Abstract
PURPOSE
Nodular desmoplastic medulloblastoma (ND) and medulloblastoma with extensive nodularity (MBEN) have been associated with a more favorable outcome in younger children. However, treatment-related neurotoxicity remains a significant concern in this vulnerable group of patients.
PATIENTS AND METHODS
ACNS1221 was a prospective single-arm trial of conventional chemotherapy for nonmetastatic ND and MBEN based on a modified HIT SKK 2000 regimen excluding intraventricular methotrexate, aiming to achieve similar outcome (2-year progression-free survival [PFS] ≥ 90%) with reduced treatment-related neurotoxicity. Secondary objectives included feasibility of timely central pathology review and evaluation of tumor molecular profile.
RESULTS
Twenty-five eligible patients (15 males and 10 females; median age, 18.7 months) were enrolled. Eighteen patients had ND and 7 had MBEN histology. Three patients had residual disease at baseline. The study closed early because of a higher than expected relapse rate. Twelve patients experienced relapse—local (n= 6), distant (n = 3), and combined (n = 3)—at a median of 9.8 months from diagnosis (range, 8.9-13.7 months), and 2 patients died of disease. Two-year PFS and overall survival rates were 52% (95% CI, 32.4% to 71.6%) and 92% (95% CI, 80.8% to 100.0%) respectively. Patients older than 12 months of age (P = .036) and ND histology (P = .005) were associated with worse PFS. No patients with MBEN histology experienced relapse. All tumor samples clustered within the sonic hedgehog (SHH) group. Methylation analysis delineated 2 subgroups, SHH-I and SHH-II, which were associated with 2-year PFS rates of 30.0% (95% CI, 1.6% to 58.4%) and 66.7% (95% CI, 44.0% to 89.4%), respectively (P = .099).
CONCLUSION
The proposed modified regimen of conventional systemic chemotherapy without serial intraventricular methotrexate injection failed to achieve the targeted 2-year PFS of 90%. With this cohort, we prospectively confirmed the existence of two SHH subgroups and observed a trend toward worse outcome for SHH-I patients.
INTRODUCTION
Medulloblastoma in early childhood constitutes a significant therapeutic challenge because of the greater vulnerability of the developing brain to cranial irradiation. The past 3 decades of clinical trials for young children with brain tumors have explored alternatives to delay or avoid the use of cranial radiation to preserve neurocognitive function and brought to light prognostic factors such as the nodular desmoplastic (ND) histology for medulloblastoma to risk-stratify therapy.1,2 Two reports by the German Pediatric Brain Study Group first described the favorable outcome associated with ND histology, characterized by a nodular architecture and a network of internodular collagen fibers, in younger children treated with chemotherapy including systemic high-dose methotrexate and intraventricular methotrexate (HIT SKK), with 5-year progression-free survival (PFS) rates of 95% and 34% for classic and large-cell anaplastic medulloblastoma subtypes, respectively.3,4 The prognostic value of ND histology was further confirmed retrospectively in several North American trials using different treatment modalities (high-dose chemotherapy or conventional chemotherapy with or without focal irradiation).5-7 At the time the ACNS1221 study was written, the HIT SKK 2000 regimen was deemed to offer the best efficacy with a shorter duration of treatment. However, given the report of associated leukoencephalopathy described on imaging, the study team wanted to evaluate the avoidance of intraventricular methotrexate. Our primary objective was to investigate whether a PFS similar to that reported with the HIT SKK 2000 protocol could be achieved without incorporating intraventricular methotrexate. Secondary objectives included the feasibility of rapid central pathology review for treatment eligibility and prospective evaluation of the molecular profile of ND and medulloblastoma with extensive nodularity (MBEN) tumors.
PATIENTS AND METHODS
ACNS1221 was a phase II trial designed for young children with newly diagnosed localized ND medulloblastoma and MBEN (ClinicalTrials.gov identifier: NCT02017964). The study was approved by each of the participating institutions.
Central Pathology Review
After informed consent, patients diagnosed with medulloblastoma at their treating institution underwent a prescreening central pathology review using the virtual microscopy online automated pathology review system (VIPER, Deep Lens, Columbus, OH) at the Children’s Oncology Group (COG) Biopathology Center. The diagnosis of ND or MBEN was confirmed if at least 2 of the 3 pathologists on the panel (C.H., C.E., and C.H.) agreed on the diagnosis. Turnaround time was to be < 10 days.
Eligibility Criteria
Patients < 4 years of age with centrally confirmed ND or MBEN, without metastasis by CSF analysis and spinal and brain magnetic resonance imaging, regardless of the extent of resection, were eligible to enroll. Patients had to have adequate organ function, defined as absolute neutrophil count ≥ 1,000/µL, platelets ≥ 100,000/µL, hemoglobin ≥ 10 g/dL, creatinine clearance ≥ 70 mL/1.73 m2, total bilirubin ≤ 1.5× upper limit of normal (ULN), and AST or ALT ≤ 2.5× ULN, and seizure disorder well controlled on anticonvulsant. Therapy had to begin within 36 days of definitive surgery.
Exclusion Criteria
Exclusion criteria included metastatic disease, prior tumor-directed therapy, evidence of status epilepticus, coma, or requirement of assisted ventilation.
Treatment
The chemotherapy protocol was aligned exactly with the HIT SKK 2000 protocol. Patients received a total of 3-5 cycles of chemotherapy based on disease status. The 3 cycles of induction therapy included cyclophosphamide (800 mg/m2/d intravenously [IV] over 1 hour, days 1-3), vincristine (1.5 mg/m2/d IV on days 1, 15, and 29), methotrexate (5,000 mg/m2 IV over 24 hours, days 15 and 29), etoposide (150 mg/m2/dose IV over 1-2 hours, days 43-45), and carboplatin (200 mg/m2/d IV, days 43-45). Patients in complete response (CR) or continuous complete response (CCR) after 3 cycles of induction received no further treatment. In case of persistent residual disease after induction therapy, second-look surgery was recommended, and 2 additional cycles of continuation therapy including cyclophosphamide (days 1-3), vincristine (day 1), carboplatin (days 21-23), and etoposide (days 21-23) were administered at the same doses described earlier. Doses were adjusted based on the patient’s age group (≤ 6 months, 6.9-12.9 months, or ≥ 13 months). Carboplatin dose was reduced to 125 mg/m2/d if decrease threshold on audiogram was between 15 and 30 dB at 1,000-3,000 Hz or > 40 dB at 4,000-8,000 Hz. Carboplatin was replaced by cyclophosphamide for any decrease threshold > 30 dB at 1,000-3,000 Hz.
Molecular Analysis
DNA was extracted from formalin-fixed paraffin-embedded (FFPE) tissues and blood using the Maxwell RSC DNA FFPE kit (AS1450; Promega, Madison, WI), according to the manufacturer’s instructions, and quantified using Qubit (Thermo Fisher Scientific, Waltham, MA). DNA methylation-based classification was performed to confirm the diagnosis of medulloblastoma and assignment of a molecular subgroup. This was done using a previously described machine learning approach based on the random forest algorithm to compare a given diagnostic sample to a cohort of > 2,000 reference samples.8 Subtype delineation of sonic hedgehog (SHH) I and SHH-II was performed by t-distributed stochastic neighbor embedding—t-SNE—combined with density-based spatial clustering of application with noise from 25 tumor samples from this trial and 87 infant SHH medulloblastomas across the 1,000 topmost variable methylation probes.9 DNA copy number variants were inferred from DNA methylation arrays using the Conumee R package with default parameters.10 Medulloblastoma samples were exome sequenced alongside patient-matched blood samples. Tumor and germline DNA exomes were captured using the SureSelect Human All Exon V5 (Agilent Technologies, Santa Clara, CA) platform. All next-generation sequencing data were harmonized and processed with the same analysis pipelines to ensure consistent germline and somatic mutation calling.11 All mutations and copy number variation calls were manually curated by inspecting sequence alignments and genome-wide copy number plots.
Statistical Considerations
Although the primary objective was to estimate the PFS distribution associated with the proposed treatment, the design used a binomial end point to estimate sample size based on a 1-sided test with a 2-year success rate of at least 90% (null). Because the desirable outcome was stated in the null hypothesis consistent with the treatment reduction objective, 16% type I error and 95% power were used to maximize the likelihood of detecting erosion in PFS. Thirty-seven patients were needed for the associated 1-sided hypothesis to declare the proposed regimen as promising if no more than 5 failures were observed during the first 2 years from diagnosis; otherwise, the proposed regimen would be rejected. One interim analysis was planned after 15 patients had been enrolled and observed for 2 years; if ≤ 10 patients completed the 2-year period without progression or death, it would suggest the therapy was inferior. In addition, to declare the proposed strategy a failure, accrual would stop if at any point 6 treatment failures were observed within the first 2 years from diagnosis.
PFS was defined as the time interval from diagnosis to disease progression, relapse, or death from any cause or to the date of last contact for patients without one of these events. OS was defined as the time from diagnosis to death from any cause or to the date of last contact for survivors. Overall survival (OS) and PFS were estimated using the Kaplan-Meier method. Outcome estimates are reported with 95% CIs. Differences in PFS distributions among groups were examined using the exact log-rank test. Fisher’s exact test was used to examine associations among categorical variables. The exact Wilcoxon-Mann-Whitney test was used to examine associations between methylation subgroup and histology with age.
RESULTS
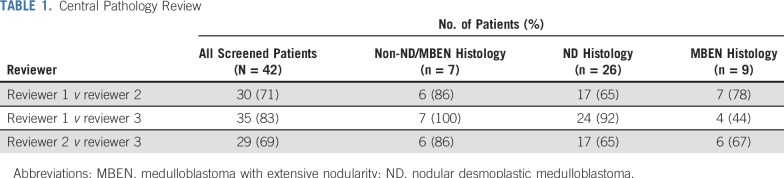
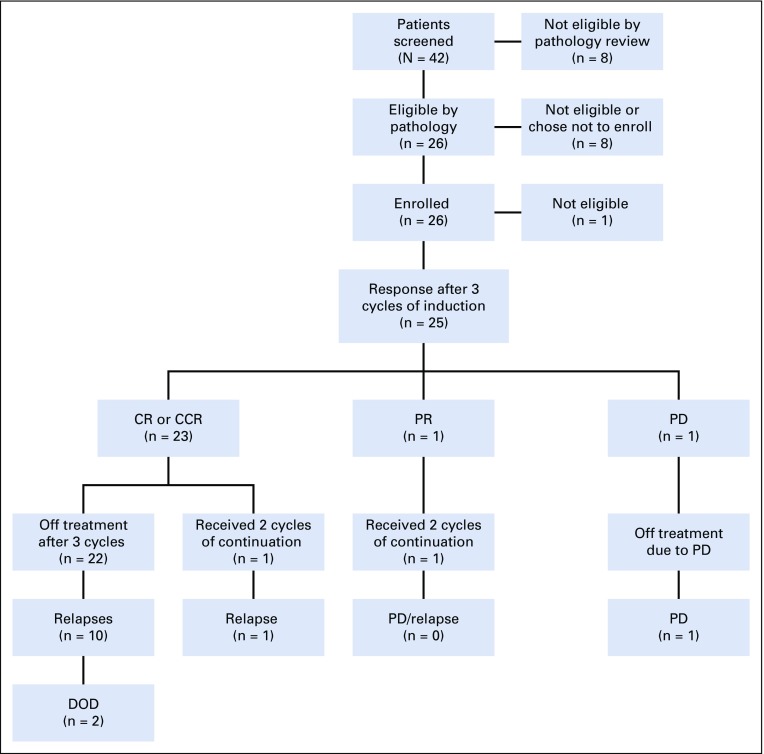
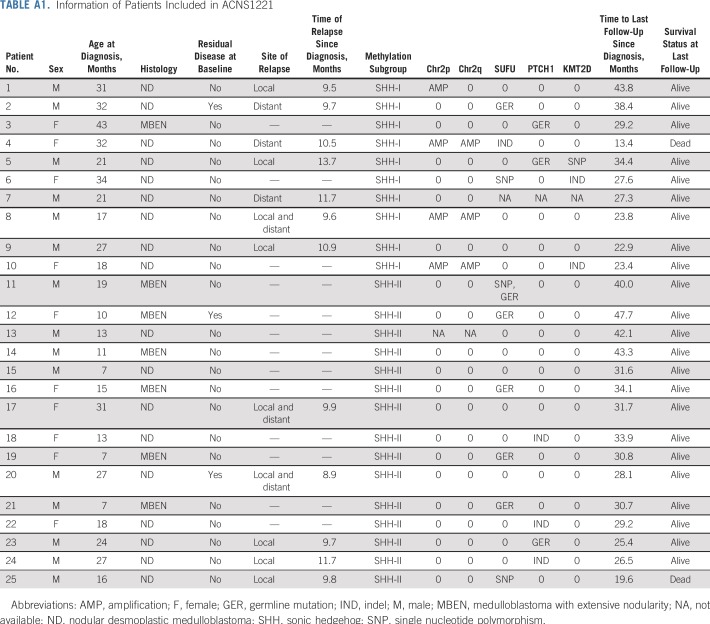
From December 12, 2013 to July 7, 2016, 42 patients underwent central pathology prescreening, and 34 were deemed eligible. All central pathology reviews were completed within the specified 10 days. Among the 34 patients deemed eligible, 2 patients had discordant histologies between the central pathology review and the institutional diagnosis. Both were considered as having ND by the institution but MBEN by central review. The overall agreement rates between pairs of the 3 central pathologists for eligibility were 69%, 71%, and 83%, whereas lower rates of agreement were observed in distinguishing ND from MBEN (Table 1). Twenty-six of the 34 patients eligible by pathology subsequently fulfilled all required criteria to enroll in the study. One patient was later found ineligible as a result of organ function requirements, leaving 25 eligible patients for the analyses (Fig 1; Appendix Table A1, online only).
TABLE 1.
Central Pathology Review
FIG 1.
Study flow. CCR, continuous complete response; CR, complete response; DOD, dead of disease; PD: progressive disease; PR, partial response.
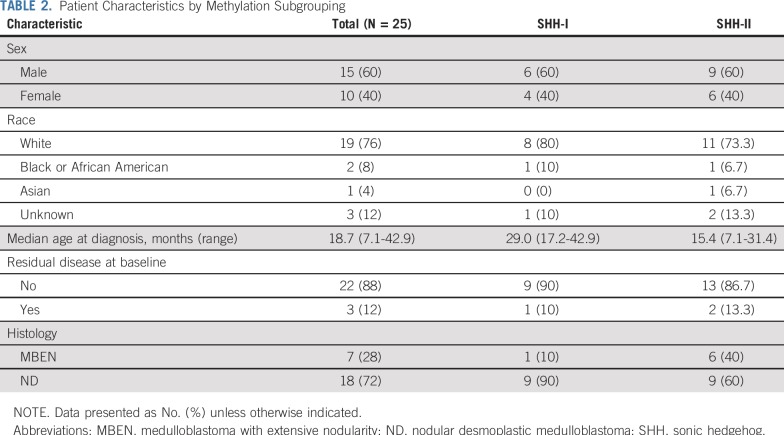
Patient characteristics are listed in Table 2. MBEN histology accounted for 28% of medulloblastomas. The median ages at diagnosis for patients with MBEN and ND were 10.8 months and 22.8 months, respectively (P = .064). Four (57%) of 7 patients with MBEN were < 12 months old, compared with 1 (6%) of 18 patients with ND (P = .012). Three patients had residual disease at baseline. All 25 patients received 3 cycles of induction; 23 (92%) of 25 patients achieved CR or CCR, 1 had partial response, and 1 had progressive disease. No patients underwent second-look surgery. Two patients received 2 additional cycles of continuation therapy.
TABLE 2.
Patient Characteristics by Methylation Subgrouping
Molecular Analysis
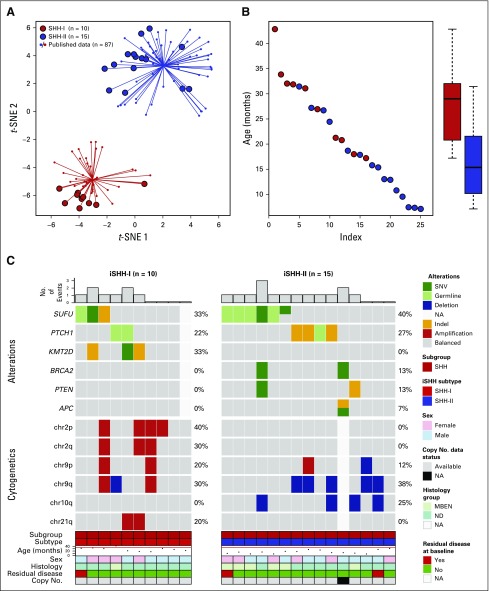
Twenty-five tumor samples underwent DNA-based methylation analysis, and 24 of 25 tumors underwent successful whole-exome sequencing with matched patient-derived blood samples. Methylation array analysis assigned all tumors to the SHH subgroup. Subgroup analysis separated these tumors into SHH-I (n = 10; 40%) and SHH-II (n = 15; 60%) subgroups (Fig 2). SHH-I patients were older than SHH-II patients (median age at diagnosis, 29.0 v 15.4 months, respectively; P = .002). Although 6 of 7 patients with MBEN were SHH-II (86%), no significant association was found between histology and methylation subgrouping (P = .18; Table 2).
FIG 2.
(A) t-distributed stochastic neighbor embedding (t-SNE) plot showing that all samples analyzed segregated into two methylation subgroups sonic hedgehog (SHH) I (n = 10) and SHH-II (n = 15). (B) SHH-I patients were older than SHH-II patients (exact Wilcoxon-Mann-Whitney test, P = .002; n = 25). (C) Oncoprint summary describing recurrent genetic alterations and cytogenetic events observed in each methylation subgroup. iSHH, infant sonic hedgehog; MBEN, medulloblastoma with extensive nodularity; NA, not available; ND, nodular desmoplastic medulloblastoma; SNV, single nucleotide variant.
Chromosome 2 gain was exclusively described in SHH-I tumors (40% of amplified chromosome 2p and 30% of amplified chromosome 2q). The most commonly mutated genes were SUFU (n = 9; 38%), PTCH1 (n = 6; 25%), and KMT2D (n = 3; 13%). Germline sequencing analysis identified 38% of patients with pathogenic mutations in PTCH1 (n = 3) and SUFU (n = 6). No significant difference was seen in the distribution of mutations between SHH-I and SHH-II. TP53 mutations, MYCN amplifications, and GLI2 amplifications were not observed in any tumor (Fig 2).
Outcome and Prognostic Factors
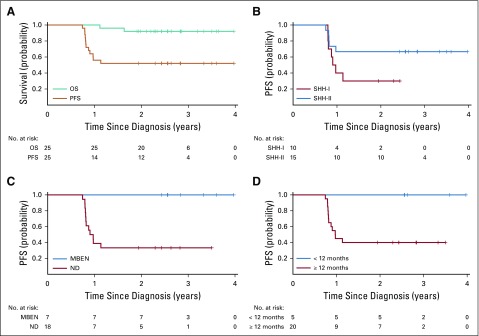
The study prematurely closed in July 2016 after an interim evaluation; at that time, 5 of the 15 first enrolled patients had experienced disease progression, meeting criteria for early closure. Twelve patients in total have since experienced relapse or progression at a median of 9.8 months from diagnosis (range, 8.9-13.7 months). The pattern of relapse was mixed and included 6 local (50%), 3 distant (25%), and 3 combined relapses (25%). Two patients died of disease, and 23 patients were still alive at a median follow-up of 2.6 years (range, 1.9-4.0 years). No second malignancies were reported. The 2-year PFS and OS rates for the entire cohort were 52% (95% CI, 32.4% to 71.6%) and 92% (95% CI, 80.8% to 100.0%), respectively (Fig 3A).
FIG 3.
(A) Progression-free survival (PFS) and overall survival (OS) for the entire cohort. (B) PFS according to methylation subgroup. (C) PFS according to histologic subtype. (D) PFS according to age group. MBEN, medulloblastoma with extensive nodularity; ND, nodular desmoplastic medulloblastoma; SHH, sonic hedgehog.
Information on treatment at time of relapse was available for 10 of 12 patients. Four patients underwent repeat surgery. All patients received conventional chemotherapy. Six patients subsequently received high-dose chemotherapy (HDC), and 5 underwent radiotherapy.
Median times to relapse for the SHH-I and SHH-II patients were 10.5 months (range, 9.5-13.7 months) and 9.8 months (range, 8.9-11.7 months), respectively. Seven of 10 SHH-I patients experienced relapse (locally [n = 3], distally [n = 3], and combined [n = 1]). Five of 15 SHH-II patients experienced relapse (locally [n = 3] and combined [n = 2]). Two-year PFS rates for SHH-I and SHH-II patients were 30.0% (95% CI, 1.6% to 58.4%) and 66.7% (95% CI, 44.0% to 89.4%), respectively (P = .099; Fig 3B).
Higher PFS was observed in female patients versus male patients (80% v 33.3% [95% CI, 11.5% to 55.1%], respectively; P = 0.025), children < 12 versus > 12 months old (100% v 40% [95% CI, 18.4% to 61.6%], respectively; P = .036), and children with MBEN versus ND (100% v 33.3% [95% CI, 11.5% to 55.1%], respectively; P = .005; Figs 3C and 3D).
Toxicity
No toxic death was reported. Grade 4 neutropenia and thrombocytopenia were observed in 56% and 28% of patients, respectively. One patient developed meningitis during induction chemotherapy. One patient developed a grade 4 ototoxicity during the first cycle of induction. Dose modifications per protocol occurred in 9 (11.4%) of 79 chemotherapy courses. Sixteen patients (64%) had delays in at least 1 course of their chemotherapy.
DISCUSSION
Medulloblastoma in young children has historically been associated with poorer outcome than in older children, but the identification of the ND medulloblastoma subtype in young children led to a refinement of this overall statement by describing a favorable outcome for ND and MBEN in the absence of adjuvant craniospinal irradiation.12-14 Building on the promising results from the HIT SKK 92 and 2000 trials, ACNS1221 was a radiation-sparing strategy designed for young patients with ND or MBEN, aimed at avoiding neurotoxicity associated with intraventricular methotrexate.3,4 However, the study failed to achieve the specified target of 2-year PFS ≥ 90% and closed prematurely, showing a concerning pattern of disseminated relapse in half of the patients who experienced progression.
Although the central pathology review process was proven feasible, the results confirmed the difficulty of obtaining consensus for the histologic diagnosis of ND or MBEN. The reported rates of ND and MBEN have varied widely among studies, ranging from 29% to 75% even with central pathology process, highlighting the challenge in relying on pathologic diagnosis to tailor therapy.5,6 In our study, lower agreement rate was primarily for the diagnosis of the MBEN versus ND subtype, as opposed to difficulty excluding non-ND/MBEN. Of interest, the patients considered as having MBEN in our study had a better PFS compared with patients with ND histology (P = .005) and were significantly younger than those with ND medulloblastoma (P = .012), as previously described by Garrè et al.15 It is worth noting that the distribution of ND and MBEN was similar in both the HIT SKK 2000 and ACNS1221 trials (70% and 30% v 72% and 28%, respectively).4
As expected for ND or MBEN in young children, all tumors clustered within the SHH subgroup.16 With this cohort, we prospectively validated that they further segregated into 2 subgroups (SHH-I and SHH-II), as recently reported by Robinson et al.9 This also aligned with the retrospective analysis by Cavalli et al,17 which described 2 infant SHH subclusters (SHH-β and SHH-γ) within the SHH group. The SHH-γ tumors were enriched with MBEN subtype as in our SHH-II subgroup. Nevertheless, although 6 of 7 MBENs were SHH-II tumors, the distribution of ND medulloblastoma and MBEN was not statistically different between SHH-I and SHH-II. Although the methylation profiling did not discriminate histologic subtype, the recent transcriptome analysis of a large cohort of patients with MBEN and ND medulloblastoma may provide a new tool to accurately differentiate these 2 histologic entities.18 We also observed a similar pattern of cytogenetic alterations with enrichment of chromosome 2 gain in the SHH-I subgroup as observed in the SHH-β group. Important from a prognostic standpoint is that the tumors within this trial were devoid of any TP53 mutations and MYCN or GLI2 amplifications, which, if present, herald a poor prognosis.19,20
In our study, patients in the SHH-II subgroup showed a trend toward better outcome compared with patients in the SHH-I subgroup (2-year PFS, 66.7% v 30.0%, respectively; P = .099). Although the lack of statistical significance may be a result of the small sample size, a similar analysis by methylation subgroup from several HIT SKK trials, with a comparable distribution of SHH-I and SHH-II (46% SHH-II), showed no significant difference in PFS (P = .76) or OS (P = .43).21 These results contrast with the findings from the SJYC07 trial, where significant differences in 5-year PFS were observed between the SHH-II and SHH-I subgroups (75.4% v 27.8%, respectively; P = .0028). The difference in PFS between the ACNS1221 and the HIT SKK cohorts seems to be related to treatment intensity, and the role of the serial intraventricular injections of methotrexate to prevent relapse, more specifically disseminated relapse, warrants future attention.
Although previous clinical trials for young children with MB did not include characterization by methylation array and used instead ND or MBEN histology as a surrogate of the SHH subgroup, several HDC protocols have reported excellent outcome for this subgroup of patients. The 5-year event-free survival (EFS) rate for the 13 patients with localized ND or MBEN treated on the CCG 99703 protocol was 84.6%.7 Similarly, in a retrospective study of patients treated as per the CCG 99703 trial, 24 SHH patients had a 5-year PFS of 86.2%.22 With such high survival associated with HDC, a significant survival difference between methylation subgroups is unlikely to be detected. In addition, the 5-year EFS of 95% reported for ND medulloblastoma on the HeadStart III protocol may suggest the possible contribution of a higher dose of systemic methotrexate.23 The outcomes observed both in the ACNS1221 and the SJYC07 trials for the SHH-II subgroup (2-year EFS of 66.7% and 5-year PFS of 75.4%, respectively) may not be satisfactory enough for these patients in light of the high cure rate associated with HDC. The interplay between methylation subgrouping and treatment intensity for SHH medulloblastoma still needs to be better deciphered through pooled data analysis of international cohorts.
Although HDC allows for high cure rate in SHH medulloblastoma, its associated toxicities, such as infertility and ototoxicity, may not be justified for all patients. Our current understanding of the SHH molecular landscape in young children, along with clinical criteria, may help define a low-risk group of patients for whom HDC may not be required. For instance, the limited group of 11 SHH-II low-risk patients (M0 and gross total resection) treated on SJYC07 had a 5-year PFS of 90.9%.9 As for patients not meeting low-risk criteria, given that both HIT SKK and HDC regimens report high survival in ND and MBEN, a comparison of the 2 regimens would address the issue of acceptable treatment-related toxicity, which ideally should be addressed in a randomized manner through international collaboration with emphasis placed on the preservation of neurocognitive function.
Our 38% rate (9 of 24 tumors) of germline mutations in PTCH1 and SUFU was higher than the 21% rate (17 of 80 tumors) recently reported by Waszak et al,24 where the infant population was defined by a lower age cutoff than ours (< 3 years). Distinct from Robinson et al,9 we found no difference in SUFU mutation distribution among the SHH-I and SHH-II subgroups. This high rate of germline mutation further supports the recommendation for genetic screening of all patients with SHH medulloblastoma. Because these patients are inherently more susceptible to radiation toxicity, radiation-sparing strategies should further be pursued.25
Finally, this trial contributes to the discussion of the age cutoff for so-called infant medulloblastomas in the molecular era. ACNS1221 was the first COG trial to raise the age eligibility to 4 years, aligning with the HIT SKK criteria. Because children with SHH-II tumors were significantly younger than those with SHH-I tumors, the age cutoff for infant medulloblastoma may need to be tied to specific methylation subgroups.
The proposed regimen without intraventricular methotrexate injections failed to achieve a favorable outcome for young patients with ND medulloblastoma or MBEN. In this prospective cohort, we separated the patients into the 2 SHH methylation subtypes (SHH-I and SHH-II) and described a trend toward worse outcome in SHH-I patients when treated with conventional chemotherapy. Future clinical trials for SHH medulloblastoma in young children should evaluate the impact of methylation subgrouping in the context of treatment intensity.
APPENDIX
TABLE A1.
Information of Patients Included in ACNS1221
PRIOR PRESENTATION
Presented, in part, at 53rd Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 2-6, 2017; 49th Congress of the International Society of Pediatric Oncology, Washington, DC, October 12-15, 2017; 50th Congress of the International Society of Pediatric Oncology, Kyoto, Japan, November 16-19, 2018; and 18th International Symposium on Pediatric Neuro-Oncology, Denver, CO, June 29-July 3, 2018.
SUPPORT
Supported by the Children’s Oncology Group, the St Baldrick’s Foundation, and the National Cancer Institute of the National Institutes of Health under Grants No. U10CA180886, U10CA180899, U10CA098543, and U10CA098413.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AUTHOR CONTRIBUTIONS
Conception and design: Lucie Lafay-Cousin, Eric Bouffet, Douglas Strother, Charles Eberhart, Mark Souweidane, Chris Williams-Hughes, Arzu Onar-Thomas, Amar Gajjar
Financial support: Paul Northcott
Administrative support: Amar Gajjar
Provision of study materials or patients: Paul Northcott
Collection and assembly of data: Lucie Lafay-Cousin, Eric Bouffet, Charles Eberhart, Craig Horbinski, Paul Northcott, Giles Robinson, Amar Gajjar
Data analysis and interpretation: Lucie Lafay-Cousin, Eric Bouffet, Douglas Strother, Vasilisa Rudneva, Cynthia Hawkins, Charles Eberhart, Linda Heier, Mark Souweidane, Arzu Onar-Thomas, Catherine A. Billups, Maryam Fouladi, Paul Northcott, Giles Robinson, Amar Gajjar
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Study of Nonmetastatic Desmoplastic Medulloblastoma in Children Younger Than 4 Years of Age: A Report of the Children’s Oncology Group (ACNS1221)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Eric Bouffet
Research Funding: Roche (Inst), Bristol-Myers Squibb (Inst)
Cynthia Hawkins
Consulting or Advisory Role: Bayer
Patents, Royalties, Other Intellectual Property: IP for low-grade glioma and sarcoma fusion panels as well as medulloblastoma subgrouping panel
Charles Eberhart
Travel, Accommodations, Expenses: Bayer
Mark Souweidane
Consulting or Advisory Role: Aesculap
Travel, Accommodations, Expenses: Aesculap
Arzu Onar-Thomas
Honoraria: Eli Lilly
Research Funding: Novartis (Inst), Apexigen (Inst), Pfizer (Inst), Celgene (Inst), Novartis (Inst), Merck (Inst)
Travel, Accommodations, Expenses: Eli Lilly
Giles Robinson
Consulting or Advisory Role: Eli Lilly, Genentech
Research Funding: Novartis (Inst), Genentech (Inst), Novartis (Inst)
Amar Gajjar
Research Funding: Genentech (Inst), Kazia Pharmaceutical (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Rutkowski S, Cohen B, Finlay J, et al. Medulloblastoma in young children. Pediatr Blood Cancer. 2010;54:635–637. doi: 10.1002/pbc.22372. [DOI] [PubMed] [Google Scholar]
- 2.Rutkowski S, von Hoff K, Emser A, et al. Survival and prognostic factors of early childhood medulloblastoma: An international meta-analysis. J Clin Oncol. 2010;28:4961–4968. doi: 10.1200/JCO.2010.30.2299. [DOI] [PubMed] [Google Scholar]
- 3.Rutkowski S, Bode U, Deinlein F, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352:978–986. doi: 10.1056/NEJMoa042176. [DOI] [PubMed] [Google Scholar]
- 4.von Bueren AO, von Hoff K, Pietsch T, et al. Treatment of young children with localized medulloblastoma by chemotherapy alone: Results of the prospective, multicenter trial HIT 2000 confirming the prognostic impact of histology. Neuro-oncol. 2011;13:669–679. doi: 10.1093/neuonc/nor025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashley DM, Merchant TE, Strother D, et al. Induction chemotherapy and conformal radiation therapy for very young children with nonmetastatic medulloblastoma: Children’s Oncology Group study P9934. J Clin Oncol. 2012;30:3181–3186. doi: 10.1200/JCO.2010.34.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leary SES, Zhou T, Holmes E, et al. Histology predicts a favorable outcome in young children with desmoplastic medulloblastoma: A report from the Children’s Oncology Group. Cancer. 2011;117:3262–3267. doi: 10.1002/cncr.25856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen BH, Geyer JR, Miller DC, et al. Pilot study of intensive chemotherapy with peripheral hematopoietic cell support for children less than 3 years of age with malignant brain tumors, the CCG-99703 phase I/II study: A report from the Children’s Oncology Group. Pediatr Neurol. 2015;53:31–46. doi: 10.1016/j.pediatrneurol.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capper D, Jones DTW, Sill M, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555:469–474. doi: 10.1038/nature26000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson GW, Rudneva VA, Buchhalter I, et al. Risk-adapted therapy for young children with medulloblastoma (SJYC07): Therapeutic and molecular outcomes from a multicentre, phase 2 trial. Lancet Oncol. 2018;19:768–784. doi: 10.1016/S1470-2045(18)30204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hovestadt V, Remke M, Kool M, et al. Robust molecular subgrouping and copy-number profiling of medulloblastoma from small amounts of archival tumour material using high-density DNA methylation arrays. Acta Neuropathol. 2013;125:913–916. doi: 10.1007/s00401-013-1126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Northcott PA, Buchhalter I, Morrissy AS, et al. The whole-genome landscape of medulloblastoma subtypes. Nature. 2017;547:311–317. doi: 10.1038/nature22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walter AW, Mulhern RK, Gajjar A, et al. Survival and neurodevelopmental outcome of young children with medulloblastoma at St Jude Children’s Research Hospital. J Clin Oncol. 1999;17:3720–3728. doi: 10.1200/JCO.1999.17.12.3720. [DOI] [PubMed] [Google Scholar]
- 13.Duffner PK, Horowitz ME, Krischer JP, et al. The treatment of malignant brain tumors in infants and very young children: An update of the Pediatric Oncology Group experience. doi: 10.1093/neuonc/1.2.152. Neuro Oncol 1:152-161, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geyer JR, Sposto R, Jennings M, et al. Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: A report from the Children’s Cancer Group. J Clin Oncol. 2005;23:7621–7631. doi: 10.1200/JCO.2005.09.095. [DOI] [PubMed] [Google Scholar]
- 15.Garrè ML, Cama A, Bagnasco F, et al. Medulloblastoma variants: Age-dependent occurrence and relation to Gorlin syndrome—A new clinical perspective. Clin Cancer Res. 2009;15:2463–2471. doi: 10.1158/1078-0432.CCR-08-2023. [DOI] [PubMed] [Google Scholar]
- 16.Northcott PA, Korshunov A, Witt H, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29:1408–1414. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavalli FMG, Remke M, Rampasek L, et al. Intertumoral heterogeneity within medulloblastoma subgroups. Cancer Cell. 2017;31:737–754.e6. doi: 10.1016/j.ccell.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korshunov A, Sahm F, Okonechnikov K, et al. Desmoplastic/nodular medulloblastomas (DNMB) and medulloblastomas with extensive nodularity (MBEN) disclose similar epigenetic signatures but different transcriptional profiles. Acta Neuropathol. 2019;137:1003–1015. doi: 10.1007/s00401-019-01981-6. [DOI] [PubMed] [Google Scholar]
- 19.Zhukova N, Ramaswamy V, Remke M, et al. Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J Clin Oncol. 2013;31:2927–2935. doi: 10.1200/JCO.2012.48.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwalbe EC, Lindsey JC, Nakjang S, et al. Novel molecular subgroups for clinical classification and outcome prediction in childhood medulloblastoma: A cohort study. Lancet Oncol. 2017;18:958–971. doi: 10.1016/S1470-2045(17)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mynarek M, Kool M, Schueller U, et al. MBCL-21: Early childhood medulloblastoma—Subgroup specific survival in patients treated with systemic chemotherapy and intraventricular MTX to avoid craniospinal radiotherapy. Neuro Oncol 20:i121, 2018 (suppl 2; abstr) [Google Scholar]
- 22.Lafay-Cousin L, Smith A, Chi SN, et al. Clinical, pathological, and molecular characterization of infant medulloblastomas treated with sequential high-dose chemotherapy. Pediatr Blood Cancer. 2016;63:1527–1534. doi: 10.1002/pbc.26042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dhall G, Ji L, Haley K, et al: Outcome of infants and young children with newly diagnosed medulloblastoma treated on Head Start III protocol. J Clin Oncol 29, 2011 (suppl 15; abstr 2011) [Google Scholar]
- 24.Waszak SM, Northcott PA, Buchhalter I, et al. Spectrum and prevalence of genetic predisposition in medulloblastoma: A retrospective genetic study and prospective validation in a clinical trial cohort. Lancet Oncol. 2018;19:785–798. doi: 10.1016/S1470-2045(18)30242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guerrini-Rousseau L, Dufour C, Varlet P, et al. Germline SUFU mutation carriers and medulloblastoma: Clinical characteristics, cancer risk, and prognosis. Neuro Oncol. 2018;20:1122–1132. doi: 10.1093/neuonc/nox228. [DOI] [PMC free article] [PubMed] [Google Scholar]