Abstract
PURPOSE
The National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH) trial, the largest national precision oncology study to date (> 1,100 sites) of patients with relapsed or refractory malignancies, assigned patients to targeted therapy in parallel phase II studies based on tumor molecular alterations. The anti–programmed death receptor 1 inhibitor nivolumab previously showed activity in mismatch repair (MMR)–deficient colon cancer. We hypothesized that nivolumab would have activity in patients with MMR-deficient, noncolorectal tumors.
PATIENTS AND METHODS
Eligible patients with relapsed or refractory tumors, good end-organ function, and Eastern Cooperative Oncology Group performance status of ≤ 1 underwent tumor biopsy for centralized screening of molecular alterations. MMR deficiency was defined by complete loss of nuclear expression of MLH1 or MSH2 MMR gene products by immunohistochemistry (IHC). Patients with MMR-deficient colorectal cancer were excluded. Nivolumab, 3 mg/kg every 2 weeks (28-day cycles) and 480 mg every 4 weeks after cycle 4, was administered intravenously. Disease reassessment was performed every 2 cycles. The primary end point was RECIST 1.1 objective response rate (ORR).
RESULTS
Two percent of 4,902 screened patients had an MMR-deficient cancer by IHC. Forty-two evaluable patients were enrolled, with a median age of 60 years and a median of 3 prior therapies. The most common histologies were endometrioid endometrial adenocarcinoma (n = 13), prostate adenocarcinoma (n = 5), and uterine carcinosarcoma (n = 4). ORR was 36% (15 of 42 patients). An additional 21% of patients had stable disease. The estimated 6-, 12-, and 18-month progression-free survival rates were 51.3% (90% CI, 38.2% to 64.5%), 46.2% (90% CI, 33.1% to 59.3%), and 31.4% (90% CI, 18.7% to 44.2%), respectively. Median overall survival was 17.3 months. Toxicity was predominantly low grade.
CONCLUSION
A variety of refractory cancers (2.0% of those screened) had MMR deficiency as defined in NCI-MATCH. Nivolumab has promising activity in MMR-deficient noncolorectal cancers of a wide variety of histopathologic types.
INTRODUCTION
Immune checkpoint inhibitor therapy directed against programmed death receptor 1 (PD-1) or its ligand (PD-L1) has dramatically altered treatment of large numbers of patients with advanced cancer.1,2 Mismatch repair (MMR)–deficient cancers have a defect in DNA damage repair, acquired either through hereditary or sporadic abnormalities in MMR genes. This inability to repair primarily single base pair mismatches in DNA results in the accumulation of DNA mutations and provides MMR-deficient cancers with one of the highest tumor mutation burdens (TMBs) among cancers, which has been correlated with clinical response to anti–PD-1/PD-L1 therapy.3,4
Given these data, clinical trials testing the PD-1 inhibitor pembrolizumab in MMR-deficient cancers of various types and nivolumab with or without ipilimumab in MMR-deficient colorectal cancers were conducted and showed striking clinical activity.5-7 On May 23, 2017, the US Food and Drug Administration (FDA) approved pembrolizumab for the treatment of MMR-deficient solid tumors, the first tumor agnostic approval for an anticancer agent. Subsequently, both nivolumab and the combination of nivolumab and ipilimumab were FDA approved for the treatment of MMR-deficient advanced colorectal cancer.8 However, the clinical activity of nivolumab in patients with MMR-deficient cancers other than colorectal cancer has not been explored extensively. Based on the activity seen with pembrolizumab in patients with noncolorectal cancer, we hypothesized that nivolumab would also be an effective anticancer therapy for these patients.
The National Cancer Institute (NCI) Molecular Analysis for Therapy Choice (NCI-MATCH or EAY131) trial (ClinicalTrials.gov identifier: NCT02465060) is a large, national trial led by the Eastern Cooperative Oncology Group (ECOG)–American College of Radiology Imaging Network Cancer Research Group through the NCI-supported National Clinical Trials Network and NCI Community Oncology Research Program. The trial aimed to find signals of efficacy for treatments targeted to actionable molecular alterations found in any tumor type. A master screening protocol profiled cancers from patients with refractory solid tumors, lymphoma, or myeloma for molecular abnormalities with multiple concurrently enrolling subprotocols, each of which targeted a particular actionable mutation of interest. Those with tumor molecular alterations that were addressed by subprotocols in NCI-MATCH were enrolled based on their genotype or phenotype.9 We report the results of NCI-MATCH phase II clinical trial subprotocol Z1D, which evaluated the activity of nivolumab in patients with MMR-deficient, noncolorectal cancer.
PATIENTS AND METHODS
Subprotocol Overview
NCI-MATCH (EAY131) functioned as a screening master protocol, under which were arrayed a number of phase II signal-finding subprotocols. The Z1D subprotocol, reported here, was an open-label, single-arm, phase II clinical trial to investigate the use of nivolumab in patients with MMR-deficient, noncolorectal cancers. The study was reviewed and approved by the NCI Central Institutional Review Board, and all patients signed written informed consent.
Patient Selection
Adult patients with any solid tumor, lymphoma, or myeloma who experienced progression on standard treatment or who had a cancer for which there was no known effective therapy were eligible. Adequate hematopoietic, liver, and kidney function; an ECOG performance status of ≤ 1; and a recent biopsy were required. Patients were excluded if they had colorectal cancer, prior history of severe autoimmune disease, or prior treatment with an immune checkpoint inhibitor.
Tumor Profiling
Tumor profiling was accomplished with a targeted investigational 143-gene Oncomine assay (Thermo Fisher Scientific, Waltham, MA), as previously described.10 MMR deficiency was assessed using an immunohistochemistry (IHC) assay for MLH1 or MSH2 expression.11 Eligible patients had complete loss of nuclear expression of either MLH1 (clone G168-728; MilliporeSigma/Cell Marque, Rocklin CA) or MSH2 (clone FE11; EMD Millipore/Calbiochem, San Diego, CA) by IHC. All tumors with loss of expression had confirmatory IHC performed for the corresponding binding partner protein: PMS2 (clone A16-4; BD Pharmingen, San Diego, CA) for LH1 or MSH6 (clone 44MSH6; BD Transduction Laboratories, San Jose, CA) for MSH2.
IHC was performed on a pretreatment tumor biopsy in the central Clinical Laboratory Improvement Amendments–accredited clinical IHC laboratory in the Department of Pathology at the University of Texas MD Anderson Cancer Center. This laboratory was also accredited by the New York State Department of Health. These assays have specificity for MMR deficiency of approximately 90% and sensitivity of approximately 80%.12 Any patient whose tumor was concurrently MMR deficient and had another actionable molecular alteration was preferentially assigned to arm Z1D.
After completion of the trial, available tumor with deficient MMR by IHC was evaluated for microsatellite instability (MSI) status with a polymerase chain reaction (PCR) assay of 4 mononucleotide repeat sequences (BAT25, BAT26, BAT40, and TGFβRII) and 3 dinucleotide repeats (D2S123, D5S346, and D17S250), as described previously.13,14 Residual tumor or DNA from the NCI-MATCH biopsy specimens (n = 16), tumors from another metastasis (n = 14), or archived primary tumors (n = 14) were evaluated. DNA extracted from peripheral blood leukocytes (n = 36) or nonneoplastic tissue (n = 8) was used as a comparator.
Assignment to Treatment
Patients were assigned using a prospectively defined, NCI-designed informatics rules algorithm (MATCHbox), as previously described.9
Treatment Details
Patients were treated with nivolumab 3 mg/kg intravenously every 2 weeks for 28-day cycles. After cycle 4, patients could be switched to nivolumab 480 mg intravenously once each cycle. An optional tumor biopsy at the time of progressive disease was encouraged.
Evaluation of Response
Response was evaluated every 2 cycles using RECIST 1.1 criteria for solid tumors15 based on investigator assessment.
Toxicity Evaluation
Toxicity was evaluated using Common Terminology Criteria for Adverse Events version 4. Dose modifications were not allowed on this subprotocol.
Statistical Considerations
The primary objective was to evaluate the objective response rate (ORR) for each subprotocol. A response rate of 16% (5 of 31 patients) or greater was predefined as a signal of activity. Secondary objectives included progression-free survival (PFS) at 6 months, time to progression, toxicity assessment, and evaluation of predictive biomarkers (comutations or other factors that potentially predict which patients will respond). The original accrual goal was 35 patients, to obtain 31 eligible patients. Because of brisk enrollment, expansion criteria were triggered. In the expansion phase (beyond 35 patients), the number of patients with each tumor type that could be enrolled was capped at 10, and histologies with at least 10 patients already enrolled on the subprotocol in the initial accrual phase were excluded.
RESULTS
Patients and Treatment
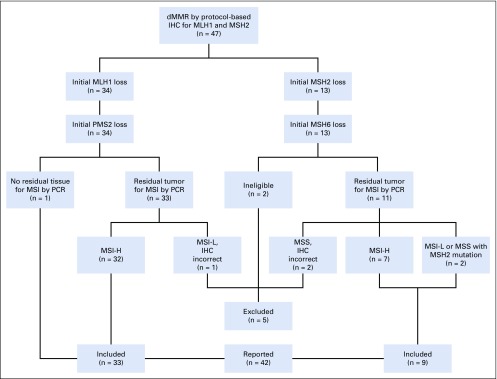
Arm Z1D opened May 31, 2016. Of 5,598 tumors submitted beginning August 12, 2015, 4,902 were successfully screened by IHC for MLH1 and MSH2, including 5 patients who were screened twice during the period; these patients were enrolled for screening, did not enroll on an arm, and at a later point were screened again under a new case number. Of these 4,902 patients, 99 (2.0%) were found to have MLH1 or MSH2 loss. Between August 1, 2016, and June 27, 2017, 47 patients of varying histologies among the 99 were enrolled in arm Z1D (Appendix Fig A1, online only). The most common reason for a patient with MLH1 or MSH2 loss not enrolling in the subprotocol was ineligible histology (colorectal cancer or endometrial cancer, the latter during expansion of accrual).
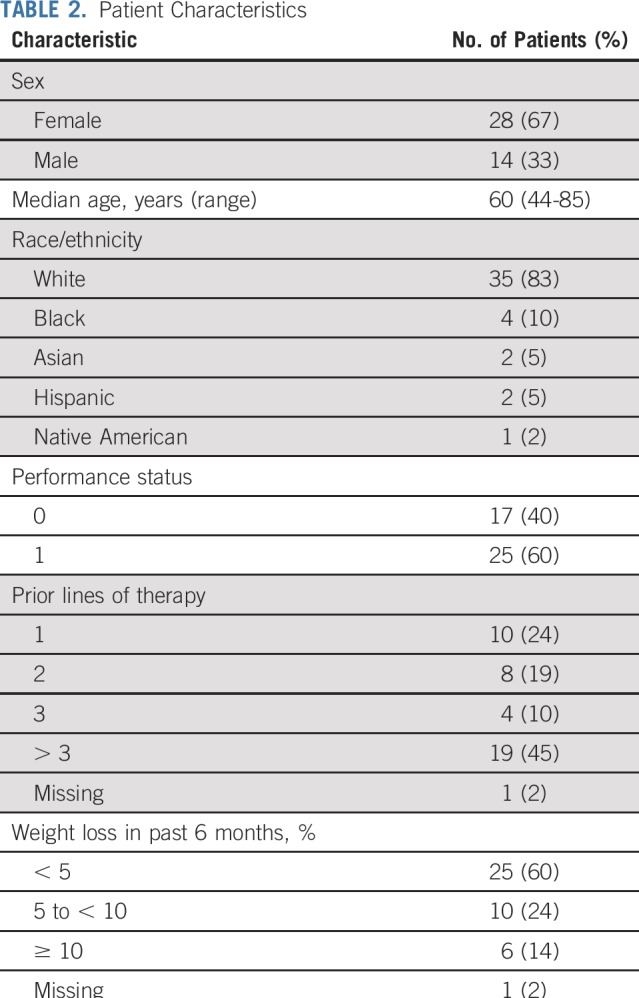
On review of the 47 enrolled patients, 2 were deemed ineligible as a result of having had treatment within the washout window or elevated creatinine at enrollment, and 3 were excluded after posttrial rereview of baseline IHC showed incomplete loss of MLH1 or MSH2 expression in their tumor. As a result, 42 patients were evaluable; 33 (79%) had loss of MLH1, and 9 (21%) had loss of MSH2 expression. Table 1 lists the histologies and Table 2 the patient characteristics for the evaluable population. All 5 patients with prostate adenocarcinoma had prior therapy with hormonal agents. Median follow-up time was 17.3 months.
TABLE 1.
Tumor Histologies of the 42 Evaluable Patients
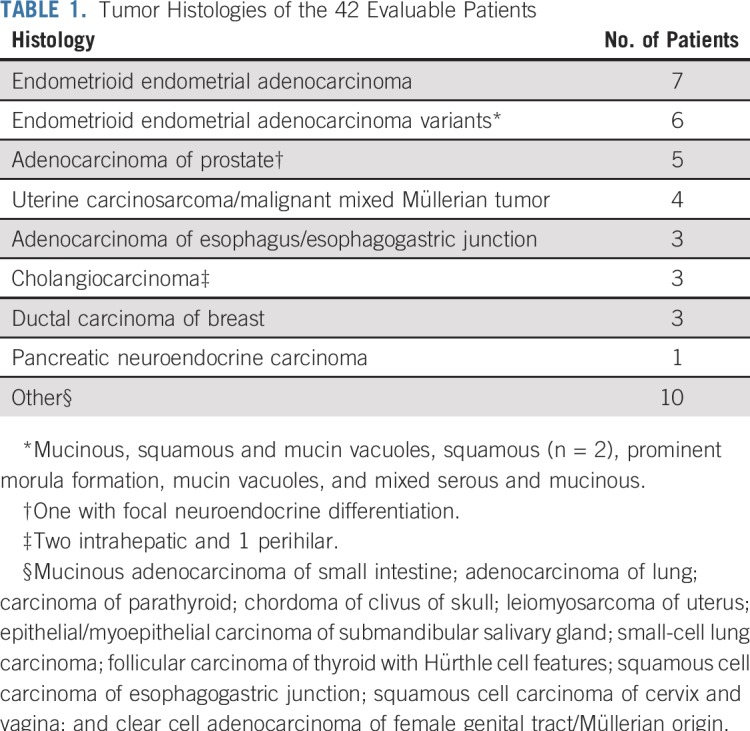
TABLE 2.
Patient Characteristics
Clinical Activity
Rates of response and survival.
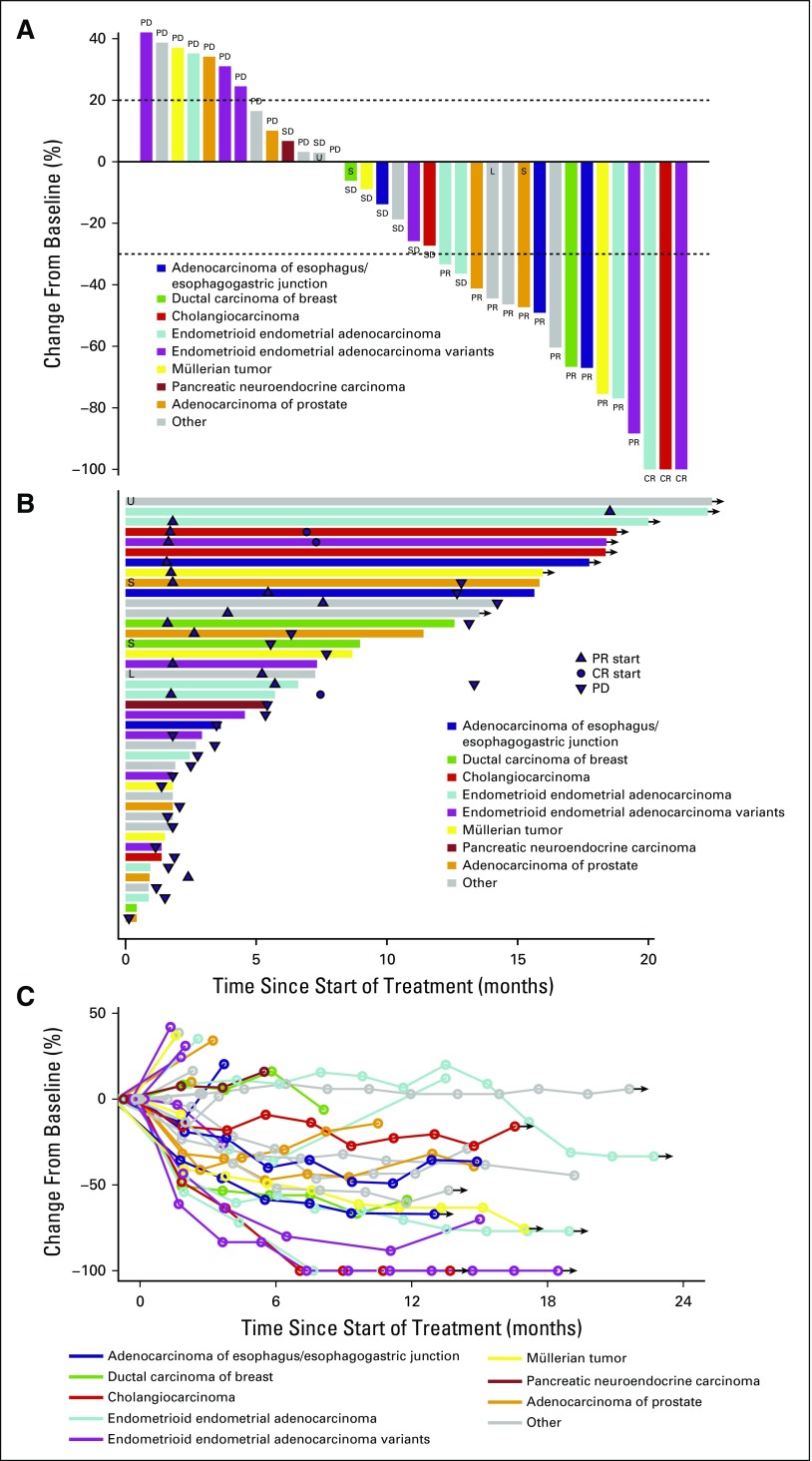
The ORR in the intent-to-treat population was 36% (15 of 42 patients; 90% CI, 23.5% to 49.5%). Three patients (7%) had complete responses (CRs), 13 patients (29%) had partial responses (PRs), 9 patients (21%) had stable disease, and 12 patients (23%) had progressive disease (Fig 1A). CRs were observed in 2 patients with endometrioid endometrial adenocarcinoma and 1 patient with cholangiocarcinoma. Seven patients were not evaluable; 5 of these patients withdrew from the study for adverse events in cycle 1 or 2 before restaging. Of the 15 patients with a confirmed best response as PR or CR, 6 developed progressive disease after their initial response.
FIG 1.
Clinical responses. (A) Depth of best clinical response by RECIST 1.1 criteria by tumor histology (35 evaluable patients included). (B) Duration of treatment in patients with stable disease (SD) or better, annotated by tumor histology. Arrows represent ongoing therapy at the time of data cutoff. (C) Spider plot of response depth and duration by tumor histology (35 evaluable patients included). Arrows represent ongoing therapy at the time of data cutoff. CR, complete response; L, microsatellite instability low; PD, progressive disease; PR, partial response; S, microsatellite instability stable; U, microsatellite instability unknown.
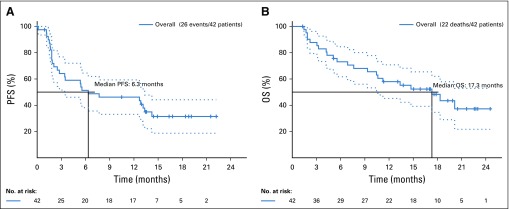
The estimated PFS distribution is provided in Figure 2A. The estimated 6-, 12-, and 18-month PFS rates were 51.3% (90% CI, 38.2% to 64.5%), 46.2% (90% CI, 33.1% to 59.3%), and 31.4% (90% CI, 18.7% to 44.2%), respectively. Twenty-two deaths were reported, with a median survival time of 17.3 months (Fig 2B)
FIG 2.
(A) Kaplan-Meier survival curve of progression-free survival (PFS) on nivolumab treatment. (B) Kaplan-Meier survival curve of overall survival (OS) on nivolumab treatment.
Durability of response.
Patients were treated for 1 to ≥ 24 cycles (median, 5 cycles). The most common reason for discontinuing study therapy was progressive disease (16 patients, 38%), followed by adverse events (10 patients, 24%). Thirty-three patients had discontinued study treatment, whereas 9 patients (21%) continued on treatment at the time of this report (Fig 1B). Those patients continuing on therapy had best responses of CR (n = 2), PR (n = 5), and stable disease (n = 2). The other patient with a CR discontinued therapy as a result of adverse events in cycle 5, remained without progression for 172 days, and then withdrew consent for follow-up. Three of the 12 patients with PR came off therapy without progression between 7 and 12 cycles. Two patients were progression free at 258 and 388 days; the third patient was lost to follow-up after cycle 12. Finally, of the 9 patients with stable disease, one patient remained progression free for 232 days after coming off therapy for adverse events.
Tolerability
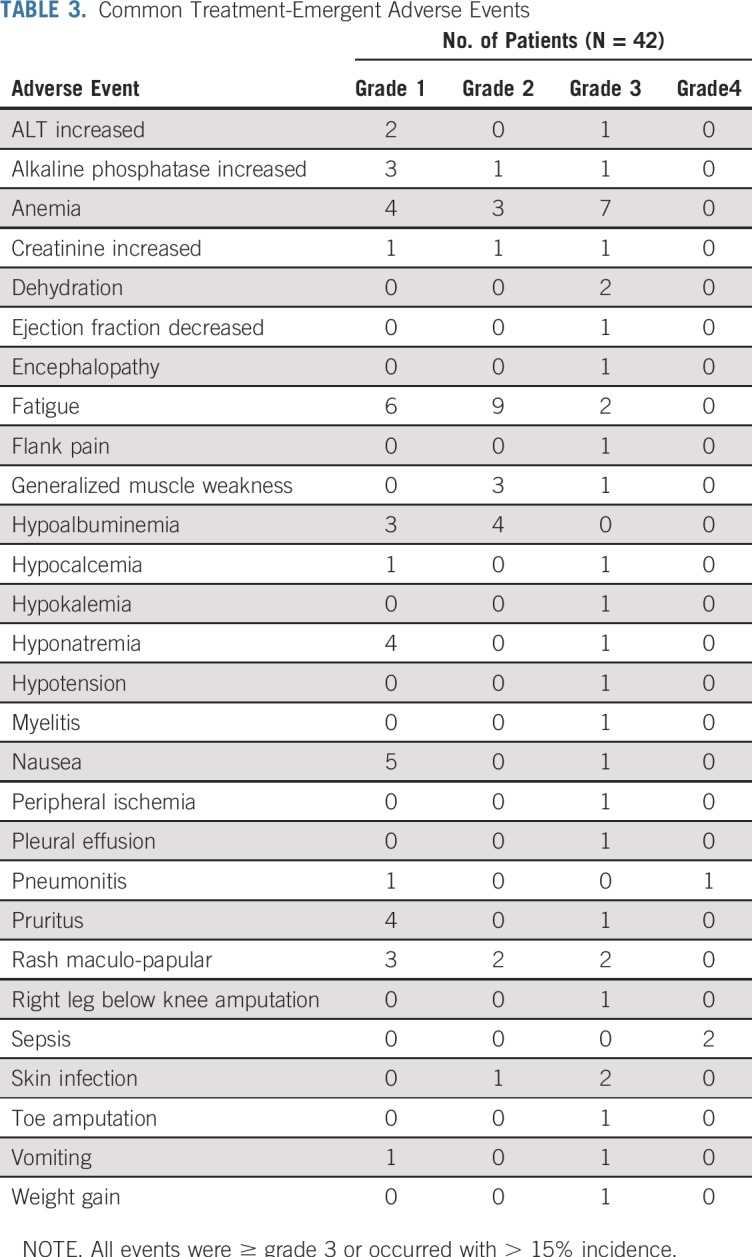
Treatment-related adverse events were generally mild and expected for this class of agents (Table 3). There were no grade 5 toxicities and only 2 grade 4 toxicities in 3 patients (sepsis in 2 patients). The most common grade 1-3 adverse events were fatigue (40%), anemia (33%), rash (17%), and hypoalbuminemia (17%).
TABLE 3.
Common Treatment-Emergent Adverse Events
Molecular Correlates
After completion of the trial, MSI testing by PCR was performed on an available tumor sample for 44 enrolled eligible patients who met the inclusion criterion of loss of IHC staining, and 39 patients (89%) had an MSI-high tumor (Appendix Fig A1). The tumors of the other 5 eligible patients were MSI low (n = 2) or microsatellite stable (MSS; n = 3). Interestingly, 2 patients with an MSI-low or MSS tumor had an MSH2 mutation in their tumor, in agreement with the IHC results, and even though their tumor was not MSI high by PCR, both had PRs to investigational therapy (Figs 1A and 1B). Among the other 3 patients, 1 had no available tissue, and the tumors from 2 patients who were ineligible based on exclusion criteria were not evaluated.
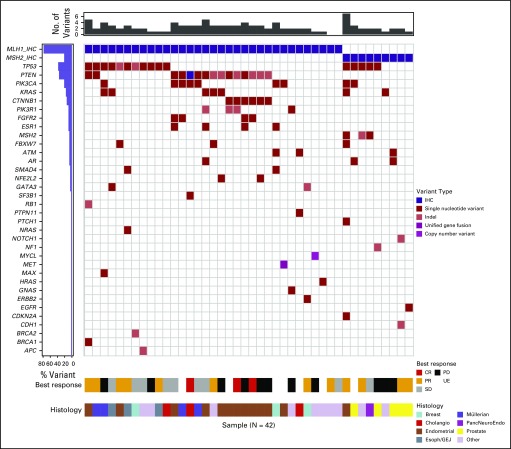
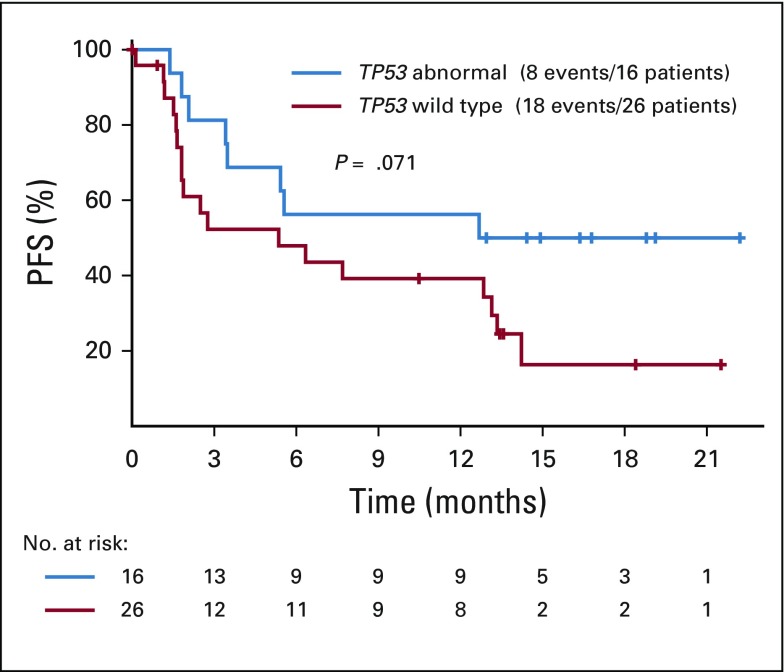
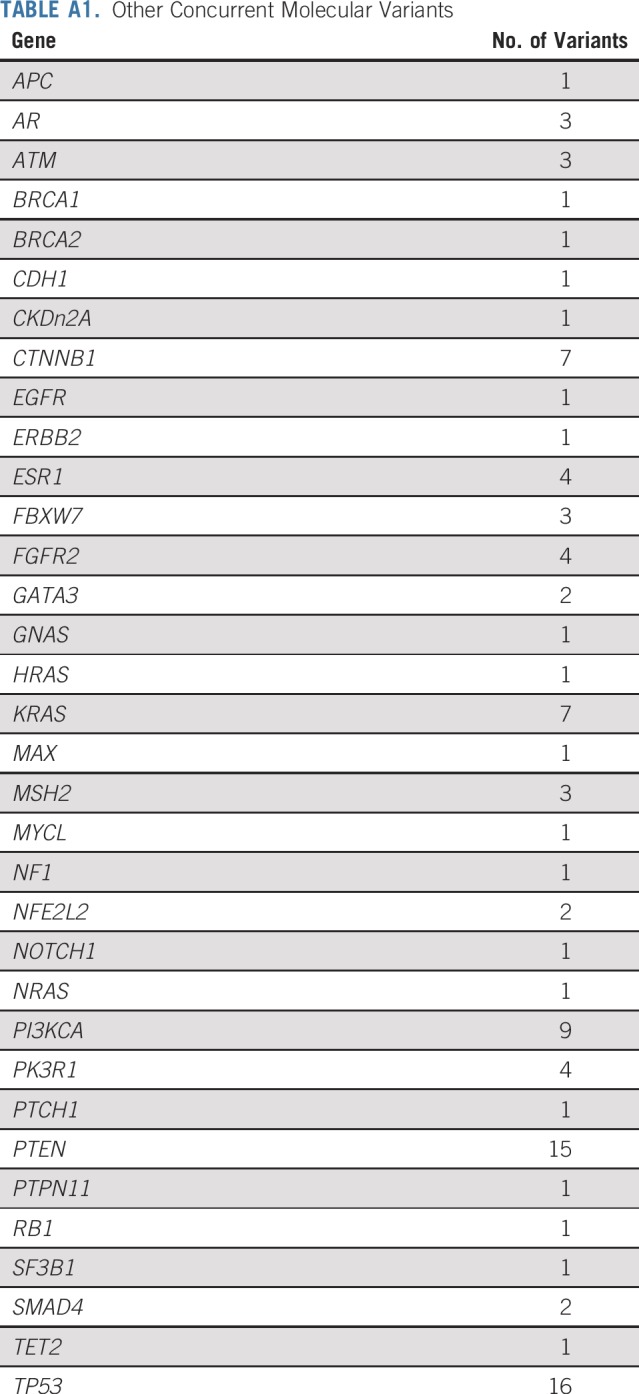
A series of post hoc exploratory analyses were undertaken to investigate possible associations with response rate and PFS using the baseline fresh tumor biopsy specimens that were taken at screening for the master protocol. There was no difference in PFS in patients with tumors with loss of MLH1 compared with loss of MSH2 (P = 1.0). The most common genes altered in the NCI-MATCH tumors that lacked nuclear expression of MLH1 or MSH2 were TP53, PTEN, PI3KCA, and CTNNB1, with multiple other genes showing variants in multiple patients (Fig 3, Appendix Table A1, online only). Clinical response, disease control, or likelihood of progression did not correlate with any particular concurrent molecular abnormality, although in patients whose tumors had TP53 aberrations, there was a trend toward improved response rate (44% v 31% in patients without TP53 aberrations) and improved PFS (P = .071 for PFS; Appendix Fig A2, online only). Similarly, an analysis using grouped molecular abnormalities by pathway or mechanism (DNA repair genes, PIK3/PTEN, RAS, and PIK3/PTEN/RAS) showed no correlation with response category on therapy.
FIG 3.
Variants by gene and patient. The genes with variants are indicated for each patient (each column represents a patient). The histograms at the top and left give the number of variants for each patient (top) and proportion of patients with variants in each gene (left). Cholangio, cholangiocarcinoma; CR, complete response; Esoph/GEJ, esophagus/gastroesophageal junction; IHC, immunohistochemistry; PancNeuroEndo, pancreatic neuroendocrine carcinoma; PD, progressive disease; PR, partial response; SD, stable disease; UE, unevaluable.
DISCUSSION
This study confirms that MMR deficiency identifies a group of tumors across a range of anatomic and histopathologic types with a substantial response rate when treated with nivolumab anti–PD-1 therapy. Patients with 19 different cancer types, none of them colorectal, were treated on this study. The response rate of 36% compares well with the previously reported 31% response rate for nivolumab in MMR-deficient colorectal cancer.16
This trial was a subprotocol of the NCI-MATCH (EAY131) trial, the largest ever trial of personalized therapy based on molecular biomarkers.9 To meet eligibility for an NCI-MATCH arm, fresh tumor biopsies were sequenced by the NCI-MATCH targeted sequencing platform and evaluated by centralized IHC testing. Tissue quantity was key to feasibility to successfully enroll on the trial; accordingly, we chose to use IHC testing for MLH1 and MSH2 rather than PCR-based testing to preserve tissue and still reach a sensitivity of 80% for MMR-deficient cancers and high specificity.17-19 For this study, we were interested in enrolling patients with MMR deficiency, not in assessing risk for MMR-deficient future cancers. Thus, the prevalence of 2% that we found is likely to be slightly lower than the actual prevalence of MMR deficiencies previously described in noncolorectal solid tumors. Although the NCI-MATCH next-generation sequencing (NGS) assay does not report MSI, multiple commercial and academic targeted NGS sequencing assays now report MSI status using proprietary algorithms and have shown excellent concordance with PCR and IHC-based testing.20-22 We found concordance of MLH1 or MSH2 loss by IHC with MSI-high status by PCR in 39 (89%) of 44 patients, showing both the utility of IHC-based testing and the potential to misidentify MMR deficiency in a small proportion of patients.
Previous studies have reported compelling activity of pembrolizumab in MMR-deficient cancers of various types and of nivolumab with or without ipilimumab in MMR-deficient colorectal cancer, with response rates of 31%-53%.5-7,12 Our trial was designed to assess the benefit of nivolumab in noncolorectal cancers. We report efficacy of nivolumab comparable to that of single-agent pembrolizumab in patients with noncolorectal cancer. Patients with endometrial cancer and prostate cancer were our most commonly enrolled patients, with usual MSI-high prevalence rates of approximately 30% and 3% reported in large series, respectively.20,23 Of note, our initial report of this trial with shorter follow-up showed a lower response rate for nivolumab (24%) compared with historical data with pembrolizumab,22 a gap that has now closed with the longer duration of follow-up of 17.3 months. These data support the premise that responses to immune therapy continue to occur over time, and patients with stable disease may eventually achieve a clinical PR or CR with continued therapy. The potentially lower response rate in arm Z1D of NCI-MATCH compared with the response rate with pembrolizumab in MMR-deficient cancers may reflect the general much wider setting of the trial, which was open in > 1,100 sites across the country. It is possible that patients were more likely to be discontinued from study therapy for immune-related toxicities, compared with patients treated at highly specialized centers with significant expertise in immunotherapy, where more experience in management of these adverse events might have allowed providers more comfort in continuing patients on therapy. In addition, 2 patients with loss of MMR proteins by IHC but who were MSS or MSI low on PCR testing had an MSH2 mutation and responded to therapy; this suggests the potential that patients with loss of MMR proteins even without a microsatellite-unstable phenotype may derive benefit from anti–PD-1 therapy.
The NGS profiling of known actionable mutations allowed us to conduct exploratory analyses examining correlations between given pathway abnormalities and immune checkpoint response in the MMR-deficient patient population and probe possible mechanisms of primary resistance. PI3 kinase pathway activation has been suggested as a mechanism of primary immunotherapy resistance.24-26 Although 9 of 23 of the MMR-deficient patients in arm Z1D did have pretreatment PIK3CA mutations, this finding did not correlate with clinical outcome in our cohort, with the requisite caveats acknowledging a small sample size in this analysis; further analysis of alterations in PIK3 signaling along the pathway (PIK3, AKT, and PTEN) did not show correlation with response to therapy. Conflicting reports exist on the effect of silencing the tumor suppressor gene TP53 on chemotherapy and immunotherapy sensitivity.27-29 Although not statistically significant, possibly because of sample size, we noted a trend toward improved response and survival in patients whose tumors had TP53 abnormalities. This finding should be explored in larger sample sets to assess the value of TP53 status as a biomarker of response to immunotherapy in patients whose tumors have MMR deficiency.
Immune checkpoint inhibitors have been approved for treatment of multiple cancers based on improved survival, including non–small-cell lung cancer (NSCLC), melanoma, urothelial cancers, and many others.2 TMB has been found to be an independent predictor of benefit for immune checkpoint therapy, with the greatest number of mutations observed in NSCLC, melanoma, cutaneous squamous cell carcinoma, and MMR-deficient cancers.3,4 TMB is increasingly being recognized as a potentially useful biomarker for identification of patients likely to respond to immune checkpoint inhibition. In a recent high-impact publication, TMB was the strongest predictor of benefit with anti–PD-1/PD-L1 therapy in NSCLC.4 Yarchoan et al3 showed a correlation between median somatic mutation burden and likelihood of response to anti–PD-1/PD-L1 therapy in 27 tumor types. The underlying hypothesis for this correlation relates to the high level of neoantigens that result from tumor types with high mutation loads.4 Interestingly, the high rate of neoantigens seen in MMR-deficient cancers relates not only to their high mutational burden but also to the nature of the mutations generated from single base pair mismatches. Frequently, single base pair mismatches result in frameshift mutations, which can result in transcription of numerous altered amino acids; therefore, each mutation has the potential to generate a greater number of neoantigens.30 Unfortunately, both limited tissue and inability of the sequencing platform used for NCI-MATCH (Oncomine) to support robust quantification of TMB as a result of insufficient number of nucleotides precluded our ability to report TMB for this study.
In conclusion, we report positive results from arm Z1D of the national NCI-MATCH precision oncology trial. These data confirm the benefit of the anti–PD-1 inhibitor nivolumab in patients with MMR-deficient, noncolorectal cancer. The rapid accrual to subprotocol Z1D also demonstrates the power of a national clinical trial to enroll patients from a rare biomarker-based population.
APPENDIX
FIG A1.
CONSORT diagram of mismatch repair (MMR) testing workflow. dMMR, deficient mismatch repair; H, high; IHC, immunohistochemistry; L, low; MSI, microsatellite instability; MSS, microsatellite stable; PCR, polymerase chain reaction.
FIG A2.
Progression-free survival (PFS) based on TP53 status on nivolumab treatment.
TABLE A1.
Other Concurrent Molecular Variants
Footnotes
Presented in part at the 32nd Annual Meeting of the Society for Immunotherapy of Cancer, National Harbor, MD, November 8-12, 2017.
Supported by the National Cancer Institute of the National Institutes of Health by Grants No. CA180820, CA180794, CA180802, CA180858, CA180867, CA180844, CA180798, CA189821, CA180868, and CA180870.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
See accompanying Editorial on page 183
AUTHOR CONTRIBUTIONS
Conception and design: Nilofer S. Azad, Robert J. Gray, Jonathan D. Schoenfeld, Edith P. Mitchell, James A. Zwiebel, Elad Sharon, Howard Streicher, Shuli Li, Lisa M. McShane, Larry Rubinstein, David R. Patton, P. Mickey Williams, Brent Coffey, Stanley R. Hamilton, Barbara A. Conley, Peter J. O'Dwyer, Alice P. Chen, Keith T. Flaherty
Financial support: Elad Sharon, Stanley R. Hamilton
Administrative support: Elad Sharon, Howard Streicher, Stanley R. Hamilton, Barbara A. Conley, Lyndsay Harris, Peter J. O'Dwyer
Provision of study materials or patients: Edith P. Mitchell, Elad Sharon, Stanley R. Hamilton, Nathan Bahary
Collection and assembly of data: Nilofer S. Azad, Robert J. Gray, Edith P. Mitchell, Elad Sharon, Shuli Li, Lisa M. McShane, David R. Patton, P. Mickey Williams, Brent Coffey, Stanley R. Hamilton, J. Marie Suga, Lyndsay Harris, Peter J. O'Dwyer
Data analysis and interpretation: Nilofer S. Azad, Robert J. Gray, Michael J. Overman, Jonathan D. Schoenfeld, Edith P. Mitchell, James A. Zwiebel, Elad Sharon, Lisa M. McShane, David R. Patton, Brent Coffey, Stanley R. Hamilton, Nathan Bahary, Hassan Hatoum, Jeffrey S. Abrams, Carlos L. Arteaga, Lyndsay Harris, Peter J. O'Dwyer, Alice P. Chen, Keith T. Flaherty
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Nivolumab Is Effective in Mismatch Repair–Deficient Noncolorectal Cancers: Results From Arm Z1D—A Subprotocol of the NCI-MATCH (EAY131) Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Nilofer S. Azad
Honoraria: AMAG Pharmaceuticals
Consulting or Advisory Role: DAVAOncology, AMAG Pharmaceuticals, Taiho Pharmaceutical
Research Funding: Celgene (Inst), Genentech (Inst), Astex Pharmaceuticals (Inst), Agios (Inst), Merck (Inst), Bristol-Myers Squibb (Inst), Syndax (Inst), Array BioPharma (Inst), Intensity Therapeutics (Inst)
Robert J. Gray
Research Funding: Agios, Amgen, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Genentech, Genomic Health, Genzyme, GlaxoSmithKline, Janssen-Ortho, Onyx, Pfizer, Sequenta, Syndax, Novartis, Takeda, AbbVie, Sanofi, Merck Sharp & Dohme
Michael J. Overman
Consulting or Advisory Role: Bristol-Myers Squibb, Genentech, Gritstone Oncology, MedImmune, Novartis, Promega, Spectrum Pharmaceuticals, Array BioPharma
Research Funding: Bristol-Myers Squibb, Merck, Roche, MedImmune
Jonathan D. Schoenfeld
Consulting or Advisory Role: Tilos Therapeutics, LEK, Catenion, ACI Clinical
Research Funding: Bristol-Myers Squibb, Merck, Regeneron
Edith P. Mitchell
Honoraria: Sanofi
Consulting or Advisory Role: Genentech, Novartis, Merck, Bristol-Myers Squib
Speakers' Bureau: Ipsen
Research Funding: Genentech (Inst), Sanofi (Inst)
James A. Zwiebel
Consulting or Advisory Role: Boston Pharmaceuticals, Scandion Oncology
P. Mickey Williams
Research Funding: Illumina (Inst)
Patents, Royalties, Other Intellectual Property: I was a coinventor of the diffuse large B-cell lymphoma cell of origin patent recently filed by the National Institutes of Health
Brent Coffey
Stock and Other Ownership Interests: Pfizer, AbbVie
Stanley R. Hamilton
Stock and Other Ownership Interests: The Johns Hopkins University School of Medicine
Consulting or Advisory Role: HalioDx, Thermo Fisher Scientific, Bristol-Myers Squibb, Loxo, Merck, Guardant Health, Cell Medica Limited
Nathan Bahary
Consulting or Advisory Role: Celgene, Bristol-Myers Squibb, AstraZeneca, Exelixis, ThermoFisher
Hassan Hatoum
Consulting or Advisory Role: Foundation Medicine
Carlos L. Arteaga
Leadership: American Association for Cancer Research
Stock and Other Ownership Interests: Provista Diagnostics, Y-Trap
Consulting or Advisory Role: Novartis, Eli Lilly, AbbVie, Sanofi, Radius Health, Taiho Pharmaceutical, Puma Biotechnology, Merck, H3 Biomedicine, Symphogen, Origimed, Petra Pharma, Third Rock Ventures, Immunomedics
Research Funding: Puma Biotechnology, Pfizer, Eli Lilly, Radius Health, Takeda, Bayer
Other Relationship: Susan G. Komen for the Cure
Lyndsay Harris
Patents, Royalties, Other Intellectual Property: Philips Healthcare
Peter J. O'Dwyer
Consulting or Advisory Role: Genentech
Research Funding: Bristol-Myers Squibb, Pfizer, Novartis, Genentech, Mirati Therapeutics, Celgene, GlaxoSmithKline, BBI Healthcare, Merck, Pharmacyclics, Bayer, Five Prime Therapeutics, Forty Seven, Amgen
Expert Testimony: Bayer
Keith T. Flaherty
Stock and Other Ownership Interests: Clovis Oncology, Loxo, X4 Pharma, Strata Oncology, PIC Therapeutics, Fount Therapeutics, Shattuck Labs, Apricity Health, Oncoceutics, Fog Pharma, Tvardi, Vivid Biosciences, Checkmate Pharmaceuticals
Consulting or Advisory Role: Novartis, Genentech, Merck, Eli Lilly, Amgen, Sanofi, Oncoceutics, Bristol-Myers Squibb, Adaptimmune, Aeglea Biotherapeutics, Loxo, Roche, Asana Biosciences, Incyte, Shattuck Labs, Tolero Pharmaceuticals, Array BioPharma, FOG Pharma, Neon Therapeutics, Tvardi, Takeda, Verastem, Boston Biomedical, Pierre Fabre, Cell Medica, Debiopharm Group
Research Funding: Novartis, Sanofi
Travel, Accommodations, Expenses: Pierre Fabre, Debiopharm Group
No other potential conflicts of interest were reported.
REFERENCES
- 1.Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18:153–167. doi: 10.1038/nri.2017.108. [DOI] [PubMed] [Google Scholar]
- 2. doi: 10.1016/j.pharmthera.2018.09.008. Constantinidou A, Alifieris C, Trafalis DT: Targeting programmed cell death-1 (PD-1) and ligand (PD-L1): A new era in cancer active immunotherapy. Pharmacol Ther 194:84-106, 2019. [DOI] [PubMed] [Google Scholar]
- 3.Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377:2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hellmann MD, Nathanson T, Rizvi H, et al. Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell. 2018;33:843–852.e4. doi: 10.1016/j.ccell.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36:773–779. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 8.Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site: When a biomarker defines the indication. N Engl J Med. 2017;377:1409–1412. doi: 10.1056/NEJMp1709968. [DOI] [PubMed] [Google Scholar]
- 9. Conley BA, Chen AP, O'Dwyer PJ, et al: NCI-MATCH (Molecular Analysis for Therapy Choice): A national signal finding trial. J Clin Oncol 34, 2017 (suppl 15; abstr TPS2606) [Google Scholar]
- 10.Lih CJ, Harrington RD, Sims DJ, et al. Analytical validation of the next-generation sequencing assay for a nationwide signal-finding clinical trial: Molecular Analysis for Therapy Choice clinical trial. J Mol Diagn. 2017;19:313–327. doi: 10.1016/j.jmoldx.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khoury JD, Wang WL, Prieto VG, et al. Validation of immunohistochemical assays for integral biomarkers in the NCI-MATCH EAY131 clinical trial. Clin Cancer Res. 2018;24:521–531. doi: 10.1158/1078-0432.CCR-17-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartley AN, Luthra R, Saraiya DS, et al. Identification of cancer patients with Lynch syndrome: Clinically significant discordances and problems in tissue-based mismatch repair testing. Cancer Prev Res (Phila) 2012;5:320–327. doi: 10.1158/1940-6207.CAPR-11-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 14.Earle JSL, Luthra R, Romans A, et al. Association of microRNA expression with microsatellite instability status in colorectal adenocarcinoma. J Mol Diagn. 2010;12:433–440. doi: 10.2353/jmoldx.2010.090154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hissong E, Crowe EP, Yantiss RK, et al. Assessing colorectal cancer mismatch repair status in the modern era: A survey of current practices and re-evaluation of the role of microsatellite instability testing. Mod Pathol. 2018;31:1756–1766. doi: 10.1038/s41379-018-0094-7. [DOI] [PubMed] [Google Scholar]
- 18.Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome: Part I. The utility of immunohistochemistry. J Mol Diagn. 2008;10:293–300. doi: 10.2353/jmoldx.2008.080031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome: Part II. The utility of microsatellite instability testing. J Mol Diagn. 2008;10:301–307. doi: 10.2353/jmoldx.2008.080062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanderwalde A, Spetzler D, Xiao N, et al. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med. 2018;7:746–756. doi: 10.1002/cam4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Azad NS, Overman M, Gray R, et al: Nivolumab in mismatch-repair deficient (MMR-d) cancers: NCI-MATCH Trial (Molecular Analysis for Therapy Choice) arm Z1D preliminary results. J Immunother Cancer 5:89, 2017 (suppl 3; abstr O37) [Google Scholar]
- 23.Abida W, Cheng ML, Armenia J, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019;5:471–478. doi: 10.1001/jamaoncol.2018.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins DC, Chenard-Poirier M, Lopez JS. The PI3K pathway at the crossroads of cancer and the immune system: Strategies for next generation immunotherapy combinations. Curr Cancer Drug Targets. 2018;18:355–364. doi: 10.2174/1568009617666170927114440. [DOI] [PubMed] [Google Scholar]
- 25. Collins NB, Manguso R, Pope H, et al: Defining molecular mechanisms of resistance to tumor immunity. Cancer Immunol Res 5, 2017 (suppl 3; abstr A16) [Google Scholar]
- 26.O’Donnell JS, Massi D, Teng MWL, et al. PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux. Semin Cancer Biol. 2018;48:91–103. doi: 10.1016/j.semcancer.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Jiang Z, Liu Z, Li M, et al. Immunogenomics analysis reveals that TP53 mutations inhibit tumor immunity in gastric cancer. Transl Oncol. 2018;11:1171–1187. doi: 10.1016/j.tranon.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao W, Du N, Huang T, et al. TP53 mutation as potential negative predictor for response of anti-CTLA-4 therapy in metastatic melanoma. EBioMedicine. 2018;32:119–124. doi: 10.1016/j.ebiom.2018.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. doi: 10.18632/oncotarget.19048. Zheng HC: The molecular mechanisms of chemoresistance in cancers. Oncotarget 8:59950-59964, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giannakis M, Mu XJ, Shukla SA, et al. Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Reports. 2016;15:857–865. doi: 10.1016/j.celrep.2016.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]