Abstract
Introduction
This study evaluated long-term reductions in intraocular pressure (IOP) and medication following implantation of 2 second-generation trabecular micro-bypass stents (iStent inject®) in eyes with open-angle glaucoma (OAG) not controlled on 1 preoperative medication.
Material and Methods
In this prospective interventional multi-surgeon study, standalone implantation of 2 iStent inject stents was performed in 57 eyes of 57 subjects with OAG, preoperative IOP of 18–30 mmHg on 1 medication, and preoperative post-washout IOP of 22–38 mmHg. The main outcome measures included the proportions of eyes achieving medication-free IOP ≤18 mmHg, IOP ≤15 mmHg, or ≥20% IOP reduction versus preoperative unmedicated IOP. Assessments included IOP, medications, visual acuity, visual field, pachymetry, complications, and interventions. Subjects were followed for 48 months with follow-up continuing in all eyes.
Results
At Month 48 (n=57), 95% of eyes achieved an IOP reduction of ≥20% without medication versus preoperative washout IOP; and although they had eliminated medication, 81% of eyes still had an IOP reduction of ≥20% versus preoperative IOP on 1 medication. Mean 48-month unmedicated IOP decreased by 46% to 13.2±1.6 mmHg vs 24.4±1.3 mmHg preoperatively (p<0.0001), with 95% of medication-free eyes having IOP ≤18mmHg and 82% having IOP ≤15mmHg. Over the course of follow-up, 3 eyes had medication added and 1 eye underwent a secondary glaucoma surgery, and safety parameters were favorable.
Discussion
Standalone iStent inject implantation in OAG patients on 1 preoperative medication resulted in average IOP reduction to ≤15 mmHg with the elimination of medication and favorable safety through 48 months.
Trial Registration
ClinicalTrials.gov identifier, NCT02868190.
Keywords: glaucoma, MIGS, trabecular micro-bypass, second-generation, prostaglandin, iStent inject
Introduction
Glaucoma is the leading cause of irreversible blindness worldwide, affecting 70 million people globally.1,2 Elevated intraocular pressure (IOP) remains the major risk factor for glaucoma,3 and current glaucoma treatment options are centered around lowering IOP. Treatments include a variety of life-long therapies including pharmacologic, laser, or surgical interventions. Ocular hypotensive medications are reasonably effective and safe, but their pressure-lowering effects may decrease over time with chronic usage, and/or they can lead to ocular surface damage and conjunctival inflammation.4–6 In addition, it is estimated that almost 40% of glaucoma patients in the United States require more than one pharmaceutical agent to effectively lower IOP,7 which likely impacts treatment consistency given the well-known decline in adherence with more eye drops.8
When medications are not sufficient, laser trabeculoplasty is often employed. This intervention reliably reduces IOP, but can lose effectiveness over time and can induce inflammation in the immediate-term.9 In cases insufficiently treated by the above therapies, incisional surgeries such as trabeculectomy and tube shunt implantation may be employed. While such surgeries can produce dramatic IOP reductions, they also open the patient up to a host of safety risks, many of which persist for the duration of their lifespan.10–12
In the past decade, the development of micro-invasive glaucoma surgery (MIGS) has increased the surgical options available for clinicians treating OAG. These MIGS procedures typically yield more modest IOP reductions than traditional filtering surgeries, but their high safety profile may yield a preferable benefit-risk profile for patients earlier in the disease process. Importantly, ab interno MIGS surgery preserves conjunctival tissue in case future surgery is needed (as is frequent in this lifelong, progressive condition). Micro-invasive glaucoma surgery (MIGS) also is able to reduce medication burden. The reduction in medications lessens dependency on patient adherence, the lack of which is thought to be a contributor to glaucoma progression.10
The first-generation trabecular micro-bypass stent, iStent® (Glaukos Corporation, San Clemente, CA, USA) was the first US FDA-approved MIGS device. It is a single-piece titanium stent designed to enhance aqueous outflow via direct access into Schlemm’s canal through the trabecular meshwork, the primary site of aqueous outflow resistance and a major cause of IOP elevation in OAG.11,13 The iStent has a well-established favorable safety profile and sustained effectiveness through up to 5 years postoperative in patients with various types of OAG, either with or without cataract surgery.14–27 The second-generation iStent inject Trabecular Micro-Bypass (Glaukos Corporation) was developed to reduce IOP in a manner similar to the iStent: by creating a patent pathway for aqueous humor to exit the anterior chamber through the trabecular meshwork into Schlemm’s canal.28 Numerous studies have demonstrated substantial IOP and medication reductions following implantation of iStent inject during cataract surgery or in standalone procedures.28–37 In addition to its clinical effectiveness, iStent inject has shown a favorable safety profile over the long term, which is an important advantage over traditional surgeries such as trabeculectomy or tube placement.
To prospectively study the performance of both devices (iStent and iStent inject) in OAG, the MIGS Study Group was established, consisting of experienced glaucoma surgeons from multiple countries. Authors from this Group previously reported that standalone iStent inject implantation resulted in IOP reduction and reduced medication dependence in OAG eyes on 1 or 2 preoperative glaucoma medication(s) through 18 months postoperative.36,37 The present report shows extended follow-up through 4 years after standalone iStent inject implantation in OAG eyes on 1 preoperative glaucoma medication. Follow-up is continuing through 5 years postoperative.
Materials and Methods
Study Design and Participants
This prospective, single-arm study was designed to enroll phakic or pseudophakic subjects with OAG (including primary open-angle, pseudoexfoliative, or pigmentary glaucoma), treatment with 1 topical ocular hypotensive medication, medicated IOP at screening of 18–30 mmHg, and unmedicated (post-washout) IOP of 22–38 mmHg. Other inclusion criteria in the study eye included cup-to-disc (C:D) ratio of ≤0.9, best-corrected visual acuity (BCVA) of 20/100 or better, and normal angle anatomy. Exclusion criteria included uveitic, traumatic, neovascular, or angle-closure glaucoma; glaucoma associated with vascular disorders; and prior laser or incisional glaucoma surgery (with the exception of prior SLT if completed >90 days prior to screening).
The study was conducted at S.V. Malayan Ophthalmological Center in Yerevan, Armenia. All surgeries were performed by the US glaucoma fellowship-trained staff surgeon (L.V.) and 10 visiting surgeons from the MIGS Study Group (listed in Appendix 1 in prior publication36). All examinations were completed at the Center by the staff surgeon or glaucoma-trained ophthalmologists. The trial was approved by the Armenian Ministry of Health and was performed in accordance with the Helsinki Declaration. Written informed consent was obtained from all patients included in the study. The ClinicalTrials.gov registration number for this study is NCT02868190.
Surgical Device and Implantation Technique
The second-generation iStent inject device consists of a single-use stainless steel injector pre-loaded with two micro-scale stents, each of which is a titanium, heparin-coated stent with 360 um length, 230 um width, and multiple lateral outlet lumens to facilitate aqueous outflow [Figure 1]. After making a temporal clear corneal incision and filling the anterior chamber with viscoelastic, the injector is advanced ab internally to the nasal anterior chamber angle, where the stents are placed through the trabecular meshwork and into Schlemm’s canal at approximately 2 clock-hours apart. After stent implantation, irrigation/aspiration is used to remove the viscoelastic. Postoperatively, patients received topical anti-microbial medication (for 1 week) and anti-inflammatory medication (for 4 weeks).
Figure 1.
iStent inject® trabecular micro-bypass stent system: stents and injector.
Outcomes and Statistical Analyses
Study visits occurred preoperatively and at postoperative Day 1, Week 1, and Months 1, 3, 6, and every 6 months thereafter; all evaluations included IOP, BCVA (Snellen equivalent), slit lamp microscopy, medications, ocular complications, and secondary surgical interventions. In addition, stent position and patency were assessed by gonioscopy at screening and at every postoperative study visit from Week 1 onward. Indirect ophthalmoscopy examination, C:D ratio, visual field (Swedish Interactive Thresholding Algorithm Standard 24–2 automated perimetry), and pachymetry were recorded at screening and at every 6 months postoperatively. Diurnal IOP measurement (consisting of the average of three IOP measures at 9am, 12pm, and 4pm in a single day) was completed at Baseline and at every postoperative visit from Month 1 onward. IOP was measured by Goldmann applanation with the 2-observer masked method commonly used in clinical trials and described in the Ocular Hypertension Treatment Study, in which one observer (masked to readings) completes the IOP measurement while a second observer (masked to measurement) records the readings. Throughout follow-up, ocular hypotensive medication was to be started if postoperative IOP exceeded 21 mmHg and/or in the case of concerning optic nerve findings per investigator discretion.
The protocol-defined efficacy endpoints were the proportion of eyes with Month 12 unmedicated IOP reduced by ≥20% versus baseline unmedicated IOP (primary efficacy), and the proportion of eyes with Month 12 unmedicated IOP of ≤18 mmHg (secondary efficacy). These endpoints also were evaluated through Month 48. Additional endpoints included mean IOP over time, the percent of eyes on no medications postoperatively, and the proportion of eyes with unmedicated IOP of ≤15 mmHg. Eyes that needed medication or additional glaucoma surgery were considered non-responders in proportional analyses. Mean and standard deviation were calculated for continuous variables, and proportions were calculated for categorical variables. Pre- and post-operative mean values were compared using a paired t-test, and proportions were compared using a McNemar test; calculations were completed using MedCalc statistical software. A p-value < 0.05 was considered statistically significant.
Results
Subject Accountability, Demographics and Preoperative Parameters
A total of 57 qualified subjects underwent ab interno implantation of iStent inject (containing 2 stents) in a standalone procedure. All subjects reached 48 months postoperative, and follow-up is ongoing. Demographic and preoperative parameters are shown in Table 1. Although the study protocol allowed for either phakic or pseudophakic lens status, only phakic subjects presented for participation and were enrolled. All subjects were phakic and were on 1 preoperative medication (a prostaglandin analogue in 86% of eyes). Preoperative mean medicated IOP was 19.5 ± 1.5 mmHg on 1 medication, and mean unmedicated (post-washout) IOP was 24.4 ± 1.3 mmHg.
Table 1.
Demographic and Preoperative Ocular Characteristics
| 57 Eyes of 57 Subjects | ||
|---|---|---|
| Gender Male/Female | 30/27 | |
| Race | % Caucasian | 100% (n=57) |
| Age (years) | Mean ± SD | 65.3 ± 9.0 |
| C:D ratio | Mean ± SD | 0.7 ± 0.1 |
| Medicated IOP (mmHg) | Mean ± SD | 19.5 ± 1.5 |
| Preoperative # medications | Mean ± SD | 1 ± 0 |
| Medication classes | % of eyes (n) | |
| Prostaglandin | 86% (n=49) | |
| Beta-blocker | 7% (n=4) | |
| Carbonic anhydrase inhibitor | 7% (n=4) | |
| Unmedicated IOP (mmHg) | Mean ± SD | 24.4 ± 1.3 |
| Glaucoma severity (available in 56 eyes)a | n (%) | |
| Mild | 5 (8.9%) | |
| Moderate | 15 (26.8%) | |
| Severe | 36 (64.3%) | |
Notes: aSeverity according to the Hodapp Parrish Anderson (HPA) glaucoma severity scale (Mild: mean deviation (MD) no worse than −6 dB; Moderate: MD worse than −6 dB but no worse than −12 dB; Severe: MD worse than −12 dB). Adapted from Lindstrom R, Lewis R, Hornbeak DM, et al. Outcomes Following Implantation of Two Second-Generation Trabecular Micro-Bypass Stents in Patients with Open-Angle Glaucoma on One Medication: 18-Month Follow-Up. Adv Ther. 2016;33(11):2082-90.36 This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/).
Efficacy
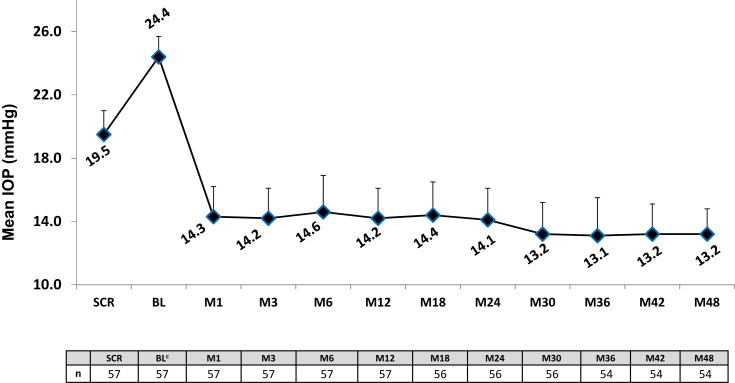
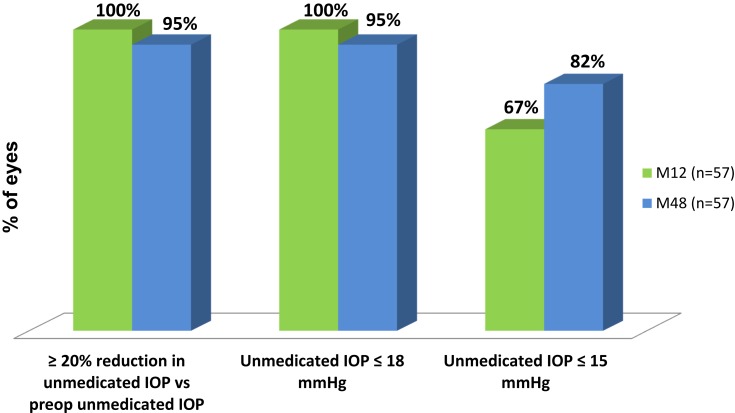
Figure 2 shows the mean IOP over time for the 48-month period after implantation of iStent inject. At Month 48, mean unmedicated IOP had decreased by 46% to 13.2 ± 1.6 mmHg vs 24.4 ± 1.3mmHg preoperatively (p<0.0001, significant). As shown in Figure 3, 95% of eyes achieved a Month 48 IOP reduction of ≥20% without medication versus preoperative washed-out IOP; and even though these eyes had eliminated their topical medication, 81% of them still achieved a ≥20% IOP reduction versus their preoperative IOP on 1 medication. In addition, 95% of eyes at Month 48 had IOP ≤18mmHg and 82% had IOP ≤15mmHg without medication [Figure 3]. At 12 months, the time point for the primary and secondary efficacy endpoints, 100% of eyes had achieved an IOP reduction of ≥20% without medication versus preoperative unmedicated IOP; and even though these eyes had eliminated their medication, 75% of them still achieved an IOP reduction of ≥20% versus preoperative medicated IOP. In addition, 100% of eyes had Month 12 unmedicated IOP ≤18 mmHg and 67% had unmedicated IOP ≤15 mmHg. Three subjects were placed on medication at Months 18, 30, and 32, respectively, but all remaining subjects remained free of medications.
Figure 2.
Mean intraocular pressure through 48 months postoperative.a,b
Notes: aVertical bars represent standard deviation. bEyes with secondary surgery or addition of medication were counted as non-responders, and their subsequent IOP data were excluded from mean IOP calculation. cUnmedicated/Post-washout. A 1-month medication washout was completed preoperatively.
Abbreviations: SCR, Screening; BL, Baseline; M, Month; IOP, intraocular pressure; Preop, preoperative; Med, medication.
Figure 3.
Proportional Analysis of Medication-Free IOP and IOP Reduction at 12 and 48 Months Postoperative (n=57 for each category at both time points).a
Note: aEyes with secondary surgery or addition of medication were counted as non-responders.
Abbreviations: IOP, intraocular pressure; SCR, Screening; BL, Baseline; M, Month; Preop, preoperative; Med, medication.
Safety
All subjects underwent ab interno placement of 2 iStent inject devices in a standalone procedure, with no intraoperative adverse events reported. Postoperative adverse events occurred in 3 eyes during the 4 years of follow-up, as detailed in Table 2. Two subjects had progression of preexisting cataract with corresponding BCVA loss >1 line. Another subject had elevated IOP and BCVA loss at Month 32 and was prescribed ocular hypotensive medication; this subject returned with high IOP 1 week later and underwent trabeculectomy. All adverse effects were noted as “definitely unrelated” to study treatment.
Table 2.
Postoperative Ocular Adverse Events Through Month 48
| Intraoperative Adverse Events |
|---|
|
| Postoperative Ocular Adverse Events in 3 Eyes |
| Case 1: |
|
| Case 2: |
|
| Case 3: |
|
Visual acuity was stable over time (Month 48 BCVA of 20/25 or better in 68% of eyes, 20/40 or better in 93% of eyes, and 20/100 or better in 98% of eyes, similar to preoperative values)[Table 2]. The mean C:D ratio (0.7 ± 0.1), visual field mean deviation (−5.0 ± 6.2) and pattern standard deviation (3.1 ± 2.6), and central corneal thickness (544.7 ± 30.2) also remained stable over the 48-month follow-up with no notable change from screening [Table 3].
Table 3.
Preoperative and Month 48 Best-Corrected Visual Acuity, Cup-to-Disc Ratio, Visual Field, and Central Corneal Thickness
| Screening (n=57) |
Month 48 (n=57) |
||
|---|---|---|---|
| BCVAa | |||
| 20/25 or better | n (%) | 41 (72%) | 39 (68%) |
| 20/40 or better | n (%) | 53 (93%) | 53 (93%) |
| 20/100 or better | n (%) | 57 (100%) | 56 (98%) |
| C:D ratiob | Mean (SD) | 0.7 (0.1) | 0.7 (0.1) |
| Visual fieldb | |||
| Mean deviation (dB) | Mean (SD) | −4.9 (5.3) | −5.0 (6.2) |
| Pattern standard deviation (dB) | Mean (SD) | 3.3 (2.4) | 3.1 (2.6) |
| Central corneal thickness (µm)b | Mean (SD) | 544.9 (30.3) | 544.7 (30.2) |
Notes: aMeasured at screening and at every postoperative visit. bMeasured at screening and at every postoperative visit from 6 months onward.
Abbreviations: BCVA, best-corrected visual acuity; C:D, cup-to-disc.
Discussion
Micro-invasive trabecular bypass stent implantation aims to improve aqueous outflow through the natural physiologic pathway and consequently reduce IOP in eyes with OAG. Evidence has shown it can provide an alternative treatment modality to anti-glaucoma medication or laser procedures. In addition, although the IOP reductions with MIGS procedures are typically more modest than invasive filtering surgeries, their favorable safety profile may make them an attractive treatment option for patients with less advanced disease. This prospective long-term study demonstrated safe and durable four-year IOP and medication reductions after implantation of second-generation iStent inject stents in eyes with OAG. Since stent implantation was completed as a standalone procedure, it was possible to assess the impact of the device alone, independent from the IOP-reducing impact of cataract surgery. The study intervention also included cessation of medication postoperatively. Due to these specifications, the observed 46% reduction in medication-free IOP can be attributed to the stents alone, without the confounding factors of cataract surgery or medication. The study’s outcomes are comparable to prior studies showing durable effectiveness and safety of iStent inject implantation both as a standalone surgery and with concomitant cataract surgery.28–37 Importantly, the IOP-reducing effect of iStent inject was consistent over time in this study, with 95–100% of eyes achieving a medication-free IOP reduction of ≥20% versus preoperative unmedicated IOP at both Month 12 and Month 48. The proportion of eyes with IOP ≤ 18 mmHg also remained high at both time points (95–100%). This durable IOP reduction contrasts with the declining effects of laser trabeculoplasty or cataract surgery over time.9,38–40
Long-lasting surgical interventions have inherent benefits over medication treatments as well. The utility of medication is limited by local and systemic side effects, difficulty with instillation, and costs. Since patient adherence is widely known to be low,7,8,41,42 reduced dependence on adherence is an important characteristic of trabecular bypass stents, as it may promote long-term treatment consistency and hence disease stabilization.7,23–25 Furthermore, even if perfect medication adherence were achieved, diurnal peaks and troughs of medication activity could conceivably place additional stress on an already-compromised optic nerve.43 In this study, all but 3 eyes were able to remain medication-free for the 4 years of follow-up, whereas all eyes were on 1 medication preoperatively. These outcomes support existing literature showing substantial reductions in medication after iStent and iStent inject implantation.13–37,44–46
The iStent inject device is designed to have three general advantages over the iStent, as highlighted in a recent comparative study by Guedes et al.30 First, the iStent inject injector is pre-loaded with two stents (rather than one) in order to bypass two separate areas of the trabecular meshwork. Each stent is precisely designed to facilitate the needed flow of aqueous through the trabecular meshwork, which in a normal healthy eye averages 2.5 µL/ min. Second, each iStent inject stent includes four outlet lumens allowing for multidirectional flow, with the goal to maximize the number of clock-hours of aqueous egress. Third, iStent inject implantation is designed for greater procedural efficiency, thereby easing the learning curve for surgeons and conceivably resulting in more straightforward, uncomplicated implantation.
The IOP reductions achieved with iStent inject in this study are supported by clinical and laboratory studies evaluating aqueous humor outflow after implantation of 2 trabecular micro-bypass stents. Huang et al showed substantial aqueous angiographic outflow improvement after iStent inject implantation with concomitant cataract surgery, including the reactivation of formerly dormant outflow areas and the possibility of accessing up to 6 clock hours of collector channels for efficient aqueous humor outflow.47 Computational fluid dynamics (CFD) corroborated engineering approximations (Hagen–Poiseuille) that resistance through the stents is extremely low, even despite their micro-size design. The CFD models also confirmed that flow through the iStent and iStent inject lumens is smooth and laminar. Using CFD, Hunter et al48 determined that the flow resistance of a single iStent inject stent is only 0.057 mmHg/µL/minute. For two stents, the resistance is even less, 0.0285 mmHg/µL/minute. At the latter resistance, only 0.071 mmHg pressure would be needed to drive the entire 2.5 µL/minute of aqueous humor production through a pair of iStent inject stents.48 This pressure is clinically insignificant compared to a normal intraocular pressure of 15 mmHg.
The use of multiple versus single stents is supported by both preclinical and clinical data. In two studies using anterior segment perfusion models, Bahler et al evaluated single versus multiple trabecular micro-bypass stents (either iStent or iStent inject). In the first study, IOP reduced from 19.7 to 13.6 mmHg after one iStent insertion, and reduced further to 10.0 mmHg after the second iStent insertion (P< 0.05 for both).49 In the second study, insertion of one iStent inject into the nasal or superior quadrant of the trabecular meshwork increased outflow facility from 0.16 ± 0.05 to 0.38 ± 0.23 µL/min/mmHg, with concurrent IOP reduction from 16.7 ± 5.4 to 8.6 ± 4.4 mmHg. Addition of a second iStent inject further increased outflow facility to 0.78 ± 0.66 µL/min/mmHg (n=2).50 Using a whole eye perfusion model, Hunter et al showed that a single iStent reduced IOP by 6.0 mmHg from baseline, while a second iStent decreased IOP by an additional 2.9 mmHg, for a total IOP reduction of 8.9 mmHg from baseline. In the clinical realm, a prospective randomized trial by Katz et al26 compared one, two, and three iStents in a standalone procedure, and Belovay et al51 and El Wardani et al52 evaluated two or three iStents with concomitant cataract surgery. Together, these clinical and laboratory studies confirm that most IOP reduction comes from the first stent and that additional stent(s) produce further IOP reductions. Therefore, iStent and iStent inject therapy appears to be titratable, allowing physicians to reach lower target IOPs with the implantation of additional stents, likely through the accessing of additional regions of the distal outflow pathway.
Alongside IOP-reducing performance, the safety profile of a given treatment intervention is paramount. In this study, all subjects were successfully implanted with 2 iStent inject stents, with no intraoperative complications. Over 4 years of follow-up, adverse events occurred in only 3 eyes, and no events were device-related. Only 1 eye underwent a secondary glaucoma surgery during follow-up. Visual acuity, C:D ratio, visual fields, and central corneal thickness remained stable over the 48 months of follow-up. There were no reports of hypotony, stent obstruction, or any of the complications seen with traditional filtering surgeries (eg, endophthalmitis, choroidal detachment or hemorrhage, bleb-related complications). There also were no cases of peripheral anterior synechiae (PAS) or inflammation (eg, uveitis, iritis). This benign safety profile is not surprising given that iStent inject (as well as iStent) stents are made of biocompatible titanium and have a well-established track record of non-inflammatory usage. The stents have been the subject of over 100 peer-reviewed clinical publications to-date, and have shown an excellent safety profile, while a single ex vivo histology study has shown fibrous material in tissue samples.53 There are limitations and strengths which may be highlighted in this open-label, single-arm study. The study’s single-site design prevents consideration of site-specific effects. However, the involvement of multiple surgeons provides variability, and the consistent outcomes across surgeons validate the stent implantation technique and overall safety profile. Given that all surgeons were glaucoma specialists, the absence of intraoperative complications may not be observed by non-glaucoma-trained surgeons. As all eligible subjects needed further glaucoma treatment (in addition to their 1 preoperative medication), a sham surgical control group (consisting of merely injecting and removing viscoelastic) was not considered for ethical reasons. One of the proposed mechanisms for postsurgical IOP reduction is lavage of the outflow pathway by irrigation/aspiration itself, which is used during stent implantation as well as cataract surgery. Due to the lack of a control group, this potential confounder cannot be accounted for. However, given that this mechanism has not been conclusively established, it is still reasonable to consider patients’ preoperative data as their own control. The patient population was 100% Caucasian and all eyes were phakic, precluding stratification by race or lens status. Multiple IOP measurements were not taken on consecutive days, making regression to the mean a possibility. However, the validity of the IOP data is strengthened by the completion of diurnal IOP measurements and 2-observer masked IOP assessments. The study’s 100% accountability through 48 months also adds to the validity of the data.
Conclusions
This prospective study collected valuable long-term data in a consistent cohort with excellent accountability through 48 months. The results suggest that safe and durable IOP reduction to ≤15 mmHg is possible without medication after standalone ab interno implantation of iStent inject in eyes with mild to moderate OAG. Outcomes were consistent with previous literature on iStent inject implantation either with or without concomitant cataract surgery. The positive benefit-to-risk profile supports the consideration of iStent inject in providing a safe, long-term, effective treatment option for patients with mild to moderate glaucoma.
Acknowledgments
We thank the participants in this study. The iStent inject device is manufactured by Glaukos Corporation.
Funding Statement
Operational support for completion of this study, assistance with data analysis and manuscript drafting, and article processing fees were funded by Glaukos Corporation (San Clemente, CA, USA).
Data Sharing Statement
The study protocol and answers to specific data-related queries will be supplied by the corresponding author on reasonable request.
Author Contributions
All authors made substantial contributions to the design and conception of the study, and acquisition, analysis and interpretation of data, and took part in either drafting or revising the manuscript. All authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics Approval and Informed Consent
The trial was approved by the Armenian Ministry of Health and was performed in accordance with the Helsinki Declaration. Written informed consent was obtained from all individual participants included in the study.
Disclosure
R. Lindstrom reports personal fees, non-financial support from Glaukos Corporation, during the conduct of the study. R. Lewis is consultant for Allergan, Alcon, AVS, ELTSight, Equinox, Glaukos, Ivantis, Kedalion, SimpleContacts, ViaLase, and Zeiss; Chief Medical Officer for Aerie. He reports grants from Glaukos, during the conduct of the study; grants from Ivantis, Glaukos, ELT Sight, Alcon, New World Medical, Equinox, Aerie Pharmaceuticals, MicroOptyx, and Sanoculis, outside the submitted work. S.R. Sarkisian reports grants, lecture fees from and is consultant for Alcon Laboratories, Inc. He is consultant for and received lecture fees from Allergan. He is also consultant for Beaver-Visitec International, Inc., Katena Products, Inc., New World Medical Inc., Santen, Inc., Omeros, Glaukos Corporation, Bausch & Lomb, and Sight Sciences, Inc. He received grant from and is equity owner of Sight Sciences, Inc. He also received grants from Glaukos Corporation and Bausch & Lomb, outside the submitted work. L. Voskanyan received financial support from Glaukos for her work as an investigator in this study. J. Hovanesian is a consultant to and a stockholder of Glaukos. The authors report no other conflicts of interest in this work.
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi: 10.1016/j.ophtha.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 3.Quigley HA. Open-angle glaucoma. N Engl J Med. 1993;328(15):1097–1106. doi: 10.1056/NEJM199304153281507 [DOI] [PubMed] [Google Scholar]
- 4.Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29(4):312–334. doi: 10.1016/j.preteyeres.2010.03.001 [DOI] [PubMed] [Google Scholar]
- 5.Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17(5):350–355. doi: 10.1097/IJG.0b013e31815c5f4f [DOI] [PubMed] [Google Scholar]
- 6.Newman-Casey PA, Robin AL, Blachley T, et al. The most common barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology. 2015;122(7):1308–1316. doi: 10.1016/j.ophtha.2015.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nordstrom BL, Friedman DS, Mozaffari E, et al. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140:598–606. doi: 10.1016/j.ajo.2005.04.051 [DOI] [PubMed] [Google Scholar]
- 8.Robin AL, Novack GD, Covert DW, Crockett RS, Marcic TS. Adherence in glaucoma: objective measurements of once-daily and adjunctive medication use. Am J Ophthalmol. 2007;144(4):533–540. doi: 10.1016/j.ajo.2007.06.012 [DOI] [PubMed] [Google Scholar]
- 9.Glaucoma Laser Trial Research Group. The Glaucoma Laser Trial (GLT) and glaucoma laser trial follow-up study: 7. Results. Am J Ophthalmol. 1995;120:718–731. doi: 10.1016/S0002-9394(14)72725-4 [DOI] [PubMed] [Google Scholar]
- 10.Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC. Tube versus trabeculectomy study group. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153:804–814. doi: 10.1016/j.ajo.2011.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rulli E, Biagioli E, Riva I, et al. Efficacy and safety of trabeculectomy vs nonpenetrating surgical procedures: a systematic review and meta-analysis. JAMA Ophthalmol. 2013;131(12):1573–1582. doi: 10.1001/jamaophthalmol.2013.5059 [DOI] [PubMed] [Google Scholar]
- 12.Jampel HD, Musch DC, Gillespie BW, et al. Perioperative complications of trabeculectomy in the collaborative initial glaucoma treatment study (CIGTS). Am J Ophthalmol. 2005;140(1):16–22. doi: 10.1016/j.ajo.2005.02.013 [DOI] [PubMed] [Google Scholar]
- 13.Popovic M, Campos-Moller X, Saheb H, Ahmed IIK. Efficacy and adverse event profile of the iStent and iStent inject trabecular micro-bypass for open-angle glaucoma: a meta-analysis. J Curr Glaucoma Pract. 2018;12(2):67–84. doi: 10.5005/jp-journals-10028-1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallardo MJ, Supnet RA, Giamporcaro JE, Hornbeak DM. Outcomes of combined trabecular micro-bypass and phacoemulsification in a predominantly Hispanic patient population. Clin Ophthalmol. 2016;10:1931–1937. doi: 10.2147/OPTH [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neuhann TH. Trabecular micro-bypass stent implantation during small-incision cataract surgery for open-angle glaucoma or ocular hypertension: long-term results. J Cataract Refract Surg. 2015;41:2664–2671. doi: 10.1016/j.jcrs.2015.06.032 [DOI] [PubMed] [Google Scholar]
- 16.Craven ER, Katz LJ, Wells JM, Giamporcaro JE. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012;38:1339–1345. doi: 10.1016/j.jcrs.2012.03.025 [DOI] [PubMed] [Google Scholar]
- 17.Fechtner RD, Voskanyan L, Vold SD, et al. Five-year, prospective, randomized, multi-surgeon trial of two trabecular bypass stents versus prostaglandin for newly-diagnosed open-angle glaucoma. Ophthalmol Glaucoma. 2019;2(3):156–166. doi: 10.1016/j.ogla.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 18.Chang DF, Donnenfeld ED, Katz LJ, et al. Efficacy of two trabecular micro-bypass stents combined with topical travoprost in open-angle glaucoma not controlled on two preoperative medications: 3-year follow-up. Clin Ophthalmol. 2017;11:523–528. doi: 10.2147/OPTH.S121041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donnenfeld ED, Solomon KD, Voskanyan L, et al. A prospective 3-year follow-up trial of implantation of two trabecular microbypass stents in open-angle glaucoma. Clin Ophthalmol. 2015;9:2057–2065. doi: 10.2147/OPTH.S91732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arriola-Villalobos P, Martinez-de-la-Casa J, Diaz-Valle D, Fernández-Pérez C, García-Sánchez J, García-Feijoó J. Combined iStent trabecular micro-bypass stent implantation and phacoemulsification for coexistent open-angle glaucoma and cataract: a long-term study. Br J Ophthalmol. 2012;96:645–649. doi: 10.1136/bjophthalmol-2011-300218 [DOI] [PubMed] [Google Scholar]
- 21.Fea AM, Consolandi G, Zola M, et al. Micro-bypass implantation for primary open-angle glaucoma combined with phacoemulsification: 4-year follow-up. J Ophthalmology. 2015;2015:795357. doi: 10.1155/2015/795357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferguson TJ, Ibach M, Schweitzer J, et al. Trabecular microbypass stent implantation in pseudophakic eyes with open-angle glaucoma: long-term results. J Cataract Refract Surg. 2019;45(4):414–420. doi: 10.1016/j.jcrs.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 23.Ferguson TJ, Swan R, Ibach M, et al. Trabecular microbypass stent implantation with cataract extraction in pseudoexfoliation glaucoma. J Cataract Refract Surg. 2017;43(5):622–626. doi: 10.1016/j.jcrs.2017.02.029 [DOI] [PubMed] [Google Scholar]
- 24.Ferguson TJ, Berdahl JP, Schweitzer JA, Sudhagoni RG. Clinical evaluation of a trabecular micro-bypass stent with phacoemulsification in patients with open-angle glaucoma and cataract. Clin Ophthalmol. 2016;10:1767–1773. doi: 10.2147/OPTH [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferguson T, Swan R, Ibach M, et al. Evaluation of a trabecular microbypass stent with cataract extraction in severe primary open-angle glaucoma. J Glaucoma. 2018;27(1):71–76. doi: 10.1097/IJG.0000000000000825 [DOI] [PubMed] [Google Scholar]
- 26.Katz LJ, Erb C, Carceller Guillamet A, et al. Long-term titrated IOP control with one, two, or three trabecular micro-bypass stents in open-angle glaucoma subjects on topical hypotensive medication: 42-month outcomes. Clin Ophthalmol. 2018;12:255–262. doi: 10.2147/OPTH [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samuelson TW, Katz LJ, Wells JM, Duh YJ, Giamporcaro JE. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118:459–467. doi: 10.1016/j.ophtha.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 28.Samuelson TW, Sarkisian SR Jr., Lubeck DM, et al. for the iStent inject study group, prospective, randomized, controlled pivotal trial of iStent inject trabecular micro-bypass in primary open-angle glaucoma and cataract: two-year results. Ophthalmology. 2019;126(6):811–821. doi: 10.1016/j.ophtha.2019.03.006 [DOI] [PubMed] [Google Scholar]
- 29.Clement CI, Howes F, Ioannidis AS, Shiu M, Manning D. One-year outcomes following implantation of second-generation trabecular micro-bypass stents in conjunction with cataract surgery for various types of glaucoma or ocular hypertension: multicenter, multi-surgeon study. Clin Ophthalmol. 2019;13:491–499. doi: 10.2147/OPTH.S187272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guedes RAP, Gravina DM, Lake JC, Guedes VMP, Chaoubah A. Intermediate results of iStent or iStent inject implantation combined with cataract surgery in a real-world setting: a longitudinal retrospective study. Ophthalmol Ther. 2019;8(1):87–100. doi: 10.1007/s40123-019-0166-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hengerer FH, Auffarth GU, Riffel C, Conrad-Hengerer I. Second-generation trabecular micro-bypass stents as standalone treatment for glaucoma: a 36-month prospective study. Adv Ther. 2019;36(7):1606–1617. doi: 10.1007/s12325-019-00984-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hengerer FH, Auffarth GU, Riffel C, Conrad-Hengerer I. Prospective, non-randomized, 36-month study of second-generation trabecular micro-bypass stents with phacoemulsification in various types of glaucoma. Ophthalmol Ther. 2018;7(2):405–415. doi: 10.1007/s40123-018-0152-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harasymowycz P Single surgeon evaluation of second-generation trabecular micro-bypass stents in patients with mild to severe glaucoma. Platform presentation at: Annual meeting of the American Society of Cataract and Refractive Surgeons; 13–17April 2018; 2018; Washington, DC. [Google Scholar]
- 34.Voskanyan L, Garcia-Feijoo J, Belda J, Fea A, Jünemann A, Baudouin C, Synergy Study Group. Prospective, unmasked evaluation of the iStent inject system for open-angle glaucoma: synergy trial. Adv Ther. 2014;31(2):189–201. doi: 10.1007/s12325-014-0095-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fea AM, Belda JI, Rekas M, et al. Prospective unmasked randomized evaluation of the iStent inject ((R)) versus two ocular hypotensive agents in patients with primary open-angle glaucoma. Clin Ophthalmol. 2014;8:875–882. doi: 10.2147/OPTH.S59932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindstrom R, Lewis R, Hornbeak DM, et al. Outcomes following implantation of two second-generation trabecular micro-bypass stents in patients with open-angle glaucoma on one medication: 18-month follow-up. Adv Ther. 2016;33(11):2082–2090. doi: 10.1007/s12325-016-0420-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berdahl J, Voskanyan L, Myers JS, et al. Implantation of two second-generation trabecular micro-bypass stents and topical travoprost in open-angle glaucoma not controlled on two preoperative medications: 18-month follow-up. Clin Exp Ophthalmol. 2017;45(8):797–802. doi: 10.1111/ceo.2017.45.issue-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juzych MS, Chopra V, Banitt MR, et al. Comparison of long-term outcomes of selective laser trabeculoplasty versus argon laser trabeculoplasty in open-angle glaucoma. Ophthalmology. 2004;111(10):1853–1859. doi: 10.1016/j.ophtha.2004.04.030 [DOI] [PubMed] [Google Scholar]
- 39.Mansberger SL, Gordon MO, Jampel H, et al. Reduction in intraocular pressure after cataract extraction: the ocular hypertension treatment study. Ophthalmology. 2012;119:1826–1831. doi: 10.1016/j.ophtha.2012.02.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedman DS, Jampel HD, Lubomski LH, et al. Surgical strategies for coexisting glaucoma and cataract: an evidence-based update. Ophthalmology. 2002;109:1902–1913. doi: 10.1016/S0161-6420(02)01267-8 [DOI] [PubMed] [Google Scholar]
- 41.Friedman DS, Quigley HA, Gelb L, et al. Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the Glaucoma Adherence and Persistency Study (GAPS). Invest Ophthalmol Vis Sci. 2007;48(11):5052–5057. doi: 10.1167/iovs.07-0290 [DOI] [PubMed] [Google Scholar]
- 42.Tsai JC. A comprehensive perspective on patient adherence to topical glaucoma therapy. Ophthalmology. 2009;116:S30–36. doi: 10.1016/j.ophtha.2009.06.024 [DOI] [PubMed] [Google Scholar]
- 43.Nouri-Mahdavi K, Medeiros FA, Weinreb RN. Fluctuation of intraocular pressure as a predictor of visual field progression. Arch Ophthalmol. 2008;126:1168–1169. author reply 1169-1170. doi: 10.1001/archopht.126.8.1168 [DOI] [PubMed] [Google Scholar]
- 44.Macher T, Häberle H, Wächter J, Thannhäuser C, Aurich H, Pham DT. Trabecular microbypass stents as minimally invasive approach after conventional glaucoma filtration surgery. J Cataract Refract Surg. 2018;44(1):50–55. doi: 10.1016/j.jcrs.2017.10.039 [DOI] [PubMed] [Google Scholar]
- 45.Pillunat LE, Erb C, Jünemann AG, Kimmich F. Micro-invasive glaucoma surgery (MIGS): a review of surgical procedures using stents. Clin Ophthalmol. 2017;11:1583–1600. doi: 10.2147/OPTH [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ansari E. An update on implants for minimally invasive glaucoma surgery (MIGS). Ophthalmol Ther. 2017;6(2):233–241. doi: 10.1007/s40123-017-0098-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang AS, Penteado RC, Papoyan V, Voskanyan L, Weinreb RN. Aqueous angiographic outflow improvement after trabecular micro-bypass in glaucoma patients. Ophthalmol Glaucoma. 2019;2:11–21. doi: 10.1016/j.ogla.2018.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hunter K, Fjield T, Heitzmann H, Shandas R, Kahook M. Characterization of micro- invasive trabecular bypass stents by ex vivo perfusion and computational flow modeling. Clin Ophthalmol. 2014;8:499–506. doi: 10.2147/OPTH.S56245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bahler C, Smedley G, Zhou J, Johnson D. Trabecular bypass stents decrease intraocular pressure in cultured human anterior segments. Am J Ophthal. 2004;138:988–994. doi: 10.1016/j.ajo.2004.07.035 [DOI] [PubMed] [Google Scholar]
- 50.Bahler C, Hann C, Fjield T, Haffner D, Heitzmann H, Fautsch MP. Second-generation trabecular meshwork bypass stent (iStent inject) increases outflow facility in cultured human anterior segments. Am J Ophthal. 2012;153:1206–1213. doi: 10.1016/j.ajo.2011.12.017 [DOI] [PubMed] [Google Scholar]
- 51.Belovay GW, Naqi A, Chan BJ, Rateb M, Ahmed II. Using multiple trabecular micro- bypass stents in cataract patients to treat open-angle glaucoma. J Cataract Refract Surg. 2012;38(11):1911–1917. doi: 10.1016/j.jcrs.2012.07.017 [DOI] [PubMed] [Google Scholar]
- 52.El Wardani M, Bergin C, Achache F, Sharkawi E. Evaluating the trabecular micro-bypass stent combined with phacoemulsification compared to phacoemulsification alone. Klin Monatsbl Augenheilkd. 2015;232:442–445. doi: 10.1055/s-00000031 [DOI] [PubMed] [Google Scholar]
- 53.Capitena Young CE, Ammar DA, Seibold LK, Pantcheva MB, SooHoo JR, Kahook MY. Histopathologic examination of trabecular meshwork changes after trabecular bypass stent implantation. J Glaucoma. 2018;27(7):606–609. doi: 10.1097/IJG.0000000000000968 [DOI] [PubMed] [Google Scholar]