ABSTRACT
Background: In the USA cancer is the second leading cause of mortality, as such, primary prevention of cancer is a major public health concern. Vitamin D supplementation has been studied as a primary prevention method for multiple diseases including cardiovascular disease, osteoporosis, diabetes mellitus and cancer. The role of Vitamin D as primary prevention of cancer is still controversial. With fast emergence of large randomized controlled trials (RCTs) in that regards, we aimed to evaluate the efficacy of Vitamin D supplementation as primary prophylaxis for cancer.
Methods: A comprehensive electronic database search was conducted for all RCTs where comparison of Vitamin D supplementation versus placebo for the prevention of any type of disease with at least 3 years of Vitamin D supplementation was used and where cancer incidence or mortality was reported. The primary outcome was cancer-related mortality and cancer incidence. We calculated risk ratios (RRs) and 95% confidence intervals (CIs) using a random-effects model at the longest follow-up.
Results: We included 10 RCTs with 79,055 total patients, mean age of 68.07 years, a female percentage of 78.02% and a minimum follow-up of 4 years and more. Vitamin D was associated with significant reduction of cancer-related mortality compared with placebo (RR 0.87; 95% CI: 0.79–0.96; P = 0.05: I2 = 0%). Compared with placebo, Vitamin D was not associated with significant reduction of cancer incidence (RR: 0.96; 95% CI: 0.86–1.07; P = 0.46; I2 = 31%).
Conclusion: With inclusion of studies, which did not primarily examine vitamin D for the purpose of preventing cancer or reducing cancer mortality our meta-analysis highlights that the use of vitamin D supplementation for primary prevention of cancer is encouraged as it does possibly decrease cancer-related mortality once cancer is diagnosed; however, it has no role or effect on cancer incidence.
KEYWORDS: Vitamin D, cancer, primary prevention, mortality, incidence
1. Introduction
Epidemiological studies showed that vitamin D deficiency is associated with increased mortality; however, there was not enough evidence that vitamin D status is inversely associated with cancer mortality [1]. The association between cancer risk and vitamin D has been studied in many epidemiologic studies, while data from interventional studies remain insufficient [2]. Almost all studies have proven that vitamin D has a strong and beneficial effect antagonizing and blocking multiple mitogenic processes related to tumorigenesis [2]. The association between solar ultraviolet-B exposure and cancer was proven, and it was stronger for mortality than for incidence for many cancers in the USA and China [3,4].
Vitamin D is highly important for bone health and mineral metabolism, and it is quite known that vitamin D deficiency can lead to rickets, osteomalacia and many other diseases [5]. In the recent past, however, vitamin D has been studied for the prevention of many highly prevalent cancer types. One of the most important studies was a randomized controlled trial (RCT) in 2003 that provided good evidence of the antineoplastic effect that vitamin D had in the colon, in addition to the role of vitamin D in reducing the recurrence of colorectal adenoma [6]. In a recent meta-analysis of observational studies, low 25-hydroxy vitamin D level was directly related to breast cancer, while total vitamin D and supplemental vitamin D intake had an inverse relationship with breast cancer [7].
Although The USA Preventive Services Task Force stated in 2014 that data were insufficient to confirm the effectiveness of vitamin D supplementation for cardiovascular disease or cancer prevention [8], yet, the role of vitamin D supplementation in primary prevention for cancer is promising [9]. With rapidly surfacing large randomized controlled trials (RCTs) studying this subject [10–13], we aimed to evaluate the efficacy and safety of vitamin D supplementation as a means of primary prevention of cancer.
2. Methods
2.1. Data sources
The study was performed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) Statement 2015 [14]. A comprehensive search of literature using PubMed, Embase, and the Cochrane Collaboration Central Register of Controlled Trials from inception to December 2018 was performed by TH, IG and YZ. Any disagreements were resolved via consensus. The search terms and their substitutes used were as follows: vitamin D, primary prevention, mortality, cancer incidence, bleeding and cancer.
PRISMA checklist was completed (Table 1).
Table 1.
PRISMA 2009 checklist.
| Section/topic | # | Checklist item | Reported on page # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 2 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 3 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 3 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g. Web address), and, if available, provide registration information including registration number. | NA |
| Eligibility criteria | 6 | Specify study characteristics (e.g. PICOS, length of follow-up) and report characteristics (e.g. years considered, language, publication status) used as criteria for eligibility, giving rationale. | 4 |
| Information sources | 7 | Describe all information sources (e.g. databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 4 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 4 |
| Study selection | 9 | State the process for selecting studies (i.e. screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 4 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g. piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 4 |
| Data items | 11 | List and define all variables for which data were sought (e.g. PICOS, funding sources) and any assumptions and simplifications made. | 4 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 5 |
| Summary measures | 13 | State the principal summary measures (e.g. risk ratio, difference in means). | 5 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g. I2) for each meta-analysis. | 5 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g. publication bias, selective reporting within studies). | 5 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g. sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | 5 |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | Figure 1 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g. study size, PICOS, follow-up period) and provide the citations. | Tables 2 & 3 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | 5,6 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | 5,6 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | 5,6 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | 6 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g. sensitivity or subgroup analyses, meta-regression [see Item 16]). | 6 |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g. healthcare providers, users, and policy makers). | 7 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g. risk of bias), and at review-level (e.g. incomplete retrieval of identified research, reporting bias). | 7,8 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 8 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g. supply of data); role of funders for the systematic review. | NA |
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097. For more information, visit www.prisma-statement.org.
2.2. Selection criteria and data extraction
The study inclusion criteria were as follows: (1) All studies are RCTs; (2) Vitamin D is used for primary prevention; (3) Vitamin D is compared to placebo; (4) Cancer mortality or cancer incidence is reported; (5) The vitamin D supplementation is for at least a period of 3 years for all patients. From each eligible study, two authors, TH and IG, extracted the data and a third author, HD, resolved any discrepancies.
2.3. Outcomes
Our primary outcomes were cancer-related mortality and cancer incidence.
2.4. Quality assessment
The quality of the included studies was assessed independently by two authors, TH and VS, based on the Jadad scoring system (Table 2).
Table 2.
Jadad scoring of included studies.
| Studies | Jadad score |
|---|---|
| Gallagher 2001 | 5 |
| Trivedi 2003 | 4 |
| Lappe 2007 | 5 |
| Lacroix 2009 | 5 |
| Sanders 2010 | 4 |
| Avenell 2012 | 4 |
| Baron 2015 | 4 |
| Jorde 2016 | 4 |
| Lappe 2017 | 5 |
| Manson 2018 | 5 |
2.5. Statistical analysis
We calculated summary risk ratios (RRs) and 95% confidence intervals (CIs) using the Mantel–Haenszel method for dichotomous data. We used a random-effects model to account for the between-study heterogeneity. Heterogeneity was measured by the Cochran’s Q statistic and I2 statistic test. Publication bias was assessed by visual inspection of the funnel plot. Furthermore, we explained any heterogeneity (≥20%) by performing sensitivity and meta-regression analyses. Sensitivity analyses were performed by removing trials sequentially and by removing small trials with a patient population less than 1000 patients, or based on follow-up period (< or > 5 years). We performed meta-regression analysis based on age, body mass index (BMI), therapy duration, follow-up duration, initial vitamin D level, and vitamin D dose. Analysis was performed using RevMan v5.3 Windows and Comprehensive Meta-Analysis software v3.
3. Results
3.1. Study selection and trial characteristics
Figure 1 illustrates the study selection process. We included 10 RCTs [10,11,12,13;15,16,17,18,19,20] with 79,055 total patients, mean age of 68.07 years, a female percentage of 78.02% and a minimum follow-up of 3 years. Tables 3 and 4 illustrate the characteristics of the included trials and patient demographics, respectively.
Figure 1.
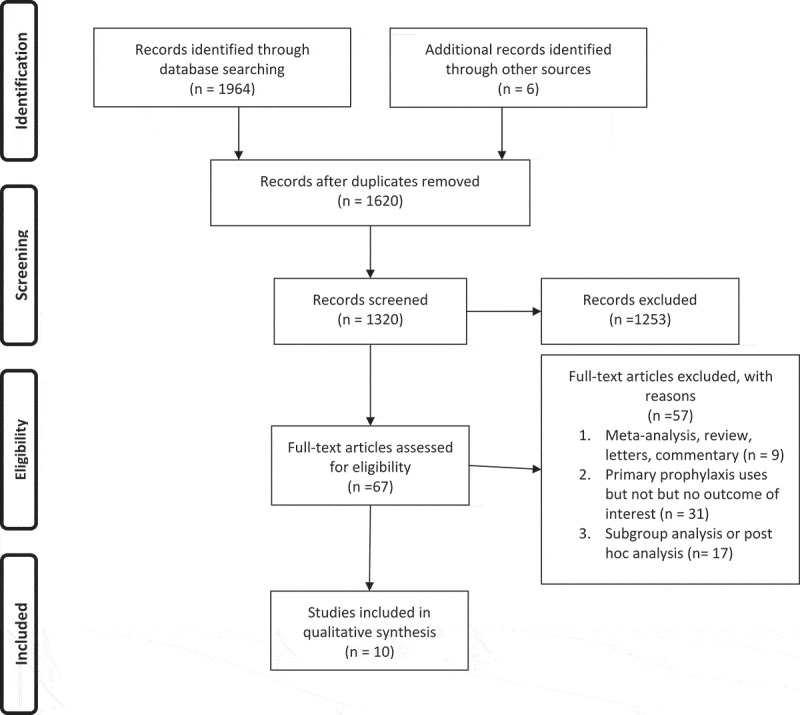
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Table 3.
Details of the randomized clinical trials.
| Studies | Country/Sites | Total number of patients/Subgroups | 25- hydroxy vitD level (standard deviation in nmol/liter) | Study design | Follow-up | Vit D form and dose | Duration of therapy | Primary outcomes | Secondary outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Gallagher 2001 | USA | Vit-D: 203 Placebo: 213 Total: 416 |
Initial: Vit-D: 79 (25.5) Placebo: 78.9 (25.9) |
Randomized, double blinded, placebo controlled trial | 3 years | Calcitriol (0.25ug twice daily), | 3 years | Bone mineral density of: -Femoral neck - Spine. |
Bone mineral density of: -Trochanter -Total hip -Total body -Radius |
| Trivedi 2003 | UK | Vit-D: 1,345 Placebo: 1,341 Total: 2,686 |
Post treatment levels: Vit D: 72.0 (22.5) Placebo: 45.37 (17.6) |
Randomized, double blinded, Placebo controlled trial | 5 years | 100,000 IU cholecalciferol every 4 months | 5 years | -Fracture incidence –Total mortality by cause |
NA |
| Lappe 2007 | USA | Vit-D: 446 Placebo: 733 Total: 1,179 |
Initial: Vit-D: 71.8 (20) Placebo: 71.9 (20.6) 12 month change: Vit D: 96.0 (21.4) Placebo: 71 (20.1) |
Randomized, double blinded, placebo-controlled trial. | 4 years | 1100 IU Cholecaliferol every 6 months | 7 years | Reduction in total mortality. | Reduction in total mortality. |
| Lacroix 2009 | USA | Vit-D: 18,176 Placebo: 18,106 Total: 36,282 |
- | Randomized, double blinded, placebo-controlled trial | 7 years | 400 IU Cholecaliferol daily | 4 years | Hip fracture prevention | -Other fracture prevention -Colorectal cancer prevention |
| Sanders 2010 | Australia | Vit-D: 1,131 Placebo: 1,125 Total: 2,256 |
Initial: Vit-D Median (IQR): 53 (40–65) Placebo Medium (IQR): 45 (40–57) |
Randomized, double blinded, placebo controlled trial | 5 years | 500,000 IU Cholecalciferol per year | 3–5 years | Incidence of falls and fractures | Incidence of falls |
| Avenell 2012 | UK | Vit-D: 2,649 Placebo: 2,643 Total: 5,292 |
Initial: accumulative 38 nmol/liter Post treatment levels in Vit D group: 62 nmol/liter |
2X2 factorial, randomized controlled trial | 3 years | 800 IU Cholecalciferol per day | 3 years | All cause mortality, vascular disease mortality, cancer mortality and cancer incidence. | Mortality from -Cardiovascular -Cerebrovascular |
| Baron 2015 | USA | Vit-D: 1,130 Placebo: 1,129 Total: 2,259 |
Initial: Vit-D: 59 (22.2) Placebo: 60.2 (21.9) |
Randomized, double-blinded, placebo controlled trial | 5 years | 1,000 IU Cholecalciferol per day | 3 or 5 years | Recurrent colorectal adenomas | NA |
| Jorde 2016 | Norway | Vit-D: 256 Placebo: 255 Total: 511 |
Initial: Vit-D: 59.9 (21.9) Placebo: 61.1 (21.2) 5-year visit: Vit D: 122.3 (25.3) Placebo: 66.7 (18.6) |
Randomized, double blinded, placebo controlled trial | 5 years | 20,000 IU Cholecalciferol per week | 5 years | Progression to Diabetes Mellitus Type II | Change in -Glucose levels, -Insulin resistance, -Serum lipids, -Blood pressure. |
| Lappe 2017 | USA | Vit-D: 1,156 Placebo: 1,147 Total: 2,303 |
Initial: Vit-D Median (IQR): 82 (80–84) Placebo Medium (IQR): 82 (80–85) Post treatment: Vit-D Median (IQR): 108 (107–111) Placebo Medium (IQR): 79 (77–80) |
Randomized double blinded, placebo-controlled trial | 4 years | 2,000 IU Cholecalciferol per week | 4 years | Incidence of all-type cancer | Hypertension, cardiovascular disease, osteoarthritis, colonic adenomas and diabetes, upper respiratory tract infections, and falls. |
| Manson 2018 | USA | Vit-D: 12,927 Placebo: 12,944 Total: 25,871 |
Initial: Accumulated: 77 (24.1) 1-year visit: Vit D: 104 (25.3) Placebo: 75.3 (23.8) |
2X2 factorial, randomized placebo controlled trial | 5.3 years | 2,000 IU Cholecalciferol per day | 5.3 years | Invasive cancer of any type and major cardiovascular events | Included site-specific cancers, death from cancer, and additional cardiovascular events |
Abbreviations: Vit D: Vitamin D; IQR: interquartile range; IU: international unit; NA: not applicable.
Table 4.
Patient demographics.
| Studies | Age (years) | Sex (female pts) | Race (no. of pts) | Hormonal use (no. of pts) | BMI | Smoking Hx (no. of pts) | HTN (no. of pts) | DM (no. of pts) |
Cardiac disease (no. of pts) | Cancer Hx (no of pts) | Alcohol use (no. of pts) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gallagher 2001 | Vit-D: 71.5(3.5) Placebo: 71.5(4) |
Vit-D: 203 Placebo: 213 |
Total: White 480 Black 6 Asian 2 |
Vit-D: 102 Placebo: 101 |
Vit-D: 27.1(4.1) Placebo: 27.2(3.9) |
- | - | - | - | - | - |
| Trivedi 2003 | Vit-D: 73.7 (4.5) Placebo: 73.6 (4.6) |
Vit-D: 326 Placebo: 323 |
- | Vit-D: 21 Placebo: 21 |
Vit-D: 24.4 (3.8) Placebo: 24.3 (3.8) |
Vit-D: 20 Placebo: 24 |
- | - | Vit-D: 65 Placebo: 55 |
Vit-D: 15 Placebo: 10 |
Vit-D: 268 Placebo: 260 |
| Lappe 2007 | Total: 66.7(7.3) | Vit-D: 446 Placebo: 733 |
Total: White 100% | Total: 543 | Total: 29 (5.7) | - | - | - | - | Vit-D: 0 Placebo: 0 |
- |
| Lacroix 2009 | Vit-D: 62.4 (7.0) Placebo: 62.4 (6.9) |
Vit-D: 18,176 Placebo: 18,106 |
Vit-D: White 15,047 Non-white 3,129 Placebo: White 15,106 Non-white 3,000 |
Vit-D: Past user 3,004 Current user 9,358 Placebo: Past user 2,932 Current user 9,484 |
Vit-D: 29.1 (5.9) Placebo: 29.0 (5.9) |
Vit-D: Past smoker 7,133 Current 1,356 Placebo: Past smoker 7,255 Current 1,405 |
Vit-D: 11,232 Placebo: 11,181 |
Vit-D: 885 Placebo: 875 |
Vit-D: 1,173 Placebo: 1,221 |
Vit-D: 0 Placebo: 0 |
Vit-D: Past drinker 3,192 Current 12,985 Placebo: Past drinker 3,209 Current 12,884 |
| Sanders 2010 | Vit-D: 76 Placebo: 76.1 |
Vit-D: 1,131 Placebo: 1,125 |
- | - | - | - | - | - | - | - | - |
| Avenell 2012 | Vit-D: 77(6) Placebo: 77(6) |
Vit-D: 2,240 Placebo: 2,241 |
Vit-D: White 99% Placebo: White 99% |
- | - | Vit-D: 298 Placebo: 320 |
- | Vit-D: 208 Placebo: 212 |
- | - | - |
| Baron 2015 | Vit-D: 58(6.8) Placebo: 57.8(6.6) |
Vit-D: 418 Placebo: 418 |
Vit-D: White- 951 Black-96 Asian 25 Other- 7 Placebo: White- 949 Black-88 Asian 28 Other- 16 |
- | Vit-D: 28.9(5.5) Placebo: 29.1(5.3) |
Vit-D: Former: 421 Current: 119 Placebo: Former: 429 Current Smoker: 96 |
- | - | - | Vit-D: 0 Placebo: 0 |
- |
| Jorde 2016 | Vit-D: 62.3 (8.1) Placebo: 61.9 (9.2) |
Vit-D: 95 Placebo: 102 |
- | - | Vit-D: 30.1(4.1) Placebo: 29.8(4.4) |
Vit-D: 59 Placebo: 47 |
Vit-D: 121 Placebo: 119 |
100% pre-diabetic | Vit-D: 0 Placebo: 0 |
Vit-D: 0 Placebo: 0 |
- |
| Lappe 2017 | Vit-D: 65.2 (6.9) Placebo: 65.2 (7.1) |
Vit-D: 1,156 Placebo: 1,147 |
Vit-D: White 99.4% Placebo: White 99.6% |
Vit-D: 186 Placebo: 168 |
Vit-D: 29.9(6.6) Placebo: 30.2(6.5) |
Vit-D: 75 Placebo: 66 |
- | - | - | Vit-D: 0 Placebo: 0 |
- |
| Manson 2018 | Vit-D: 67.1(7) Placebo: 67.1(7.1) |
Vit-D: 6547 Placebo: 6538 |
Vit-D: White 9,013 Black 2,553 Other 1,081 Placebo: White 9,033 Black 2,553 Other 1,071 |
- | Vit-D: 28.1 (5.7) Placebo: 28.1 (5.8) |
Vit-D: 921 Placebo: 915 |
Vit-D: 6352 Placebo: 6439 |
Vit-D: 1,812 Placebo: 1,737 |
Vit-D: 0 Placebo: 0 |
Vit-D: 0 Placebo:0 |
- |
Abbreviations: Vit-D: Vitamin D; pts: patients; no: number; BMI: body mass index; Hx: history; HTN: hypertension; DM: diabetes mellitus;
In the 10 included studies, 5 studies explored the role of vitamin D to decrease fracture risk and increase bone health, 3 assessed vitamin D for primary prevention of cancer, 1 study assessed vitamin D’s role in colorectal adenoma and 1 study assessed aspirin for use in prevention of diabetes mellitus progression. All studies were randomized controlled trials. Almost all studies were assessed to be of moderate to high quality (Table 2). Nine of the 10 studies used cholecalciferol as the mean for vitamin D replacement, whereas 1 study used calcitriol; the doses and frequency of supplementation varied from 400 international units (IU) daily to 2000 IU, with reported regimens either daily, weekly, monthly or yearly. Follow-up duration ranged from 3 years to 7 years, while duration of therapy varied from 3 to 6 years. All studies compared vitamin D to placebo.
3.2. Primary outcome
Vitamin D was associated with significant reduction of cancer-related mortality compared with placebo (RR 0.87; 95% CI: 0.79–0.96; P = 0.05: I2 = 0%). Compared with placebo, vitamin D was not associated with significant reduction of cancer incidence (RR: 0.96; 95% CI: 0.86–1.07; P = 0.46; I2 = 31%) (Figure 2). Examination of the funnel plot did not suggest any publication bias (Figure 3). Sensitivity analysis by removing each trial sequentially demonstrated consistent results.
Figure 2.
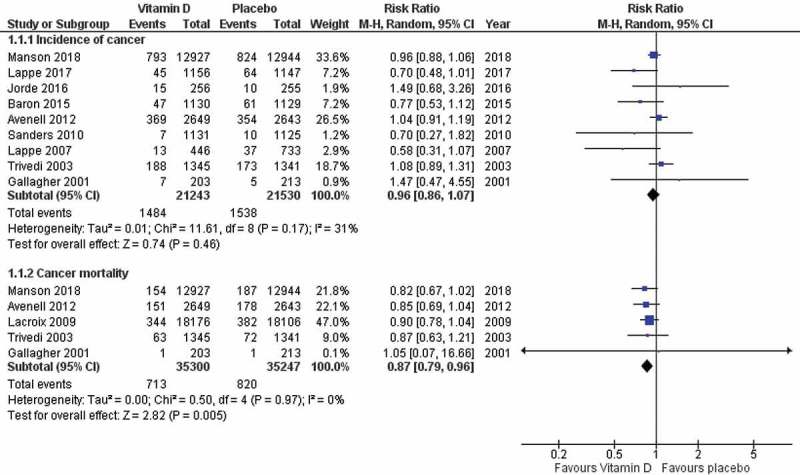
Forest plot of primary outcome (cancer-related mortality and cancer incidence).
Figure 3.
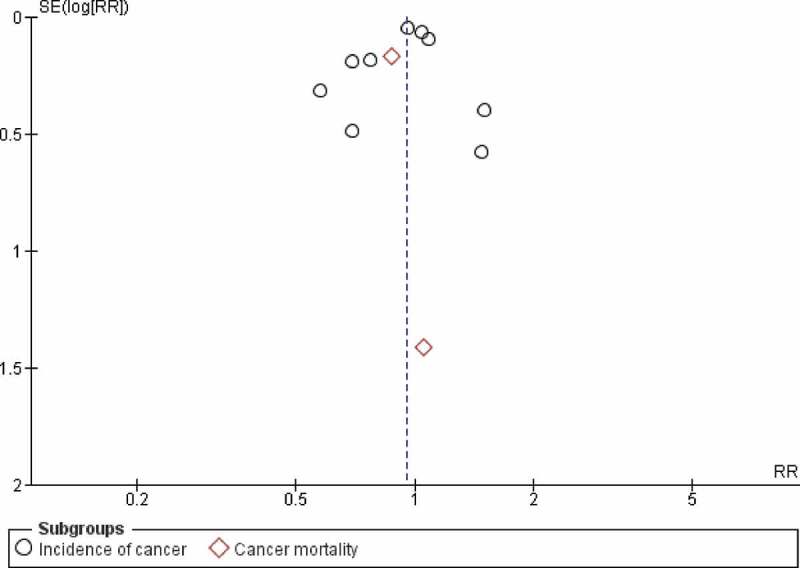
Funnel plot for primary outcome (cancer-related mortality).
A subgroup analysis including the three RCTs that included cancer as a primary outcome only was also conducted where vitamin D was associated with significant reduction of cancer-related mortality when compared to placebo (RR 0.84; 95% CI: 0.72–0.97; P = 0.02: I2 = 0%). However, when compared with placebo, vitamin D in this subgroup of RCTs was not associated with significant reduction of cancer incidence (RR: 0.96; 95% CI: 0.84–1.09; P = 0.54; I2 = 51%) (Figure 4).
Figure 4.
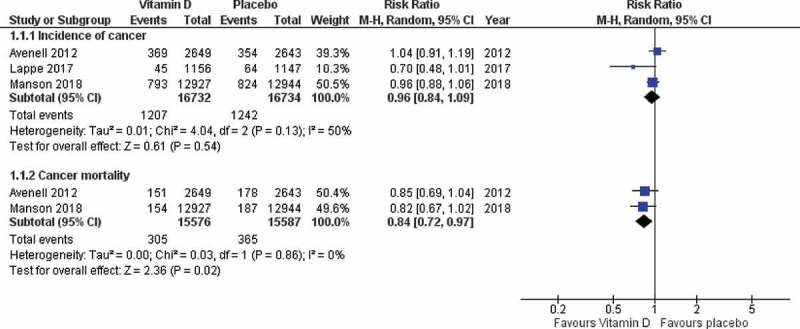
Forest plot for subgroup analysis (cancer-related mortality and cancer incidence).
For cancer incidence, meta-regression analysis based on age (R2 = 46%; b = 0.01; SE < 0.01; P = 0.09), BMI (R2 = 28%; b = −0.06; SE= 0.04;P = 0.12), therapy duration (R2 = 0%; b = 0.01; SE = 0.06; P = 0.81), follow-up duration (R2 = 0%; b <-0.01; SE = 0.07; P = 0.91), initial vitamin D level (R2 = 0%; b < -0.01; SE < 0.01; P = 0.47), and vitamin D dose (R2 = 0%; b < -0.01; SE < 0.01; P = 0.51) did not significantly explain the heterogeneity.
4. Discussion
In this meta-analysis of 10 RCTs, vitamin D supplementation was compared to placebo. With the use of vitamin D supplementation for at least 3 years, it was found to have benefit in reducing cancer-related mortality, however, it had no effect on cancer incidence. And when conducting a subgroup analysis including the three RCTs where cancer was reported as a primary outcome, the results were also consistent with the initial analysis results.
Several retrospective studies, large RCTs and meta-analyses have evaluated the role of vitamin D in cancer primary prevention. According to the last review that studied the role of vitamin D in primary prevention of cancer, it was proven that vitamin D supplementation alone as primary prevention had no effect on cancer mortality and incidence. And that was after including 30 RCTs that reported cancer in their outcomes and despite including those that had long-term follow-up [21].
Keum et al., in their 2014 review, which included four RCTs with a minimum of 5 years of vitamin D supplementation, proved that long-term vitamin D supplementation did have a benefit in cancer prevention, however, only limited to cancer-related mortality [22].
In 2014, a Cochrane review also concluded that there could be decreases in all-cause mortality and cancer-related mortality among vitamin D–treated people in comparison with those who never received it. However, these results could be due to random errors [23].
Keum et al. recently reanalyzed their initial meta-analysis by adding newer RCTs with longer follow-up, which proved that vitamin D supplementation significantly reduced total cancer mortality but did not reduce total cancer incidence [24].
The strengths of our meta-analysis include an extensive search of the available literature. Furthermore, we included only RCTs, which helps eliminate the likelihood of confounding bias from nonrandomized studies. However, there are several limitations in the included clinical trials. First, over half of the included trials were not primarily studying vitamin D with the intent of preventing cancer and rather all the results were obtained by examining other reported primary outcomes. Second, due to various trial designs and protocols, there were major differences in the vitamin D forms and dosing. Third, only a few clinical trials reported all the predetermined outcomes of our study, and some trials reported only one of the two outcomes either directly or indirectly. Fourth, the follow-up period was short in some of the trials. Fifth, not all trials reported the end of trial 25-hydroxy vitamin D level to examine if the blood vitamin D levels had any effect on cancer mortality or incidence.
5. Conclusion
With inclusion of studies, which did not primarily examine vitamin D for the purpose of preventing cancer or reducing cancer mortality our meta-analysis highlights that the use of vitamin D supplementation for primary prevention of cancer is encouraged as it does possibly decrease cancer-related mortality once cancer is diagnosed; however, it has no role or effect on cancer incidence. However, this also opens questions for the future with the need for clinical trials that can account for all the limitations of our study including vitamin D form and dosing, length of therapy and exact therapeutic vitamin D levels, to provide stronger evidence and recommendations for the future.
Disclosure statement
No potential conflict of interest was reported by the authors.
Financial disclosure
No financial disclosure to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- [1].Heath AK, Kim IY, Hodge AM, et al. Vitamin D status and mortality : a systematic review of observational studies. Int J Environ Res Public Health. 2019;16:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Haidari F, Abiri B, Iravani M, et al. The effects of UVB and Vitamin D on decreasing risk of colorectal cancer incidence and mortality : a review of the epidemiology, clinical trials, and mechanisms the effects of UVB and Vitamin D on decreasing risk of colorectal cancer.2018. Nutr Cancer. 2019;71:1–9. [DOI] [PubMed] [Google Scholar]
- [3].Boscoe FP, Schymura MJ.. Solar ultraviolet-B exposure and cancer incidence and mortality in the USA, 1993–2002. BMC Cancer. 2006;6:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chen W, Clements M, Rahman B, et al. Relationship between cancer mortality/incidence and ambient ultraviolet B irradiance in China. Cancer Causes Control. 2010;21(10):1701–1709. [DOI] [PubMed] [Google Scholar]
- [5].Pilz S, Zittermann A, Trummer C, et al. Vitamin D testing and treatment : a narrative review of current evidence. Endocr Connect. 2019;30:27–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Grau MV, Baron JA, Sandler RS, et al. Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J Natl Cancer Inst. 2003. December 3;95(23):1765–1771. [DOI] [PubMed] [Google Scholar]
- [7].Hossain S, Beydoun MA, Beydoun HA, et al. Clinical nutrition ESPEN Vitamin D and breast cancer : A systematic review and meta-analysis of observational studies. Clin Nutr ESPEN. 2019;30:170–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Moyer VA. U.S. Preventive services task force. Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer: U.S. Preventive services task force recommendation statement. Ann Intern Med. 2014;160(8):558–564. [DOI] [PubMed] [Google Scholar]
- [9].Krstic MN, Mijac DD, Popovic DD. General aspects of primary cancer prevention. Dig Dis. 2019;37:406–415. [DOI] [PubMed] [Google Scholar]
- [10].Baron JA, Barry EL, Mott LA, et al. A trial of calcium and vitamin D for the prevention of colorectal adenomas. N Engl J Med. 2015;373(16):1519–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jorde R, Sollid ST, Svartberg J, et al. Vitamin D 20 000 IU per week for five years does not prevent progression from prediabetes to diabetes. Journal of clinical endocrinology and metabolism. 2016;25(April):1647–1655. [DOI] [PubMed] [Google Scholar]
- [12].Lappe J, Watson P, Travers-Gustafson D, et al. Effect of Vitamin D and calcium supplementation on cancer incidence in older women: a randomized clinical trial. JAMA. 2017;317(12):1234–1243. [DOI] [PubMed] [Google Scholar]
- [13].Manson JE, Cook NR, Lee I-M, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2018;380(1):33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gallagher JC, Fowler SE, Detter JR, et al. Combination treatment with estrogen and calcitriol in the prevention of age-related bone loss. J Clin Endocrinol Metab. 2001;86(8):3618–3628. [DOI] [PubMed] [Google Scholar]
- [16].Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D 3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326(March):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lappe JM, Travers-gustafson D, Davies KM, et al. Vitamin D and calcium supplementation reduces cancer risk : results of a randomized trial 1, 2. Am J Clin Nutr. 2007;85:1586–1591. [DOI] [PubMed] [Google Scholar]
- [18].Gerontol J, Sci AB, Sci M, et al. Calcium plus Vitamin D supplementation and mortality in postmenopausal women : the women s health initiative calcium – Vitamin D randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2009;64(5):559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral Vitamin D and falls and fractures in older women. JAMA. 2010;303(18):1815–1822. [DOI] [PubMed] [Google Scholar]
- [20].Avenell A, Maclennan GS, Jenkinson DJ, et al. Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of Vitamin D 3 and/or calcium (RECORD trial). J Clin Endocrinol Metab. 2012;97(February):614–622. [DOI] [PubMed] [Google Scholar]
- [21].Goulão B, Stewart F, Ford JA, et al. Cancer and vitamin D supplementation : a systematic review and meta-analysis. Am J Clin Nutr. 2018;25(1):652–663. [DOI] [PubMed] [Google Scholar]
- [22].Keum N, Giovannucci E. Vitamin D supplements and cancer incidence and mortality : a meta-analysis. Br J Cancer. 2014;111(5):976–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014;6:CD007469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Keum N, Lee DH, Greenwood DC, et al. Vitamin D supplementation and total cancer incidence and mortality : a meta-analysis of randomized controlled trials. Ann Oncol. 2019;30(5):733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]


