Abstract
Background
Colorectal cancer is a leading cause of cancer-related death in the world. Despite cisplatin is a commonly used chemotherapeutic drug for the colorectal cancer treatment, resistance of cancer cells to cisplatin restricts its clinical efficacy. It is important to explore the potential mechanisms and take strategies to sensitize colorectal cancer cells to cisplatin treatment.
Methods
Differences of TRIM32 and miR-519d expression between colorectal cancer cells and human normal colon epithelial cells were evaluated by qRT-PCR and Western blot assays. Cytotoxicity of cisplatin against colorectal cancer cells was tested by CCK-8 assay. Generation of reactive oxygen species (ROS), mitochondrial membrane potential and apoptosis was measured by flow cytometry. Dual-luciferase reporter assay was used to validate the association between miR-519d and TRIM32.
Results
Significant increase of TRIM32 expression in colorectal cancer tissues and cell lines was observed. TRIM32 negatively regulated the cisplatin sensitivity in colorectal cancer cells. Mechanically, overexpression of TRIM32 was induced by decrease of miR-519d. Exogenous miR-519d can inhibit the expression of TRIM32 and thus promoted the cisplatin-induced apoptosis through the mitochondrial pathway.
Conclusion
Overexpression of TRIM32 was induced by the absence of miR-519d in colorectal cancer. MiR-519d can be used as a sensitizer during the cisplatin-based chemotherapy of colorectal cancer.
Keywords: miR-519d, TRIM32, cisplatin, colorectal cancer
Introduction
Colorectal cancer (CRC) is one of the most prevalent malignant tumors and the incidence of CRC continues to increase every year.1,2 Because of the high recurrence and high metastasis of CRC, mortality rate of CRC is still very high nowadays.3,4 In the treatment of CRC, surgical resection is the most effective therapeutic approach. however, it is only suitable for patients with early- to mild-stage CRC. For patients with the advanced CRC, chemotherapy is still an irreplaceable and important strategy in the treatment course.5–7 However, the resistance of cancer cells to chemotherapeutic drugs restricts the clinical efficacy of chemotherapy.8,9 There is an urgent need to identify novel targets and improve clinical outcome.
MicroRNAs (miRNAs) are an enormous group of non-coding RNAs with short nucleotides. MiRNAs can decrease expression of more than 30% of human genes through binding to 3ʹuntranslated region (3ʹ UTR) of targeted mRNAs at the post-transcriptional or translational level.10,11 Thus, miRNAs can mediate abundant biological processes, including cell proliferation, differentiation, apoptosis and tumorigenesis.12–14 In cancer cells, it has been found that miRNAs are usually dysregulated. Furthermore, previous studies have demonstrated that aberrant expression of miRNAs is an important cause of chemoresistance in cancers including CRC.15–17 Therefore, miRNAs can be considered as a potential target for sensitizing the chemotherapy.
TRIM32 is a protein that belongs to the tripartite motif (TRIM) family. As a member of TRIM family, TRIM32 has E3 ubiquitinase activity that promotes post-translational modifications of various substrates.18,19 TRIM32 is involved in a wide range of biological processes, including cell growth, differentiation and apoptosis. In cancer cells, it has been reported that TRIM32 is usually overexpressed and promotes cell proliferation, migration and invasion.20,21 Thus, TRIM32 is suggested to exert oncogenic functions. However, the potential role of TRIM32 in chemoresistance is still unclear. In this study, we found that TRIM32 was overexpressed and was targeted by miR-519d in CRC cells. The aim of this study is to investigate the potential role of miR-519d/TRIM32 axis in sensitizing CRC cells to cisplatin.
Materials and Methods
Cell Culture and Patients’ Specimens
Human CRC cell lines HT29 and SW480 and human normal colon epithelial cell line FHC were purchased from American Type Culture Collection (ATCC, Manassas, Virginia, USA) and maintained in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). All of these cells were maintained at 37°C in a humidified 5% CO2 incubator. To detect the expression of miR-519d in CRC in vivo, 25 pairs of CRC tumor tissues and corresponding paracancerous tissues were obtained from Hainan General Hospital between May 2016 and December 2018 from patients who had undergone surgical resection. The study was approved by the Ethics Committee and Institutional Review Board of Hainan General Hospital. We have obtained the written informed consent from all of the patients.
RNA Extraction and Quantitative Reverse Transcriptase Real-Time PCR (qRT-PCR)
Total RNAs from tissues or cells were isolated by using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer’s instruction. Subsequently, One Step PrimeScript miRNA cDNA Synthesis Kit (Takara Bio, Inc., Otsu, Japan) was applied to synthesize cDNA according to the manufacturer’s instruction. Afterward, qPCR was carried out by using the SYBR Premix Ex Taq II (Takara Bio, Inc., Otsu, Japan) based on the manufacturer’s protocols. GAPDH was used as the internal reference for normalization of TRIM32 mRNA expression. Meanwhile, the expression of miR-519d was determined according to the internal control of U6 snRNA. Relative gene expression was calculated by using the 2−ΔΔCt method.
Cell Transfection
Human miR-519d mimics, negative control oligonucleotide (NCO) and TRIM32 short interfering RNA (siRNA) were purchased from GenePharma (Shanghai, China). pcDNA3.1 plasmid was also obtained from GenePharma (Shanghai, China). The TRIM32 plasmid was constructed by inserting the open reading frame of TRIM32 gene into the pcDNA3.1 vector. For transfection, cancer cells with the density of 4×105 cells/well were seeded into six-well plates, then the miR-519d mimics (50 pmol/mL), antisense oligonucleotide (anti-miR-519d), NCO (50 pmol/mL), TRIM32 siRNA (50 pmol/mL) and TRIM32 plasmid (2 μg/mL) were transfected into cells by using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the instruction of the manufacturer.
Cell Viability and IC50
The cytotoxicity of cisplatin (Sigma-Aldrich; Merck, Germany) against SW480 and HT29 cells was determined by using a cell counting kit-8 (CCK-8; Beyotime, Shanghai, China) assay. Briefly, transfected cells were seeded into a 96-well plate at 5 × 103 cells/well and cultured overnight for adherence. Subsequently, cells with different treatments were treated with various concentrations of cisplatin for 48 hrs. After treatment, cells were incubated by 10 μL CCK-8 for 2 hrs according to the manufacturer’s protocol. The absorbance was determined at 450 nm by using a microplate reader (Sunrise Microplate Reader, TECAN, Switzerland). Half maximal inhibitory concentration (IC50) of cisplatin was calculated according to the cell viability curve. All the procedures were conducted in triplicate.
Western Blot Analysis
Proteins were extracted from cells by using RIPA buffer (Cell Signaling Technology, Beverly, USA). Equal amount of protein samples were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The membranes were blocked with 5% non-fat milk and subsequently incubated with primary antibodies of TRIM32, Bcl-2, caspase-9, caspase-3 and GAPDH (Cell Signaling Technology, Beverly, USA) overnight. The membranes were then probed with corresponding secondary antibodies at room temperature for 1 hr. Immunoreactive protein bands were visualized by using an enhanced chemiluminescent substrate (Thermo Fisher Scientific, Inc, Waltham, MA, USA).
Luciferase Reporter Assay
TRIM32 3ʹ UTR fragment was amplified from cDNA and cloned into the pMIR-REPORT™ miRNA Expression Reporter Vector (Thermo Fisher Scientific, Inc, Waltham, MA, USA) according to the manufacturer’s instruction. The recombinant plasmid was named as pMIR-wtTRIM32. pMIR-REPORT with mutant TRIM32 3ʹ UTR (pMIR-mtTRIM32) was obtained by using QuikChange Site-Directed Mutagenesis kit (Stratagene, Missouri, Texas, USA). After co-transfection with miR-519d mimics, pMIR-REPORT and Renilla luciferase pRL-TK vectors (Promega, Madison, WI, USA) for 48 hrs, luciferase activities were measured by using Dual-Luciferase Reporter assay system (Promega, Madison, WI, USA) according to the manufacturer’s instruction.
Flow Cytometry
Mitochondrial membrane potential (MMP), reactive oxygen cluster (ROS) and cell apoptosis were detected by flow cytometry analysis. Cells were harvested and washed twice with PBS. For detection of MMP, cells were resuspended in PBS and stained with 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethyl imidacarbo cyanine iodide (JC-1) (Molecular Probes; Waltham, MA, USA) as an indicator. Cells with high MMP emits red fluorescence and cells with low MMP emits green fluorescence. For the detection of ROS, cells were stained with dihydroethidium (DHE) (Molecular Probes; Waltham, MA, USA) for 15 min at room temperature. For measurement of cell apoptotic rate, Annexin V-FITC Apoptosis Detection Kit (Sigma-Aldrich; Merck, Germany) was used according to the manufacturer’s instruction.
Animal Model
Lentivirus carrying miR-519d precursor was conducted by Genechem Co., Ltd (Shanghai, China). For xenograft, 5×106 HT29 cells transfected with lentivirus carrying miR-519d precursor (HT29-miR-519d) or HT29 cells transfected with empty lentivirus (HT29-control) were injected subcutaneously into the right armpit of mice (four-week-old and female immunodeficient nude BALB/c mice; Shanghai Super-B&K Laboratory Animal Corp., Ltd., Shanghai, China). Cisplatin (2 mg/kg) was administrated by intraperitoneal injection twice a week when the xenografts reached 0.5 cm in diameter. The volume of the tumour was monitored every 3 days and calculated by using the following formula: volume = (length × width2)/2. Twenty-eight days post-inoculation, mice were sacrificed and the tumors were extracted for further analysis. All animal procedures and experimental protocols were approved by the Animal Care Committee of Hainan General Hospital.
Statistical Analysis
Data are represented as mean ± standard deviation (SD) and obtained from three independent experiments. For comparison analysis, two-tailed Student’s t-tests were used to estimate the statistical differences between two groups. One-way analysis of variance (ANOVA) and Bonferroni’s post hoc test were used to determine the differences between three or more groups. Statistical analyses were performed with SPSS software, version 16.0 (IBM, Armonk, NY). P<0.05 was considered to indicate a statistically significant difference.
Results
Increase of TRIM32 Expression in CRC
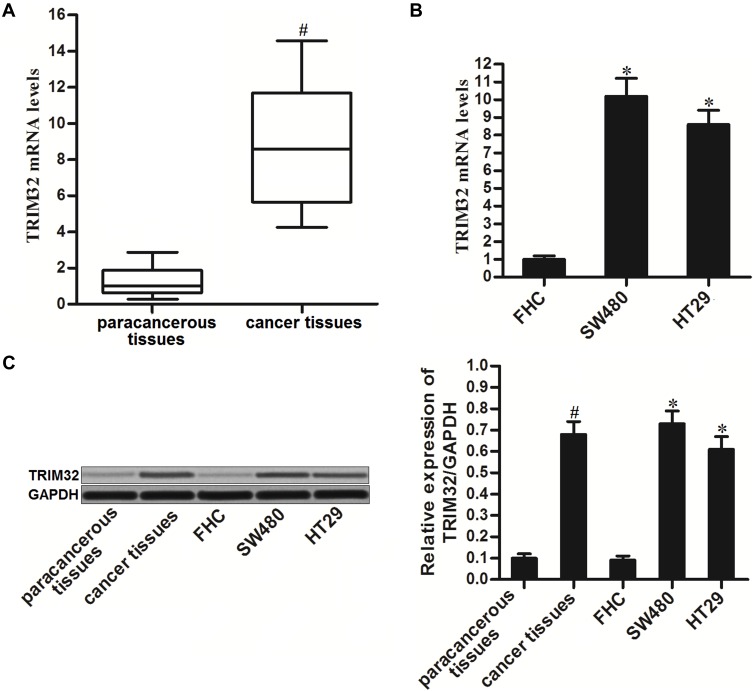
To investigate the potential role of TRIM32 in CRC, we first detected the expression of TRIM32 in CRC patients’ samples and CRC cell lines. Results of qRT-PCR analysis showed that TRIM32 expression was upregulated in colorectal tumor tissues compared to the paracancerous tissues at the mRNA level (P<0.05) (Figure 1A). Similarly, we found that mRNA expression of TRIM32 in SW480 and HT29 cells was higher than that in human normal colon epithelial cell line FHC (P<0.05) (Figure 1B). Additionally, results of Western blot assays showed that both CRC tissues and cell lines expressed higher amount of TRIM32 at the protein level (P<0.05) (Figure 1C). These results indicated the increase of TRIM32 expression in CRC.
Figure 1.
Increase of TRIM32 expression in colorectal cancer. (A) mRNA expression levels of TRIM32 in 25 pairs of colorectal cancer patients’ tumor tissues and paracancerous tissues were evaluated by qRT-PCR analysis. (B) mRNA expression levels of TRIM32 in FHC, SW480 and HT29 cells were detected by qRT-PCR analysis. (C) Protein levels of TRIM32 in colorectal cancer tissues, paracancerous tissues, FHC, SW480 and HT29 were detected by Western blot assays.
Notes: Data was expressed as mean±SD. #P<0.05 vs paracancerous tissues, *P<0.05 vs FHC group.
Abbreviations: TRIM32, tripartite motif 32; mRNA, messenger RNA; qRT-PCR, quantitative real-time polymerase chain reaction.
Knockdown of TRIM32 Increases the Sensitivity of CRC Cells to Cisplatin
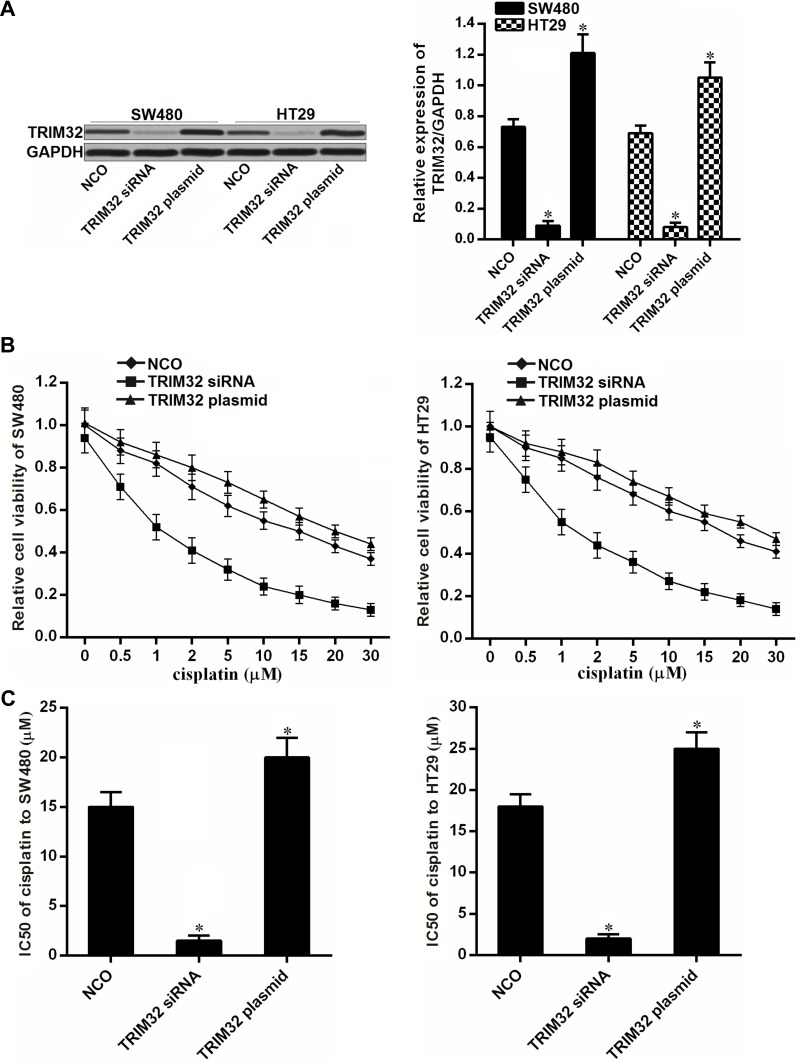
To investigate the association between TRIM32 and cisplatin sensitivity in CRC, we first changed the expression of TRIM32 in SW480 and HT29 cells by using TRIM32 plasmid and siRNA. The transfection efficiency of TRIM32 siRNA and plasmid on changing the TRIM32 protein level was shown in Figure 2A. Next, we performed CCK-8 assays to evaluate the effect of TRIM32 plasmid and siRNA on changing the sensitivity of SW480 and HT29 cells to cisplatin. As shown in Figure 2B, TRIM32 plasmid slightly decreased the sensitivity of SW480 and HT29 cells to cisplatin, whereas knockdown of TRIM32 obviously enhanced the cytotoxicity of cisplatin against SW480 and HT29. After analysis of the cell viability curve, we indicated that TRIM32 siRNA reduced the IC50 of cisplatin by 83.5% to SW480 and 74.3% to HT29, respectively (P<0.05) (Figure 2C). We thus demonstrated that the expression level of TRIM32 partially determined the cisplatin sensitivity of SW480 and HT29. Knockdown of TRIM32 can enhance the cytotoxicity of cisplatin against CRC.
Figure 2.
Knockdown of TRIM32 increases the sensitivity of colorectal cancer cells to cisplatin. (A) Effect of TRIM32 siRNA and plasmid on changing the TRIM32 expression was detected by Western blot assay. (B) SW480 and HT29 cells were transfected with TRIM32 siRNA or plasmid. 24 hrs later, these cells were treated with different concentrations of cisplatin (0–30 μM) for another 48 hrs. CCK-8 assays were performed to measure the cell viability of these cells. (C) Effect of TRIM32 siRNA and plasmid on changing the IC50 of cisplatin to SW480 and HT29.
Notes: Data was expressed as mean±SD. *P<0.05 vs NCO group.
Abbreviations: TRIM32, tripartite motif 32; CCK-8, Cell Counting Kit-8; IC50, 50% inhibiting concentration; siRNA, small interfering RNA.
Overexpression of TRIM32 Is Induced by Downregulation of miR-519d in CRC
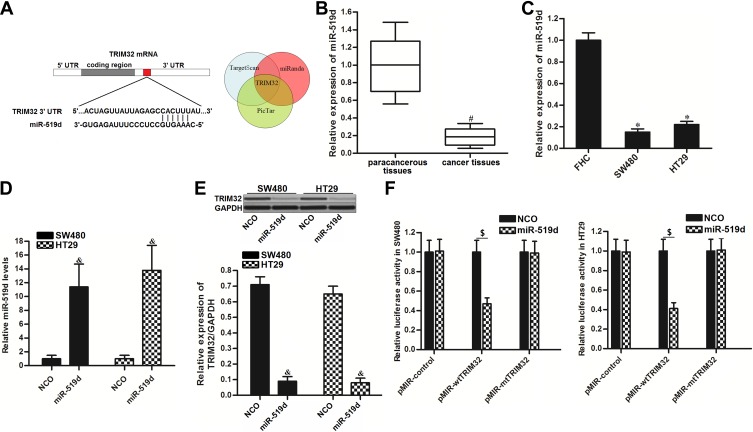
To investigate the mechanism by which TRIM32 was overexpressed in CRC cells, we searched the upstream miRNA of TRIM32 through the online bioinformatics tools TargetScan (http://www.targetscan.org), miRanda (http://www.microrna.org/microrna/microrna/home.do) and PicTar (http://www.pictar.org). All of these databases commonly predicted that TRIM32 may be targeted by miR-519d (Figure 3A). Additionally, we found obvious downregulation of miR-519d in CRC tissues (P<0.05) (Figure 3B) and CRC cell lines (P<0.05) (Figure 3C). We thus predicted that Overexpression of TRIM32 is associated with the downregulation of miR-519d in CRC. To confirm this speculation, we detected the expression level of TRIM32 after transfection with miR-519d mimics in SW480 and HT29 cells. Effect of miR-519d mimics on changing the miR-519d level in SW480 and HT29 was shown in Figure 3D. Next, results of Western blot analysis revealed that transfection with miR-519d mimics can decrease the protein level of TRIM32 obviously in both SW480 and HT29 cells (P<0.05) (Figure 3E). Furthermore, results of luciferase reporter assays showed that transfection with miR-519d mimics significantly decreased the luciferase activities of pMIR-Reporters carrying wild type TRIM32 3ʹ UTR (P<0.05) (Figure 3F). Taken together, we demonstrated that TRIM32 was targeted by miR-519d. Overexpression of TRIM32 is induced by downregulation of miR-519d in CRC cells.
Figure 3.
TRIM32 is targeted by miR-519d in colorectal cancer. (A) The bioinformatics software TargetScan, miRanda and PicTar were used to identify the binding region of miR-519d in the TRIM32 3ʹ UTR. (B) Expression levels of miR-519d in 25 pairs of colorectal cancer patients’ tumor tissues and paracancerous tissues were evaluated by qRT-PCR analysis. (C) Expression levels of miR-519d in FHC, SW480 and HT29 were measured by qRT-PCR analysis. (D) Transfection with miR-519d mimics significantly increased the miR-519d levels in SW480 and HT29 cells. (E) Effect of miR-519d mimics on changing the protein expression of TRIM32 was evaluated by Western blot assays. (F) Effect of miR-519d mimics on changing the luciferase activities of pMIR-Reporters carrying wild type or mutant type of TRIM32 3ʹ UTR.
Notes: Data was expressed as mean±SD. #P<0.05 vs paracancerous tissues, *P<0.05 vs FHC group, &P<0.05 vs NCO group, $P<0.05.
Abbreviations: TRIM32, tripartite motif 32; 3ʹ UTR, 3ʹuntranslated region; qRT-PCR, quantitative real-time polymerase chain reaction.
Inhibition of TRIM32 Induced by miR-519d Increases the Sensitivity of CRC Cells to Cisplatin
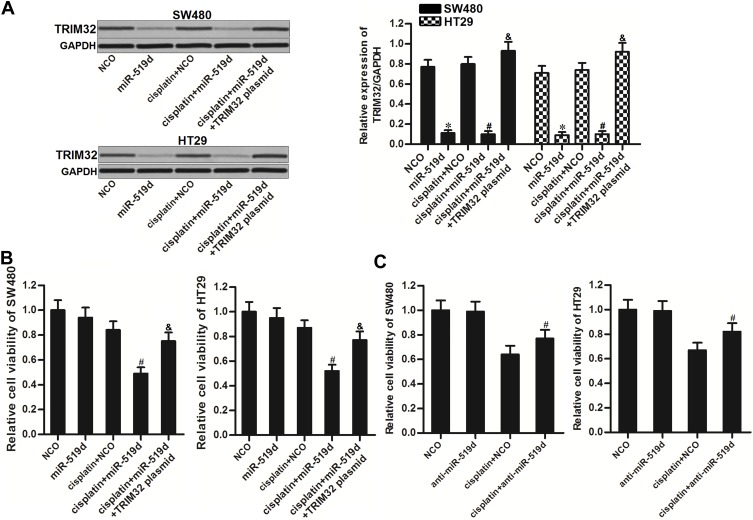
To explore whether restore of miR-519d expression can increase the sensitivity of CRC cells to cisplatin through inhibition of TRIM32, we co-treated the SW480 and HT29 cells with miR-519d, TRIM32 plasmid and cisplatin. Effect of miR-519d, TRIM32 plasmid and cisplatin on changing the TRIM32 expression was shown in Figure 4A. Results of CCK-8 assays showed that restore of miR-519d significantly increased the cytotoxicity of cisplatin to SW480 and HT29 cells (P<0.05). However, co-transfection with TRIM32 plasmid abolished the effect of miR-519d on cisplatin against SW480 and HT29 (P<0.05) (Figure 4B). On the other hand, despite anti-miR-519d single treatment had no effect on SW480 and HT29 cells, it decreased the cytotoxicity of cisplatin against the CRC cells (P<0.05) (Figure 4C). Our data indicated that combination with miR-519d can sensitize the CRC cells to cisplatin through inhibition of TRIM32.
Figure 4.
Inhibition of TRIM32 induced by miR-519d increases the sensitivity of colorectal cancer cells to cisplatin. (A) Effect of miR-519d and TRIM32 plasmid on changing the TRIM32 expression was evaluated by Western blot assay. (B) SW480 and HT29 cells were transfected with miR-519d and TRIM32 plasmid. 24 hrs later, these cells were treated with cisplatin (1 μM) for another 48 hrs. CCK-8 assays were performed to measure the cell viability of these cells. (C) SW480 and HT29 cells were transfected with anti-miR-519d. 24 hrs later, these cells were treated with cisplatin (5 μM) for another 48 hrs. CCK-8 assays were performed to measure the cell viability of these cells.
Notes: Data was expressed as mean±SD. *P<0.05 vs NCO group, #P<0.05 vs cisplatin + NCO group, &P<0.05 vs cisplatin + miR-519d group.
Abbreviations: TRIM32, tripartite motif 32; CCK-8, Cell Counting Kit-8.
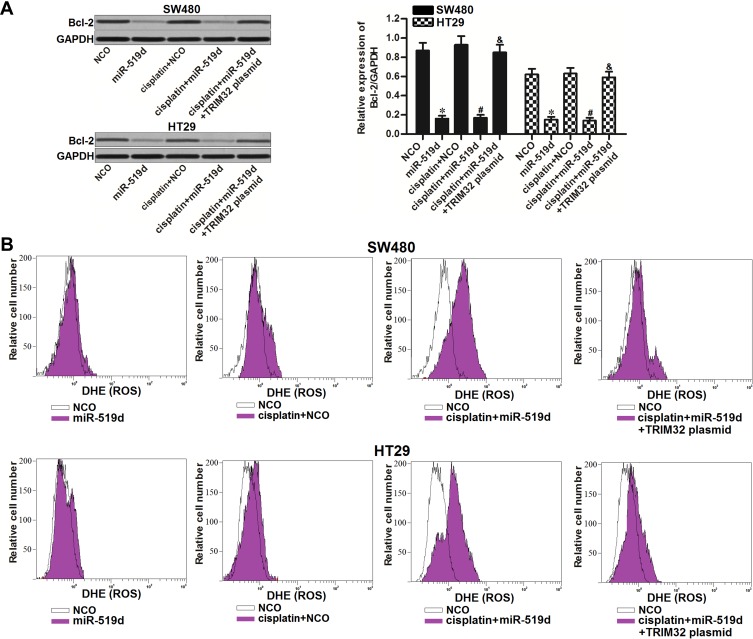
Inhibition of TRIM32 Induced by miR-519d Suppresses Bcl-2 Expression and Promotes Cisplatin-Induced Generation of ROS
As TRIM32 has been shown to decrease the cisplatin sensitivity in CRC, we next screened a panel of apoptosis-related proteins to elucidate the potential mechanisms. As a result, we found that treatment with miR-519d decreased the expression of Bcl-2 in SW480 and HT29 cells. However, co-transfection with TRIM32 plasmid in miR-519d-treated CRC cells rebounds the protein level of Bcl-2 (P<0.05) (Figure 5A). Besides, although miR-519d single treatment can not induce obvious generation of ROS, it promoted the production of ROS in cisplatin-treated SW480 and HT29 cells. However, overexpression of TRIM32 in SW480 and HT29 cells made the ROS generation at a low level even the cells were co-treated with cisplatin and miR-519d (Figure 5B). Taken together, we found that miR-519d can suppress Bcl-2 expression and promote the cisplatin-induced generation of ROS through inhibition of TRIM32.
Figure 5.
Inhibition of TRIM32 induced by miR-519d suppresses Bcl-2 expression and promotes the cisplatin-induced generation of ROS. (A) Effect of miR-519d and TRIM32 plasmid on changing the Bcl-2 expression was evaluated by Western blot assay. (B) SW480 and HT29 cells were transfected with miR-519d and TRIM32 plasmid. 24 hrs later, these cells were treated with cisplatin (1 μM) for another 48 hrs. Flow cytometry analysis was performed to detect the generation of ROS.
Notes: Data was expressed as mean±SD. *P<0.05 vs NCO group, #P<0.05 vs cisplatin + NCO group, &P<0.05 vs cisplatin + miR-519d group.
Abbreviations: TRIM32, tripartite motif 32; ROS, reactive oxygen species.
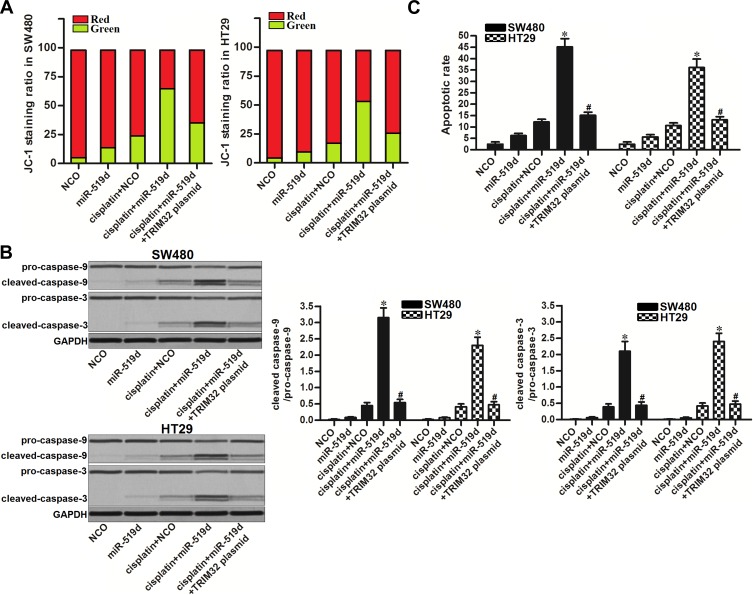
Inhibition of TRIM32 Induced by miR-519d Promotes Mitochondria-Mediated Intrinsic Apoptosis Induced by Cisplatin
To investigate the effect of miR-519d/TRIM32 axis on mitochondria-mediated apoptosis induced by cisplatin, we detected the mitochondrial membrane potential (MMP, Δφ) of SW480 and HT29 cells. Results of flow cytometry showed that combination with miR-519d increased the effect of cisplatin on MMP collapse. However, co-transfection with TRIM32 plasmid attenuated the collapse of MMP in the cisplatin and miR-519d co-treated CRC cells (Figure 6A). It suggested that miR-519d can enhance the cisplatin-induced damage of mitochondria. Results of Western blot analysis showed that combination with cisplatin and miR-519d induced significant activation of caspase-9 and caspase-3 (P<0.05) which are the markers of intrinsic apoptosis (Figure 6B). Furthermore, the cisplatin-induced apoptosis was expanded due to the miR-519d co-treatment (P<0.05) (Figure 6C). These data showed that inhibition of TRIM32 induced by miR-519d can promote mitochondria-mediated intrinsic apoptosis induced by cisplatin.
Figure 6.
Inhibition of TRIM32 induced by miR-519d promotes mitochondria-mediated intrinsic apoptosis induced by cisplatin. (A) SW480 and HT29 cells were transfected with miR-519d and TRIM32 plasmid. 24 hrs later, these cells were treated with cisplatin (1 μM) for another 48 hrs. After staining with JC-1, flow cytometry analysis was performed to detect the MMP (Δφ) of cells. (B) Activation of caspase-9 and caspase-3 in SW480 and HT29 cells was detected by Western blot assay. (C) Apoptosis of SW480 and HT29 cells was detected by flow cytometry analysis.
Notes: Data was expressed as mean±SD. *P<0.05 vs cisplatin + NCO group, #P<0.05 vs cisplatin + miR-519d group.
Abbreviations: TRIM32, tripartite motif 32; MMP, mitochondrial membrane potential.
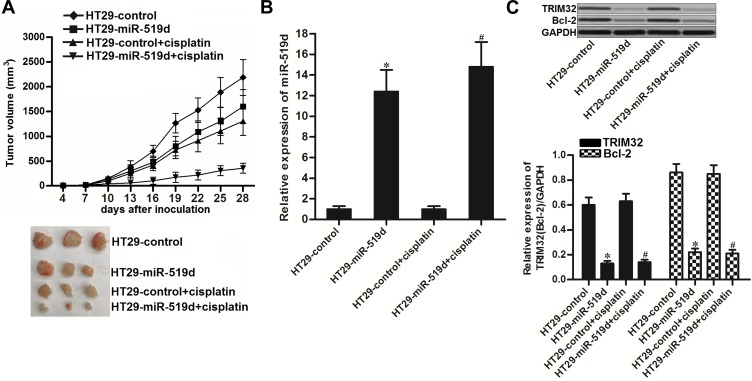
Overexpression of miR-519d Sensitizes CRC to Cisplatin Treatment in vivo
To investigate the effect of miR-519d on cisplatin treatment in CRC in vivo, we inoculated the nude mice with HT29 cells transfected with lentivirus carrying miR-519d precursor (HT29-miR-519d) or HT29 cells transfected with empty lentivirus (HT29-control). Under the administration of equal dose of cisplatin (2 mg/kg, twice a week), the tumor growth of HT29-miR-519d was obviously slower than the HT29-control (Figure 7A). The results indicated the sensitization of miR-519d on cisplatin treatment in CRC in vivo. After euthanasia of mice, we found significant increase of miR-519d expression in HT29-miR-519d tumors (P<0.05) (Figure 7B). On the contrary, expression of TRIM32 and Bcl-2 was decreased in HT29-miR-519d tumors compared to the HT29-control tumors (P<0.05) (Figure 7C). These results indicated that TRIM32 was also targeted by miR-519d in CRC in vivo.
Figure 7.
Overexpression of miR-519d sensitizes colorectal cancer to cisplatin treatment in vivo. (A) Tumor growth of miR-519d-overexpressed or control HT29 tumors which were treated with cisplatin (2 mg/kg) twice a week. (B) Expression of miR-519d in resected tumor tissues. (C) Expression of TRIM32 and Bcl-2 in resected tumor tissues.
Notes: Data was expressed as mean±SD. *P<0.05 vs HT29-control group, #P<0.05 vs HT29-control + cisplatin group.
Abbreviations: TRIM32, tripartite motif 32; Bcl-2, B-cell lymphoma-2.
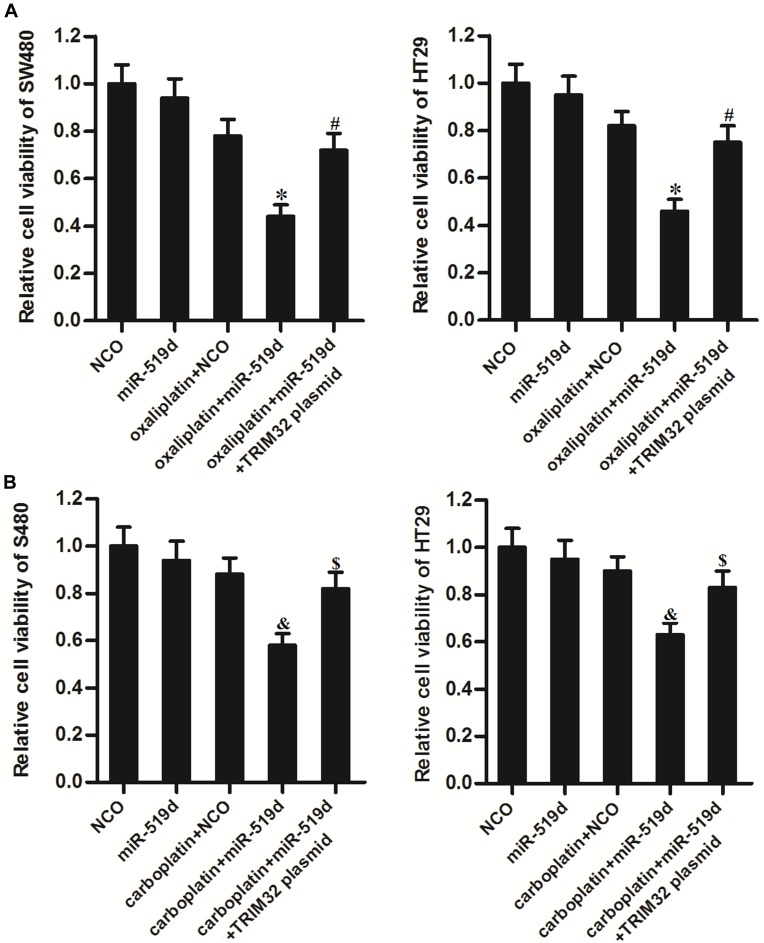
Inhibition of TRIM32 Induced by miR-519d Increases the Sensitivity of CRC Cells to Other Platinum-Based Drugs
To explore whether restore of miR-519d expression can increases the sensitivity of CRC cells to some other platinum-based drugs including oxaliplatin and carboplatin, we transfected the SW480 and HT29 cells with miR-519d and TRIM32 before treatment with oxaliplatin and carboplatin. Results of CCK-8 assays showed that restore of miR-519d significantly increased the cytotoxicity of oxaliplatin to SW480 and HT29 cells (P<0.05) (Figure 8A). Similarly, miR-519d also enhanced the effect of carboplatin on CRC cells (P<0.05) (Figure 8B). However, enforced expression of TRIM32 protected these CRC cells from oxaliplatin and carboplatin (Figure 8A and B). These results indicated that the miR-519d had a common activity on platinum-based drugs through the TRIM32 pathway.
Figure 8.
Inhibition of TRIM32 induced by miR-519d increases the sensitivity of colorectal cancer cells to oxaliplatin and carboplatin. (A) SW480 and HT29 cells were transfected with miR-519d and TRIM32 plasmid. 24 hrs later, these cells were treated with oxaliplatin (1 μM) for another 48 hrs. CCK-8 assays were performed to measure the cell viability of these cells. (B) SW480 and HT29 cells were transfected with miR-519d and TRIM32 plasmid. 24 hrs later, these cells were treated with carboplatin (1 μM) for another 48 hrs. CCK-8 assays were performed to measure the cell viability of these cells.
Notes: Data was expressed as mean±SD. *P<0.05 vs oxaliplatin + NCO group, #P<0.05 vs oxaliplatin + miR-519d group, &P<0.05 vs carboplatin + NCO group, $P<0.05 vs carboplatin + miR-519d group.
Abbreviations: TRIM32, tripartite motif 32; CCK-8, Cell Counting Kit-8.
Discussion
Cisplatin is a broad-spectrum antineoplastic drug that is commonly used for the treatment of CRC.22,23 Cisplatin can bind to DNA in cancer cells and causes cross-linking of DNA. Thus, cisplatin destroys the normal function of DNA in cancer cells and induces cells to enter the apoptotic process.24,25 However, high-dose treatment of cisplatin causes severe side effects, including neurotoxicity, nephrotoxicity, gastrointestinal reactions and myelosuppression.26,27 Therefore, reducing the dosage of cisplatin and increasing the sensitivity of CRC cells to cisplatin is important to improve the chemotherapy effect and patient compliance.
Dysregulation of miRNAs usually induces chemoresistance in various cancers including CRC.15,16 Nowadays, some specific miRNAs have been considered as novel targets for improving the effect of chemotherapeutic drugs on cancers.28,29 Among these cancer-related miRNAs, miR-519d has been reported to act as a tumor suppressor in some cancers such as breast cancer and hepatocellular carcinoma.30,31 Furthermore, previous studies have indicated that miR-519d can improve chemotherapy on some cancers.32,33 It suggests that overexpression of miR-519d may be a potential strategy in cancer therapy.
In the present study, we found that overexpression of TRIM32 was responsible for the decrease of cisplatin sensitivity in CRC. Knockdown of TRIM32 obviously improved the cytotoxicity of cisplatin against CRC. After exploring the association between TRIM32 and miR-519d, we found that overexpression of TRIM32 in CRC was induced by insufficiency of miR-519d. We then proved that restore of miR-519d can sensitize the CRC cells to cisplatin treatment through suppression of TRIM32 in vitro and in vivo. Thus, we demonstrated that overexpression of miR-519d may represent a novel strategy to reduce the dosage of cisplatin through improving the chemotherapy effect of it.
In cancer cells, cisplatin or other platinum-based drugs induces DNA damage which is an important apoptosis signal,34 under the effect of cisplatin-dependent apoptosis signal, mitochondria-mediated intrinsic apoptosis pathway, which represents a collapse of mitochondria and activation of caspase-9 and caspase-3,35,36 will be triggered. In the mitochondria apoptosis pathway, Bcl-2 and reactive oxygen species (ROS) are important regulators. High level of Bcl-2, which is a key anti-apoptotic protein, attenuates the cisplatin-induced apoptosis.37 On the contrary, ROS expands the apoptosis induced by cisplatin.38
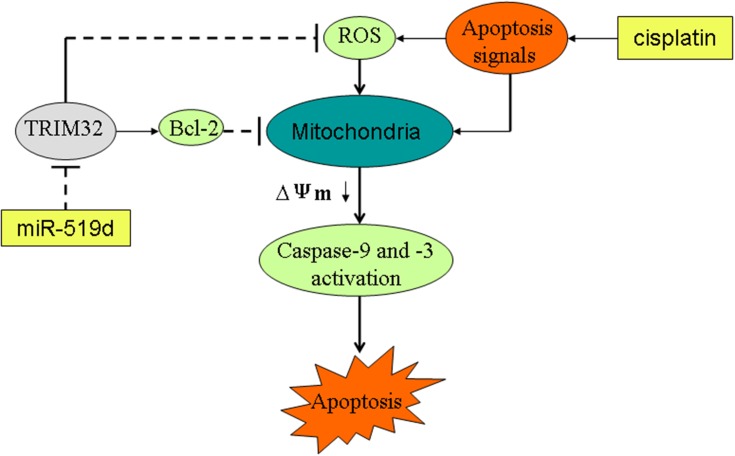
In our further studies on miR-519d/TRIM32 axis, we demonstrated that miR-519d inhibited TRIM32 expression and thus suppressed the expression of Bcl-2 and promotes the cisplatin-caused generation of ROS. Because of the high level of ROS and inhibition of Bcl-2, apoptosis signals produced by cisplatin were expanded to trigger the mitochondria-mediated intrinsic apoptosis pathway. As a result, mitochondrial membrane potential was decreased and the effector caspases (caspase-9 and caspase-3) are triggered to cause the apoptotic cell death of SW480 and HT29 cells (Figure 9).
Figure 9.
Schema of the predicted mechanisms implicated in SW480 and HT29 cells response to cisplatin and miR-519d. MiR-519d inhibits TRIM32 expression and thus suppresses the expression of Bcl-2 and promotes the cisplatin-caused generation of ROS. Because of the high level of ROS and inhibition of Bcl-2, apoptosis signals produced by cisplatin were expanded to trigger the mitochondria-mediated intrinsic apoptosis pathway. As a result, MMP (Δφ) was decreased and the effector caspases (caspase-9 and caspase-3) are triggered to cause the apoptotic cell death of SW480 and HT29 cells.
Abbreviations: TRIM32, tripartite motif 32; Bcl-2, B-cell lymphoma-2; MMP, mitochondrial membrane potential, ROS, reactive oxygen species.
Taken together, this study indicates that inhibition of TRIM32 induced by miR-519d increases the sensitivity of CRC cells to cisplatin. Despite further studies are required, the miR-519d/TRIM32 axis may represent a potential target for improving the chemotherapy effect on CRC.
Acknowledgements
This study is supported by 2017 key research and development projects in social development field of Hainan Province (grant no: ZDYF2017090).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 3.Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254–259. doi: 10.1097/01.sla.0000217629.94941.cf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao Y, Rao C, Zheng G, Wang S. Luteolin suppresses colorectal cancer cell metastasis via regulation of the miR-384/pleiotrophin axis. Oncol Rep. 2019;42:131–141. doi: 10.3892/or.2019.7136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LCiombor KK, Wu C, Goldberg RM. Recent therapeutic advances in the treatment of colorectal cancer. Annu Rev Med. 2015;66:83–95. doi: 10.1146/annurev-med-051513-102539 [DOI] [PubMed] [Google Scholar]
- 6.Kuipers EJ, Grady WM, Lieberman D, et al. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. doi: 10.1038/nrdp.2015.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JH. Chemotherapy for colorectal cancer in the elderly. World J Gastroenterol. 2015;21:5158–5166. doi: 10.3748/wjg.v21.i17.5158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S, Li J, Yang X. Long non-coding RNA LINC00525 promotes the stemness and chemoresistance of colorectal cancer by targeting miR-507/ELK3 axis. Int J Stem Cells. 2019;12:347–359. doi: 10.15283/ijsc19041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Li C, Li D, Yang L, Jin J, Zhang B. lncRNA KCNQ1OT1 enhances the chemoresistance of oxaliplatin in colon cancer by targeting the miR-34a/ATG4B pathway. Onco Targets Ther. 2019;12:2649–2660. doi: 10.2147/OTT.S188054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chi YD, Zhou DM. MicroRNAs in colorectal carcinoma–from pathogenesis to therapy. J Exp Clin Cancer Res. 2016;35:43. doi: 10.1186/s13046-016-0320-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2007;131:11–29. doi: 10.1016/s0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- 12.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iorio MV, Croce CM. microRNA involvement in human cancer. Carcinogenesis. 2012;33:1126–1133. doi: 10.1093/carcin/bgs140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287–314. doi: 10.1146/annurev-pathol-012513-104715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H, Deng Z, Chen X, et al. Downregulation of miR-222-3p reverses doxorubicin-resistance in lovo cells through upregulating forkhead box protein P2 (FOXP2) protein. Med Sci Monit. 2019;25:2169–2178. doi: 10.12659/MSM.913325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang H, Xu Y, Zhang Q, et al. MiR-483-3p regulates oxaliplatin resistance by targeting FAM171B in human colorectal cancer cells. Artif Cells Nanomed Biotechnol. 2019;47:725–736. doi: 10.1080/21691401.2019.1569530 [DOI] [PubMed] [Google Scholar]
- 17.Lei W, Yan C, Ya J, Yong D, Yujun B, Kai L. MiR-199a-3p affects the multi-chemoresistance of osteosarcoma through targeting AK4. BMC Cancer. 2018;18:631. doi: 10.1186/s12885-018-4460-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cambiaghi V, Giuliani V, Lombardi S, Marinelli C, Toffalorio F, Pelicci PG. TRIM proteins in cancer. Adv Exp Med Biol. 2012;770:77–91. doi: 10.1007/978-1-4614-5398-7_6 [DOI] [PubMed] [Google Scholar]
- 19.Ozato K, Shin DM, Chang TH, Morse HC. TRIM family proteins and their emerging roles in innate immunity. Nat Rev Immunol. 2008;8:849. doi: 10.1038/nri2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C, Xu J, Fu H, et al. TRIM32 promotes cell proliferation and invasion by activating β-catenin signalling in gastric cancer. J Cell Mol Med. 2018;22:5020–5028. doi: 10.1111/jcmm.13784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Zhang C, Wang XL, et al. E3 ubiquitin ligase TRIM32 negatively regulates tumor suppressor p53 to promote tumorigenesis. Cell Death Differ. 2014;21:1792–1804. doi: 10.1038/cdd.2014.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao H, Sun Q, Zhu J. miR-1271 enhances the sensitivity of colorectal cancer cells to cisplatin. Exp Ther Med. 2019;17:4363–4370. doi: 10.3892/etm.2019.7501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin A, Jiang Y, Zhang X, Zhao J, Luo H. Transfection of PDCD5 sensitizes colorectal cancer cells to cisplatin-induced apoptosis in vitro and in vivo. Eur J Pharmacol. 2010;649:120–126. doi: 10.1016/j.ejphar.2010.09.040 [DOI] [PubMed] [Google Scholar]
- 24.Li S, Ren B, Shi Y, et al. Notch1 inhibition enhances DNA damage induced by cisplatin in cervical cancer. Exp Cell Res. 2019;376:27–38. doi: 10.1016/j.yexcr.2019.01.014 [DOI] [PubMed] [Google Scholar]
- 25.Jin X, Tian S, Li P, et al. Growth arrest-specific 5 attenuates cisplatin-induced apoptosis in cervical cancer by regulating STAT3 signaling via miR-21. J Cell Physiol. 2019;234:9605–9615. doi: 10.1002/jcp.27647 [DOI] [PubMed] [Google Scholar]
- 26.Trendowski MR, El Charif O, Dinh PC Jr, Travis LB, Dolan ME. Genetic and modifiable risk factors contributing to cisplatin-induced toxicities. Clin Cancer Res. 2019;25:1147–1155. doi: 10.1158/1078-0432.CCR-18-2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Surnar B, Kolishetti N, Basu U, et al. Reduction of cisplatin-induced ototoxicity without compromising its antitumor activity. Biochemistry. 2018;57:6500–6513. doi: 10.1021/acs.biochem.8b00712 [DOI] [PubMed] [Google Scholar]
- 28.Bian WG, Zhou XN, Song S, Chen HT, Shen Y, Chen P. Reduced miR-363-3p expression in non-small cell lung cancer is associated with gemcitabine resistance via targeting of CUL4A. Eur Rev Med Pharmacol Sci. 2019;23:649–659. doi: 10.26355/eurrev_201901_16879 [DOI] [PubMed] [Google Scholar]
- 29.Turato C, Fornari F, Pollutri D, et al. MiR-122 targets SerpinB3 and is involved in sorafenib resistance in hepatocellular carcinoma. J Clin Med. 2019;8:E171. doi: 10.3390/jcm8020171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng X, Zhao Y, Wang B. miR-519d-mediated downregulation of STAT3 suppresses breast cancer progression. Oncol Rep. 2015;34:2188–2194. doi: 10.3892/or.2015.4160 [DOI] [PubMed] [Google Scholar]
- 31.Hou YY, Cao WW, Li L, et al. MicroRNA-519d targets MKi67 and suppresses cell growth in the hepatocellular carcinoma cell line QGY-7703. Cancer Lett. 2011;307:182–190. doi: 10.1016/j.canlet.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 32.Xie Q, Wang S, Zhao Y, Zhang Z, Qin C, Yang X. MiR-519d impedes cisplatin-resistance in breast cancer stem cells by down-regulating the expression of MCL-1. Oncotarget. 2017;8:22003–22013. doi: 10.18632/oncotarget.15781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pang Y, Mao H, Shen L, Zhao Z, Liu R, Liu P. MiR-519d represses ovarian cancer cell proliferation and enhances cisplatin-mediated cytotoxicity in vitro by targeting XIAP. Onco Targets Ther. 2014;7:587–597. doi: 10.2147/OTT.S60289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao JX, Liu H, Lv J, Yang XJ. Wortmannin enhances cisplatin-induced apoptosis in human ovarian cancer cells in vitro. Eur Rev Med Pharmacol Sci. 2014;18:2428–2434. [PubMed] [Google Scholar]
- 35.Liu J, Tang Q, Li S, Yang X. Inhibition of HAX-1 by miR-125a reverses cisplatin resistance in laryngeal cancer stem cells. Oncotarget. 2016;7:86446. doi: 10.18632/oncotarget.13424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang G, Pan J, Ye Z, Fang B, Cheng W, Cao Z. Overexpression of miR-216b sensitizes NSCLC cells to cisplatin-induced apoptosis by targeting c-Jun. Oncotarget. 2017;8:104206–104215. doi: 10.18632/oncotarget.22171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park SB, Lee JH, Jeong WW, et al. TTP mediates cisplatin-induced apoptosis of head and neck cancer cells by down-regulating the expression of Bcl-2. J Chemother. 2015;27:174–180. doi: 10.1179/1973947814Y.0000000234 [DOI] [PubMed] [Google Scholar]
- 38.Zhang L, He L, Zhang H, Chen Y. Knockdown of MiR-20a enhances sensitivity of colorectal cancer cells to cisplatin by increasing ASK1 expression. Cell Physiol Biochem. 2018;47:1432–1441. doi: 10.1159/000490834 [DOI] [PubMed] [Google Scholar]