Abstract
PURPOSE
Operable triple-negative breast cancers (TNBCs) have a higher risk of relapse than non-TNBCs with standard therapy. The GEICAM/2003-11_CIBOMA/2004-01 trial explored extended adjuvant capecitabine after completion of standard chemotherapy in patients with early TNBC.
PATIENTS AND METHODS
Eligible patients were those with operable, node-positive—or node negative with tumor 1 cm or greater—TNBC, with prior anthracycline- and/or taxane-containing chemotherapy. After central confirmation of TNBC status by immunohistochemistry, patients were randomly assigned to either capecitabine or observation. Stratification factors included institution, prior taxane-based therapy, involved axillary lymph nodes, and centrally determined phenotype (basal v nonbasal, according to cytokeratins 5/6 and/or epidermal growth factor receptor positivity by immunohistochemistry). The primary objective was to compare disease-free survival (DFS) between both arms.
RESULTS
Eight hundred seventy-six patients were randomly assigned to capecitabine (n = 448) or observation (n = 428). Median age was 49 years, 55.9% were lymph node negative, 73.9% had a basal phenotype, and 67.5% received previous anthracyclines plus taxanes. Median length of follow-up was 7.3 years. DFS was not significantly prolonged with capecitabine versus observation [hazard ratio (HR), 0.82; 95% CI, 0.63 to 1.06; P = .136]. In a preplanned subgroup analysis, nonbasal patients seemed to derive benefit from the addition of capecitabine with a DFS HR of 0.53 versus 0.94 in those with basal phenotype (interaction test P = .0694) and an HR for overall survival of 0.42 versus 1.23 in basal phenotype (interaction test P = .0052). Tolerance of capecitabine was as expected, with 75.2% of patients completing the planned 8 cycles.
CONCLUSION
This study failed to show a statistically significant increase in DFS by adding extended capecitabine to standard chemotherapy in patients with early TNBC. In a preplanned subset analysis, patients with nonbasal phenotype seemed to obtain benefit with capecitabine, although this will require additional validation.
INTRODUCTION
Early triple-negative breast cancer (TNBC) can be cured with local–regional therapy plus adjuvant chemotherapy, usually anthracycline- and/or taxane-based combinations. However, despite these therapies, a proportion of patients eventually experience relapse and die. A recent analysis of data from the National Cancer Institute SEER reported a 3-year relapse rate of approximately 8%, 15%, and 40% for patients with stages I, II, and III TNBC,1 respectively; therefore, new adjuvant options are necessary to improve the prognosis of this breast cancer subtype.
Capecitabine is an oral prodrug of fluorouracil approved for the treatment of metastatic breast cancer in patients with prior progression after anthracyclines and taxanes and is therefore partially non–cross resistant with these two classes of agents.2 On the basis of this concept, we carried out a trial in which capecitabine was sequentially added to standard (neo)adjuvant chemotherapy in operable TNBC to explore the ability of the drug to reduce the rate of relapse and increase the survival of this disease.
PATIENTS AND METHODS
The GEICAM/2003-11_CIBOMA/2004-01 trial is an open-label, randomized phase III study that was conducted in compliance with the International Council for Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki. The study was reviewed and approved by the independent ethics committees or institutional review boards of all participating institutions. Written informed consent was obtained from all patients before any study-related procedures were performed.
Patient Eligibility
Eligible patients included women with hormone receptor–negative (immunohistochemistry staining of estrogen and progesterone receptors < 1%) and human epidermal growth factor 2–negative operable breast cancer, with invasive adenocarcinoma histologically confirmed. Patients had received 6 to 8 cycles of standard anthracycline- and/or taxane-containing chemotherapy in the (neo)adjuvant setting, followed by radiation therapy according to institutional guidelines. In the case of node-negative disease, 4 cycles of doxorubicin and cyclophosphamide were allowed. Eligible patients were those with ipsilateral axillary node involvement classified as pN1a, pN2a, or pN3a—excluding metastatic infraclavicular lymph nodes—according to the American Joint Committee on Cancer 2002 staging system. Patients without axillary node involvement (N0) were also eligible provided the primary tumor measured 1 cm or greater in diameter. Patients were ineligible in the case of bilateral invasive breast cancer, absence of surgical treatment with curative intent, resection of fewer than 6 lymph nodes when axillary lymph node dissection was performed, or pregnancy or breastfeeding. Triple-negative and basal versus nonbasal status were determined centrally by a GEICAM Spanish Breast Cancer Group pathologist (F.R.). Tumors with any staining for epidermal growth factor receptor and/or cytokeratins 5/6 were considered basal. Patients with no staining for both biomarkers were considered nonbasal.3
Study Procedures
Baseline assessments performed before patient randomization in centrally confirmed eligible patients included mammography, chest radiography, abdominal ultrasonography and/or computed tomography (CT), bone scan (if bone pain or increased alkaline phosphatase), and bone X-ray (if suspicious lesions on the bone scan). Hematology, biochemistry, and pregnancy test—potentially fertile women only—were also completed before randomization.
Eligible patients were stratified according to basal status (yes v no), institution, number of axillary lymph nodes (0 v 1-3 v 4 or more), and type of adjuvant chemotherapy (anthracyclines plus taxanes v anthracyclines alone). Patients were randomly assigned on a one-to-one basis to eight cycles of capecitabine 2,000 mg/m2 (1,000 mg/m2 administered orally two times per day) on days 1 to 14 every 3 weeks, or observation. Two dose reductions were permitted—75% and 50% of initial dose—on the basis of hematologic or nonhematologic adverse events (AEs) observed. Randomization was centralized at GEICAM headquarters.
AEs were assessed during the study period and graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. Serious AEs (SAEs) were reported during the study treatment and within 30 days of the end of this time period.
Physical examination, menopausal status, and presence of amenorrhea were assessed at baseline, at every cycle during the treatment period, every 3 months during years 1 to 2, every 6 months during years 3 to 5, and yearly afterward. Mammograms were performed yearly. Chest X-ray, abdominal ultrasounds, CT scan, or bone scans were performed if clinically indicated in the case of suspicion of disease recurrence. A complete follow-up on vital status was obtained until April 11, 2018, for all patients.
Statistical Analysis
Primary end point was disease-free survival (DFS), which was measured from the date of random assignment in the intent-to-treat (ITT) population to locoregional or distant recurrence, second primary malignancy, or death date, whichever occurred first. Secondary end points included 5-year DFS, overall survival (OS), safety, and analyses by subgroups and of biomarkers.
According to the GEICAM El Álamo registry,4 estimated 5-year DFS for a similar population of patients with TNBC was 64.7%. The aim was to detect an increase in DFS to 73.7% with capecitabine, corresponding to a hazard ratio (HR) of 0.701 with a power of 80% using a two-tailed log-rank test at 0.05 and considering 4 years of recruitment and 3 years of follow-up. Two hundred fifty-five events were projected and 834 eligible patients were needed. Assuming a drop-out rate of 5%, 876 patients were to be enrolled—438 patients in each arm. The sample size calculation was performed using EAST version 5.2.
The initial protocol established the main DFS analysis to be performed after 255 events; however, the number of DFS events was much lower than expected and the steering committee of the study—with the advice of the independent data monitoring committee—therefore decided to perform the analysis after a median follow-up of more than 7 years after a total of 225 events and when the rate of annual recurrences was low in both arms of the study.
We used the Kaplan-Meier limit-product method to estimate DFS and OS and comparison between the two study arms was performed using the stratified log-rank test using the stratification factors [basal status, number of axillary lymph nodes, and type of (neo)adjuvant chemotherapy]. All tests of hypotheses were two sided. In addition, we performed a multivariable Cox proportional hazard model analysis for DFS and OS to adjust for major prognostic factors: age, menopausal status, histopathologic findings, tumor size, disease stage, type of surgery, region, and the stratification factors for randomization.
The safety analysis was performed in all patients who had received at least 1 cycle of study treatment. The worst AE grade per category for each patient was reported.
RESULTS
Study Patients
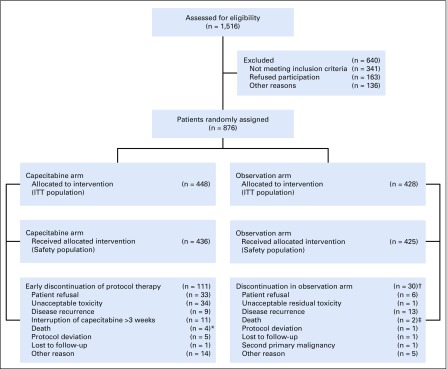
Between October 2006 and September 2011, 876 patients were recruited across 80 institutions in 8 countries (Spain, Brazil, Chile, Colombia, Ecuador, Mexico, Peru, and Venezuela; Fig 1).
FIG 1.
All patients enrolled (N = 876) were included in the efficacy analyses. All patients who had received at least 1 cycle of study treatment (n = 861) were evaluated for safety. Safety population: In the capecitabine arm, all patients who have completed at least one cycle of study treatment and in the observation arm, all patients with a follow-up period ≥ 14 days.
*Reasons of death on these patients: psychiatric disorder, cerebral hemorrhage, septic shock secondary to respiratory infection and stroke (not related with capecitabine).
†Discontinuation of initial follow-up period (equivalent to treatment period in capecitabine arm).
‡Reasons of death on these patients: acute myocardial infarction and pulmonary sepsis.
Demographics and baseline disease characteristics were balanced, with no statistical differences between the two arms but with a slightly numerically higher proportion of poor prognosis features in the capecitabine arm (Table 1). The majority of patients were white and postmenopausal, and median age was 49 years. Most frequently, tumors were of grade 3 (71%), basal phenotype (73.9%), stage II at diagnosis (61.8%), and node negative (55.9%). Small differences were found in relation to disease stage at diagnosis—based on American Joint Committee on Cancer 2002—and the number of involved lymph nodes.
TABLE 1.
Patients’ Demographics and Baseline Disease Characteristics According to Study Arm (intention-to-treat population)
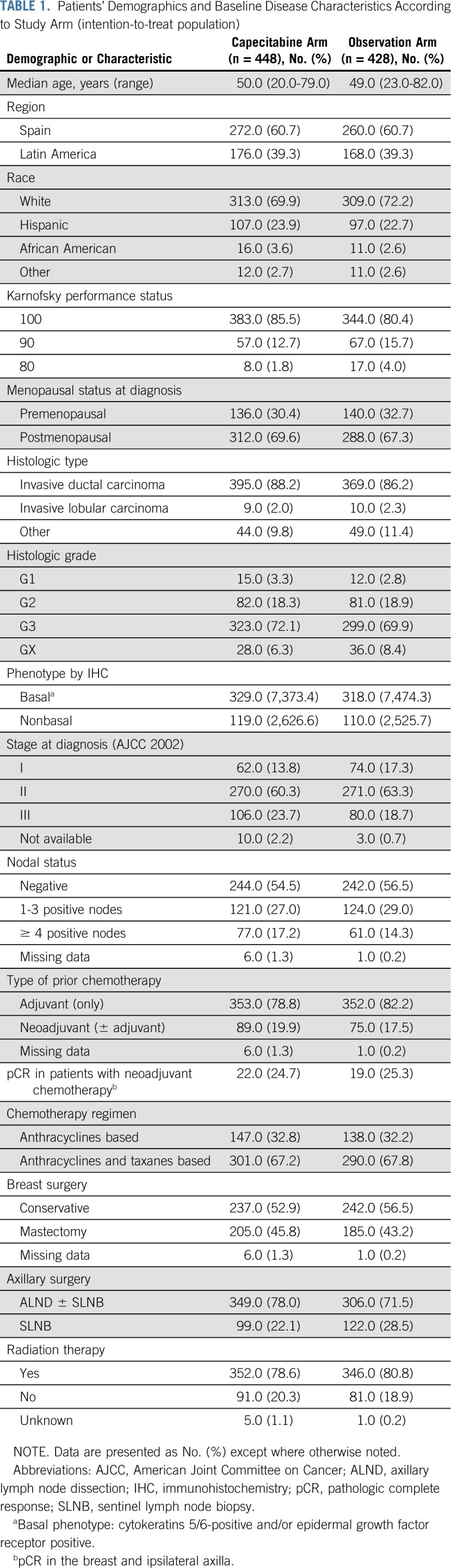
Most patients in both arms had received adjuvant chemotherapy with anthracyclines and taxanes (67.5%), had breast-conservative surgery (54.7%) and axillary lymph node dissection with or without sentinel lymph node biopsy (74.8%), and received radiation therapy (79.7%).
Drug Exposure and Discontinuations
Of patients who were assigned to capecitabine, 75.2% (n = 337) completed 8 cycles of treatment. Median number of cycles was 8 (range, 1 to 8 cycles). A few patients (n = 12; 2.7%) did not complete at least 1 cycle of treatment and were excluded from the safety analysis as per protocol requirement. Four percent (n = 18) of patients completed 1 cycle of capecitabine, 10.3% (n = 46) of patients completed 2 to 4 cycles, and 7.8% (n = 35) of patients completed 5 to 7 cycles. Median dose intensity was 86.3% (range, 0.86% to 136.2%). Dose intensity rate was between 110% and 80% in 55.8% (n = 250) of patients and less than 75% in 33.5% (n = 150) of patients. Five patients were reported to have a dose intensity rate between 110% and 136.2%. In four of these patients, it was because of a mistake or rounding the capecitabine dose and in the fifth patient the reason is unknown. Dose reductions of capecitabine were reported in 161 patients (36.9%).
Main reasons for discontinuation of capecitabine (n = 97) were patient refusal, unacceptable toxicity, disease recurrence, or interruption of capecitabine for more than 3 weeks because of AEs (Fig 1).
Efficacy
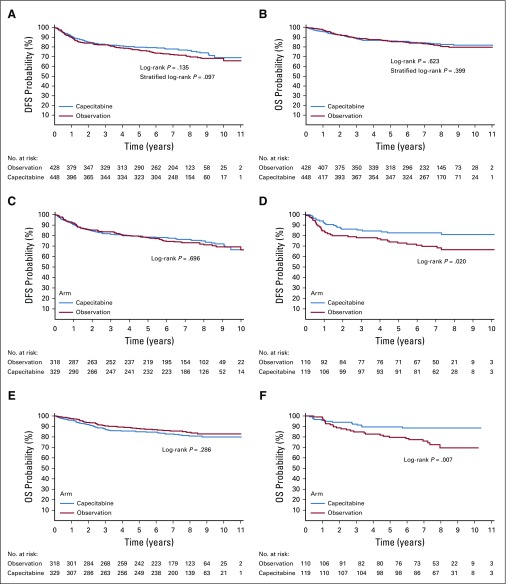
At the time of data cutoff, median follow-up was 7.4 years and 7.2 years in the capecitabine and observation arms, respectively. A total of 225 events were observed, 105 (23.4%) in the capecitabine arm and 120 (28%) in the observation arm. In the ITT analysis (n = 876), results of the Cox proportional hazards regression model did not demonstrate a statistically significant difference in DFS between the capecitabine and observation arms (unadjusted HR, 0.82; 95% CI, 0.63 to 1.06; P = .136; adjusted HR according to stratification factors, 0.79; 95% CI, 0.61 to 1.03; P = .082; and fully adjusted HR, 0.77; 95% CI, 0.59 to 1.00; P = .051). Five-year DFS rates were 79.6% (95% CI, 75.8% to 83.4%) in the capecitabine arm and 76.8% (95% CI, 72.7% to 80.9%) in the observation arm. Figure 2A shows the Kaplan-Meier curves for DFS. In addition, there was no statistically significant difference in OS between study arms (unadjusted HR, 0.92; 95% CI, 0.66 to 1.28; P = .623; adjusted HR according to stratification factors, 0.88; 95% CI, 0.64 to 1.23; P = .4562; and fully adjusted HR, 0.86; 95% CI, 0.6262 to 1.20; P = .371). Five-year OS rates were 86.2% (95% CI, 82.9% to 89.4%) in the capecitabine arm and 85.9% (95% CI, 82.4% to 89.3%) in the observation arm. Figure 2B shows the Kaplan-Meier curves for OS.
FIG 2.
(A, C, D) Disease-free survival (DFS) and (B, E, F) overall survival (OS) Kaplan-Meier curves on the intention-to-treat population and subpopulations based on the immunohistochemistry phenotype.
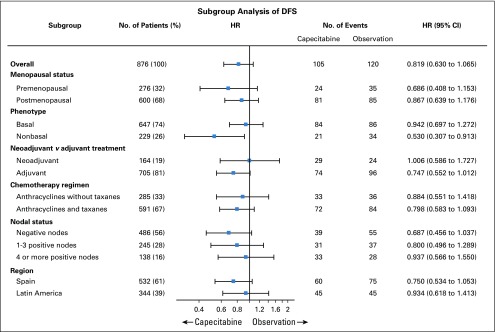
An exploratory subgroup analysis for DFS in the ITT population (Fig 3) showed similar treatment effects by menopausal status (pre- v postmenopausal), phenotype (basal v nonbasal), nodal status (negative v 1 to 3 positive nodes, 4 or more positive nodes), type of previous CT scan (neoadjuvant v adjuvant), prior administration of taxanes (yes v no) and region (Spain v Latin America). Patients with a nonbasal phenotype had a statistically significant increase in DFS (HR, 0.53; 95% CI, 0.31 to 0.91; P = .022) and OS (HR, 0.42; 95% CI, 0.21 to 0.81; P = .0095) with capecitabine. Five-year DFS rates were 82.6% (95% CI, 75.7% to 89.5%) with capecitabine and 72.9% (95% CI, 64.4% to 81.3%) in the observation arm. Five-year OS rates were 89.5% (95% CI, 83.9% to 95.1%) with capecitabine and 79.6% (95% CI, 71.7% to 87.4%) in the observation arm. The interaction tests treatment/nonbasal status had adjusted P values of .0694 for DFS and .0052 for OS. Figures 2C and 2D show the Kaplan-Meier curves for DFS and OS, respectively, in the basal and nonbasal phenotype subpopulations.
FIG 3.
Subgroup analysis for disease-free survival (DFS) on the intention-to-treat (ITT) population. HR, hazard ratio.
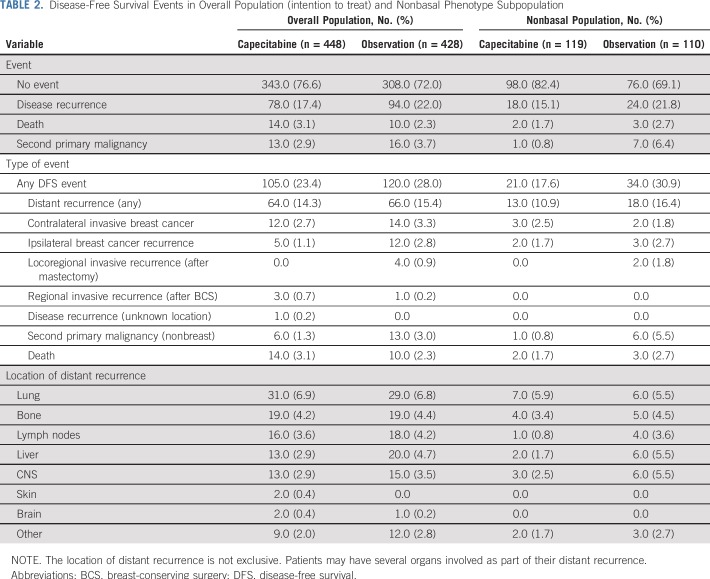
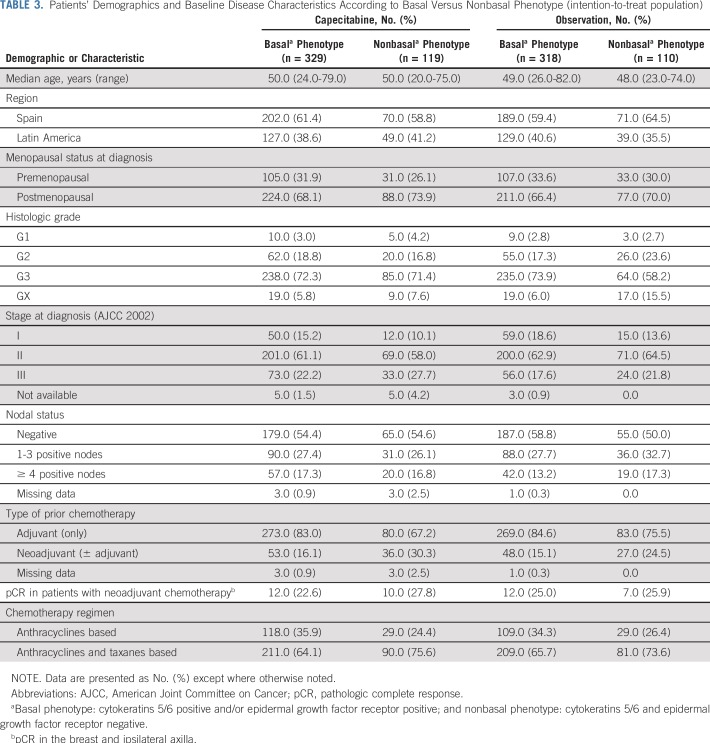
The number and type of DFS events in the overall and nonbasal populations are shown in Table 2. Of note, in the nonbasal subpopulation, and in agreement with DFS and OS data, DFS events were more frequent in the observation arm [30.9% (n = 34)] compared with the capecitabine arm [17.6% (n = 21)]. Remarkably, in this subtype the reduction of DFS events with capecitabine was mainly a result of distant relapses, particularly in liver, CNS, and lymph nodes. Demographics and baseline disease characteristics according to basal versus nonbasal phenotype are included in Table 3.
TABLE 2.
Disease-Free Survival Events in Overall Population (intention to treat) and Nonbasal Phenotype Subpopulation
TABLE 3.
Patients’ Demographics and Baseline Disease Characteristics According to Basal Versus Nonbasal Phenotype (intention-to-treat population)
Safety
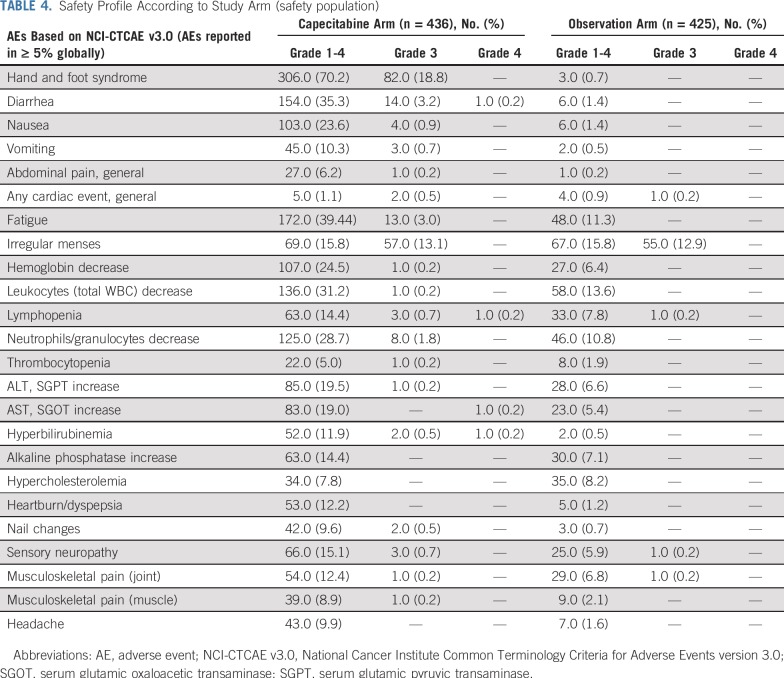
Safety was assessed in 861 patients of the study population: 436 patients (97.3%) in the capecitabine arm and 425 patients (99.3%) in the observation arm. AEs were reported in 95.4% (n = 416) of patients in the capecitabine arm and 63.8% (n = 271) in the observation arm (Table 4). In addition, 40.6% (n = 177) and 15.5% (n = 66) of patients had AEs of grade 3 or greater in the capecitabine and observation arms, respectively. With capecitabine, 92.4% (n = 403) of patients had AEs related to study treatment, and in 30% (n = 131) of patients these AEs were of grade 3 or greater. Patients who experienced at least one SAE were 5.3% (n = 23) in the capecitabine arm and 1.4% (n = 6) in the observation arm. In addition, there were a few patients with SAEs that caused death: 1.1% (n = 5) in the capecitabine arm and 0.5% (n = 2) in the observation arm. Two deaths in the capecitabine arm were probably related to study treatment according to investigator’s criteria. These SAEs included one case of septic shock in the absence of neutropenia and one case of grade 4 hyperbilirubinemia and systemic organ failure.
TABLE 4.
Safety Profile According to Study Arm (safety population)
DISCUSSION
Our study failed to show a statistically significant improvement in DFS by adding 8 cycles of extended capecitabine to standard (neo)adjuvant chemotherapy for operable TNBC after a median follow-up of more than 7 years. Adjusted HR, considering the stratification factors, was 0.79 (P = .082); therefore, the study was formally negative in accordance with the statistical assumption made when the trial was designed. The role of capecitabine in combination with other drugs as adjuvant therapy of operable breast cancer is still unclear.5-7 As a single agent, adjuvant capecitabine was inferior to standard adjuvant therapy—either cyclophosphamide, methotrexate, fluorouracil; or doxorubicin plus cyclophosphamide—in patients with breast cancer age 65 years or older.8 A recent meta-analysis of (neo)adjuvant capecitabine trials, including 8 trials and 9,302 patients, found that globally, capecitabine did not improve DFS9; however, in trials in which capecitabine was added to standard adjuvant chemotherapy (in contrast to those trials in which capecitabine replaced standard agents), a significant DFS advantage was found. Of note, in this meta-analysis the benefit of adding capecitabine to standard chemotherapy was mainly observed in patients with TNBC. The GEICAM-CIBOMA study added sequential capecitabine to standard (neo)adjuvant chemotherapy in patients with operable TNBC but was unable to show a statistically significant improvement in DFS in the overall population. The results of our study were therefore apparently different from those of the meta-analysis and, in particular, from those of the the Capecitabine for Residual Cancer as Adjuvant Therapy (CREATE-X) trial,10 which addressed a similar question but in a population at higher risk of relapse. The CREATE-X trial randomly assigned patients with breast cancer with residual disease at surgery after standard neoadjuvant chemotherapy to 6 to 8 cycles of capecitabine versus observation—plus hormone therapy in both arms for patients with hormone receptor–positive tumors—and found a statistically significant increase in DFS and OS with capecitabine. The effect was particularly remarkable in TNBC. Compared with our trial, the populations in both studies were significantly different, as Asian patients had a significantly higher risk of relapse, as shown by the 56.1% 5-year DFS for patients with TNBC in the control arm of the CREATE-X trial. In contrast, DFS in the control arm in our trial was better than expected: DFS at 5 years was 76.8%, while our statistical hypothesis assumed a 5-year DFS of 64.7% on the basis of historical controls.
Moreover, the selection criteria in the CREATE-X trial limited to patients with residual disease after neoadjuvant treatment suggest that capecitabine could have a more relevant role in patients with tumors that are less sensitive or partially resistant to regimens containing anthracyclines and taxanes.
The DFS events in our trial were numerically higher in the control group (n = 120) than in the capecitabine group (n = 105), arguably because of the apparent efficacy of capecitabine in a predefined subgroup of patients—being a stratification factor as well—with nonbasal phenotype, as defined by central immunohistochemistry (lack of staining for epidermal growth factor receptor and cytokeratins 5/6). In these patients, both DFS and OS were statistically superior with capecitabine. TNBC is a heterogeneous disease that encompasses a wide spectrum of clinical and molecular subtypes with different sensitivity to standard therapies,11 The results in the nonbasal subgroup suggest that the activity of capecitabine might be selective for this particular subset of patients, although a validation of this hypothesis in other TNBC adjuvant trials exploring capecitabine is necessary to confirm the finding. Perhaps capecitabine is less effective in basal-like tumors as these are highly proliferative tumors and more sensitive to taxanes carboplatin and eribulin, as seen in the TNT trial comparing docetaxel with carboplatin12 and the 301 study comparing eribulin with capecitabine,13 whereas nonbasal tumors could be more sensitive to an antimetabolite drug, such as capecitabine, as they have a lower proliferation index.
The tolerability of capecitabine was as expected, with a median dose intensity of 86.3% and 75.2% of patients receiving the planned 8 cycles of therapy.
Among the limitations of this trial, we can include the fact that it was an open-label study. In addition, the population enrolled demonstrated a much lower recurrence rate than expected. The latter finding has been observed in other recent adjuvant trials and compromises the ability to show a difference between treatment strategies. In contrast, designed in 2002 to 2003, this was one of the first trials, to our knowledge, devoted to this specific subtype of breast cancer.
In conclusion, the GEICAM-CIBOMA study failed to show a statistically significant improvement in DFS by adding capecitabine to standard (neo)adjuvant chemotherapy for operable TNBC. An apparent improvement in DFS and OS was observed in the nonbasal subset of patients, although the real implication of this finding should be further confirmed with the analysis of other (neo)adjuvant capecitabine trials.
ACKNOWLEDGMENT
The authors thank the investigators involved in the GEICAM/2003-11_CIBOMA/2004-01 study: J. Corona, C. Jara, B. Cardemil, R. Toro, C. Pimentel, B. Hernando, E. Vicente, L. Zagame, M. Gil, L. García Estévez, C. Rodríguez, M.A. de la Cruz, J.M. Tello, S. Campos, M. Lomas, D. Capdevile, M. Campos, M. Margelí, R. Andrés, I. Tusquets, A. Ballesteros, A. Guerrero, M. Arguello, J.L. Rodríguez, M. Muñoz, J. Florian, S. Azevedo, R. Mondragón, J. Peralta, A.E. Palomo, L.J. Barajas, A. Arcusa, H. Carranza, C. García, C. Umbría, José E. Alés, J.M. López Vega, M. Romeo, J. Valero, J.L. Alonso, C. Mathias, F. Gutierrez, E. Adrover, P. Nuñez, C. Mendiola, and J. Cassinello, A. de la Huerta. We acknowledge Dr Javier Castellanos and his contribution to this study in life.
Footnotes
Presented in part at the 15th European Cancer Organization and 34th European Society for Medical Oncology Multidisciplinary Congress, Berlin, Germany, September 20-24, 2009; the 33rd Annual San Antonio Breast Cancer Symposium, San Antonio, TX, December 8-12, 2010; the 47th American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 3-7, 2011; the 8th European Breast Cancer Conference, Vienna, Austria, March 21-24, 2012; the 36th Annual San Antonio Breast Cancer Symposium, San Antonio, TX, December 10-14, 2013; and 2018 San Antonio Breast Cancer Symposium, San Antonio, TX, December 4-8, 2018.
Written on behalf of GEICAM Spanish Breast Cancer Group, CIBOMA (Iberoamerican Coalition for Research in Breast Oncology), and LACOG (Latin American Cooperative Oncology Group). Funded by F Hoffmann-La Roche. The company also contributed with the study drug (capecitabine; Xeloda), but were not involved in study design, data collection, data analyses or interpretation, or writing of this report.
See accompanying Editorial on page 179
AUTHOR CONTRIBUTIONS
Conception and design: Ana Lluch, Carlos H. Barrios, Laura Torrecillas Miguel Martín
Provision of study materials or patients: Ana Lluch, Carlos H. Barrios, Laura Torrecillas, Manuel Ruiz-Borrego, Jose Bines, Jose Segalla, Ángel Guerrero-Zotano, Jose A. García-Sáenz, Roberto Torres, Juan de la Haba, Elena García-Martínez, Henry L. Gómez, Antonio Llombart, Javier Salvador Bofill, José M. Baena-Cañada, Agustí Barnadas, Lourdes Calvo, Laura Pérez-Michel, Manuel Ramos, Isaura Fernández, Álvaro Rodríguez-Lescure, Jesús Cárdenas, Jeferson Vinholes, Eduardo Martínez de Dueñas, Maria J. Godes, Miguel A. Seguí, Antonio Antón, Pilar López-Álvarez, Jorge Moncayo, Gilberto Amorim, Esther Villar, Salvador Reyes, Carlos Sampaio, Bernardita Cardemil, Miguel Martín
Collection and assembly of data: Ana Lluch, Carlos H. Barrios, Laura Torrecillas, Manuel Ruiz-Borrego, Jose Bines, Jose Segalla, Ángel Guerrero-Zotano, Jose A. García-Sáenz, Roberto Torres, Juan de la Haba, Elena García-Martínez, Henry L. Gómez, Antonio Llombart, Javier Salvador Bofill, José M. Baena-Cañada, Agustí Barnadas, Lourdes Calvo, Laura Pérez-Michel, Manuel Ramos, Isaura Fernández, Álvaro Rodríguez-Lescure, Jesús Cárdenas, Jeferson Vinholes, Eduardo Martínez de Dueñas, María J. Godes, Miguel A. Seguí, Antonio Antón, Pilar López-Álvarez, Jorge Moncayo, Gilberto Amorim, Esther Villar, Salvador Reyes, Carlos Sampaio, Bernardita Cardemil, Miguel Martín
Data analysis and interpretation: Ana Lluch, Carlos H. Barrios, Laura Torrecillas, Maria J. Escudero, Susana Bezares, Eva Carrasco, Miguel Martín
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase III Trial of Adjuvant Capecitabine After Standard Neo-/Adjuvant Chemotherapy in Patients With Early Triple-Negative Breast Cancer (GEICAM/2003-11_CIBOMA/2004-01)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ana Lluch
Consulting or Advisory Role: Novartis, Pfizer, Genentech, Eisai, Celgene
Research Funding: Roche (Inst), AstraZeneca (Inst), Merck (Inst), PharmaMar (Inst), Boehringer Ingelheim (Inst), Amgen (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Pfizer (Inst), Eisai (Inst), Celgene (Inst), Pierre Fabre (Inst)
Travel, Accommodations, Expenses: Genentech, Novartis
Carlos H. Barrios
Stock and Other Ownership Interests: Biomarker, MedSIR, Tummi
Honoraria: Novartis, Genentech, Pfizer, GlaxoSmithKline, Sanofi, Boehringer Ingelheim, Eisai
Consulting or Advisory Role: Boehringer Ingelheim, Genentech, Novartis, GlaxoSmithKline, Eisai, Pfizer, AstraZeneca, Libbs, MSD Oncology, United Medical
Research Funding: Pfizer, Novartis, Amgen, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Genentech, Eli Lilly, Sanofi, GlaxoSmithKline, Taiho Pharmaceutical, Mylan, Merrimack Pharmaceuticals, Merck, AbbVie, Astellas Pharma, Biomarin, Bristol-Myers Squibb, Daiichi Sankyo, Abraxis BioScience, AB Science, Asana Biosciences, Medivation, Daiichi Sankyo, Exelixis, ImClone Systems, LEO Pharma, Millennium Pharmaceuticals, Janssen Pharmaceuticals, Atlantis Clinica, INC Research, Halozyme, Covance, Celgene, Celgene, inVentiv Health
Travel, Accommodations, Expenses: Genentech, Novartis, Pfizer, Bristol-Myers Squibb, AstraZeneca, MSD Oncology
Laura Torrecillas
Consulting or Advisory Role: Pfizer, Roche, Merck, Bristol-Myers Squibb, Bayer
Speakers' Bureau: Pfizer, Roche, Eli Lilly, Amgen, Bayer
Travel, Accommodations, Expenses: Pfizer, Roche, Merck, Ipsen
Manuel Ruiz-Borrego
Honoraria: Genentech
Consulting or Advisory Role: Roche
Jose Bines
Honoraria: Roche
Consulting or Advisory Role: Roche
Travel, Accommodations, Expenses: Roche
Ángel Guerrero-Zotano
Consulting or Advisory Role: AstraZeneca, Novartis
Speakers' Bureau: Novartis, Roche, Pfizer, AstraZeneca
Research Funding: Pfizer (Inst)
Travel, Accommodations, Expenses: Roche, Pfizer, Novartis
Jose A. García-Sáenz
Consulting or Advisory Role: Novartis, Celgene, AstraZeneca, Eli Lilly
Research Funding: AstraZeneca (Inst)
Travel, Accommodations, Expenses: Roche, Novartis
Juan de la Haba
Speakers' Bureau: Roche, Novartis, Pfizer
Travel, Accommodations, Expenses: Roche, Novartis
Elena García-Martínez
Consulting or Advisory Role: AstraZeneca, Roche
Travel, Accommodations, Expenses: Roche, PharmaMar, AstraZeneca, Pfizer, Bristol-Myers Squibb
Henry L. Gómez
Speakers' Bureau: Roche, Novartis, AstraZeneca, Bristol-Myers Squibb
Antonio Llombart
Honoraria: Roche, Eli Lilly, AstraZeneca, Amgen, Novartis, Pfizer
Consulting or Advisory Role: Novartis, Pfizer, Roche, Eli Lilly, AstraZeneca, Eisai
Research Funding: Pfizer, Genentech, Tesaro, Novartis, Eisai
Travel, Accommodations, Expenses: Roche, Pfizer, Celgene, Eli Lilly
Other Relationship: MedSIR
José M. Baena-Cañada
Consulting or Advisory Role: Roche, Pfizer, Novartis, Grünenthal Group, Eisai
Travel, Accommodations, Expenses: Roche, Pfizer, MSD Oncology
Agustí Barnadas
Honoraria: Pfizer
Consulting or Advisory Role: Pfizer, Novartis, Eli Lilly
Speakers' Bureau: Roche, Pfizer, Novartis, Genomic Health International
Travel, Accommodations, Expenses: Roche, Pfizer
Manuel Ramos
Consulting or Advisory Role: Novartis
Speakers' Bureau: AstraZeneca, Roche, Novartis, Pfizer
Travel, Accommodations, Expenses: Pfizer
Álvaro Rodríguez-Lescure
Honoraria: Roche, Pfizer, Novartis, Mylan
Consulting or Advisory Role: Roche, Pfizer, Novartis, AstraZeneca, Mylan
Speakers' Bureau: Pfizer, Novartis, Roche, Kern Pharma
Research Funding: Roche (Inst), Novartis (Inst), Pfizer (Inst), Eli Lilly (Inst)
Expert Testimony: Pfizer, Novartis, Roche, Kern Pharma
Travel, Accommodations, Expenses: Roche, Pfizer
Jeferson Vinholes
Research Funding: Roche
Travel, Accommodations, Expenses: Roche
Eduardo Martínez de Dueñas
Consulting or Advisory Role: Novartis, Roche
Travel, Accommodations, Expenses: Roche
Miguel A. Seguí
Consulting or Advisory Role: Roche, Pfizer, Novartis, Amgen, Eisai, Eli Lilly
Speakers' Bureau: Roche, Pfizer, Amgen
Research Funding: Roche (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Roche, Pfizer, Novartis, Amgen
Antonio Antón
Consulting or Advisory Role: Bayer
Gilberto Amorim
Honoraria: Roche, Novartis, Eli Lilly, Sanofi
Consulting or Advisory Role: Novartis, Roche
Travel, Accommodations, Expenses: Roche
Carlos Sampaio
Employment: Clinica AMO
Leadership: Clinica AMO
Stock and Other Ownership Interests: Clinica AMO
Consulting or Advisory Role: Pneuma Respiratory
Susana Bezares
Employment: Eli Lilly (I)
Stock and Other Ownership Interests: Eli Lilly (I)
Eva Carrasco
Stock and Other Ownership Interests: Eli Lilly
Consulting or Advisory Role: Bristol-Myers Squibb (I), Novartis (I), Celgene (I), Roche (I), Janssen Pharmaceuticals (I), Amgen (I), Pfizer (I), Incyte (I), AbbVie (I)
Research Funding: Genentech (Inst), Novartis (Inst), Pfizer (Inst), Bristol-Myers Squibb (Inst), Celgene (Inst), AstraZeneca (Inst), Merck Sharp & Dohme (Inst), AstraZeneca (Inst), Pierre Fabre (Inst), Takeda (Inst), Celgene (I), Janssen Pharmaceuticals (I), Genentech (I), Novartis (I), Bristol-Myers Squibb (I), Amgen (I), Pfizer (I), AbbVie (I)
Patents, Royalties, Other Intellectual Property: PAM 50 taxane predictor
Travel, Accommodations, Expenses: Roche, Novartis (I), Bristol-Myers Squibb (I), Celgene (I)
Miguel Martín
Consulting or Advisory Role: Genentech, Novartis, Pfizer, Eli Lilly, AstraZeneca, Taiho Pharmaceutical, PharmaMar
Research Funding: Novartis (Inst), Roche (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Li X, Yang J, Peng L, et al. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res Treat. 2017;161:279–287. doi: 10.1007/s10549-016-4059-6. [DOI] [PubMed] [Google Scholar]
- 2.Walko CM, Lindley C. Capecitabine: A review. Clin Ther. 2005;27:23–44. doi: 10.1016/j.clinthera.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Cheang MC, Voduc D, Bajdik C, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 4.GEICAM Spanish Breast Cancer Group . Proyecto El Álamo III. Encuesta de Evolución de Pacientes Con Cáncer de Mama en Hospitales del Grupo GEICAM (1998-2001) Madrid, Spain: GEICAM Spanish Breast Cancer Group; 2014. [Google Scholar]
- 5.Martín M, Ruiz Simón A, Ruiz Borrego M, et al. Epirubicin plus cyclophosphamide followed by docetaxel versus epirubicin plus docetaxel followed by capecitabine as adjuvant therapy for node-positive early breast cancer: Results from the GEICAM/2003-10 study. J Clin Oncol. 2015;33:3788–3795. doi: 10.1200/JCO.2015.61.9510. [DOI] [PubMed] [Google Scholar]
- 6.Joensuu H, Kellokumpu-Lehtinen PL, Huovinen R, et al. Adjuvant capecitabine, docetaxel, cyclophosphamide, and epirubicin for early breast cancer: Final analysis of the randomized FinXX trial. J Clin Oncol. 2012;30:11–18. doi: 10.1200/JCO.2011.35.4639. [DOI] [PubMed] [Google Scholar]
- 7.O’Shaughnessy J, Koeppen H, Xiao Y, et al. Patients with slowly proliferative early breast cancer have low five-year recurrence rates in a phase III adjuvant trial of capecitabine. Clin Cancer Res. 2015;21:4305–4311. doi: 10.1158/1078-0432.CCR-15-0636. [DOI] [PubMed] [Google Scholar]
- 8.Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360:2055–2065. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Natori A, Ethier JL, Amir E, et al. Capecitabine in early breast cancer: A meta-analysis of randomised controlled trials. Eur J Cancer. 2017;77:40–47. doi: 10.1016/j.ejca.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 10.Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147–2159. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 11.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 12.Tutt A, Tovey H, Cheang MCU, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: The TNT Trial. Nat Med. 2018;24:628–637. doi: 10.1038/s41591-018-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufman PA, Awada A, Twelves C, et al. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2015;33:594–601. doi: 10.1200/JCO.2013.52.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]