Tukey’s correction for multiple comparisons. (B) The expression of qacC relative to that of the 16S rRNA gene was examined in the isogenic strains RN4220 (pCN38-qacC [pSK41]) and RN4220 (pCN38-qacC [pSK89]). A statistically significant difference between strains (P < 0.001) is indicated by triple asterisks. Statistical analysis was conducted using a Student t test. Bar graphs indicate the geometric means from 3 biologically independent replicates, and error bars represent the geometric SD. (C) Growth curve of isogenic strains. Overnight cultures were subcultured and strains were grown for 7 h. CFU were determined at 0, 1, 3, 5, and 7 h. The geometric means from at least 2 biologically independent replicates are plotted.
To enable direct comparison between plasmid-carried efflux genes, we cloned qacA and qacC variants into pCN38 with their native promoters (30, 34). For the qacA isogenic strain, qacA and qacR, which encodes qacA’s regulator, QacR, were cloned into pCN38 to create RN4220 (pCN38-qacA qacR). As for the qacC isogenic strains, previous reports have shown qacC to have two different promoter sequences; these sequences vary based on the plasmid backbone that qacC is carried on. To determine if these promoter differences result in different phenotypic effects, we synthesized qacC and its promoter from pSK41 (a large conjugative plasmid) (42) and qacC plus 133 bp upstream of qacC from pSK89; this promoter sequence is still undefined (43). Both qacC variants were cloned into pCN38 to create RN4220 (pCN38-qacC [pSK41]) and RN4220 (pCN38-qacC [pSK89]). Expression of qacC in logarithmically grown cells was different between the two promoters; RN4220 (pCN38-qacC [pSK41]) showed a 10-fold increase in expression compared to that of RN4220 (pCN38-qacC [pSK89]) (Fig. 1B). Finally, to characterize the phenotype that results from harboring multiple pumps in a single strain, we also created strains that carried various combinations of the efflux pumps described above (Table 1). In the isogenic strains that were designed to carry multiple qac genes, qacA, qacR, and qacC were cloned into the same plasmid.
To ensure that genetic manipulation of RN4220 did not result in any obvious fitness defects of the constructed strains, levels of growth of the strains were compared in BBL Mueller-Hinton II (cation-adjusted) broth (MHB) across a standard growth curve; no significant differences in growth were observed (Fig. 1C). In addition, to determine if the various efflux systems altered antibiotic resistance of the strains, all of the isogenic strains were tested for antibiotic resistance using the BD Phoenix system. Resistance profiles for ampicillin-sulbactam, cefazolin, cefoxitin, clindamycin, daptomycin, erythromycin, gentamicin, levofloxacin, linezolid, minocycline, moxifloxacin, nitrofurantoin, oxacillin, penicillin G, rifampin, tetracycline, trimethoprim-sulfamethoxazole, and vancomycin were defined, and no significant changes in antibiotic resistance were observed between the isogenic strains (data not shown). Taken together, the basic characterizations indicate that the isogenic strains are comparable to wild-type RN4220 in terms of growth and antibiotic resistance.
Isogenic strain MICs and MBCs to various antiseptic compounds.
To determine the relative contribution of the various efflux pumps to antiseptic resistance, we next determined the MIC and MBC to CHX, CHX digluconate, BKC, cetrimide, pentamidine isethionate, and ethidium bromide for each of the isogenic strains. Two forms of CHX were tested since literature has reported MICs using both pure CHX and the more soluble CHX digluconate (37, 44). BKC and cetrimide are QACs that represent commonly used antiseptics, and pentamidine isethionate and ethidium bromide are known substrates for NorA, QacA, and QacC (28, 37). To facilitate MIC and MBC comparisons to an additional strain background, we also included the recent qacA-positive isolate S. aureus 2014.C02 (C02) (44).
The MICs of the individual compounds against the various strains are presented in Table 2. In terms of NorA, deleting norA resulted in increased susceptibility to all of the tested compounds. This strongly suggests that norA is important for basal resistance to all of the tested compounds. Furthermore, strains that expressed higher levels of norA showed higher levels of resistance to the majority of antiseptics (Table 2). Of note, after several failed attempts to clone norA and its native promoter into pCN38, the functional complementation of norA was accomplished by fusing norA to the cadmium-inducible promoter carried in pCN51. The leaky expression of this promoter provided functional complementation and resulted in a phenotype similar to that of the isogenic strains that overexpress norA (Table 2). Thus, in terms of NorA, the results demonstrated increased susceptibility to antiseptics in RN4220 ΔnorA juxtaposed with increased antiseptic resistance in strains overexpressing norA (Table 2). Taken together, the data indicate that NorA is a key efflux pump for antiseptic resistance.
TABLE 2.
Isogenic strain MICs
| Strain | MIC (μg/ml)a
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chlorhexidine |
Chlorhexidine digluconate |
Benzalkonium chloride |
Cetrimide |
Pentamidine isethionate |
Ethidium bromide |
|||||||
| Median | Max | Median | Max | Median | Max | Median | Max | Median | Max | Median | Max | |
| RN4220 | 1.6 | 1.6 | 2 | 4 | 4 | 4 | 2 | 2 | 25 | 25 | 8 | 8 |
| RN4220 ΔnorA | 0.8 | 0.8 | 1 | 2 | 1 | 2 | 0.5 | 0.5 | 1.56 | 1.56 | 2 | 2 |
| RN4220 ΔnorA (pCN51-norA) | 3.2 | 3.2 | 4 | 4 | 8 | 8 | 8 | 8 | 50 | 50 | 16 | 16 |
| RN4220 N1 | 1.6 | 1.6 | 4 | 4 | 8 | 8 | 4 | 4 | 25 | 50 | 16 | 16 |
| RN4220 N2 | 3.2 | 3.2 | 4 | 4 | 8 | 8 | 4 | 4 | 50 | 50 | 32 | 32 |
| RN4220 (pCN38) | 1.6 | 1.6 | 2 | 2 | 4 | 4 | 2 | 2 | 12.5 | 12.5 | 8 | 8 |
| RN4220 (pCN38-qacC [pSK89]) | 1.6 | 1.6 | 2 | 2 | 4 | 4 | 2 | 2 | 12.5 | 12.5 | 8 | 8 |
| RN4220 (pCN38-qacC [pSK41]) | 1.6 | 1.6 | 3 | 4 | 16 | 16 | 8 | 8 | 12.5 | 25 | 128 | 128 |
| RN4220 (pCN38-qacA qacR) | 3.2 | 3.2 | 4 | 4 | 16 | 16 | 4 | 4 | 400 | 400 | 128 | 128 |
| RN4220 ΔnorA (pCN38-qacA qacR) | 3.2 | 3.2 | 4 | 4 | 16 | 16 | 4 | 8 | 300 | 400 | 128 | 128 |
| RN4220 (pCN38-qacA qacR qacC [pSK41]) | 3.2 | 3.2 | 4 | 4 | 16 | 16 | 16 | 16 | 200 | 400 | 128 | 128 |
| RN4220 (pCN38-qacA qacR qacC [pSK89]) | 3.2 | 3.2 | 4 | 4 | 8 | 16 | 8 | 8 | 200 | 400 | 128 | 128 |
| RN4220 N1 (pCN38-qacA qacR) | 3.2 | 3.2 | 4 | 4 | 16 | 16 | 8 | 8 | 400 | 400 | 128 | 256 |
| S. aureus 2014.C02 | 3.2 | 3.2 | 4 | 8 | 16 | 16 | 8 | 8 | 400 | 400 | 256 | 256 |
Values are from ≥3 independent replicates. Values in italics indicate a MIC less than that of the wild type. Bold values indicate MIC greater than that of the wild type. Max, maximum.
Two distinct phenotypes were observed with the two qacC variants (Table 2). The strains harboring qacC [pSK41] showed increased resistance to BKC, cetrimide, and ethidium bromide. In comparison, strains carrying qacC [pSK89] exhibited no increases in antiseptic resistance compared to RN4220.
Similar to the case with norA, carriage of qacA was associated with increased resistance to the majority of tested antiseptics (Table 2). Strains containing qacA displayed increased resistance to CHX, BKC, and the highest observed resistance to pentamidine isethionate and ethidium bromide. Unexpectedly, in RN4220 ΔnorA (pCN38-qacA qacR), the absence of norA appeared inconsequential; carriage of qacA provided levels of resistance similar to those of the strain that carried both qacA and norA [RN4220 (pCN38-qacA qacR)]. Similarly, even compared to those of the isogenic strains overexpressing norA, the isogenic qacA-containing strains demonstrated enhanced resistances to several of the compounds. Of note, the isogenic RN4220 qacA-containing strains were phenotypically similar to the previously characterized naturally isolated qacA-positive strain C02.
Among the strains that carried multiple efflux pumps, RN4220 N1 (pCN38-qacA qacR) demonstrated a resistance phenotype similar to that of strain C02, which naturally harbors both norA and qacA (Table 2). The functional compatibility of qacA, qacC, and norA in a single strain was most evident in RN4220 (pCN38-qacA qacR qacC [pSK41]); this strain displayed the highest resistance to cetrimide (Table 2). Thus, strains that carry multiple antiseptic efflux pumps may show increased resistance to some antiseptics.
The MBC data (Table 3) showed a trend similar to that of the MIC data (Table 2). However, similar to prior reports (37, 45), there was increased variability in the MBC data. For example, RN4220 N1 (pCN38-qacA qacR) displayed the highest resistance to CHX; however, this observation was not maintained with CHX gluconate. Overall, when combined with the MIC data, these results demonstrated that qacA provided the highest level of resistance; however, strains with promoter mutations in norA also showed enhanced resistance compared to that of wild-type RN4220. Additionally, the data suggest that strains carrying all three efflux pumps may show increased resistance to some antiseptics.
TABLE 3.
Isogenic strain MBCs
| Strain | MBC (μg/ml)a
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chlorhexidine |
Chlorhexidine digluconate |
Benzalkonium chloride |
Cetrimide |
Pentamidine isethionate |
Ethidium bromide |
|||||||
| Median | Max | Median | Max | Median | Max | Median | Max | Median | Max | Median | Max | |
| RN4220 | 3.2 | 3.2 | 2 | 8 | 8 | 8 | 4 | 4 | 25 | 50 | 16 | 32 |
| RN4220 ΔnorA | 3.2 | 3.2 | 4 | 4 | 8 | 8 | 2 | 2 | 3.1 | 3.1 | 2 | 8 |
| RN4220 ΔnorA (pCN51-norA) | 3.2 | 3.2 | 4 | 4 | 8 | 8 | 8 | 8 | 50 | 50 | 16 | 16 |
| RN4220 N1 | 3.2 | 3.2 | 4 | 4 | 8 | 8 | 4 | 8 | 50 | 50 | 32 | 32 |
| RN4220 N2 | 3.2 | 3.2 | 8 | 16 | 16 | 16 | 8 | 16 | 100 | 100 | 32 | 64 |
| RN4220 (pCN38) | 3.2 | 3.2 | 4 | 4 | 4 | 8 | 2 | 4 | 25 | 25 | 16 | 16 |
| RN4220 (pCN38-qacC [pSK89]) | 3.2 | 3.2 | 2 | 4 | 8 | 8 | 4 | 4 | 25 | 25 | 32 | 32 |
| RN4220 (pCN38-qacC [pSK41]) | 3.2 | 3.2 | 4 | 8 | 32 | 32 | 8 | 16 | 25 | 25 | 256 | 256 |
| RN4220 (pCN38-qacA qacR) | 3.2 | 3.2 | 4 | 8 | 16 | 16 | 8 | 16 | 400 | 800 | 256 | 256 |
| RN4220 ΔnorA (pCN38-qacA qacR) | 3.2 | 3.2 | 8 | 16 | 16 | 32 | 4 | 8 | 400 | 400 | 128 | 128 |
| RN4220 (pCN38-qacA qacR qacC [pSK41]) | 3.2 | 6.4 | 8 | 8 | 16 | 16 | 16 | 16 | 200 | 200 | 256 | 256 |
| RN4220 (pCN38-qacA qacR qacC [pSK89]) | 3.2 | 3.2 | 8 | 8 | 32 | 64 | 16 | 16 | 200 | 200 | 128 | 128 |
| RN4220 N1 (pCN38-qacA qacR) | 6.4 | 6.4 | 4 | 8 | 16 | 16 | 16 | 16 | 400 | 400 | 256 | 256 |
| S. aureus 2014.C02 | 3.2 | 3.2 | 8 | 8 | 16 | 16 | 8 | 16 | 400 | 800 | 256 | 512 |
Values are from 3 independent replicates. Values in italics indicate an MBC less than that of the wild type. Bold values indicate an MBC greater than that of the wild type.
Fitness cost of efflux gene overexpression.
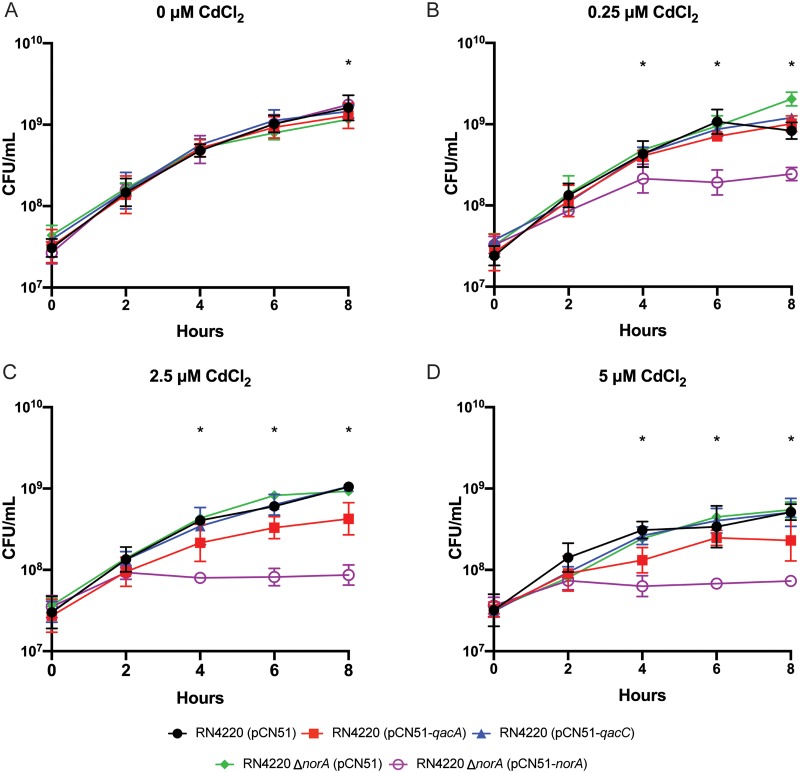
As described above, we were unsuccessful in our initial attempts to complement the RN4220 ΔnorA strain by cloning norA with its native promoter into pCN38. On the few occasions where pCN38-norA-containing S. aureus colonies were obtained, sequencing of norA revealed nonsynonymous mutations in the coding sequence (data not shown). This difficulty led us to hypothesize that norA expression is limited in S. aureus by a fitness cost associated with norA overexpression. This led us to wonder if overexpression of qacA and qacC could also affect the fitness of S. aureus. To test the effects of efflux gene overexpression, we generated isogenic strains that carried norA, qacA, and qacC under the control of the cadmium-inducible promoter found in pCN51; empty-pCN51-containing control strains were also constructed [RN4220 (pCN51) and RN4220 ΔnorA (pCN51)] and analyzed. Even low concentrations of cadmium chloride resulted in a significant decrease in growth of RN4220 (pCN51-norA) (Fig. 2). For the strain overexpressing qacA, a slight, but statistically insignificant, growth defect was evident at 2.5 μM and higher concentrations of cadmium chloride. Of note, we did not observe any growth inhibition associated with overexpression of qacC at any concentration of cadmium chloride. Taken together, these data indicated that overexpression of norA resulted in a pronounced fitness cost, overexpression of qacA resulted in a mild defect, and overexpression of qacC showed no growth phenotype. Thus, overall levels of expression of norA and qacA in S. aureus may be limited by a cost to host fitness.
FIG 2.
Overexpression of norA, qacA, and qacC. Promotorless norA, qacA, and qacC were transcriptionally fused to a cadmium-inducible promoter in pCN51. Overnight cultures were diluted to an OD600 of 0.05, and strains were grown for 8 h in increasing concentrations of cadmium chloride. CFU were determined every 2 h for 8 h. Each panel indicates a growth curve in the presence of the indicated concentration of cadmium chloride. The geometric mean and geometric SD are plotted from ≥3 biologically independent replicates. *, P < 0.05 between RN4220 ΔnorA (pCN51) and RN4220 ΔnorA (pCN51-norA). In panel A, the observed significance between RN4220 ΔnorA (pCN51) and RN4220 ΔnorA (pCN51-norA) was due to the nearly identical CFU counts between replicates and likely not biologically relevant. Significant differences were determined by two-way ANOVA with Tukey’s correction for multiple comparisons.
Efflux pump expression after antiseptic exposure.
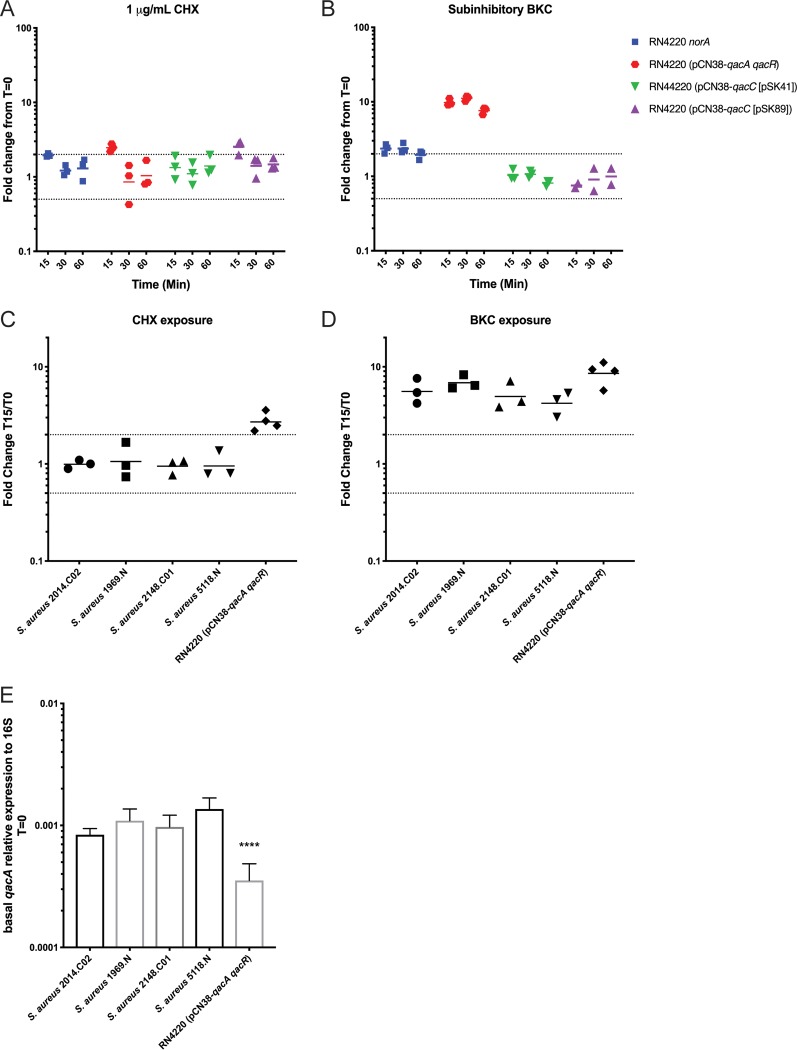
Although a few studies have looked at efflux gene expression in response to antiseptic exposure (30, 33, 46), there is currently a poor overall understanding of how antiseptic exposure may affect transcription of norA, qacA, and qacC. To address this gap, the isogenic strains were exposed to subinhibitory concentrations of CHX and BKC and expression of the various genes was monitored every 15 min for 1 h. After exposure to CHX, qacC [pSK41] showed no change in expression. In comparison, qacA, norA, and qacC [pSK89] showed a transient 2-fold increase in expression at 15 min, but expression levels returned to basal levels by 30 min postexposure (Fig. 3A). In comparison, exposure to BKC resulted in a sustained 10-fold increase in qacA expression (Fig. 3B). Similarly, norA showed a sustained 2-fold increase in expression (Fig. 3B). Expression was unchanged for qacC [pSK41] and qacC [pSK89]. Taken together, these observations support previous reports that indicate that CHX is not a strong inducer of efflux gene expression compared to BKC, which is known to induce both qacA and norA expression (33).
FIG 3.
Efflux pump gene expression before or after antiseptic exposure. (A and B) The expression of norA, qacA, and the two qacC variants after exposure to 1 μg/ml of CHX or subinhibitory concentrations of BKC {2.5 μg/ml for RN4220 and RN4220 (pCN38-qacC [pSK89]) or 5 μg/ml for RN4220 (pCN38-qacA qacR) and RN4220 (pCN38-qacC [pSK41])} was examined. Fold differences in gene expression compared to expression prior to the addition of antiseptic were calculated at 15, 30, and 60 min. The geometric means from ≥3 biologically independent replicates are plotted (except for panel B; two replicates were completed for RN4220 (pCN38-qacC [pSK89]), as no change was observed). The dotted lines indicate a 2-fold increase or decrease in expression. (C and D) The expression of qacA in S. aureus USA300 strains was examined after exposure to 1 μg/ml of CHX or 5 μg/ml of BKC. For comparison, expression results from RN4220 (pCN38-qacA qacR) were included. Fold differences in gene expression compared to expression prior to the addition of antiseptic were calculated at 15, 30, and 60 min. The geometric means from ≥3 biologically independent replicates are plotted. (E) The basal level relative expression of qacA compared to the 16S gene was examined in the indicated strains. The geometric mean is plotted, and the error bars indicate the geometric SD. ****, P < 0.0001 compared to the values for all other strains. Significant differences were determined by one-way ANOVA with Tukey’s correction for multiple comparisons.
Though isogenic strains are a valuable tool that can mitigate strain background issues and allow one to compare various strains with a greater understanding of exact strain differences, the genetic content found in various clinical isolates likely affects expression of genes of interest. Furthermore, little is known about qacA expression when the gene is carried on native plasmids; the majority of transcriptional studies have involved cloning of qacA and qacR into shuttle vectors (30, 46). Given these facts and the apparent importance of QacA in antiseptic resistance (Tables 2 and 3), we next assessed qacA expression changes in response to CHX and BKC exposure in a small collection of previously described S. aureus USA300 isolates (18). Unlike the constructed RN4220 strain, no qacA expression changes were observed in the USA300 strains after the addition of CHX (Fig. 3C). In contrast, a 3- to 7-fold increase in qacA expression was clearly evident after BKC induction (Fig. 3D). Given the differences in the transcriptional changes observed in the various strains, we reasoned that the basal levels of qacA expression might also differ across the various strains. Indeed, compared to that of the RN4220 isogenic strain, the USA300 isolates all showed increased basal levels of qacA expression (Fig. 3E). Though not completely clear, these differences are likely affected by the basic plasmid characteristics of the plasmids on which qacA is carried. For example, pCN38 and pC02 show differences in copy number (47) and replication initiation genes (47). Furthermore, gene copy number likely affects the basal level of expression and ultimate potential for induction of qacA within a strain. Taken together, the results indicate that though there are strain differences, early transcriptional activation of efflux genes can occur with different compounds but is most robust and sustained upon exposure to BKC.
Overexpression of qacA increases CHX resistance.
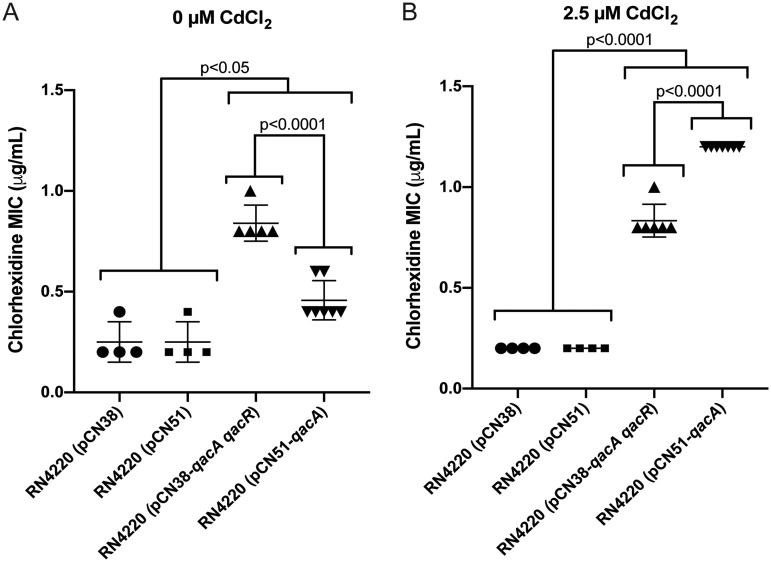
The expression differences observed with BKC and CHX suggested that qacA-mediated CHX resistance may be limited by overall weak CHX-mediated induction of qacA. Thus, we sought to determine if increased production of qacA could further enhance CHX resistance using the isogenic strain carrying qacA and qacR [RN4220 (pCN38-qacA qacR)] compared to an isogenic strain carrying qacA under the control of a cadmium-inducible promoter [RN4220 (pCN51-qacA)]. A CHX broth macrodilution assay was performed with these strains in increasing concentrations of CHX with or without the addition of exogenous cadmium chloride; appropriate vector controls were included. In the absence of cadmium chloride, RN4220 (pCN38-qacA qacR) showed the highest MIC to CHX (∼0.8 μg/ml), followed by RN4220 (pCN51-qacA), which had a MIC of ∼0.4 μg/ml (Fig. 4A). When 2.5 μM cadmium chloride was added to the medium, the MIC of RN4220 (pCN38-qacA qacR) was unchanged (Fig. 4B). However, RN4220 (pCN51-qacA) showed a MIC that increased to 1.2 μg/ml. Thus, overexpression of qacA in RN4220 (pCN51-qacA) significantly increased CHX resistance compared to qacA expressed from its native promoter. This increase suggests that qacA-mediated resistance to CHX may be limited by the overall induction potential of CHX and that a potential mechanism for increased CHX resistance in qacA harboring isolates would be to alter regulation of qacA expression.
FIG 4.
Overexpression of qacA increases CHX resistance. The MIC to CHX was determined for RN4220 (pCN38-qacA qacR), RN4220 (pCN51-qacA), and the indicated RN4220 vector controls in the presence of 0 μM or 2.5 μM cadmium chloride. The data from at least 4 biologically independent replicates are plotted with the mean and standard deviation indicated by error bars. The error bars represent the SD. The P values above the graphs indicate statistically significant differences between the indicated groups as determined by one-way ANOVA with Tukey’s correction for multiple comparisons.
Preexposure to BKC increases CHX tolerance.
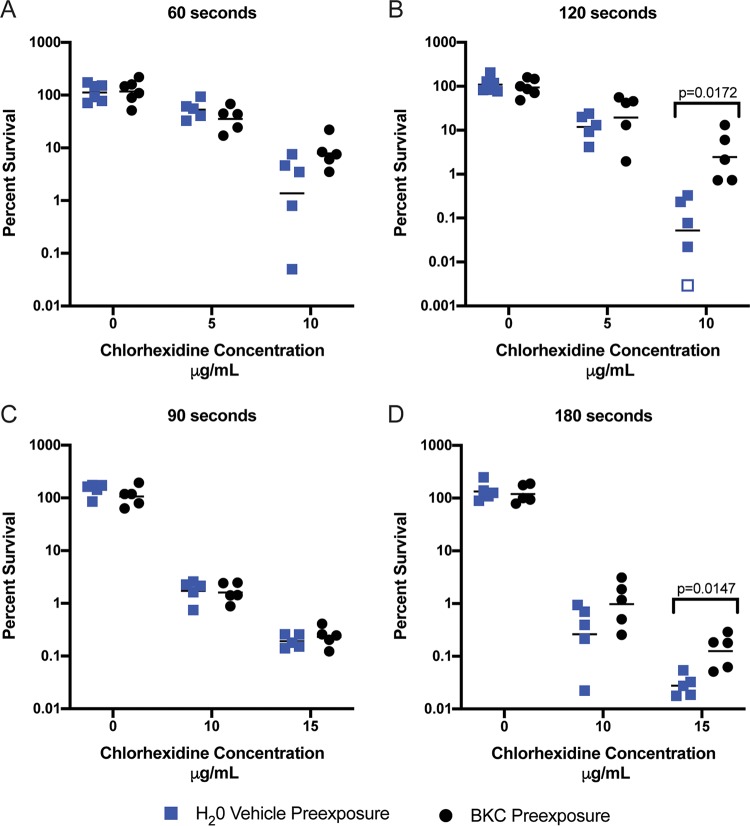
The sustained increases in norA and qacA induced by BKC (Fig. 3B and D) combined with the abundance of BKC and other QACs found in personal-care and household products present a possible source for selection for increased CHX tolerance in S. aureus strains that harbor both genes. Indeed, we reasoned that even transient low-level exposure to these ubiquitous compounds may decrease the killing potential of other antiseptics. To investigate this possibility, we next assessed if preexposure to BKC would increase CHX tolerance. To this end, RN4220 (pCN38-qacA qacR) cells were exposed to water (vehicle control) or a subinhibitory concentration of BKC for 60 min. Cells were then washed and inoculated into inhibitory concentrations of CHX and the percent survival was determined at different time points after CHX exposure. Though no dramatic differences between treatment groups were seen at 5 μg/ml of CHX, differences in survival were apparent at 10 μg/ml of CHX; the tendency of BKC-treated cells to be more tolerant to CHX exposure was evident at 60 s (Fig. 5A) and statistically significant at 120 s (Fig. 5B).
FIG 5.
Preexposure to BKC increases CHX resistance. (A and B) RN4220 (pCN38-qacA qacR) was grown to log phase and exposed to either H2O (vehicle control) or BKC for 60 min. Cells were then washed and inoculated into the indicated concentration of CHX. CFU were determined immediately following inoculation and at 60 and 120 s. (C and D) C02 was grown to log phase and exposed to either H2O (vehicle control) or BKC for 60 min. Cells were then washed and inoculated into the indicated concentration of CHX. CFU were determined immediately following inoculation and at 90 and 180 s. Percent survival was calculated as (CFU at N seconds/CFU of input) × 100, where N equals indicated chlorhexidine exposure times. Graphed values for percent survival are from ≥5 independent replicates, and the bars indicate the geometric means. The open square represents a replicate for which no bacteria were recovered and is graphed at the limit of detection. The P values above the graphs indicate statistically significant differences as determined by Student t test with a correction for multiple comparisons using the Holm-Sidak method.
We observed the same enhanced tolerance in C02, a clinical MRSA isolate, albeit with a higher concentration and longer exposure times (Fig. 5C and D). C02 exposed to BKC displayed enhanced survival in 15 μg/ml of CHX (Fig. 5D). Indeed, the increase in survival seen with BKC-exposed cells was approximately 15-fold the survival seen with control cells. Thus, preexposure to a subinhibitory concentration of BKC can increase tolerance to CHX in S. aureus strains that contain norA and qacA.
DISCUSSION
Given the skyrocketing rates of antibiotic resistance, infection prevention has become even more important than in the decades before widespread resistance. Among prevention approaches, antiseptics are crucial in the battle against hospital-acquired infections and pan-drug-resistant bacteria. Consequently, a number of these compounds have come into common use in the hospital as well as community setting. For example, CHX is a well-tolerated antiseptic that has been shown to be effective as a presurgical skin preparation. Though prior thought suggested that antiseptic resistance was unlikely (7), the ubiquitous use of CHX in hospitals and the community has led to the appearance of strains of bacteria, including S. aureus, that are more resistant to CHX (9, 48). Furthermore, the increasing presence of QACs such as BKC in everyday personal-care products appears to have provided an additional selection for efflux-mediated antiseptic resistance in bacteria. In S. aureus, the functional redundancy of efflux pumps associated with antiseptic resistance highlights the importance of these pumps within this pathogen. Moreover, the presence of multiple pumps provides multiple pathways for resistance evolution. Using a panel of isogenic S. aureus strains, we investigated the contribution of norA, qacA, and qacC to antiseptic resistance. Though qacA imparted the highest level of resistance of an individual pump, all three genes could individually alter antiseptic resistance. Moreover, strains that carried multiple efflux pumps showed the highest level of achieved resistance for some antiseptics. Overexpression of qacA or norA further increased CHX resistance. Alarmingly, preexposure of two different norA- and qacA-positive strains to subinhibitory concentrations of BKC subsequently increased their resistance to CHX. Given the widespread prevalence of these antiseptics in common-use items, this finding presents a potential cause for concern.
In terms of evolution of antiseptic resistance, it is important to consider that while qacA and qacC are typically horizontally acquired factors, norA exists as part of the core genome of S. aureus; qacA and qacC typically reside on a variety of conjugative and nonconjugative plasmids (18, 42, 49, 50). Importantly, naturally occurring S. aureus isolates that concomitantly carry norA, qacA, and qacC have been documented (20–22, 51). Unfortunately, the genome sequences of these strains are not available, so it is impossible to know whether genomic localization of the three genes follows the expected paradigm. Interestingly, no available plasmid sequence in the NCBI GenBank database contains both qacA and qacC. However, there are several examples of Staphylococcus haemolyticus isolates (GenBank accession numbers CP011116.1, CP025396.1, and CP024809.1) that harbor qacA and an smr family efflux pump within the chromosome. In all three of these genomes, the two genes are separated by >200 kb, suggesting that separate integration events are responsible for dual carriage. While the majority of S. aureus literature indicates that qacA typically resides on a plasmid (23), a recent publication from Australia described stable chromosomal integration of a qacA-positive plasmid in S. aureus clinical isolates (48). The integration event was linked to increased CHX tolerance and was hypothesized to result in a change in qacA expression. Thus, genomic location likely affects qacA expression, which, in turn, affects downstream antiseptic resistance. Indeed, our data that show that basal-level qacA expression differs depending on the plasmid on which the gene is carried (Fig. 3E) appear to support this hypothesis.
Given the complete conservation of the qacA promoter sequence in the strains that we examined, it is not completely clear at a mechanistic level why the basal levels of qacA expression differ across the examined strains. One possibility could be plasmid copy number. However, the four clinical isolates that showed the highest levels of basal qacA expression all harbor RepA_N replication initiation proteins, which are believed to mediate theta plasmid replication (52) and to be carried at lower copy numbers than RCR plasmids like pCN38-qacA qacR. Indeed, we previously showed that pC02 has a copy number of 3 or 4, while pCN38 has a copy number of 9 to 18 (47). Thus, counterintuitively, the higher levels of basal qacA expression were seen in the strains with a lower plasmid copy number and thus lower qacA copy number. This finding likely suggests that the difference in qacA expression is intricately tied to the amount of the QacR regulator that is produced in the various strain backgrounds. Prior work indicates that qacR is carried adjacent to qacA and is either regulated by an unknown mechanism or constitutively expressed (53, 54). Furthermore, QacR represses qacA by binding to the qacA promoter as a pair of dimers (31). Thus, if expression of qacR is directly proportional to its copy number in the genome, additional copies of qacR would increase the number of QacR proteins available to form dimers. Thus, a strain with a higher plasmid copy number would produce more QacR, which could then function to turn off qacA expression. Conversely, a strain with a lower plasmid copy number would produce less QacR, and thus qacA expression could be higher due to a decrease in the amount of the repressor. Intriguingly, this regulatory model also explains the increased resistance seen upon qacA and qacR integration into the chromosome (48); in a single copy, smaller amounts of functional QacR dimers would lead to increased expression of qacA and thus increased CHX resistance. Future targeted mechanistic studies will seek to investigate the proposed regulatory model.
The importance of evolutionary changes in gene expression as a means to increase antiseptic resistance is clearly supported by data from Hardy et al. (9), who proposed that the temporal evolving increase in S. aureus CHX MBC may be associated with mutations in norA or in norB, which is another broad-substrate efflux pump. In this as well is in other studies (37, 38), increased expression of norA was associated with enhanced resistance (Tables 2 and 3). Indeed, prior reports have identified numerous norA promoter mutations that are associated with increased expression (38–41). However, our data strongly suggest that the extent of norA expression within S. aureus is limited by a previously unrecognized fitness cost (Fig. 2). Indeed, we were unable to move a plasmid containing norA under the control of its own promoter into S. aureus. Conversely, we were able to clone norA under the control of the cadmium-inducible promoter in pCN51, but we then observed a clear and significant dose-dependent growth defect of RN4220 (pCN51-norA) upon addition of cadmium chloride. This finding suggests a delicate balance in the tolerable expression levels of norA, which, in turn, places a theoretical limit to the degree of norA overexpression-mediated resistance that can occur in a strain. Beyond norA expression, it is worth mentioning that a recent report defined three possible norA alleles (17). It is intriguing to speculate that these various alleles may have different functional consequences in terms of norA-mediated antiseptic resistance; however, this topic remains to be explored.
Beyond S. aureus, antiseptic resistance has been observed in other bacterial species. For example, in vitro evolution experiments with vancomycin-resistant Enterococcus faecium and Pseudomonas stutzeri demonstrated that stable CHX resistance development was associated with efflux pump mutations and/or alterations in cellular membrane lipid composition (55, 56). Furthermore, it is known that Gram-negative cells are inherently more resistant to CHX than Gram-positive cells (6); this resistance is likely attributable to the additional lipid membrane. Nonetheless, increased resistance to CHX relative to wild-type susceptibilities has been demonstrated for numerous Gram-negative species (6). For example, a multidrug-resistant isolate of Klebsiella pneumoniae that was obtained from a CHX soap dispenser was able to grow in 1% CHX soap (57). Clearly, increased CHX tolerance is evident in clinically relevant bacterial species and is likely being selected for by QACs that have found their ways into many common-use items and thus into the environment.
Little is currently known about the possible effects of exposure to subinhibitory concentrations of antiseptics on bacterial cellular processes. However, a recent study demonstrated that subinhibitory CHX exposure can increase vancomycin and daptomycin resistance gene expression in Enterococcus faecium (58). Furthermore, induction of biofilm formation by subinhibitory QAC exposure provides another potential mechanism for increased antibiotic and antiseptic resistance (59). To date, multiple studies have shown that increased expression of qacA and norA can be induced by subinhibitory exposure to various antiseptic QACs (33, 46). These prior findings led us to the discovery that preexposure to BKC could reduce CHX-mediated killing of S. aureus (Fig. 5). Given this finding, one has to wonder if other QACs can promote a similar phenotype in S. aureus or other bacterial pathogens. Since they are important cationic surfactants, QACs are incorporated into numerous personal-care products, such as makeup, soaps, shampoos, conditioners, and lotions. Given this, it is not difficult to imagine that a patient using several of these products prior to a surgical intervention might “prime” bacterial cells to exhibit enhanced tolerance to a CHX presurgical skin preparation. This could then increase the chance of a subsequent surgical site infection. Indeed, future studies should be directed to examine the phenotypic effect of cosmetic QACs on efflux pump expression in S. aureus and other bacteria. Given that it is imperative that antiseptics remain highly effective at preventing infections in this age of increased antibiotic resistance, it is likely time to consider greater antiseptic stewardship.
MATERIALS AND METHODS
Bacterial growth conditions and information.
Strains were cultured in BBL Mueller-Hinton II (cation-adjusted) broth (MHB), 0.1× concentrated MHB, tryptic soy broth (TSB; Becton, Dickinson), or lysogeny broth (LB; Becton, Dickinson) as indicated for the individual experiments. Bacteria were grown at 37°C stationary or shaking at 190 rpm as indicated. When solid medium was used, broth was supplemented with 1.7% agar (Alfa Aesar). Antiseptic neutralizing broth was made as previously described (60) and contained 3% asolectin (Sigma), 10% Tween 80 (Sigma), and 1% peptone (Fisher). Neutralizing agar was modified from reference 60 and contained 1.5% TSB, 0.3% azolectin, 1% Tween 80, and 1.7% agar. All isogenic strains were constructed in the S. aureus RN4220 background (61, 62). Primer sequences are listed in Table 4.
TABLE 4.
Primer and Gblock sequences
| Primer | Primer sequence (5′–3′)a | Reference |
|---|---|---|
| 38qacA-For | AAAGGATCCAGATCTTCTCACAGCGTCCTAAA | This study |
| 38qacA-Rev | AATTGTCGACTTTCAAACCTAGTCATCCTTGCC | This study |
| qacAF | GCTGCATTTATGACAATGTTTG | 51 |
| qacAseq-Rev | GATAAAATTGTAGAAGGAATATCCC | This study |
| 51qacA-For | AATAGTCGACTATAGACCGATCGCACGGT | This study |
| 51qacA-Rev | AATAGGATCCCTACAATATCTAAAAATATATGTTTAGTAC | This study |
| 51-seqFor | GCAGATAATGATGATCGCCCTAG | This study |
| 51-seqRev | TCTGTTAACTTATTAACTCTTTCCGC | This study |
| 51norA-For | AATAGTCGACGGAGAAAAAAGAGGTGAGCAT | This study |
| 51norA-Rev | AATAGGATCCACACCAAAATACTTATGCTACATATT | This study |
| 51norA-seqFor | ATGATACGACCAGCCATTAC | This study |
| 51norA-seqRev | CTAGTAATCCTAAATCACTACCAG | This study |
| Smr-F | ATAAGTACTGAAGTTATTGGAAGT | 51 |
| Smr-R | TTCCGAAAATGTTTAACGAAACTA | 51 |
| pCN38-For | GCTCACATGTTCTTTCCTGC | 47 |
| pCN38-Rev | ATTAAGTTGGGTAACGCCAG | 47 |
| qRT 16S rRNA FWD | GTGGAGGGTCATTGGAAACT | 68 |
| qRT 16S rRNA REV | CACTGGTGTTCCTCCATATCTC | 68 |
| qRTqacA-For | GTTGCATCTGCTCTAATAATG | 69 |
| qRTqacA-Rev | GGCTACCAAGTACTGCTA | 69 |
| qRTqacC-For | GCCATAAGTACTGAAGTTATTGG | This study |
| qRTqacC-Rev | TAGTGGTAGGTGTTGCATTG | This study |
| qRTnorA-For | GGTGGTATGAGTGCTGGTATGG | 44 |
| qRTnorA-Rev | GCATACGATGTGAAACTTCTGCC | 44 |
| Nup-For | GGGGACAAGTTTGTACAAAAAAGCAGGCTCCGCTAAGAAGACACCAAGC | This study |
| Nup-Rev | CAATATACACCAAAATACTTATGCTAATGCTCACCTCTTTTTTCTCCATGTC | This study |
| Ndn-For | GACATGGAGAAAAAAGAGGTGAGCATTAGCATAAGTATTTTGGTGTATATTG | This study |
| Ndn-Rev | GGGGACCACTTTGTACAAGAAAGCTGGGTGACCAGATAAATGTTCTTCTGGTAC | This study |
| Ncon-For | GCCAACGGTGAGAAGTCAATG | This study |
| Ncon-Rev | CATTTGCTTGTATGAGTTCTGCGAC | This study |
| N1up-Rev | GTTTCTATATTATATTACAACATTGCTAC | This study |
| N1dn-For | GTAGCAATGTTGTAATATAATATAGAAAC | This study |
| N12dn-Rev | GGGGACCACTTTGTACAAGAAAGCTGGGTCAATATACACCAAAATACTTATGCTA | This study |
| N2up-Rev | CGTAAGAAGTTTCTATATTGTATTACAACATTGC | This study |
| N2dn-For | GCAATGTTGTAATACAATATAGAAACTTCTTACG | This study |
| Nseq-For | GGTCATCTGCAAAGGTTGTTATAC | This study |
| Nseq-Rev | CTCCATGTCATGCTTAAAGCTG | This study |
| pCN51-qacC gBlock | AATAGTCGACCGAAAATTAAAAGGAGTTAAAAATGCCTTATATTTATTTAATAATAGCCATAAGTACTGAAGTTATTGGAAGTGCATTTCTTAAATCTTCAGAAGGCTTTTCAAAATTTATACCATCCTTAGGAACAATAATTTCATTTGGAATTTGTTTCTATTTTTTAAGTAAAACAATGCAACACCTACCACTAAATATAACTTATGCAACTTGGGCGGGACTAGGTTTAGTCTTAACAACCGTAGTCTCAATAATTATTTTCAAAGAACAAATAAATCTAATAACTATAGTATCTATAGTTTTAATCATAGTCGGCGTAGTTTCGTTAAACATTTTCGGAACATCGCATTAAGGATCCAATA | This study |
| qacC(pSK41) gBlock | AAAAGAGCTCATAAATTTTCTCGGCATAAATGCATGTTTACTGTAAAATTGATACTGATACAAATAAAAAATAAAAGGATAGTTGCAAATGAAAAATACTTAGAATAAAATTAAATAAAATACGAAAATTAAAAGGAGTTAAAAATGCCTTATATTTATTTAATAATAGCCATAAGTACTGAAGTTATTGGAAGTGCATTTCTTAAATCTTCAGAAGGCTTTTCAAAATTTATACCATCCTTAGGAACAATAATTTCATTTGGAATTTGTTTCTATTTTTTAAGTAAAACAATGCAACACCTACCACTAAATATAACTTATGCAACTTGGGCGGGACTAGGTTTAGTCTTAACAACCGTAGTCTCAATAATTATTTTCAAAGAACAAATAAATCTAATAACTATAGTATCTATAGTTTTAATCATAGTCGGCGTAGTTTCGTTAAACATTTTCGGAACATCGCATTAATTGCTTTATTCCAATTGCTTTCCCGGGAAAA | This study |
| qacC(pSK89) gBlock | AAAAGAGCTCACTCGTTTCAAAAACCTTTCAAAAACCATCAATCCACAAAAATACCACGCGAATGACACTCAAAATACAAGACTACAATTAAAAAATACTTAGAATAAAATTAAATAAAATACGAAAATTAAAAGGAGTTAAAAATGCCTTATATTTATTTAATAATAGCCATAAGTACTGAAGTTATTGGAAGTGCATTTCTTAAATCTTCAGAAGGCTTTTCAAAATTTATACCATCCTTAGGAACAATAATTTCATTTGGAATTTGTTTCTATTTTTTAAGTAAAACAATGCAACACCTACCACTAAATATAACTTATGCAACTTGGGCGGGACTAGGTTTAGTCTTAACAACCGTAGTCTCAATAATTATTTTCAAAGAACAAATAAATCTAATAACTATAGTATCTATAGTTTTAATCATAGTCGGCGTAGTTTCGTTAAACATTTTCGGAACATCGCATTAATTGCTTTATTCCAATTGCTTTCCCGGGAAAA | This study |
Underlining indicates restriction enzyme sites for cloning and additional nucleotides for increased restriction digestion efficiencies.
Construction of S. aureus RN4220 ΔnorA.
norA, which is located in the chromosome of S. aureus, was deleted by allelic exchange using pKOR1 (63). To construct the pKOR1-ΔnorA vector, splicing by overlap extension (SOE) PCR was used to generate the vector insert. First, the region upstream of norA (1,153 bp) was amplified from S. aureus RN4220 genomic DNA using primers Nup-For and Nup-Rev, and the region downstream of norA (1,024 bp) was amplified using primers Ndn-For and Ndn-Rev. The primers included a 26-nucleotide overlapping region between the upstream and downstream regions of norA. Following amplification of the regions, the upstream fragment was fused to the downstream fragment by SOE PCR using Nup-For and Ndn-Rev. The resulting fragment was cloned into pKOR1 via Gateway cloning (Thermo Fisher Scientific). The resulting plasmid, pKOR1-ΔnorA, was transformed into chemically competent Escherichia coli TOP10 cells, and transformants were selected on LB agar supplemented with 100 μg/ml of ampicillin (Thermo Fisher Scientific). Colonies were selected and the insert was verified by PCR using the primers Nup-For and Ndn-Rev. The resulting pKOR1-ΔnorA plasmid was used to transform S. aureus RN4220, and the subsequent deletion of norA was completed by following the described protocol (63) to create S. aureus RN4220 ΔnorA. The deletion of norA was verified by PCR using primers Ncon-For and Ncon-Rev.
Construction of S. aureus RN4220 N1.
To construct S. aureus RN4220 N1, site-directed mutagenesis by allelic exchange was used to create a point mutation in the −10 region of the norA promoter. First, the region upstream of the N1 mutation was amplified with primers Nup-For and N1up-Rev using S. aureus RN4220 DNA as a template. The sequence of primer N1up-Rev contained a C-to-T transition mutation in the −10 region of norA at nucleotide 24793 of GenBank sequence NZ_AFGU01000065.1. The downstream region of N1 was amplified with primers N1dn-For and N12dn-Rev. Similarly, the sequence of primer N1dn-For contained a C-to-T transition mutation in the −10 region of norA. The two amplified regions contained a 30-bp overlap and were joined by SOE PCR using primers Nup-For and N12dn-Rev. The resulting fragment was cloned into pKOR1 via Gateway cloning. The constructed plasmid, pKOR1-N1, was used to create the point mutation in the promoter region of norA in S. aureus RN4220 following the protocol described above. To verify the mutation, the DNA was PCR amplified with primers Nup-For and Nup-Rev and sequenced with primers Nseq-For and Nseq-Rev.
Construction of S. aureus RN4220 N2.
To construct the S. aureus RN4220 N2 strain, site-directed mutagenesis by allelic exchange was used to create a point mutation in the 5′ UTR of the norA promoter. First, the region upstream of norA was amplified with primers Nup-For and N2up_Rev using S. aureus RN4220 DNA as a template. The sequence of primer N2up-Rev contained a T-to-C transition mutation in the 5′ UTR of norA at nucleotide 24779 of GenBank sequence NZ_AFGU01000065.1. The downstream region of N2 was amplified with primers N2dn-For and N12dn-Rev. Similarly, the sequence of primer N2dn-For contained the transition mutation in the 5′ UTR of norA. The two amplified regions contained a 35-bp overlap and were joined by SOE PCR using primers Nup-For and N12dn-Rev. The resulting fragment was cloned into pKOR1 via Gateway cloning. The constructed plasmid, pKOR1-N2, was used to create the point mutation in the promoter region of norA in S. aureus RN4220 by following the protocol described above. To verify the mutation, the DNA was PCR amplified with primers Nup-For and Nup-Rev and sequenced with primers Nseq-For and Nseq-Rev.
Construction of isogenic S. aureus RN4220 strains carrying qacA and qacC.
pCN38 was selected as the backbone to carry the plasmid-located efflux genes. qacA and its native regulator along with qacR and its native promoter were PCR amplified using primers 38qacA-For and 38qacA-Rev. The resulting product was digested with BamHI and SalI enzymes and ligated into a similarly digested pCN38. pCN38-qacA qacR was transformed into E. coli. Transformants were plated on LB agar supplemented with 100 μg/ml of ampicillin. Single colonies were picked and were grown overnight in LB supplemented with ampicillin. Plasmids were isolated from the overnight cultures with the QIAprep spin miniprep kit, and the insert was verified by amplification with 38qacA-For and 38qacA-Rev. The resulting plasmid was electroporated into S. aureus RN4220 to create S. aureus RN4220 (pCN38-qacA qacR). The sequence of pCN38-qacA qacR was confirmed by sequencing with primers qacAF, qacAseq-Rev, pCN38-For, and pCN38-Rev.
Gblocks (Integrated DNA Technologies) were synthesized to create sequences for the two qacC variants: qacC from pSK41 (GenBank accession number AF051917.1) and qacC from pSK89 (GenBank accession number M37889.1). The synthesized products were digested with SmaI and SacI enzymes and individually ligated into the similarly digested pCN38 vector. The qacC-containing plasmids were individually transformed into E. coli and plasmids were obtained with the QIAprep spin miniprep kit. To verify the insert, recovered plasmids were amplified with pCN38-For and pCN38-Rev. The resulting qacC-containing plasmids were electroporated into S. aureus RN4220 to create strains RN4220 (pCN38-qacC [pSK41]) and RN4220 (pCN38-qacC [pSK89]). The correct sequences of pCN38-qacC [pSK41] and pCN38-qacC [pSK89] were confirmed by sequencing with primers pCN38-For and pCN38-Rev.
To create the strains carrying qacA and qacC, pCN38-qacA qacR was isolated from S. aureus RN4220 (pCN38-qacA qacR) and was digested with SmaI and SacI. The synthesized Gblocks qacC [pSK41] and qacC [pSK89] were similarly digested and independently ligated into the digested pCN38-qacA qacR plasmid. The pCN38-qacA qacR qacC [pSK41] and pCN38-qacA qacR qacC [pSK89] plasmids were processed through E. coli and S. aureus as described above to generate strains RN4220-pCN38 (qacA qacR qacC [pSK41]) and RN4220-pCN38 (qacA qacR qacC [pSK89]).
pCN38-qacA qacR was isolated from S. aureus RN4220 (pCN38-qacA qacR) and was electroporated into electrocompetent S. aureus RN4220 N1 and S. aureus RN4220 ΔnorA to generate S. aureus RN4220 N1 (pCN38-qacA qacR) and S. aureus RN4220 ΔnorA (pCN38-qacA qacR).
Construction of S. aureus RN4220 strains with controllable expression of qacA, qacC, and norA.
pCN51 was used as the vector to carry promoterless qacA, qacC, and norA. The individual efflux genes were cloned downstream of a cadmium-inducible promoter in the multicloning site of pCN51. qacA was PCR amplified from pC02 (GenBank accession number CP012121.2) template DNA using primers 51qacA-For and 51qacA-Rev. norA was PCR amplified from RN4220 template DNA using primers 51norA-For and 51norA-Rev. A Gblock for qacC was synthesized using the pSK41 sequence (GenBank accession number AF051917.1) qacC sequence. All efflux gene products were digested with SalI and BamHI and were ligated into a similarly digested pCN51. The pCN51 constructs were transformed into E. coli and correct inserts were confirmed as follows: in the pCN51-qacA plasmid using the 51qacA-For and 51qacA-Rev primers, in the pCN51-norA plasmids using the 51norA-For and 51norA-Rev primers, and in the pCN51-qacC plasmids using the Smr-F and Smr-R primers. pCN51-qacA and pCN51-qacC were electroporated into S. aureus RN4220 to create S. aureus RN4220 (pCN51-qacA) and S. aureus RN4220 (pCN51-qacC). pCN51-norA was electroporated into S. aureus RN4220 ΔnorA to create RN4220 ΔnorA (pCN51-norA). The sequence of pCN51-qacA was confirmed by sequencing with primers 51-seqFor, 51-seqRev, and qacAseq-Rev. The sequence of pCN51-norA was confirmed by sequencing with primers 51-SeqFor, 51seqRev, 51norA-seqFor, and 51norA-seqRev. The sequence of pCN51-qacC was confirmed by sequencing with primers 51-seqFor and 51-seqRev.
Isogenic strain growth curves.
Cultures of the isogenic strains were grown overnight in MHB with the appropriate antibiotic selection added for plasmid maintenance. The following day, overnight cultures were subcultured to an optical density at 600 nm (OD600) of 0.05 in 2 ml of MHB without antibiotics and strains were grown for 7 h with shaking at 37°C. After inoculation and at hours 1, 3, 5, and 7 of the growth curves, 10 μl was removed from the cultures and was serially diluted and plated to enumerate CFU. A similar method was used to determine the growth kinetics of strains that harbored pCN51 containing qacA, qacC, or norA. Isogenic strains were grown overnight in MHB with added erythromycin for plasmid maintenance. The following day, overnight cultures were subcultured to an OD600 of 0.05 in 2 ml of MHB without antibiotics and containing 0, 0.25, 2.5, or 5 μM cadmium chloride. Strains were grown for 8 h with shaking at 37°C and CFU were determined at time points 0, 2, 4, 6, and 8.
Phoenix antibiotic susceptibility testing.
Strains were inoculated from freezer stocks onto Mueller-Hinton agar (MHA) or MHA plus antibiotics for plasmid maintenance, and individual colonies were chosen for susceptibility testing using the Becton, Dickinson Phoenix automated identification and susceptibility system (Franklin Lakes, NJ), following the manufacturer protocol and reagents. Briefly, pure colonies from overnight culture plates were suspended in the Phoenix ID broth and vortexed to generate a uniform suspension to a density of 0.5 McFarland as determined using the BD PhoenixSpec nephelometer. Twenty-five microliters of the 0.5 McFarland suspension was added to the antibiotic susceptibility testing (AST) broth and mixed gently by inversion to avoid generating bubbles. The ID and AST broths were added by pouring into the respective inoculation port of the PMIC/ID-107 panel (number 448607). Panels were placed into the instrument and run to completion (18 to 24 h). Inocula were plated on TSA overnight to ensure culture purity prior to interpretation of results. MICs to ampicillin-sulbactam, cefazolin, cefoxitin, clindamycin, daptomycin, erythromycin, gentamicin, levofloxacin, linezolid, minocycline, moxifloxacin, nitrofurantoin, oxacillin, penicillin G, rifampin, tetracycline, trimethoprim-sulfamethoxazole, and vancomycin were calculated by the BD Phoenix instrument software, with interpretations determined according to Clinical and Laboratory Standards Institute (CLSI) M100 guidelines based on the bacterial identification (64).
Microbroth antiseptic MIC dilutions.
Antiseptic MICs were determined by following the CLSI broth microdilution protocol, with minor modifications (65). Cultures of S. aureus were grown overnight in MHB with the appropriate antibiotic selection added for plasmid maintenance. Overnight cultures were diluted to an OD600 of 0.063 in MHB without antibiotics (which is equivalent to a McFarland dilution of 0.5). A subsequent 1/20 dilution was performed in MHB without antibiotics, and from that, 10 μl was inoculated into microtiter wells containing 2-fold dilutions of chlorhexidine (0.2 μg/ml to 6.4 μg/ml; Sigma), chlorhexidine digluconate solution (20%; 0.5 μg/ml to 64 μg/ml; Sigma), benzalkonium chloride (0.5 μg/ml to 64 μg/ml; Sigma), alkyltrimethylammonium bromide (cetrimide; 0.5 μg/ml to 32 μg/ml; Sigma), pentamidine isethionate salt (two separate dilution series: 1.156 to 100 μg/ml and 500 μg/ml to 1,600 μg/ml; Sigma), or ethidium bromide (2 μg/ml to 1,024 μg/ml; VWR). The inoculum was ∼5 × 105 CFU/ml. MICs were determined after 16 to 20 h of incubation at 37°C without shaking.
To determine MBCs, 10 μl was collected from wells that did not show any turbidity changes and plated on MHA to check for killing. The MBC was calculated as described previously (37).
CHX macrobroth dilutions.
CHX macrobroth dilutions were performed as previously described (18, 44), with minor modifications; the 1-ml broth macrodilution increased chlorhexidine concentrations by intervals of 0.2 μg/ml (0.2 μg/ml to 1.4 μg/ml) with or without the addition of 2.5 μM cadmium chloride and an inoculum of ∼5 × 104 CFU/ml.
RNA expression analysis.
Bacterial strains were grown overnight in MHB supplemented with antibiotics for plasmid maintenance. The next day, cells were subcultured 1/100 in 10 ml of MHB without antibiotics and grown to OD600 of ∼0.500. One milliliter of the preinduced culture was collected at the initial time point (time zero). The remaining cells were exposed to a subinhibitory concentration of antiseptic CHX (1 μg/ml) or BKC {2.5 μg/ml for RN4220 and RN4220 (pCN38-qacC [pSK89]) or 5 μg/ml for all other strains}, and 1-ml samples were collected at 15, 30, and 60 min after exposure. For collection, the samples were pelleted by centrifugation at 13,000 × g for 2 min, supernatant was removed, and the cells were immediately frozen in liquid nitrogen. Frozen cells were stored at –80°C until RNA extraction.
To isolate RNA, pelleted cells were resuspended in 200 μl of phosphate-buffered saline (PBS). Cells were mixed with 200 μl of TRIzol (Thermo Fisher Scientific) and lysed by bead beating for 4 min on setting 10 with 0.1-mm glass beads in the Bullet Blender 5 (Next Advance). After bead beating, 700 μl of TRIzol was added to the lysed cells and the solution was briefly vortexed. Subsequently, 600 μl of chloroform was added to the lysed cells and the solution was briefly vortexed. Samples were spun at 2,844 × g for 20 min at 4°C. The aqueous phase was removed and mixed with equal parts of isopropanol alcohol, and RNA was processed as directed by the EasyRNA kit (Qiagen). RNA quality was examined on a 2% agarose gel.
cDNA synthesis and quantitative reverse transcription-PCR (qRT-PCR) were performed as described previously (66). The qRT-PCRs included a 16S rRNA reference gene and the efflux gene(s) of interest (norA, qacA, or qacC). Primers are listed in Table 4 and identified by the “qRT” name. The following cycling conditions were used for all qRT-PCRs: 95°C for 10 min (1 cycle) and 40 cycles of 95°C for 10 s, 56°C for 15 s, and 72°C for 20 s. Gene expression relative to the 16S rRNA reference gene was calculated by the 2−ΔCT method; fold change in gene expression over time was calculated by the 2−ΔΔCT method (70).
Antiseptic preexposure test.
RN4220 (pCN38-qacA) was grown overnight in MHB supplemented with chloramphenicol for plasmid maintenance. The strain was subcultured 1/50 in 25 ml of MHB without antibiotics and grown to an OD600 of ∼0.500. At that time, the culture was split into two 10-ml cultures and exposed to 5 μg/ml of BKC or to water as a vehicle control (50 μl). The cultures were grown in the presence of the inducer for 60 min. Following induction, cells were centrifuged at 2,630 × g for 5 min. Supernatant was removed and cells were resuspended in 10 ml of 0.1× concentrated MHB. Cells were then centrifuged at 2,630 × g for 5 min, and supernatant was removed from the pelleted cells. The washed cells were resuspended in 5 ml of 0.1× MHB and standardized to an OD600 of 0.400 in 0.1× MHB. A total of 250 μl of the standardized culture was aliquoted into 1.750 ml of 0.1× MHB supplemented with 0, 5, or 10 μg/ml of CHX. After inoculation, 10 μl was immediately taken from the CHX-treated cells to enumerate starting CFU (input). Subsequent 10-μl samples were removed at 60 and 120 s during CHX exposure (output). Cells were diluted in neutralizing broth and plated on neutralizing TSA. Plates were incubated at 37°C overnight and CFU were enumerated the following day.
A similar method was used for S. aureus C02, with the following modifications; C02 was grown overnight in MHB without antibiotics, and after standardization to an OD600 of 0.400 in 0.1× MHB, the cells were aliquoted into 1.750 ml of 0.1× MHB supplemented with 0, 10, or 15 μg/ml of CHX. After inoculation, 10 μl was immediately taken from the CHX-treated cells to enumerate starting CFU (input). Subsequent 10-μl samples were removed at 90 and 180 s after CHX exposure (output).
Percent survival was calculated using the following equation: (CFU at N seconds/CFU of input) × 100, where N equals indicated chlorhexidine exposure times.
ACKNOWLEDGMENTS
These studies were supported by a U.S. Department of Defense Program project grant (HT9404-12-1-0019) and a Military Infectious Diseases Research Program award (HU0001-15-2-0031).
The views expressed in this article reflect the results of research conducted by the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, The Uniformed Services University, the Henry M. Jackson Foundation, or the U.S. government. Some of the authors are either a military service member (M.P.S.) or federal/contracted employee of the United States government (J.S.). This work was prepared as part of their official duties. Title 17 U.S.C. 105 provides that “copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. 101 defines a U.S. government work as work prepared by a military service member or employee of the U.S. government as part of that person’s official duties.
REFERENCES
- 1.Bhalla A, Pultz NJ, Gries DM, Ray AJ, Eckstein EC, Aron DC, Donskey CJ. 2004. Acquisition of nosocomial pathogens on hands after contact with environmental surfaces near hospitalized patients. Infect Control Hosp Epidemiol 25:164–167. doi: 10.1086/502369. [DOI] [PubMed] [Google Scholar]
- 2.Vestergaard M, Frees D, Ingmer H, Vestergaard M, Frees D, Ingmer H. 2019. Antibiotic resistance and the MRSA problem. Microbiol Spectr 7:GPP3-0057-2018. doi: 10.1128/microbiolspec.GPP3-0057-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kourtis AP, Emerging Infections Program MRSA author group, Hatfield K, Baggs J, Mu Y, See I, Epson E, Nadle J, Kainer MA, Dumyati G, Petit S, Ray SM, Ham D, Capers C, Ewing H, Coffin N, McDonald LC, Jernigan J, Cardo D. 2019. Vital signs: epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections—United States. MMWR Morb Mortal Wkly Rep 68:214–219. doi: 10.15585/mmwr.mm6809e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang SS, AHRQ DECIDE Network and Healthcare-Associated Infections Program, Septimus E, Kleinman K, Moody J, Hickok J, Avery TR, Lankiewicz J, Gombosev A, Terpstra L, Hartford F, Hayden MK, Jernigan JA, Weinstein RA, Fraser VJ, Haffenreffer K, Cui E, Kaganov RE, Lolans K, Perlin JB, Platt R. 2013. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med 368:2255–2265. doi: 10.1056/NEJMoa1207290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Echols K, Graves M, LeBlanc KG, Marzolf S, Yount A. 2015. Role of antiseptics in the prevention of surgical site infections. Dermatol Surg 41:667–676. doi: 10.1097/DSS.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 6.Kampf G. 2016. Acquired resistance to chlorhexidine—is it time to establish an ‘antiseptic stewardship’ initiative? J Hosp Infect 94:213–227. doi: 10.1016/j.jhin.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Sheldon AT. 2005. Antiseptic “resistance”: real or perceived threat? Clin Infect Dis 40:1650–1656. doi: 10.1086/430063. [DOI] [PubMed] [Google Scholar]
- 8.Davies GE, Francis J, Martin AR, Rose FL, Swain G. 1954. 1:6-Di-4′-chlorophenyldiguanidohexane (hibitane); laboratory investigation of a new antibacterial agent of high potency. Br J Pharmacol Chemother 9:192–196. doi: 10.1111/j.1476-5381.1954.tb00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardy K, Sunnucks K, Gil H, Shabir S, Trampari E, Hawkey P, Webber M. 2018. Increased usage of antiseptics is associated with reduced susceptibility in clinical isolates of Staphylococcus aureus. mBio 9:e00894-18. doi: 10.1128/mBio.00894-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicoletti G, Boghossian V, Borland R. 1990. Hygienic hand disinfection: a comparative study with chlorhexidine detergents and soap. J Hosp Infect 15:323–337. doi: 10.1016/0195-6701(90)90089-7. [DOI] [PubMed] [Google Scholar]
- 11.Jennings MC, Buttaro BA, Minbiole KPC, Wuest WM. 2015. Bioorganic investigation of multicationic antimicrobials to combat QAC-resistant Staphylococcus aureus. ACS Infect Dis 1:304–309. doi: 10.1021/acsinfecdis.5b00032. [DOI] [PubMed] [Google Scholar]
- 12.Domagk G. 1935. Eine neue Klasse von Desinfektionsmitteln. Dtsch Med Wochenschr 61:829–832. doi: 10.1055/s-0028-1129654. [DOI] [Google Scholar]
- 13.US Food and Drug Administration. 2019. FDA issues final rule on safety and effectiveness of consumer hand sanitizers. https://www.fda.gov/news-events/press-announcements/fda-issues-final-rule-safety-and-effectiveness-consumer-hand-sanitizers. Accessed 23 September 2019.
- 14.McDonnell G, Russell AD. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 12:147–179. doi: 10.1128/CMR.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buffet-Bataillon S, Tattevin P, Bonnaure-Mallet M, Jolivet-Gougeon A. 2012. Emergence of resistance to antibacterial agents: the role of quaternary ammonium compounds—a critical review. Int J Antimicrob Agents 39:381–389. doi: 10.1016/j.ijantimicag.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Costa SS, Viveiros M, Amaral L, Couto I. 2013. Multidrug efflux pumps in Staphylococcus aureus: an update. Open Microbiol J 7:59–71. doi: 10.2174/1874285801307010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costa SS, Sobkowiak B, Parreira R, Edgeworth JD, Viveiros M, Clark TG, Couto I. 2018. Genetic diversity of norA, coding for a main efflux pump of Staphylococcus aureus. Front Genet 9:710. doi: 10.3389/fgene.2018.00710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaBreck PT, Rice GK, Paskey AC, Elassal EM, Cer RZ, Law NN, Schlett CD, Bennett JW, Millar EV, Ellis MW, Hamilton T, Bishop-Lilly KA, Merrell DS. 2018. Conjugative transfer of a novel staphylococcal plasmid encoding the biocide resistance gene, qacA. Front Microbiol 9:2664. doi: 10.3389/fmicb.2018.02664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Firth N, Ridgway KP, Byrne ME, Fink PD, Johnson L, Paulsen IT, Skurray RA. 1993. Analysis of a transfer region from the staphylococcal conjugative plasmid pSK41. Gene 136:13–25. doi: 10.1016/0378-1119(93)90442-6. [DOI] [PubMed] [Google Scholar]
- 20.Ho C-M, Li C-Y, Ho M-W, Lin C-Y, Liu S-H, Lu J-J. 2012. High rate of qacA- and qacB-positive methicillin-resistant Staphylococcus aureus isolates from chlorhexidine-impregnated catheter-related bloodstream infections. Antimicrob Agents Chemother 56:5693–5697. doi: 10.1128/AAC.00761-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taheri N, Ardebili A, Amouzandeh-Nobaveh A, Ghaznavi-Rad E. 2016. Frequency of antiseptic resistance among Staphylococcus aureus and coagulase-negative staphylococci isolated from a university hospital in central Iran. Oman Med J 31:426–432. doi: 10.5001/omj.2016.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNeil JC, Kok EY, Vallejo JG, Campbell JR, Hulten KG, Mason EO, Kaplan SL. 2016. Clinical and molecular features of decreased chlorhexidine susceptibility among nosocomial Staphylococcus aureus isolates at Texas Children’s Hospital. Antimicrob Agents Chemother 60:1121–1128. doi: 10.1128/AAC.02011-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wassenaar TM, Ussery D, Nielsen LN, Ingmer H. 2015. Review and phylogenetic analysis of qac genes that reduce susceptibility to quaternary ammonium compounds in Staphylococcus species. Eur J Microbiol Immunol (Bp) 5:44–61. doi: 10.1556/EuJMI-D-14-00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J-T, Sheng W-H, Wang J-L, Chen D, Chen M-L, Chen Y-C, Chang S-C. 2008. Longitudinal analysis of chlorhexidine susceptibilities of nosocomial methicillin-resistant Staphylococcus aureus isolates at a teaching hospital in Taiwan. J Antimicrob Chemother 62:514–517. doi: 10.1093/jac/dkn208. [DOI] [PubMed] [Google Scholar]
- 25.Sommer LM, Krauss JL, Hultén KG, Dunn JJ, Kaplan SL, McNeil JC. 2019. The prevalence of antiseptic tolerance genes among staphylococci and enterococci in a pediatric population. Infect Control Hosp Epidemiol 40:333–340. doi: 10.1017/ice.2019.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bischoff M, Bauer J, Preikschat P, Schwaiger K, Mölle G, Hölzel C. 2012. First detection of the antiseptic resistance gene qacA/B in Enterococcus faecalis. Microb Drug Resist 18:7–12. doi: 10.1089/mdr.2011.0092. [DOI] [PubMed] [Google Scholar]
- 27.Liu WJ, Fu L, Huang M, Zhang JP, Wu Y, Zhou YS, Zeng J, Wang GX. 2017. Frequency of antiseptic resistance genes and reduced susceptibility to biocides in carbapenem-resistant Acinetobacter baumannii. J Med Microbiol 66:13–17. doi: 10.1099/jmm.0.000403. [DOI] [PubMed] [Google Scholar]
- 28.Littlejohn TG, Paulsen IT, Gillespie MT, Tennent JM, Midgley M, Jones IG, Purewal AS, Skurray RA. 1992. Substrate specificity and energetics of antiseptic and disinfectant resistance in Staphylococcus aureus. FEMS Microbiol Lett 95:259–265. doi: 10.1111/j.1574-6968.1992.tb05376.x. [DOI] [PubMed] [Google Scholar]
- 29.Paulsen IT, Brown MH, Littlejohn TG, Mitchell BA, Skurray RA. 1996. Multidrug resistance proteins QacA and QacB from Staphylococcus aureus: membrane topology and identification of residues involved in substrate specificity. Proc Natl Acad Sci U S A 93:3630–3635. doi: 10.1073/pnas.93.8.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grkovic S, Brown MH, Roberts NJ, Paulsen IT, Skurray RA. 1998. QacR is a repressor protein that regulates expression of the Staphylococcus aureus multidrug efflux pump QacA. J Biol Chem 273:18665–18673. doi: 10.1074/jbc.273.29.18665. [DOI] [PubMed] [Google Scholar]
- 31.Grkovic S, Brown MH, Schumacher MA, Brennan RG, Skurray RA. 2001. The staphylococcal QacR multidrug regulator binds a correctly spaced operator as a pair of dimers. J Bacteriol 183:7102–7109. doi: 10.1128/JB.183.24.7102-7109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schumacher MA, Miller MC, Grkovic S, Brown MH, Skurray RA, Brennan RG. 2001. Structural mechanisms of QacR induction and multidrug recognition. Science 294:2158–2163. doi: 10.1126/science.1066020. [DOI] [PubMed] [Google Scholar]
- 33.Cervinkova D, Babak V, Marosevic D, Kubikova I, Jaglic Z. 2013. The role of the qacA gene in mediating resistance to quaternary ammonium compounds. Microb Drug Resist 19:160–167. doi: 10.1089/mdr.2012.0154. [DOI] [PubMed] [Google Scholar]
- 34.Littlejohn TG, DiBerardino D, Messerotti LJ, Spiers SJ, Skurray RA. 1991. Structure and evolution of a family of genes encoding antiseptic and disinfectant resistance in Staphylococcus aureus. Gene 101:59–66. doi: 10.1016/0378-1119(91)90224-Y. [DOI] [PubMed] [Google Scholar]
- 35.Paulsen IT, Brown MH, Dunstan SJ, Skurray RA. 1995. Molecular characterization of the staphylococcal multidrug resistance export protein QacC. J Bacteriol 177:2827–2833. doi: 10.1128/jb.177.10.2827-2833.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bay DC, Rommens KL, Turner RJ. 2008. Small multidrug resistance proteins: a multidrug transporter family that continues to grow. Biochim Biophys Acta 1778:1814–1838. doi: 10.1016/j.bbamem.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 37.Leonardo F, Ciusa ML, Knight D, Di Lorenzo V, Tocci N, Cirasola D, Aragones L, Coelho JR, Freitas AT, Marchi E. 2013. Evaluation of reduced susceptibility to quaternary ammonium compounds and bisbiguanides in clinical isolates and laboratory-generated mutants of Staphylococcus aureus. Antimicrob Agents Chemother 57:3488–3497. doi: 10.1128/AAC.00498-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noguchi N, Okada H, Narui K, Sasatsu M. 2004. Comparison of the nucleotide sequence and expression of norA genes and microbial susceptibility in 21 strains of Staphylococcus aureus. Microb Drug Resist 10:197–203. doi: 10.1089/mdr.2004.10.197. [DOI] [PubMed] [Google Scholar]
- 39.Fournier B, Truong-Bolduc QC, Zhang X, Hooper DC. 2001. A mutation in the 5′ untranslated region increases stability of norA mRNA, encoding a multidrug resistance transporter of Staphylococcus aureus. J Bacteriol 183:2367–2371. doi: 10.1128/JB.183.7.2367-2371.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeMarco CE, Cushing LA, Frempong-Manso E, Seo SM, Jaravaza TA, Kaatz GW. 2007. Efflux-related resistance to norfloxacin, dyes, and biocides in bloodstream isolates of Staphylococcus aureus. Antimicrob Agents Chemother 51:3235–3239. doi: 10.1128/AAC.00430-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaatz GW, Thyagarajan RV, Seo SM. 2005. Effect of promoter region mutations and mgrA overexpression on transcription of norA, which encodes a Staphylococcus aureus multidrug efflux transporter. Antimicrob Agents Chemother 49:161–169. doi: 10.1128/AAC.49.1.161-169.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berg T, Firth N, Apisiridej S, Hettiaratchi A, Leelaporn A, Skurray RA. 1998. Complete nucleotide sequence of pSK41: evolution of staphylococcal conjugative multiresistance plasmids. J Bacteriol 180:4350–4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyon BR, Skurray R. 1987. Antimicrobial resistance of Staphylococcus aureus: genetic basis. Microbiol Rev 51:88–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson RC, Schlett CD, Crawford K, Lanier JB, Merrell DS, Ellis MW. 2015. Recurrent methicillin-resistant Staphylococcus aureus cutaneous abscesses and selection of reduced chlorhexidine susceptibility during chlorhexidine use. J Clin Microbiol 53:3677–3682. doi: 10.1128/JCM.01771-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koljalg S, Naaber P, Mikelsaar M. 2002. Antibiotic resistance as an indicator of bacterial chlorhexidine susceptibility. J Hosp Infect 51:106–113. doi: 10.1053/jhin.2002.1204. [DOI] [PubMed] [Google Scholar]
- 46.Galluzzi L, Virtanen P, Karp M. 2003. A real-time analysis of QacR-regulated multidrug resistance in Staphylococcus aureus. Biochem Biophys Res Commun 301:24–30. doi: 10.1016/S0006-291X(02)02992-3. [DOI] [PubMed] [Google Scholar]
- 47.LaBreck PT, Li Z, Gibbons KP, Merrell DS. 2019. Conjugative and replicative biology of the Staphylococcus aureus antimicrobial resistance plasmid, pC02. Plasmid 102:71–82. doi: 10.1016/j.plasmid.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 48.Baines SL, Jensen SO, Firth N, Gonçalves da Silva A, Seemann T, Carter GP, Williamson DA, Howden BP, Stinear TP. 2019. Remodeling of pSK1 family plasmids and enhanced chlorhexidine tolerance in a dominant hospital lineage of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 63:e02356-18. doi: 10.1128/AAC.02356-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCarthy AJ, Lindsay JA. 2012. The distribution of plasmids that carry virulence and resistance genes in Staphylococcus aureus is lineage associated. BMC Microbiol 12:104. doi: 10.1186/1471-2180-12-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jensen SO, Apisiridej S, Kwong SM, Yang YH, Skurray RA, Firth N. 2010. Analysis of the prototypical Staphylococcus aureus multiresistance plasmid pSK1. Plasmid 64:135–142. doi: 10.1016/j.plasmid.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 51.Vali L, Davies SE, Lai LLG, Dave J, Amyes S. 2008. Frequency of biocide resistance genes, antibiotic resistance and the effect of chlorhexidine exposure on clinical methicillin-resistant Staphylococcus aureus isolates. J Antimicrob Chemother 61:524–532. doi: 10.1093/jac/dkm520. [DOI] [PubMed] [Google Scholar]
- 52.Kwong SM, Ramsay JP, Jensen SO, Firth N. 2017. Replication of staphylococcal resistance plasmids. Front Microbiol 8:2279. doi: 10.3389/fmicb.2017.02279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grkovic S, Brown MH, Skurray RA. 2001. Transcriptional regulation of multidrug efflux pumps in bacteria. Semin Cell Dev Biol 12:225–237. doi: 10.1006/scdb.2000.0248. [DOI] [PubMed] [Google Scholar]
- 54.Brown MH, Skurray RA. 2001. Staphylococcal multidrug efflux protein QacA. J Mol Microbiol Biotechnol 3:163–170. [PubMed] [Google Scholar]
- 55.Bhardwaj P, Hans A, Ruikar K, Guan Z, Palmer KL, Bhardwaj P, Hans A, Ruikar K, Guan Z, Palmer KL. 2018. Reduced chlorhexidine and daptomycin susceptibility in vancomycin-resistant Enterococcus faecium after serial chlorhexidine exposure. Antimicrob Agents Chemother 62:e01235-17. doi: 10.1128/AAC.01235-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tattawasart U, Maillard J-Y, Furr J, Russell A. 1999. Development of resistance to chlorhexidine diacetate and cetylpyridinium chloride in Pseudomonas stutzeri and changes in antibiotic susceptibility. J Hosp Infect 42:219–229. doi: 10.1053/jhin.1999.0591. [DOI] [PubMed] [Google Scholar]
- 57.Brooks SE, Walczak MA, Hameed R, Coonan P. 2002. Chlorhexidine resistance in antibiotic-resistant bacteria isolated from the surfaces of dispensers of soap containing chlorhexidine. Infect Control Hosp Epidemiol 23:692–695. doi: 10.1086/501996. [DOI] [PubMed] [Google Scholar]
- 58.Bhardwaj P, Ziegler E, Palmer KL. 2016. Chlorhexidine induces VanA-type vancomycin resistance genes in enterococci. Antimicrob Agents Chemother 60:2209–2221. doi: 10.1128/AAC.02595-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martínez-Suárez JV, Ortiz S, López-Alonso V. 2016. Potential impact of the resistance to quaternary ammonium disinfectants on the persistence of Listeria monocytogenes in food processing environments. Front Microbiol 7:638. doi: 10.3389/fmicb.2016.00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheikh W. 1981. Development and validation of a neutralizer system for in vitro evaluation of some antiseptics. Antimicrob Agents Chemother 19:429–434. doi: 10.1128/AAC.19.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berscheid A, Sass P, Weber-Lassalle K, Cheung AL, Bierbaum G. 2012. Revisiting the genomes of the Staphylococcus aureus strains NCTC 8325 and RN4220. Int J Med Microbiol 302:84–87. doi: 10.1016/j.ijmm.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 62.Kreiswirth BN, Löfdahl S, Betley MJ, O’Reilly M, Schlievert PM, Bergdoll MS, Novick RP. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 63.Bae T, Schneewind O. 2006. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55:58–63. doi: 10.1016/j.plasmid.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 64.CLSI. 2019. M100 Performance standards for antimicrobial susceptibility testing, 29th ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 65.CLSI. 2018. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 11th ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 66.Servetas SL, Doster RS, Kim A, Windham IH, Cha J-H, Gaddy JA, Merrell DS. 2018. ArsRS-dependent regulation of homB contributes to Helicobacter pylori biofilm formation. Front Microbiol 9:1497. doi: 10.3389/fmicb.2018.01497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Charpentier E, Anton AI, Barry P, Alfonso B, Fang Y, Novick RP. 2004. Novel cassette-based shuttle vector system for gram-positive bacteria. Appl Environ Microbiol 70:6076–6085. doi: 10.1128/AEM.70.10.6076-6085.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hardy BL, Dickey SW, Plaut RD, Riggins DP, Stibitz S, Otto M, Merrell DS. 2019. Corynebacterium pseudodiphtheriticum exploits Staphylococcus aureus virulence components in a novel polymicrobial defense strategy. mBio 10:e02491-18. doi: 10.1128/mBio.02491-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McGann P, Milillo M, Kwak YI, Quintero R, Waterman PE, Lesho E. 2013. Rapid and simultaneous detection of the chlorhexidine and mupirocin resistance genes qacA/B and mupA in clinical isolates of methicillin-resistant Staphylococcus aureus. Diagn Microbiol Infect Dis 77:270–272. doi: 10.1016/j.diagmicrobio.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 70.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]