Abstract
Context
Resveratrol is a natural polyphenol compound. It exhibits antitumor, immunostimulatory, and antiviral activities. However, poor water solubility and structural instability limit its administration and storage.
Objective
A resveratrol dry suspension (RDS) was prepared and immunomodulatory effect in immunosuppressive mice induced by cyclophosphamide and anti-inflammatory activities in mice were evaluated.
Materials and methods
The preparation of RDS was optimized by the orthogonal design method. To evaluate the immunomodulatory effects, SPF Kunming mice were divided into seven groups comprising of nine males and nine females for each group. The RDS supplemented group was administrated doses of 3.33, 1.67, and 0.83 g/kg/d. Then visceral index, lymphocyte proliferation, the ratio of CD3+ CD4+/CD3+ CD8+, and the contents of cytokines in serum were tested. To ameliorate effects of acetic acid induced capillary permeability, xylene-based ear oedema, and cotton pellet granuloma, RDS as anti-inflammatory agent was administered at doses of 1, 0.33, and 0.1 g/kg/d as compared to indomethacin (IM) provided as a positive control at 10 mg/kg.
Results
RDS inhibited the degradation of resveratrol and enhanced the CD3+ CD4+/CD3+ CD8+ ratio, spleen index, IL-2 level, and splenic lymphocytes in immunosuppressive mice. RDS (0.1 g/kg/d) significantly inhibited the acetic acid-induced capillary permeability, and at doses of 0.33 and 1 g/kg/d repressed the ear swelling and granuloma formation in immunocompromised mice.
Discussion and Conclusion
RDS is a stable, cheaper, and suitable preparation with potent immunoregulatory and anti-inflammatory activities. Keeping in view these remarkable properties, RDS could be an appropriate preparation for clinic use of resveratrol.
Keywords: Orthogonal design, quality assessment, splenic lymphocytes, granuloma
Introduction
Many studies have shown that natural products have potential effects in the prevention and treatment of various diseases. Resveratrol (trans-3,4′,5-trihydroxystilbene), a phytoalexin produced by the injured plants, mainly exists in grapes, peanuts, and Polygonum cuspidatum Sieb. et Zucc. (Polygonaceae) (Pezzuto 2008). Traditionally it was obtained through extraction from Polygonum cuspidatum rhizome (Peng et al. 2013), nowadays it could be chemically synthesized or biosynthesized (Fan et al. 2010). Resveratrol displayed various pharmacological activities, such as anti-inflammation, cardiovascular protection, antioxidation, and antitumor, which has attracted a great deal of attention (Amri et al. 2012).
Resveratrol plays an active role in immune function. It may be involved in the specific immune response and non-specific immune response though directly regulating macrophages, lymphocytes, and dendritic cells activation (Svajger and Jeras 2012). In immunosuppressive mice, resveratrol could promote recovery of immune function by activating JNK/NF-κB pathway in splenic lymphocytes (Lai et al. 2017). Recently, it was also reported that resveratrol could improve the immune efficacy of pseudorabies virus vaccine (Chen et al. 2019).
Similarly, resveratrol has also shown the positive effect in the anti-inflammatory reactions by inhibiting the secretions of TNF-α and nitric oxide in cortical microglia and N9 microglial cells (Bergman et al. 2013). The potential of resveratrol to improve monosodium iodoacetate-induced cartilage damage was through inhibiting the expression of inflammatory mediators, proposing the possibility that resveratrol could be used as an effective therapeutic agent against osteoarthritis (Wang et al. 2016). Besides, resveratrol showed a significant protective effect on LPS-induced lung injury by inhibition of NLRP3 inflammasome (Jiang et al. 2016). Wang et al. (Wang, Hu, Fu et al. 2017; Wang, Hu, Song et al. 2017) found resveratrol could mitigate lipopolysaccharide-mediated acute inflammation in rats, and the anti-inflammatory mechanism may be due to inhibiting the TLR4/NF-κB p65/MAPKs signalling cascade.
Despite the excellent pharmacological activity, resveratrol was confined by the poor solubility, low bioavailability, easily autoxidation, and photosensitivity (Amri et al. 2012). Therefore, the objectives of the current study were to improve the solubility and stability of resveratrol by preparation of dry suspension, and to evaluate the immunomodulatory and anti-inflammatory activities of this preparation in mice.
Materials and methods
Chemicals
Resveratrol, cyclophosphamide, and levamisole were purchased from Sigma Co. Ltd. (St. Louis, MO, USA). PVP K30, SDS, and mannitol were purchased from Xilong Scientific Co., Ltd. (Guangdong, China). HPMC E5 was purchased from Guangfu Fine Chemical Research Institute (Tianjin, China). Xanthan gum and poloxamer-188 were kindly supplied by BioDuly Co., Ltd. (Nanjing, China). Astragalus polysaccharides were purchased from Centre Biology Co., Ltd. (Beijing, China). Echinacea purpurea (L.) Moench (Asteraceae) powder was purchased from Qilu Animal Health products Co., Ltd. (Jinan, China).
Animals
The SPF Kunming (KM) mice were purchased from Chengdu Dossy Experimental Animals Co., Ltd. [License No. SCXK (Sichuan) 2012-24]. The mice were reared at a temperature of 20-25 °C, a stable relative humidity of 50 ± 10% and lighting for 12 h per day. All the mice were domesticated for a week before experiments. Mice were killed by cervical dislocation after the animals have been lightly anaesthetized. The entire procedures were executed as rapidly and painlessly as possible.
All procedures involving animals and their care in this study were approved by the Ethics Committee of Sichuan Agricultural University according to the Regulation of Experimental Animal Management (State Scientific and Technological Commission of the People’s Republic of China, No. 2, 1988) and the Interim Measures of Sichuan Province Experimental Animal Management (Science and Technology Bureau of Sichuan, China, No. 25, 2013).
Resveratrol dry suspension preparation
Screening of suspending and wetting agents
Excipients are commonly used in preparation. Three kinds of suspending agents were used for the preparation of resveratrol dry suspension (RDS), including povidone (PVP), xanthan gum, and hydroxypropyl methylcellulose (HPMC). These were used alone and in combination as suspending agents. Two different kinds of wetting agents sodium dodecyl sulphate (SDS) and poloxamer-188 were compared. The types of suspending and wetting agents were determined through the settling volume ratio and the re-dispersion of the suspension.
Formulation optimization by orthogonal design method
After consideration of the results of single factor, the orthogonal design was used to determine the dosages of excipients. In the orthogonal test design, the table of L9 (34) was adopted, mannitol and sucrose were used as filler. The evaluation index was sedimentation volume and redispersion after 7 days.
Quality assessment of RDS
The quality of RDS was denoted according to the standards of the Chinese pharmacopoeia for the dry suspension (Chinese Pharmacopoeia Commission 2015). The weight loss and sedimentation volume on drying of suspension should not exceed the 2.0% and 0.90, respectively. High-performance liquid chromatography (HPLC) method was used to determine the content of resveratrol. The factors influencing the stability of the preparation were tested.
The effects of RDS on the immunologic function in immunosuppressive mice
Experimental design
The SPF Kunming mice were equally distributed into seven groups comprising of nine males and nine females for each group. The mice were intraperitoneally injected with cyclophosphamide (CY, 60 mg/kg/d) for three days in six treatment groups. At the same time, the mice in normal group were injected with normal saline. The mice in RDS-treated groups (H, M, and L) were treated with RDS at doses of 3.33, 1.67, and 0.83 g/kg/d for 7 days, respectively. Astragalus polysaccharide (APS) and Echinacea purpurea (EP) have been shown to immunomodulation activity (Jin et al. 2014; Manayi et al. 2015). Therefore, APS and EP were selected as positive controls. The dosages of APS and EP were 50 and 250 mg/kg/d, respectively. In the normal group and the model group, the mice were treated with equal volume of normal saline. The volume of intragastric administration was 0.2 mL/10 g body weight. The appropriate dose of the drug in RDS-treated groups was determined, and then RDS was added into water and shaken well for administration.
Visceral index assay
Within 24 h of the last administration, the mice were sacrificed and the immune organs were weighed, including thymus and spleen. The thymus and spleen index were calculated according to the formula: index (mg/g) = (the weight of thymus or spleen)/the body weight.
T lymphocyte subsets assay
The lymphocytes were separated by lymphocyte separation medium (Solarbio, China). The cells used to determine the rate of CD3+, CD3+ CD4+, and CD3+ CD8+ were stained with anti-CD3, anti-CD4, and anti-CD8 monoclonal antibodies (BD Biosciences, Franklin Lakes, NJ, USA) at temperature 37 °C for 0.5 h in the darkness, and followed by centrifugation. T lymphocyte subsets were analyzed by flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA).
Proliferation of splenic lymphocyte
Within 24 h of the last administration, the mice were sacrificed in a sterile environment. Spleen tissue was disrupted, and spleen cell suspensions were passed through sterile nylon mesh. Red blood cells were lysed by Erythrocyte Lysate (Solarbio, China). Cells were resuspended in RPMI 1640 medium (Solarbio, China) at the concentration of 5 × 106 cells/L. Blastogenic response of splenocytes to the mitogens, concanavalin A (ConA, Solarbio, China), and lipopolysaccharide (LPS, Solarbio, China), were assessed by CCK-8 (Dojindo Laboratories, Japan). Splenocytes suspension was incubated with ConA (10 μg/mL) or LPS (15 μg/mL) in 150 μL RPMI 1640 medium containing 10% foetal bovine serum (FBS, Gibco Company, Carlsbad, CA, USA) at 37 °C with 5% CO2. After incubation for 44 h, 10 μL CCK-8 was added to each well. After incubation for 2 h, the absorbance at 450 nm was measured by a microplate reader (Bio-Rad, Hercules, CA, USA).
Serum cytokines assay
Within 24 h of the last administration, the mice were sacrificed and the blood samples were collected and coagulated at 37 °C, followed by centrifugation for 10 min. The concentrations of IL-2 and IFN-γ in serum were analyzed by ELISA (R&D, Minneapolis, MN, USA) in accordance with the manufacturer’s instructions.
The anti-inflammatory assay in mice
Capillary permeability
Capillary permeability caused by glacial acetic acid was used to assess the anti-inflammatory activity. The male mice were equally divided into five groups (8 mice in each group). RDS at 1, 0.33, and 0.1 g/kg/d, and indomethacin (IM) as positive control at 10 mg/kg were given to the mice in treated groups by oral route, respectively. The mice in the normal group were given equal volume of normal saline.
Within 1 h of the last administration, each mouse was injected with 0.1 mL of 5% Evans blue in normal saline via tail vein. After 10 min, each mouse was injected with 0.1 mL/10 g bw of 0.6% (v/v) glacial acetic acid via intraperitoneal injection. After 20 min, the mice were sacrificed and the viscera were unwrapped and washed with normal saline, which was then transferred into 10 mL tubes and 6 mL of normal saline was added into these tubes followed by centrifugation at 3000 rpm for 10 min. The absorbance of supernatant was measured at 590 nm by ultraviolet spectrophotometer (SPECORD® 200 PLUS, Berlin, Germany).
Ear swelling test
Ear swelling, a recognized inflammatory response, is often used to evaluate the effects of anti-inflammatory drugs. The groups and method of dosing were the same as described above. To induce auricular inflammation, 0.1 mL xylene was gently applied to the inner surface of the left auricle after 1 h of last dosing. After another 1 h, mice were enforced to puncture on both sides of the ears. An 8 mm biopsy punch was used to obtain the specimens of auricular tissue. The weight difference between left (inflamed) and right (non-inflamed) specimens was used to calculate the magnitude of swelling.
Cotton pellet granuloma assay
The cotton pellet granuloma was used for establishing chronic inflammation model. The mice were anaesthetized by pentobarbital sodium at the dosage of 45 mg/kg through intraperitoneally injection. Then, cotton pellets having weight 10 ± 1 mg each and containing 0.1 mL streptomycin (798 μg/mL), were implanted in groin beneath the skin under sterile conditions. The groups and method of dosing were the same as described above. Within 24 h of the last administration, the mice were retrained and the pellets surrounded by granuloma tissues were dissected out. The cotton balls were placed in the oven at 80 °C for 8 h and weighted. The antiproliferative function was calculated by the granuloma weight.
Statistical analysis
The data of T lymphocyte subsets were presented as the mean ± standard error of mean, and other data were presented as the mean ± standard deviation. All the statistical analyses were performed using SPSS 22.0 software (SPSS, Chicago, IL, USA), and all the histograms experiments were plotted by GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA, USA). Statistical significance of the data from the control and experimental groups was compared by one-way analysis of variance (ANOVA) and the Duncan test. p < 0.05 and p < 0.01 were considered statistically significant.
Results
Preparation of RDS
Selection of excipient
The sedimentation volumes of different excipients are shown in Table 1. The mixture of HPMC and xanthan gum showed the best suspending effect. In addition, there were no differences in the redispersion of these excipients. Therefore, the mixture of HPMC and xanthan gum was used for the following orthogonal test. SDS and poloxamer 188, as wetting agents, had no differences in sedimentation volume and redispersibility. However, it was reported that poloxamer-188 had better protection compared to SDS (Rowe 2012). Therefore, poloxamer-188 was selected as the wetting agent for dry suspension.
Table 1.
Selection and evaluation of excipient.
| Species of excipient | Sedimentation volume |
|---|---|
| PVP (0.5%) | 0.67 |
| Xanthan gum (0.5%) | 0.91 |
| HPMC (0.5%) | 0.8 |
| HPMC (0.25%) + PVP (0.25%) | 0.75 |
| Xanthan gum (0.25%) + PVP (0.25%) | 0.88 |
| HPMC (0.25%) + xanthan gum (0.25%) | 0.93 |
Orthogonal test
During the orthogonal test, HPMC, xanthan gum, and poloxamer-188 were designated as A, B, and C, respectively, and these were chosen as influencing factors. Their contents were chosen as factor level. According to the orthogonal experiment table, a numerical experiment was carried out as shown in Table 2. Through visual analysis, the order of influence factors in percentage (%) was as B > C > A. Besides, there is no difference in the dispersion of these formulas. Therefore, dry suspensions were prepared as follows.
Table 2.
The orthogonal experiment table L 9(3̂4).
| Order number | Factor levels |
Result | |||
|---|---|---|---|---|---|
| A (HPMC%) | B (Xanthan gum%) | C (Poloxamer-188%) | D (Blank) | Sedimentation volume | |
| 1 | 0.2 | 0.2 | 0.1 | 0.07 | |
| 2 | 0.2 | 0.4 | 0.2 | 0.2 | |
| 3 | 0.2 | 0.8 | 0.3 | 0.94 | |
| 4 | 0.5 | 0.2 | 0.2 | 0.07 | |
| 5 | 0.5 | 0.4 | 0.3 | 0.27 | |
| 6 | 0.5 | 0.8 | 0.1 | 0.93 | |
| 7 | 1 | 0.2 | 0.3 | 0.07 | |
| 8 | 1 | 0.4 | 0.1 | 0.2 | |
| 9 | 1 | 0.8 | 0.2 | 0.95 | |
| K1 | 0.403 | 0.07 | 0.4 | 0.43 | |
| K2 | 0.423 | 0.223 | 0.407 | 0.4 | |
| K3 | 0.407 | 0.94 | 0.427 | 0.403 | |
| R | 0.02 | 0.87 | 0.027 | 0.03 | |
The formulation consist of resveratrol 3%, HPMC 0.5%, xanthan gum 0.8%, poloxamer-188 0.3%, mannitol 3%, and sugar 92.4%. All the excipients were mixed and ground, and then passed through 100 mesh sieve. Finally, the powder was packaged to give RDS. The prepared drug was placed in aluminium foil bag to keep away from the light. Water (10 mL) was used to dissolve 1 g of RDS during the treatment of animals.
Determination of resveratrol content
Validation for the HPLC method
Chromatographic condition. The C-18 chromatographic column (4.6 × 250 mm, Phenomenex, Torrance, CA, USA) was used as stationary phase and acetonitrile: 0.1% phosphoric acid (V/V) (30:70) as mobile phase. The UV detector wavelength was set to 306 nm. The injection volume was 10 μL.
Precision test. The standard solution of resveratrol (101.6 μg/mL) was continuously measured for 5 times to calculate the precision. The value of relative standard deviation (RSD) that we calculated was 0.34%.
Stability test for the HPLC method. The standard solution and sample solution were measured at 0, 2, 4, 8, 24, 48, and 72 h after the preparation to calculate the stability for the HPLC method. The value of RSD of standard solution was 0.39% and that of sample solution was 0.42%. The result showed that the standard solution and sample solution were stable and the HPLC method was suitable within 3 days after the preparation.
Sample recovery test. RDS (0.2 g) was precisely weighed and mixed with 4.7, 5.9, and 7.0 mg of reference standard resveratrol. The solutions were analyzed according to the method of sample preparation. The test was repeated three times. The average recovery rate was calculated to be 96.31% and the value of RSD was 2.64%. The result of the validation suggested that the HPLC method was suitable for the determination of resveratrol content in RDS.
Standard curve preparation. Resveratrol (12.7 mg) (≥ 99%; Sigma, St. Louis, MO, USA) was precisely weighed and dissolved in 100 mL ethanol (101.6 μg/mL). Then, different volumes of this solution (0.5, 1.0, 2.0, 4.0, and 8.0 mL) were aspirated and diluted to 10 mL. Then the standard solutions were filtered (0.45 μm; Millipore, Billerica, MA, USA) and analyzed by HPLC.
Sample preparation. RDS (0.2 g) was precisely weighed and dissolved in 50 mL ethanol. The solution was treated with ultrasonic for 40 min, then filtered (0.45 μm; Millipore, Billerica, MA) and analyzed by HPLC. The results showed that the concentration and peak area had good linear relationship (y = 633,408x − 358,526, r2 = 0.9993) when the concentration of resveratrol ranged from 10 to 100 μg/mL. The content of resveratrol in the formulation was 2.93%.
The main influence factors on the stability of RDS. It was observed that resveratrol in RDS could remain stable under high temperature, high humidity, and high-light exposure as results shown in Table 3.
Table 3.
The main influence factors on the stability of RDS.
| Temperature 60 °C |
Humidity 25 °C, RH90 ± 5% |
Light intensity 4500 ± 500LX |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 d | 5 d | 10 d | 0 d | 5 d | 10 d | 0 d | 5 d | 10 d | |
| RDS | 2.93% | 2.89% | 2.88% | 2.93% | 2.91% | 2.90% | 2.93% | 2.91% | 2.89% |
The stability study of RDS in water. The sedimentation volume, redispersion, and content were determined to evaluate the stability of the preparations. The results (Table 4) showed that the preparation was stable in water for 7 days.
Table 4.
The stability study after the preparations was added to the water.
| 3 h | 1 d | 2 d | 3 d | 5 d | 7 d | |
|---|---|---|---|---|---|---|
| Sedimentation volume | 1 | 1 | 1 | 0.99 | 0.99 | 0.99 |
| Redispersion | 0 | 0 | 0 | 1 | 2 | 3 |
| Content | 2.93% | 2.93% | 2.92% | 2.91% | 2.91% | 2.91% |
The effects of RDS on the immunologic function in immunosuppressive mice
Determination of visceral index
The results (Table 5) showed that there was no significant difference about the thymus index among all the groups, indicating that CY and treatment with RDS, APS and EP had no effects on thymus weight. The spleen indices in RDS-treated groups and APS-treated group were significantly enhanced when compared to model group (p < 0.01), suggesting that RDS and APS could recover the spleen index. In the EP-treated group, the spleen index had no significant changes compared to the model group. The RDS showed a better antagonistic action against reduction of spleen index induced by CY than EP.
Table 5.
Determination of visceral index.
| Group | Thymus index (mg/g) | Spleen index (mg/g) |
|---|---|---|
| Model (CY 60 mg/kg) | 2.39 ± 0.66 | 3.05 ± 0.70 |
| Normal | 2.42 ± 0.64 | 4.59 ± 2.09 |
| L (CY 60 mg/kg + RDS 833 mg/kg) | 2.66 ± 0.99 | 5.71 ± 1.30** |
| M (CY 60 mg/kg + RDS 1667 mg/kg) | 2.74 ± 0.70 | 6.81 ± 1.58** |
| H (CY 60 mg/kg + RDS 3333 mg/kg) | 3.2 ± 1.73 | 6.39 ± 1.89** |
| Positive I (CY 60 mg/kg + APS 50 mg/kg) | 2.70 ± 0.68 | 5.99 ± 0.99** |
| Positive II (CY 60 mg/kg + EP 150 mg/kg) | 2.55 ± 1.23 | 5.00 ± 0.62 |
Data are represented as means ± SD; n = 12; comparison was made with the model group; one-way ANOVA followed by the Duncan test. The symbols represent statistical significance at **p < 0.01.
T lymphocyte subsets assay
The CD3+ CD4+/CD3+ CD8+ ratio of normal group was remarkably higher than that of model group (p < 0.05), while treatment with RDS, APS, and EP could recover the CD3+ CD4+/CD3+ CD8+ ratio (Table 6). The level of CD4+ in the normal group was remarkably increased as compared the model group, and the level of CD8+ was remarkably decreased in normal, RDS (L and H) and APS groups (p < 0.05). These results suggested CY significantly inhibited the body's immune function, while RDS-treatment showed a trend towards antagonism.
Table 6.
T lymphocyte subsets assay.
| Group | CD3+ (%) | CD4+ (%) | CD8+ (%) | CD4+/CD8+ |
|---|---|---|---|---|
| Model (CY 60 mg/kg) | 55.82 ± 0.12 | 32.39 ± 0.56 | 25.18 ± 0.48 | 1.3 ± 0.05 |
| Normal | 65.55 ± 1.44 | 50.8 ± 1.56* | 14.93 ± 0.74* | 3.5 ± 0.21** |
| L (CY 60 mg/kg + RDS 833 mg/kg) | 52.67 ± 4.68 | 38.8 ± 3.72 | 15.17 ± 0.88* | 2.49 ± 0.15 |
| M (CY 60 mg/kg + RDS 1667 mg/kg) | 56.87 ± 0.44 | 38.53 ± 1.99 | 21.03 ± 1.61 | 2.12 ± 0.30 |
| H (CY 60 mg/kg + RDS 3333 mg/kg) | 46.13 ± 1.69 | 30.11 ± 1.00 | 15.51 ± 0.61* | 1.95 ± 0.02 |
| Positive I (CY 60 mg/kg + APS 50 mg/kg) | 46.42 ± 2.29 | 31.73 ± 1.57 | 15.37 ± 1.17* | 2.16 ± 0.12 |
| Positive II (CY 60 mg/kg + EP 150 mg/kg) | 55.8 ± 2.82 | 41.87 ± 1.38 | 20.63 ± 1.93 | 2.18 ± 0.14 |
| Pooled SD | 12.29 | 9.80 | 6.01 | 0.86 |
Data are represented as means ± SEM; n = 6; comparison was made with the Model group; one-way ANOVA followed by the Duncan test. The symbols represent statistical significance at *p < 0.05, **p < 0.01.
The contents of serum cytokines
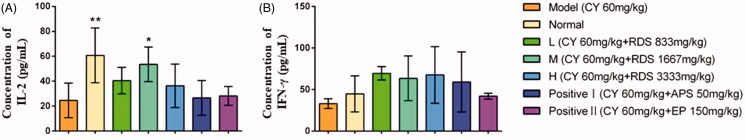
The concentrations of IL-2 and IFN-γ in serum are shown in Figure 1. CY significantly decreased (p < 0.01) the IL-2 content compared to the normal group, while RDS-treatment (H) remarkably increased the IL-2 content in comparison with the model group (p < 0.05). But no significant difference was observed in IFN-γ concentrations among all groups. Meanwhile, the positive control (APS and EP) showed no effects on IL-2 and IFN-γ levels. This study indicated that the regulatory effect of RDS on IL-2 and IFN-γ levels is superior to that of APS and EP in an appropriate dosage.
Figure 1.
Concentrations of serum cytokines. (A) Concentration of IL-2; (B) Concentration of IFN-γ. Symbols are represented as means ± SD; n = 6; comparison was made with the model group; one-way ANOVA followed by the Duncan test. The symbols represent statistical significance at *p < 0.05, **p < 0.01.
Splenic lymphocyte proliferation
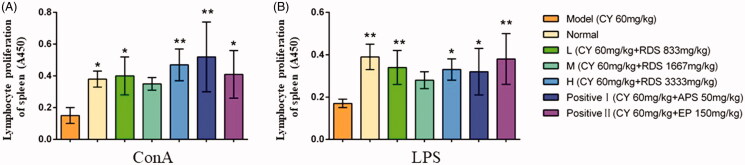
As shown in Figure 2, compared with the model group, RDS-treatment (L and H) could significantly increase the proliferation of splenic lymphocytes stimulated by ConA and LPS (p < 0.01 and p < 0.05, respectively), which indicated that RDS may enhance lymphocyte mitogenesis in vitro. There was no difference in splenic lymphocyte proliferation between RDS and the two positive controls (APS and EP).
Figure 2.
Splenic lymphocyte proliferation. (A) Splenic lymphocyte proliferation stimulated by ConA; (B) Splenic lymphocyte proliferation stimulated by LPS. Symbols are represented as means ± SD; n = 6; comparison was made with the model group; one-way ANOVA followed by the Duncan test. The symbols represent statistical significance at *p < 0.05, **p < 0.01.
The anti-inflammatory activity of RDS
Capillary permeability
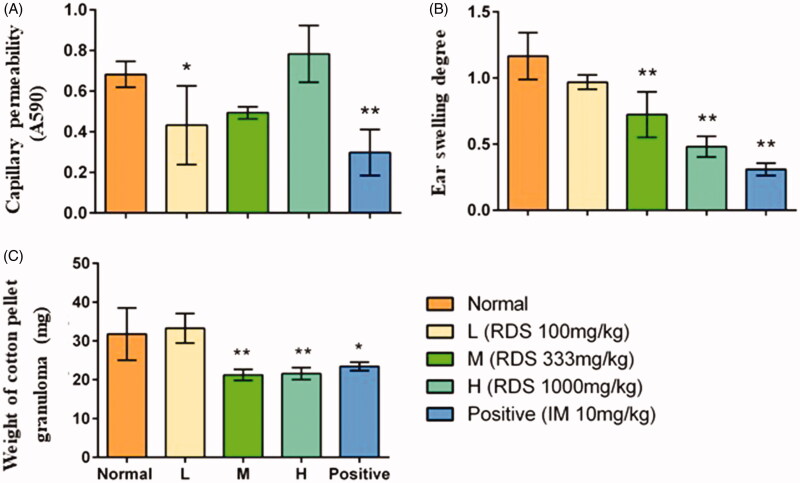
The results of capillary permeability study demonstrated that treatment with RDS and indomethacin significantly inhibited the acetic acid-induced capillary permeability in comparison with the normal group (p < 0.05 and p < 0.01, respectively) (Figure 3(A)). However, no changes were observed in medium and high doses of RDS groups (M and H), which suggested that the effect of RDS on peritoneal permeability may be related to the dose. The inhibition rates in low dose of RDS-treated group were 36.75%, and the indometacin group was 56.36%.
Figure 3.
The anti-inflammatory activity of resveratrol dry suspension. (A) Coeliac capillary permeability induced by acetic acid; (B) ear swelling induced by xylene; (C) the weight of cotton pellet granuloma. Symbols are represented as means ± SD; n = 8; comparison was made with the normal group; one-way ANOVA followed by the Duncan test. The symbols represent statistical significance at *p < 0.05, **p < 0.01.
Ear swelling
Significant inhibitory effects on ear swelling (p < 0.01) in RDS (M and H) groups and indomethacin group were observed in comparison with the normal group (Figure 3(B)). A dose-dependent relationship was found in the RDS-treated groups, and the inhibition rates of ear swelling of RDS (L, M, and H) were 17.09, 38.46, and 58.97%, respectively.
Cotton pellet granuloma
The results (Figure 3(C)) showed that RDS (M and H) and indomethacin had significant inhibitory effects on cotton pellet-induced granuloma formation in comparison with the normal group (p < 0.01). The inhibitory effects of RDS were significantly higher than that of indomethacin group. There was no significant difference observed between the low dose of the RDS group and normal group, which indicated that an obvious therapeutic effect of resveratrol for chronic inflammation required adequate dose. The inhibition rates of medium and high doses of RDS and indomethacin were 34.37%, 31.25%, and 28.12%, respectively.
Discussion
Resveratrol has been proved to possess a wide range of biological activities, including anti-inflammation, cardiovascular protection, antioxidation, as well as regulatory effects on cell proliferation and gene expression (Pervaiz 2003). In this study, for convenient use of resveratrol, a dry suspension was prepared with excellent performance. The immune regulation and anti-inflammatory activity were carried out in mice, which suggested that RDS was an appropriate preparation with potent immunoregulatory and anti-inflammatory activities.
It has showed that the relatively higher lipophilicity of resveratrol leads to poor aqueous solubility, causing low oral bioavailability (Bertacche et al. 2006; Boocock et al. 2007). In addition, its ethanol solution was unstable. UV irradiation caused the photochemical isomerization and electrocyclization, which led to different byproducts (Franciosoa et al. 2014). Therefore, we planned to prepare resveratrol as a dry suspension. Suspension is a disperse system in which the internal phase is dispersed uniformly throughout the external phase, and the internal phase consists of insoluble solid particles with the aid of single or combination of suspending agents (Zhang et al. 2019). The dry suspension has the characteristics of convenient transportation and good stability, and is suitable for patients who have difficulty in swallowing (Liu et al. 2019). In our study, a series of excipients were used to improve the solubility of resveratrol; the final content of resveratrol in the formulation was 2.93%, which was in line with the relevant standards. The preparation could remain stable under high temperature, high humidity, high-light exposure. Moreover, it had a simple preparation process and a convenient form for administration. Therefore, the dry suspension could be the promising preparation for clinic use of resveratrol.
The immunoregulatory function of resveratrol was demonstrated by various indicators in our study. Thymus and spleen are important immune organs in animals. In our previous study, resveratrol at the dosage of 30 or 60 mg/kg could recover or even increase the spleen index in immunosuppressive mice (Lai et al. 2016). In this study, RDS and APS could promote the spleen index, but no significant effect on thymus, which was corroborated by the previous study. These results suggested that RDS could strengthen the immune function through spleen function.
In various diseases, the count of CD3+ CD4+ lymphocytes can directly reflect the immune function. Due to the large fluctuation of CD3+ CD4+ absolute count in different physiological conditions, the ratio of CD3+ CD4+/CD3+ CD8+ with good stability is commonly used to evaluate the immune function. It was reported that dietary intake of resveratrol induced a significant increase in T helper cells (CD4+) in aged-rats, and the percentages of CD3+ CD4+/CD3+ CD8+ subsets was increased after resveratrol administration (Yuan et al. 2012; Wang et al. 2014). In the present study, RDS antagonized the immunosuppressive effects of cyclophosphamide by increasing the CD3+ CD4+/CD3+ CD8+ subsets ratio. In addition, previously it was also reported that intraperitoneal injection of resveratrol could reduce Treg cells (CD4+ CD25+ Foxp3+ cells) and upregulate IFN-γ-expressing CD8+ T cells (Jeong et al. 2012). These findings indicated that resveratrol had a bidirectional regulation on T lymphocyte subsets, which elucidated the underlying mechanism of immunoregulatory and anti-inflammatory activities.
Cytokines are a direct reflection of the physiological activities of body. IL-2 can enhance the vitality of NK cell, cytotoxic T cells, monocytes, macrophages, and improves the proliferation and secretions of the antibodies by the B lymphocytes (Oh et al. 2018). Resveratrol induced cytokine balance of antigen-stimulated spleen cells to Th1 type, which leads to the enhancement of IL-2, IFN-γ and IL-12 productions, while suppresses IL-10 production (Feng et al. 2002). Moreover, resveratrol could relieve symptoms of autoimmune neuroinflammation by increasing the maturation of lymphocyte subsets and cytokines, such as CD4+, IL-17+, IL-10+, and IFN-γ+ (ImLer and Petro 2009). In our study, RDS had no effect on IFN-γ, but significantly up-regulated the expression of IL-2 only in the medium dose of the RDS group. The results indicated that the immunoregulatory effect of resveratrol required appropriate dose. The enhancement of Th1 cytokine caused by resveratrol may be the interpretation of the immune response (Lai et al. 2016).
The activity of lymphocyte is directly related to the immune activity. Resveratrol appeared to protect activated human B lymphocytes from apoptosis by upregulating the antiapoptotic protein Bcl-2 (Zunino and Storms 2009). Daily addition of resveratrol could significantly increase the proliferation index of spleen in rats (Kim et al. 2014). With the stimulation by ConA and LPS, the RDS had obvious stimulation effect on the proliferation of splenic lymphocytes. Our results were in line with previous study that RDS supplementation enhanced the differentiation of lymphocytes by mitosis. In our study, APS and EP were used as positive controls which have been reported as the inducers of different cytokines and lysozyme-C genes expression in a dose-dependent manner (Park and Pezzuto 2015). The immune regulatory effect of the RDS at low and high doses was similar to APS and EP. No significantly proliferative activity was found in the mice treated with medium dose of RDS, which may be due to individual variations among the animals.
Infiltration of inflammatory substances is a common response to inflammation. It has been reported that the anti-inflammatory effect of resveratrol was achieved by ameliorating oedema and inflammatory exudation and decreasing oxidative stress and leukocyte infiltration (Yeh et al. 2014; Dong et al. 2015). Resveratrol could inhibit TNF-α-induced inflammatory exudation in vivo and modify vascular permeability (Fulgenzi et al. 2001). In our study, the low dose of RDS remarkably reduced the inflammatory response of capillary permeability by decreasing the release of inflammatory factors.
The tissue swelling has a reference value in judging the inflammatory response as well. Ear swelling is used in the screening of anti-acute inflammatory activity and in the evaluation of anti-inflammatory activity of steroids (Wang, Hu, Fu et al. 2017; Wang, Hu, Song, et al. 2017). Resveratrol was found to inhibit development of oedema and neutrophil infiltration in the 12-O-tetradecanoylphorbol-13-acetate-induced mouse ear model of topical inflammation (Bralley et al. 2008). Vaticaffinol, the tetramer of resveratrol, significantly ameliorated auricular swelling in mouse model by picryl chloride-induced contact dermatitis (Feng et al. 2013). In the present study, the medium and high doses of RDS could significantly reduce the inflammation of auricular swelling, and the higher dosage of the preparation showed higher anti-inflammatory activity.
Chronic inflammation is a common disorder in many diseases. Resveratrol regulated the chronic inflammation by decreasing the secretion of PGE2, CCL5/RANTES, CXCL8/IL-8, and by increasing the productions of IL-1β, IL-6, IL-10 (Schwager et al. 2015). Cotton pellet granuloma model was used to evaluate the treatment effect of RDS on chronic hyperplastic inflammation in our study. The process of granuloma formation involved proliferation of macrophages, neutrophils and fibroblast (Afsar et al. 2013). The medium and high doses of RDS significantly inhibited the proliferation of granuloma, and the inhibitory effect was demonstrably superior to indomethacin. These results indicated that RDS had excellent inhibitory and recovery effects on different inflammatory responses in mice.
Conclusions
The resveratrol dry suspension has excellent performance in stability. It could increase the spleen index and the level of IL-2, regulate T lymphocyte subsets, and improve the proliferation of splenic lymphocytes in immunosuppressive mice. Resveratrol dry suspension inhibited inflammatory reaction, including capillary permeability, auricular swelling response, and granuloma in chronic inflammation. Resveratrol dry suspension is an appropriate preparation for clinic use of resveratrol.
Funding Statement
This research was supported by the Sichuan Veterinary Medicine and Drug Innovation Group of China Agricultural Research System (CARS-SVDIP) and the Science and Technology Project of Sichuan Province (Grant nos. 2018NZ0043 and 2018NZ0064).
Disclosure statement
The authors declare that they have no conflict of interest.
References
- Afsar SK, Rajesh Kumar K, Venu Gopal J, Raveesha P.. 2013. Assessment of anti-inflammatory activity of Artemisia vulgaris leaves by cotton pellet granuloma method in Wister albino rats. J Pharm Res. 7:463–467. [Google Scholar]
- Amri A, Chaumeil J, Sfar S, Charrueau C.. 2012. Administration of resveratrol: what formulation solutions to bioavailability limitations? J Control Release. 158(2):182–193. [DOI] [PubMed] [Google Scholar]
- Bergman M, Levin GS, Hanna B, Djaldetti M, Salman H.. 2013. Resveratrol affects the cross talk between immune and colon cancer cells. Biomed Pharmacother. 67(1):43–47. [DOI] [PubMed] [Google Scholar]
- Bertacche V, Lorenzi N, Nava D, Pini E, Sinico C.. 2006. Host guest interaction study of resveratrol with natural and modified cyclodextrins. J Incl Phenom Macrocycl Chem. 55(3–4):279–287. [Google Scholar]
- Boocock DJ, Faust G, Patel KR, Schinas AM, Brown V, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ.. 2007. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidem Biomar. 16(6):1246–1252. [DOI] [PubMed] [Google Scholar]
- Bralley EE, Greenspan P, Hargrove JL, Wicker L, Hartle DK.. 2008. Topical anti-inflammatory activity of Polygonum cuspidatum extract in the TPA model of mouse ear inflammation. J Inflamm. 5(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Chen XX, Song X, Muhammad A, Jia RY, Zou YF, Yin LZ, Li LX, He CL, Ye G, et al. . 2019. The immune-adjuvant activity and the mechanism of resveratrol on Pseudorabies virus vaccine in a mouse model. Int Immunopharmacol. 76:105876. [DOI] [PubMed] [Google Scholar]
- Chinese Pharmacopoeia Commission. 2015. Pharmacopoeia of People’s Republic of China. China: China Medical Science Press. [Google Scholar]
- Dong WW, Liu YJ, Lv Z, Mao YF, Wang YW, Zhu XY, Jiang L.. 2015. Lung endothelial barrier protection by resveratrol involves inhibition of HMGB1 release and HMGB1-induced mitochondrial oxidative damage via an Nrf2-dependent mechanism. Free Radical Bio Med. 88(part B):404–416. [DOI] [PubMed] [Google Scholar]
- Fan EG, Zhang K, Zhu MZ, Wang Q.. 2010. Obtaining resveratrol: from chemical synthesis to biotechnological production. Mini Rev Org Chem. 7(4):272–281. [Google Scholar]
- Feng LL, Wu XF, Liu HL, Guo WJ, Luo Q, Tao FF, Ge HM, Shen Y, Tan RX, Xu Q, et al. . 2013. Vaticaffinol, a resveratrol tetramer, exerts more preferable immunosuppressive activity than its precursor in vitro and in vivo through multiple aspects against activated T lymphocytes. Toxicol Appl Pharm. 267(2):167–173. [DOI] [PubMed] [Google Scholar]
- Feng YH, Zhou WL, Wu QL, Li XY, Zhao WM, Zou JP.. 2002. Low dose of resveratrol enhanced immune response of mice. Acta Pharmacol Sin. 23(10):893–897. [PubMed] [Google Scholar]
- Franciosoa A, Mastromarino P, Masci A, d’Erme M, Mosca L.. 2014. Chemistry, stability and bioavailability of resveratrol. Med Chem. 10(3):237–245. [DOI] [PubMed] [Google Scholar]
- Fulgenzi A, Bertelli AAE, Magni E, Ferrero E, Ferrero ME.. 2001. In vivo inhibition of TNF alpha-induced vascular permeability by resveratrol. Transpl P. 33(3):2341–2343. [DOI] [PubMed] [Google Scholar]
- ImLer TJ, Petro TM.. 2009. Decreased severity of experimental autoimmune encephalomyelitis during resveratrol administration is associated with increased IL-17+ IL-10+ T cells, CD4− IFN-γ+ cells, and decreased macrophage IL-6 expression. Int Immunopharmacol. 9(1):134–143. [DOI] [PubMed] [Google Scholar]
- Jeong MH, Yang KM, Choi YJ, Kim SD, Yoo YH, Seo SY, Lee SH, Ryu SR, Lee CM, Suh Hs, et al. . 2012. Resveratrol analog, HS-1793 enhance anti-tumor immunity by reducing the CD4+ CD25+ regulatory T cells in FM3A tumor bearing mice. Int Immunopharmacol. 14(3):328–333. [DOI] [PubMed] [Google Scholar]
- Jiang L, Fei DS, Gong R, Yang W, Yu W, Pan SH, Zhao MR, Zhao MY.. 2016. CORM-2 inhibits TXNIP/NLRP3 inflammasome pathway in LPS-induced acute lung injury. Inflamm Res. 65(11):905–915. [DOI] [PubMed] [Google Scholar]
- Jin ML, Zhao K, Huang QS, Shang P.. 2014. Structural features and biological activities of the polysaccharides from Astragalus membranaceus. Int J Biol Macromol. 64:257–266. [DOI] [PubMed] [Google Scholar]
- Kim KO, Park H, Kim HS.. 2014. Effects of high-protein diet and/or resveratrol supplementation on the immune response of irradiated rats. Prev Nutr Food Sci. 19(3):156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai X, Cao M, Song X, Jia RY, Zou YF, Li LX, Liang XX, He CL, Yin LZ, Yue GZ, et al. . 2017. Resveratrol promotes recovery of immune function of immunosuppressive mice by activating JNK/NF-κB pathway in splenic lymphocytes. Can J Physiol Pharmacol. 95(6):763–767. [DOI] [PubMed] [Google Scholar]
- Lai X, Pei QS, Song X, Zhou X, Yin ZQ, Jia RY, Zou YF, Li LX, Yue GZ, Liang XX, et al. . 2016. The enhancement of immune function and activation of NF-κB by resveratrol-treatment in immunosuppressive mice. Int Immunopharmacol. 33:42–47. [DOI] [PubMed] [Google Scholar]
- Liu KT, Meng ZJ, Li Y, Liu JW, Xu Y, Wang YL, Li XM.. 2019. Preparation and evaluation of mosapride citrate dual-release dry suspension. AAPS PharmSciTech. 20(4):1–12. [DOI] [PubMed] [Google Scholar]
- Manayi A, Vazirian M, Saeidnia S.. 2015. Echinacea purpurea: pharmacology, phytochemistry and analysis methods. Pharmacogn Rev. 9:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JG, Hwang DJ, Heo TH.. 2018. Direct regulation of IL-2 by curcumin. Biochem Biophys Res Co. 495(1):300–305. [DOI] [PubMed] [Google Scholar]
- Park EJ, Pezzuto JM.. 2015. The pharmacology of resveratrol in animals and humans. Biochim Biophys Acta. 1852(6):1071–1113. [DOI] [PubMed] [Google Scholar]
- Peng W, Qin RX, Li XL, Zhou H.. 2013. Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb. et Zucc.: a review. J Ethnopharmacol. 148(3):729–745. [DOI] [PubMed] [Google Scholar]
- Pervaiz SZ. 2003. Resveratrol: from grapevines to mammalian biology. Faseb J. 17(14):1975–1985. [DOI] [PubMed] [Google Scholar]
- Pezzuto JM. 2008. Grapes and human health: a perspective. J Agric Food Chem. 56(16):6777–6784. [DOI] [PubMed] [Google Scholar]
- Rowe RC. 2012. Handbook of pharmaceutical excipients. Greyslake, IL: Pharmaceutical Press. [Google Scholar]
- Schwager J, Richard N, Riegger C, Salem N.. 2015. ω-3 PUFAs and resveratrol differently modulate acute and chronic inflammatory processes. BioMed Res Int. 2015:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svajger U, Jeras M.. 2012. Anti-inflammatory effects of resveratrol and its potential use in therapy of immune-mediated diseases. Int Rev Immunol. 31:202–222. [DOI] [PubMed] [Google Scholar]
- Wang ZM, Chen YC, Wang DP.. 2016. Resveratrol, a natural antioxidant, protects monosodium iodoacetate-induced osteoarthritic pain in rats. Biomed Pharmacother. 83:763–770. [DOI] [PubMed] [Google Scholar]
- Wang GX, Hu ZQ, Fu QT, Song X, Cui QK, Jia RY, Zou YF, He CL, Li LX, Yin ZQ.. 2017. Resveratrol mitigates lipopolysaccharide-mediated acute inflammation in rats by inhibiting the TLR4/NF-κBp65/MAPKs signaling cascade. Sci Rep. 7(1):45006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GX, Hu ZQ, Song X, Cui QK, Fu QT, Jia RY, Zou YF, Li LX, Yin ZQ.. 2017. Analgesic and anti-inflammatory activities of resveratrol through classic models in mice and rats. Evid-Based Complement Alternat. 2017:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Sun J, Li LN, Zheng J, Shi YH, Le GW.. 2014. Regulatory effects of resveratrol on glucose metabolism and T-lymphocyte subsets in the development of high-fat diet-induced obesity in C57BL/6 mice. Food Funct. 5(7):1452–1463. [DOI] [PubMed] [Google Scholar]
- Yeh DYW, Fu YH, Yang YC, Wang JJ.. 2014. Resveratrol alleviates lung ischemia and reperfusion–induced pulmonary capillary injury through modulating pulmonary mitochondrial metabolism. Transpl P. 46(4):1131–1134. [DOI] [PubMed] [Google Scholar]
- Yuan JS, Lu LL, Zhang ZL, Zhang SC.. 2012. Dietary intake of resveratrol enhances the adaptive immunity of aged rats. Rejuv Res. 15(5):507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LY, Xu CC, Mao JJ, Wang WW, Han H, Pu YQ, Zhang T.. 2019. Formulation and characterization of novel dry suspension and dry emulsion of 20(S)-protopanaxadiol. AAPS Pharmscitech. 20(7):275. [DOI] [PubMed] [Google Scholar]
- Zunino SJ, Storms DH.. 2009. Resveratrol alters proliferative responses and apoptosis in human activated B lymphocytes in vitro. J Nutr. 139(8):1603–1608. [DOI] [PubMed] [Google Scholar]